Abstract
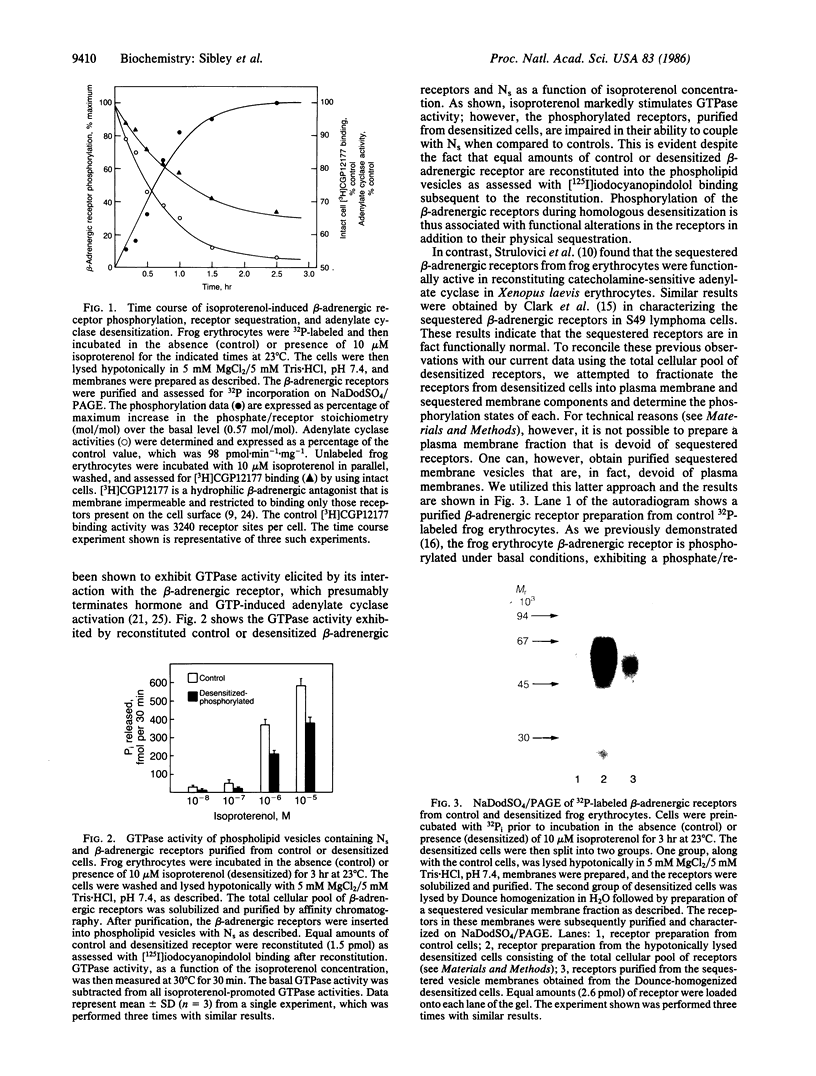
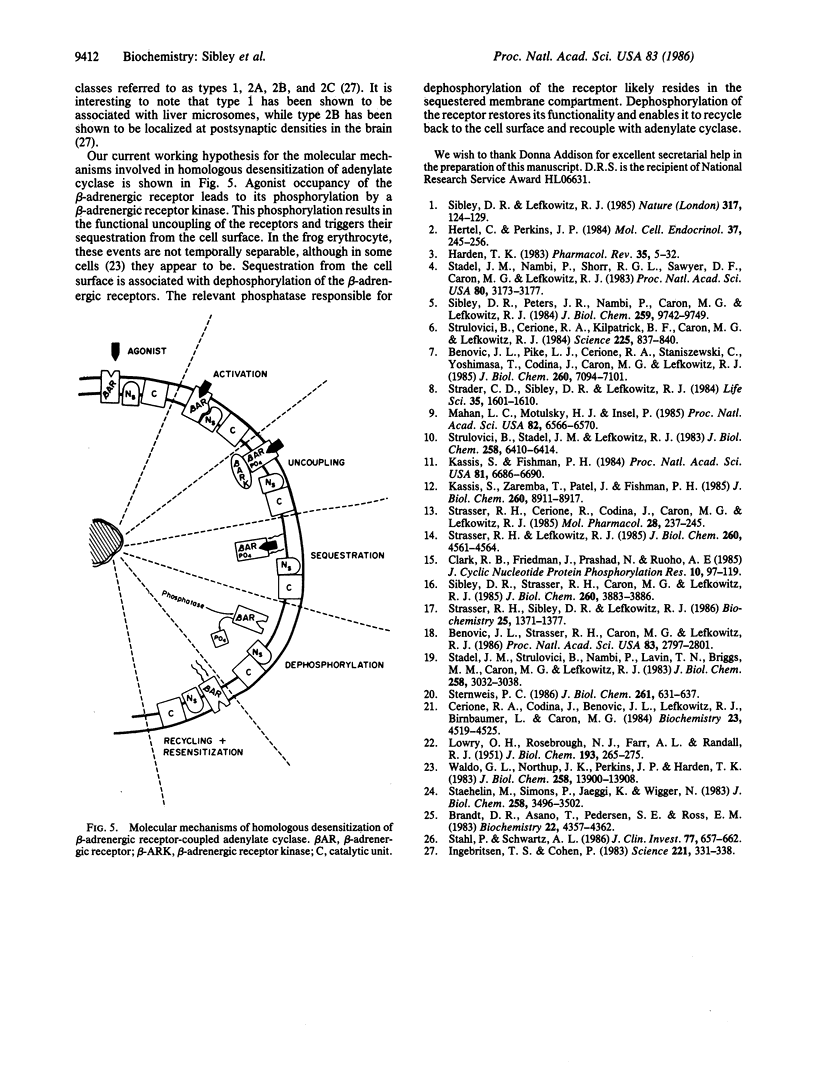
Prolonged exposure of cells or tissues to drugs or hormones such as catecholamines leads to a state of refractoriness to further stimulation by that agent, known as homologous desensitization. In the case of the beta-adrenergic receptor coupled to adenylate cyclase, this process has been shown to be intimately associated with the sequestration of the receptors from the cell surface through a cAMP-independent process. Recently, we have shown that homologous desensitization in the frog erythrocyte model system is also associated with increased phosphorylation of the beta-adrenergic receptor. We now provide evidence that the phosphorylation state of the beta-adrenergic receptor regulates its functional coupling to adenylate cyclase, subcellular translocation, and recycling to the cell surface during the process of agonist-induced homologous desensitization. Moreover, we show that the receptor phosphorylation is reversed by a phosphatase specifically associated with the sequestered subcellular compartment. At 23 degrees C, the time courses of beta-adrenergic receptor phosphorylation, sequestration, and adenylate cyclase desensitization are identical, occurring without a lag, exhibiting a t1/2 of 30 min, and reaching a maximum at approximately 3 hr. Upon cell lysis, the sequestered beta-adrenergic receptors can be partially recovered in a light membrane vesicle fraction that is separable from the plasma membranes by differential centrifugation. The increased beta-adrenergic receptor phosphorylation is apparently reversed in the sequestered vesicle fraction as the sequestered receptors exhibit a phosphate/receptor stoichiometry that is similar to that observed under basal conditions. High levels of a beta-adrenergic receptor phosphatase activity appear to be associated with the sequestered vesicle membranes. The functional activity of the phosphorylated beta-adrenergic receptor was examined by reconstituting purified receptor with its biochemical effector the guanine nucleotide regulatory protein (Ns) in phospholipid vesicles and assessing the receptor-stimulated GTPase activity of Ns. Compared to controls, phosphorylated beta-adrenergic receptors, purified from desensitized cells, were less efficacious in activating the Ns GTPase activity. These results suggest that phosphorylation of the beta-adrenergic receptor leads to its functional uncoupling and physical translocation away from the cell surface into a sequestered membrane domain. In the sequestered compartment, the phosphorylation is reversed thus enabling the receptor to recycle back to the cell surface and recouple with adenylate cyclase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benovic J. L., Pike L. J., Cerione R. A., Staniszewski C., Yoshimasa T., Codina J., Caron M. G., Lefkowitz R. J. Phosphorylation of the mammalian beta-adrenergic receptor by cyclic AMP-dependent protein kinase. Regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. J Biol Chem. 1985 Jun 10;260(11):7094–7101. [PubMed] [Google Scholar]
