Abstract
Background
Antibiotic resistance is an increasing problem, particularly in countries where antibiotic use is frequently not controlled. The aim of this study was to analyse the prevalence of the molecular mechanisms of quinolone-resistance in E. coli isolated from faeces of healthy Peruvian children or those presenting diarrhoea.
Methods
The presence of target mutations, transferable quinolone-resistance mechanisms and the role of Phe-Arg-β-Naphtylamyde inhibitible efflux pumps were studied in 96 Escherichia coli (46 diarrheogenic and 50 non-diarrheogenic) isolates exhibiting resistance or diminished susceptibility to quinolones.
Results
The most resistant phenotype, NalR and CipR, was most frequently present in isolates of healthy children. The distribution of quinolone resistance mechanisms between diarrheogenic (DEC) and commensal (non DEC) isolates was equitable, although the aac(6′)Ib-cr gene was mainly detected in DEC isolates: 17 (34%) vs non DEC isolates nine (20%). QnrB was present in five (10%) DEC vs three (6%) non DEC isolates.
Conclusions
Point mutations in gyrA and parC genes play a relevant role in quinolone resistance acquisition and highlight the role of efflux pumps also. This study provides knowledge about the molecular mechanisms involved in quinolone resistance in isolates in a non exposed population under high community antibiotic pressure.
Keywords: Antibiotic resistance, Children, Commensal, Developing countries, Diarrheogenic Escherichia coli, Quinolones
Introduction
Although antibiotic resistance is an increasing problem in both developed and developing countries,1 economical constrictions in the latter result both in empirical use of antimicrobial agents that are usually older, cheaper and sometimes unnecessary, and a lack of antibiotic alternatives resulting in high levels of antimicrobial resistance exhibited by microorganisms against the most common antibacterial agents.1 In some middle-income countries the situation has a series of particularities that lead to a unique scenario: antibiotic use is frequently not controlled either at human or animal levels and the antibiotics in use include not only those which are cheaper but also those more expensive. The social pressure on physicians is relatively high to obtain antibiotic prescriptions, and diagnostic resources are not always available.2–4 All of the above may result in the selection and development of antibiotic resistance not only to antibiotics such as ampicillin or cotrimoxazole, but also to the most modern fluoroquinolones or cephalosporins.5–7
Quinolone resistance has traditionally been related to chromosomal mutations in drug target genes, gyrA and gyrB (encoding DNA Gyrase), parC and parE (encoding Topoisomerase IV), and overexpression of efflux pumps or decreased expression of outer membrane porins.8 Of these, amino acid substitutions at GyrA and ParC are by far the most important mechanisms of quinolone resistance in clinical isolates.8
Although transferable mechanisms of quinolone resistance (TMQR) only confer a moderate reduction of quinolone susceptibility, they are increasingly being identified worldwide, mainly in Gram-negative microorganisms, especially among Enterobacteriaceae. Nonetheless, TMQR have also recently been detected in Gram-positive microorganisms.9 Thus, among Gram-negative bacteria, a series of TMQR has been described, including different Qnr protein families (QnrA, QnrB, QnrC, QnrD, QnrS and QnrVC) that protect antibiotic targets, ranking among the most prevalently described. Other TMQR such as the aac-(6′)-Ib-cr gene, which encodes an aminoglycoside acetyltransferase variant that inactivates some specific quinolones such as ciprofloxacin (CIP) or norfloxacin, and the qepA and oqxA and oqxB genes, encoding efflux systems, have been reported.10
Although the possibility of acquisition by transformation may not be ruled out, as described in some Streptococci,9 the presence of target alterations is of clinical interest when selected in pathogenic microorganisms. However, the prevalence of TMQR among non-pathogenic microorganisms is of special concern, because these mechanisms may be potentially transferred towards pathogenic microorganisms, and then, the non-pathogenic microorganisms act as antibiotic-resistant determinant reservoirs.
Given the observed impact on health, antibiotic resistance and its mechanisms should be closely followed to identify their prevalence, bacterial reservoirs and evaluate future dissemination of antibiotic resistance mechanisms. Thus, previous studies have shown a high presence of quinolone resistance in microorganisms isolated in faeces from healthy adult Peruvian volunteers.11 Moreover, despite their lack of use in young children, previous studies on healthy and diarrhoeic children under 1 year of age have shown a prevalence of 28% and 32% of nalidixic acid (NAL) resistance in diarrheogenic and commensal Escherichia coli strains, respectively.7,12
The aim of this study was, therefore, to analyse the molecular mechanisms of quinolone resistance in commensal and diarrheogenic E. coli isolated from children of less than 1 year of age.
Material and methods
Microorganisms
The E. coli strains were obtained from a previous prospective cohort study, conducted in 1034 children less than 12 months of age in a low socioeconomic community in Lima, Perú.13 This study was reviewed and approved by the Ethical Review Board of the Instituto de Investigación Nutricional and by the Ethics Committee of the Universidad Peruana Cayetano Heredia.
A total of 96 E. coli isolates exhibiting resistance or diminished susceptibility to quinolones were analysed; of these, 46 were commensal (non DEC) and 50 were diarrheogenic E. coli (DEC): nine enteropathogenic E. coli (EPEC), 10 diffusely adherent E. coli (DAEC), 29 enteroaggregative E. coli (EAEC) and two enterotoxigenic E. coli (ETEC).
Antimicrobial susceptibility
Antimicrobial susceptibility to NAL and CIP was established by the disc diffusion method in accordance with Clinical and Laboratory Standards Institute guidelines14 using the E. coli strain ATCC 25922 as quality control. The minimal inhibitory concentration (MIC) to NAL and CIP was established by agar dilution methodology as previously described.14
Effect of efflux pump inhibitor
To determine the effect of efflux pumps, the efflux pump inhibitor Phe-Arg-β-Napthylamide (PAβN) was used. Thus, the MICs of both quinolones were also established in the presence of PAβN (20 mg/L) as previously described.15
It was considered that the efflux pumps inhibitor have an effect when the MIC decreased ≥two fold.
Presence of target mutations
The Quinolone-Resistance Determining Regions (QRDR) of the gyrA and parC genes were determined by PCR (Table 1).16 Amplified PCR products were purified using a commercial kit (Wizard SV gel and PCR clean up system, Promega, Madison, WI, USA) and posterior sequencing was performed at Macrogen Service (Macrogen, Seoul, Korea).
Table 1.
Quinolone resistance primers used in this study
| Primer | Sequence | Size | Reference |
|---|---|---|---|
| gyrA-up | 5′ AAATCTGCCCGTGTCGTTGGT 3′ | 343pb | 16 |
| gyrA-low | 5′ GCCATACCTACGGCGATACC 3′ | ||
| parC-up | 5′ AAACCTGTTCAGCGCCGCATT 3′ | 395pb | 16 |