- Benovic J. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt D. R., Asano T., Pedersen S. E., Ross E. M. Reconstitution of catecholamine-stimulated guanosinetriphosphatase activity. Biochemistry. 1983 Sep 13;22(19):4357–4362. doi: 10.1021/bi00288a002. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Codina J., Benovic J. L., Lefkowitz R. J., Birnbaumer L., Caron M. G. The mammalian beta 2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry. 1984 Sep 25;23(20):4519–4525. doi: 10.1021/bi00315a003. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Friedman J., Prashad N., Ruoho A. E. Epinephrine-induced sequestration of the beta-adrenergic receptor in cultured S49 WT and cyc- lymphoma cells. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(1):97–119. [PubMed] [Google Scholar]
- Harden T. K. Agonist-induced desensitization of the beta-adrenergic receptor-linked adenylate cyclase. Pharmacol Rev. 1983 Mar;35(1):5–32. [PubMed] [Google Scholar]
- Hertel C., Perkins J. P. Receptor-specific mechanisms of desensitization of beta-adrenergic receptor function. Mol Cell Endocrinol. 1984 Oct;37(3):245–256. doi: 10.1016/0303-7207(84)90094-7. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. Protein phosphatases: properties and role in cellular regulation. Science. 1983 Jul 22;221(4608):331–338. doi: 10.1126/science.6306765. [DOI] [PubMed] [Google Scholar]
- Kassis S., Fishman P. H. Functional alteration of the beta-adrenergic receptor during desensitization of mammalian adenylate cyclase by beta-agonists. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6686–6690. doi: 10.1073/pnas.81.21.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis S., Zaremba T., Patel J., Fishman P. H. Phorbol esters and beta-adrenergic agonists mediate desensitization of adenylate cyclase in rat glioma C6 cells by distinct mechanisms. J Biol Chem. 1985 Jul 25;260(15):8911–8917. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahan L. C., Motulsky H. J., Insel P. A. Do agonists promote rapid internalization of beta-adrenergic receptors? Proc Natl Acad Sci U S A. 1985 Oct;82(19):6566–6570. doi: 10.1073/pnas.82.19.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Peters J. R., Nambi P., Caron M. G., Lefkowitz R. J. Desensitization of turkey erythrocyte adenylate cyclase. Beta-adrenergic receptor phosphorylation is correlated with attenuation of adenylate cyclase activity. J Biol Chem. 1984 Aug 10;259(15):9742–9749. [PubMed] [Google Scholar]
- Sibley D. R., Strasser R. H., Caron M. G., Lefkowitz R. J. Homologous desensitization of adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. J Biol Chem. 1985 Apr 10;260(7):3883–3886. [PubMed] [Google Scholar]