| parC-low | 5′ GTGGTGCCGTTAAGCAAA 3′ | ||
| aac(6′)Ib-cr-up | 5′ TTGCGATGCTCTATGAGTGGCTA 3′ | 482pb | 17 |
| aac(6′)Ib-cr-low | 5′ CTCGAATGCCTGGCGTGTTT 3′ | ||
| qnrA-up | 5′ AGAGGATTTCTCACGCCAGG 3′ | 580pb | 18 |
| qnrA-low | 5′ TGCCAGGCACAGATCTTGAC 3′ | ||
| qnrB-up | 5′ GGMATHGAAATTCGCCACTG 3′ | 264pb | 18 |
| qnrB-low | 5′ TTTGCYGYYCGCCAGTCGAA 3′ | ||
| qnrS-up | 5′ GCAAGTTCATTGAACAGGGT 3′ | 428pb | 18 |
| qnrS-low | 5′ TCTAAACCGTCGAGTTCGGCG 3′ | ||
| qepA-F | 5′ CGTGTTGCTGGAGTTCTTC 3′ | 403pb | 19 |
| qepA-R | 5′ CTGCAGGTACTGCGTCATG 3′ | ||
| oqxA-F | 5′ AACCTCGTCTCCCGTGAAGAG 3′ | 512pb | 20 |
| oqxA-R | 5′ TGAACGCTCTCCACCGCTTCA 3′ | ||
| oqxB-F | 5′ TTC TCC CCC GGC GGG AAG TAC3′ | 392pb | 20 |
| oqxB-R | 5′ CTC GGC CAT TTT GGC GCG TA3′ |
Analysis of transferable mechanisms of quinolone resistance
The aac(6′)-Ib gene was amplified as previously described.17 Positive PCR products were digested with BtsCI (New England Biolabs, Beijing, China) to identify the aac-(6′)-Ib-cr variant.17 The qnrA, qnrB, qnrS, qepA and oqxAB genes were also detected by conventional PCR, using the primers listed in Table 1.16–20
Phylogenetic characterisation and clonality
The E. coli phylogenetic group (A, B1, B2, D) was determined using the three-locus PCR-based method described by Clermont et al.21 Repetitive extragenic palindromic-PCR (REP-PCR) was performed to determine clonal relationships as described elsewhere.22
Statistical analysis
Proportions were compared using the χ2 test or Fisher's exact test as appropriate, using Graphpad package (www.graphpad.com); p values <0.05 were considered significant.
Results
A total of 96 strains, in which the disc diffusion test showed the presence of diminished susceptibility or resistance to any of the quinolones, were analysed (Table 2).
Table 2.
Quinolone resistance patterns associated with presence of transferable mechanisms of quinolone resistance, mutations in targets and MIC in presence or absence of eflux pump inhibitor
| Pathotype | Na | Phenotype (Nal-Cip)b | Qnr | ACC(6′)Ib-cr | (gyrA-83,87) (parC-80,84) | MIC NAL | MIC NAL+PAβN | PAβN decrease | MIC CIP | MIC CIP+PAβN |
|---|---|---|---|---|---|---|---|---|---|---|
| DAEC | 2 | R-S | − | + | (Leu,wt)(wt,wt) | 256 | 16 | 4 | 0.25 | 0.25 |
| 1 | R-S | − | + | (Leu,wt)(wt,wt) | 256 | 32 | 3 | 0.25 | 0.25 | |
| 1 | R-S | − | + | wt | 256 | 32 | 3 | 0.5 | 0.25 | |
| 1 | R-S | − | − | (Leu,wt)(wt,wt) | 256 | 128 | 1 | 0.5 | 1 | |
| 1 | R-S | − | − | (Leu,wt)(wt,wt) | >256 | 64 | >3 | 0.25 | 0.25 | |
| 1 | R-R | − | − | (Leu,wt)(wt,wt) | >256 | 128 | >2 | 64 | 64 | |
| 1 | R-R | − | − | (Leu, Asn)(Ile,Val) | >256 | 128 | >2 | 128 | 64 | |
| 2 | R-R | − | − | (Leu,wt)(wt,wt) | >256 | 256 | >1 | 64 | 64 | |
| EAEC | 1 | I-S | − | + | Wt | 32 | 4 | 3 | 0.25 | 0.25 |
| 1 | R-S | QnrB | − | Wt | 32 | 4 | 3 | 0.5 | 0.5 | |
| 2 | I-S | QnrB | − | Wt | 32 | 8 | 2 | 0.25 | 0.25 | |
| 1 | R-S | − | + | Wt | 32 | 8 | 2 | 0.25 | 0.25 | |
| 1 | R-S | − | − | (Leu,wt)(wt,wt) | 64 | 8 | 3 | 0.25 | 0.25 | |
| 1 | R-S | − | − | (Leu,wt)(wt,wt) | 64 | 16 | 2 | 0.125 | 0.25 | |
| 4 | R-S | − | − | (Leu,wt)(wt,wt) | 128 | 8 | 4 | 0.125 | 0.25 | |
| 1 | R-S | QnrB | − | (Leu,wt)(wt,wt) | 128 | 8 | 4 | 0.25 | 0.25 | |
| 3 | R-S | − | + | (Leu,wt)(wt,wt) | 128 | 16 | 3 | 0.25 | 0.25 | |
| 2 | R-S | − | − | (Leu,wt)(wt,wt) | 128 | 16 | 3 | 1 | 1 | |
| 3 | R-S | − | − | (Leu,wt)(wt,wt) | 128 | 32 | 2 | 0.25 | 0.25 | |
| 2 | R-S | − | + | (Leu,wt)(wt,wt) | 128 | 32 | 2 | 0.25 | 0.25 | |
| 1 | R-S | − | + | Wt | 128 | 64 | 1 | 0.25 | 0.25 | |
| 2 | R-S | − | + | (Leu,wt)(wt,wt) | 256 | 32 | 3 | 0.25 | 0.25 | |
| 2 | R-S | − | − | (Leu,wt)(wt,wt) | 256 | 32 | 3 | 0.5 | 0.25 | |
| 2 | R-S | − | − | (Leu,wt)(wt,wt) | >256 | 128 | >2 | 0.5 | 1 | |
| ETEC | 1 | I-S | QnrB | + | Wt | 16 | 4 | 2 | 0.25 | 0.5 |
| 1 | R-S | − | − | Wt | 256 | 32 | 3 | 0.5 | 0.25 | |
| 1 | I-S | − | − | (Ala,wt)(wt,wt) | 8 | 0.5 | 4 | 0.03 | 0.125 | |
| EPEC | 1 | I-S | − | − | Wt | 64 | 4 | 4 | 1 | 1 |
| 1 | R-S | − | + | Wt | 64 | 16 | 2 | 0.25 | 0.25 | |
| 2 | R-S | − | − | Wt | 64 | 32 | 1 | 0.25 | 0.5 | |
| 1 | R-S | − | + | (Leu,wt)(wt,wt) | 128 | 16 | 3 | 0.5 | 0.5 | |
| 2 | R-S | − | − | (Leu,wt)(wt,wt) | 128 | 32 | 2 | 0.25 | 0.5 | |
| 1 | R-S | − | − | (Leu,wt)(wt,wt) | >256 | 32 | >3 | 0.25 | 0.5 | |
| non DEC | 1 | I-S | QnrB | − | Wt | 32 | 16 | 1 | 0.125 | 0.125 |
| 1 | R-I | QnrB | − | (Leu,wt)(Ile,wt) | >256 | 256 | >1 | 8 | 16 | |
| 1 | R-S | − | − | (Leu,wt)(wt,wt) | 128 | 16 | 3 | 0.5 | 0.5 | |
| 1 | R-S | − | + | (wt,Tyr)(wt,wt) | 128 | 16 | 3 | 0.5 | 0.5 | |
| 4 | R-S | − | − | (Leu,wt)(wt,wt) | 128 | 32 | 2 | 0.25 | 0.25 | |
| 1 | R-S | − | − | (Leu,Asp)(wt,wt) | 256 | 32 | 3 | 0.25 | 0.25 | |
| 6 | R-S | − | + | (Leu,wt)(wt,wt) | 256 | 64 | 2 | 1 | 1 | |
| 1 | R-S | − | − | (Leu,wt)(wt,wt) | 256 | 64 | 2 | 0.25 | 0.25 | |
| 1 | R-S | − | + | (Leu,wt)(Ile,wt) | 256 | 64 | 2 | 1 | 1 | |
| 2 | R-S | − | − | (Leu,wt)(wt,wt) | 256 | 128 | 1 | 1 | 1 | |
| 1 | R-S | − | − | (Val, wt)(wt,wt) | 256 | 128 | 1 | 0.5 | 0.5 | |
| 1 | R-S | QnrB | + | (Leu,wt)(wt,wt) | 256 | 128 | 1 | 0.5 | 0.5 | |
| 5 | R-S | − | − | (Leu,wt)(Ile,wt) | >256 | 256 | >1 | 1 | 1 | |
| 1 | R-R | − | − | (Leu,Asn)(Arg,wt) | >256 | 128 | >2 | 64 | 32 | |
| 1 | R-R | − | − | (Leu,Asn)(Tyr,wt) | >256 | 256 | >1 | 8 | 16 | |
| 1 | R-R | − | − | (Leu,Asn)(Ile,wt) | >256 | ≥256 | 1 | 8 | 8 | |
| 1 | R-R | − | − | (Leu,Asn)(Ile,Gly) | >256 | 256 | >1 | 32 | 32 | |
| 4 | R-R | − | − | (Leu,Asn)(Ile,Val) | >256 | ≥256 | 1 | 64 | 64 | |
| 3 | R-R | − | − | (Leu,Asn)(Ile,Gly) | >256 | ≥256 | 1 | 64 | 64 | |
| 9 | R-R | − | − | (Leu,Asn)(Ile,wt) | >256 | 256 | >1 | 64 | 64 |
DAEC: diffusely adherent E. coli; DEC: diarrheogenic E. coli; EAEC: enteroaggregative E. coli; EPEC: enteropathogenic E. coli, ETEC: enterotoxigenic E. coli; MIC: minimal inhibitory concentration; PAβN: Phe-Arg-β-Napthylamide, efflux pump inhibitor; wt: wild type.