- Stadel J. M., Nambi P., Shorr R. G., Sawyer D. F., Caron M. G., Lefkowitz R. J. Catecholamine-induced desensitization of turkey erythrocyte adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3173–3177. doi: 10.1073/pnas.80.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadel J. M., Strulovici B., Nambi P., Lavin T. N., Briggs M. M., Caron M. G., Lefkowitz R. J. Desensitization of the beta-adrenergic receptor of frog erythrocytes. Recovery and characterization of the down-regulated receptors in sequestered vesicles. J Biol Chem. 1983 Mar 10;258(5):3032–3038. [PubMed] [Google Scholar]
- Staehelin M., Simons P., Jaeggi K., Wigger N. CGP-12177. A hydrophilic beta-adrenergic receptor radioligand reveals high affinity binding of agonists to intact cells. J Biol Chem. 1983 Mar 25;258(6):3496–3502. [PubMed] [Google Scholar]
- Stahl P., Schwartz A. L. Receptor-mediated endocytosis. J Clin Invest. 1986 Mar;77(3):657–662. doi: 10.1172/JCI112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C. The purified alpha subunits of Go and Gi from bovine brain require beta gamma for association with phospholipid vesicles. J Biol Chem. 1986 Jan 15;261(2):631–637. [PubMed] [Google Scholar]
- Strader C. D., Sibley D. R., Lefkowitz R. J. Association of sequestered beta-adrenergic receptors with the plasma membrane: a novel mechanism for receptor down regulation. Life Sci. 1984 Oct 8;35(15):1601–1610. doi: 10.1016/0024-3205(84)90359-x. [DOI] [PubMed] [Google Scholar]
- Strasser R. H., Cerione R. A., Codina J., Caron M. G., Lefkowitz R. J. Homologous desensitization of the beta-adrenergic receptor. Functional integrity of the desensitized receptor from mammalian lung. Mol Pharmacol. 1985 Sep;28(3):237–245. [PubMed] [Google Scholar]
- Strasser R. H., Lefkowitz R. J. Homologous desensitization of beta-adrenergic receptor coupled adenylate cyclase. Resensitization by polyethylene glycol treatment. J Biol Chem. 1985 Apr 25;260(8):4561–4564. [PubMed] [Google Scholar]
- Strasser R. H., Sibley D. R., Lefkowitz R. J. A novel catecholamine-activated adenosine cyclic 3',5'-phosphate independent pathway for beta-adrenergic receptor phosphorylation in wild-type and mutant S49 lymphoma cells: mechanism of homologous desensitization of adenylate cyclase. Biochemistry. 1986 Mar 25;25(6):1371–1377. doi: 10.1021/bi00354a027. [DOI] [PubMed] [Google Scholar]
- Strulovici B., Cerione R. A., Kilpatrick B. F., Caron M. G., Lefkowitz R. J. Direct demonstration of impaired functionality of a purified desensitized beta-adrenergic receptor in a reconstituted system. Science. 1984 Aug 24;225(4664):837–840. doi: 10.1126/science.6089331. [DOI] [PubMed] [Google Scholar]
- Strulovici B., Stadel J. M., Lefkowitz R. J. Functional integrity of desensitized beta-adrenergic receptors. J Biol Chem. 1983 May 25;258(10):6410–6414. [PubMed] [Google Scholar]
- Waldo G. L., Northup J. K., Perkins J. P., Harden T. K. Characterization of an altered membrane form of the beta-adrenergic receptor produced during agonist-induced desensitization. J Biol Chem. 1983 Nov 25;258(22):13900–13908. [PubMed] [Google Scholar]