a Number of isolates.
b Phenotype NAL (nalidixic acid) and CIP (ciprofloxacin) by disc diffusion (I: intermediate; R: resistant; S: susceptible).
c - non gene detected,+presence of acc(6′)Ib-cr gene.
Considering resistance patterns (NalR, NalI, and CipR, CipI, CipS), four profiles were described. In general the pattern most frequently observed was NalRCipS 64/96 (67%), followed by NalRCipR 24/96 (25%) and finally NalICipS 7/96 (7%). Among the DEC group the most frequent phenotype was NalRCipS 39 (78%), followed by NalICipS seven (14%). Meanwhile, the NalRCipS (24/46, 52%) and NalRCipR (20/46, 43%), phenotypes were the most frequent in the non DEC group. The NalRCipR phenotype was significantly more frequently detected amongst non-pathogenic isolates (20/46, 43% vs 4/50, 8%; p<0.001).
When the MIC to NAL and CIP was established, five out of seven isolates identified as NalI presented a NAL–MIC of 32–64 mg/L, while the remaining two cases, in which all the MICs were under the resistance breakpoints considered, the amino acid change Ser83 to Ala was detected in one case, and a qnrB gene was detected in the other (Table 2).
Two out of the TMQR-genes sought were detected among the present isolates, being the aac(6′)Ib-cr gene the most frequently found by far, followed by the qnrB genes. In total 32 (33%) of the isolates analysed presented at least one TMQR. Thus, 26 isolates presented the aac(6′)Ib-cr, 24 alone, and two concomitanly with qnrB. In addition, eight other isolates present the qnrB gene.
The results show the important role of efflux pumps in quinolone resistance acquisition. Thus, in the MIC50 of NAL, when the efflux pump inhibitor was added to the media, a reduction in at least two-fold dilutions was found. (Table 3)
Table 3.
MIC values to NAL and NAL plus efflux pump inhibitor according to phenotypes and diarrheogenic group
| Groups | Phenotypes | MIC50 NAL (mg/L) | MIC50 NAL+PAβN (mg/L) |
|---|---|---|---|
| DAEC | NalRCipS | 256 | 32 |
| DAEC | NalRCipR | >256 | 128 |
| EAEC | NalRCipS | 128 | 32 |
| EPEC | NalRCipS | 128 | 32 |
| Non DEC | NalRCipS | 256 | 64 |
| Non DEC | NalRCipR | >256 | 256 |
Cip: ciprofloxacin; DAEC: diffusely adherent Escherichia coli; DEC: diarrheogenic E. coli; EAEC: enteroaggregative E. coli; EPEC: enteropathogenic E. coli; MIC: minimal inhibitory concentration; NAL: nalidixic acid (in lower case, Nal is related to bacterial phenotype)
The unusual NalI or NalR phenotype without target mutations in gyrA and parC genes was observed, being detected in 14 isolates (15%); 13 in the DEC group vs one in the non DEC group p<0.05. Of these, the presence of TMQR was reported in 10 isolates: QnrB in four isolates, AAC(6′)Ib-cr in five isolates and both mechanisms in one isolate. In nine out of the 14 strains the MIC to NAL decreased to values ≤16 in the presence of PAβN. In the remaining five isolates the mechanisms sought were not found.
From seven isolates displaying one substitution in GyrA and one in ParC (Leu83/Ile80), six displayed the NalR CipS phenotype. Meanwhile isolates with three or more substitutions in the targets were fully resistant to both quinolones (NalR CipR; Table 2).
Although not significant, differences in the prevalence of TMQR were found between the DEC and non DEC isolates. Thus, the presence of the aac(6′)Ib-cr gene was mainly detected in DEC isolates, being found in 17 (34%) vs nine (20%) in non DEC isolates, while QnrB was present in five (10%) DEC vs three (7%) in non DEC isolates (Figure 1).
Figure 1.
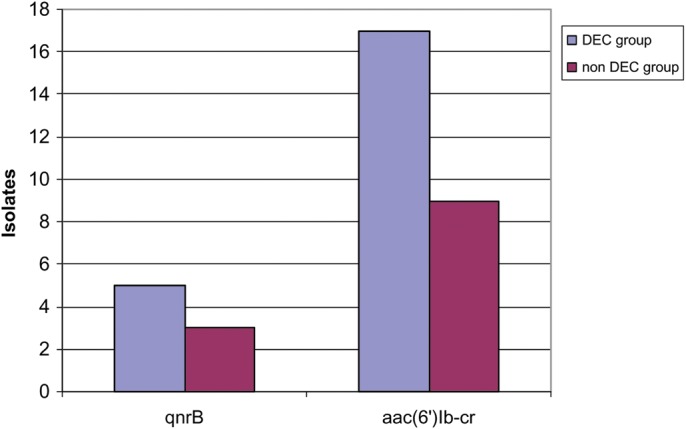
Distribution of transferable mechanisms of quinolone resistance in diarrheogenic Escherichia coli (DEC) and commensal (non DEC) groups
Thirty-seven (74%) DEC and 45 (98%) non DEC isolates presented at least one mutation in the gyrA gene. Of these, one (2%) DEC and 26 (56%) non DEC also presented substitutions in the parC gene. Finally, neither the qepA, qnrS nor oqxAB gene was detected.
No association was observed among the specific TMQR and the specific phylogenetic group. However, when all the TMQR were considered together, they were distributed differently among the different phylogenetic groups, being more prevalent in isolates belonging to the D group (p<0.05). Additionally, when all the phylotypes associated with higher virulence were considered together (B2 and D) the significance of this association was increased.
No clonal relationship was observed with REP-PCR among the strains included in the present study (data not shown).
Discussion
Although the safety of the use of quinolones in children has been demonstrated,23 their use in this population remains unusual, and limited to specific geographic areas or pathologies. Peru is not an exception, and the use of quinolones in children is not considered in clinical guidelines except for specific pathologies. However, in Peru, as in other different geographical areas, high levels of quinolone-resistance have been reported, in microorganisms isolated from ill or healthy children.7,12 Detection of high levels of resistance to quinolones in non-exposed populations such as children, and especially in non-pathogenic microorganisms, suggests high quinolone pressure in the community.24–26 Thus, in a study about trends in antibiotic utilisation in eight Latin American countries it was observed that the general antibiotic consumption in Peru increases 5.58 daily dose per 1000 inhabitants per day.26 Regarding quinolones, Llanos-Zavalaga et al.25 in a comparative study addressed to analyse the antibiotic use in different Latin American hospitals showed that CIP was by far the most prescribed antibiotic, accounting for 17.5% of the total prescriptions. Besides, the same study showed that incorrect or unnecessary antibiotic prescription rates were of 81.7%.26 Moreover, despite the dearth of data on the veterinary use of antibacterial agents, high levels of quinolone-resistance in microorganisms isolated from different marketed meats has been shown.27
The extent of the problem reflects the loss of the potential of quinolones to treat various infections in which these antimicrobial agents are the standard therapy, both because the causative microorganisms are under the same selective pressure and because of the appearance and the long dissemination of TMQR after their first description in 1998, acting as risk factors for the development of full quinolone resistance.9 The results show the presence of quinolone resistance in non clonally related strains, showing that the presence of quinolone resistant microorganisms in the area, is not due to selection and dissemination of a specific strain, but to extensive antibiotic pressure resulting in the selection of a variety of quinolone resistant mechanisms and subsequently in the spreading of multiple quinolone resistant isolates in the area.
The present data showed both the high relevance of the target mutations in the development of full resistance to fluoroquinolones28 and the presence of different TMQR in the study area. Full resistance to quinolones occurs mainly as a result of mutations in the chromosomal genes encoding quinolone targets.28,29 Thus, in accordance with the literature,8,28 mutations in the gyrA gene at position 83 (leading to Leu, Val or Ala) were the most frequent quinolone-resistance mechanism observed in the E. coli DNA Gyrase, being associated with moderate increases in the levels of quinolone resistance. Only one isolate presented the amino acid substitution Ser83 to Ala, with its MICs levels to NAL and CIP being the lowest. Previous reports have shown that the alteration in the hydrophobicity that results from this amino acid change is lesser than that due to the changes Ser-83 to Val / Leu, and this fact has been related to a lesser effect on the ability to hinder the quinolone/target interactions thereby resulting in low levels of quinolone resistance.28 Another unusual single amino acid change, Asp87 to Tyr, was also observed. Although mutations at codon 87 have been extensively related to the acquisition of quinolone resistance, alterations at codon 87 (except in specific microorganisms such as Salmonella spp.), in E. coli and other Gram-negative microorganisms appear as secondary after a primary mutation in codon 83. High levels of resistance have also been associated with the presence of multiple mutations in the QRDRs.28
The PAβN-inhibitible efflux pumps play a relevant but usually clinically hidden role in the basal levels of NAL resistance.15,30 Thus, the inhibition of this kind of efflux pump resulted in 27 isolates with a decrease in their NAL–MIC under the breakpoint considered. Independently of the presence of TMQR, these 27 isolates presented one single amino acid change in GyrA, in accordance with that described by Saenz et al.15
The QnrB, and AAC(6′)Ib-cr were detected in our isolates. Interestingly, in 10 isolates those mechanisms were not associated with target-mutations; five displayed full resistance to NAL and five were intermediate resistant to NAL. However, in four cases a single AAC(6′)Ib-cr encoding gene was detected, and this enzyme does not inactive NAL.9,31 In one of these four isolates, overexpression of PAβN-inhibitible efflux pumps may be adduced, but in the remaining three cases the isolates remained over the NAL resistance breakpoint after the addition of PAβN. It is important to mention that in four isolates no resistance mechanism was identified; these isolates remain with NAL MIC levels higher than 32 mg/L after the addition of PAβN, suggesting the presence of unusual resistance mechanisms in the study area, such as decreased uptake due to a reduction in porins expression, overexpression of efflux pumps other than those PAβN-inhibitible, mutations in the parE or gyrB genes28 or another more infrequent TMQR such as QnrD, QnrC or its recently proposed closely phylogenetically group QnrVC.32
TMQR genes confer low levels of quinolone resistance and it has been suggested that these mechanisms facilitate the selection of higher levels of resistance of quinolone resistance mutants.9,10 Thus, it has been shown that QnrA1, QnrB1 and QnrS1, may play a significant role in the acquisition of clinical resistance to fluoroquinolones, and therefore, therapeutic failure in treatment.10 The presence of a single TMQR usually does not confer full resistance to these antimicrobial agents, but rather increases the risk of developing quinolone resistance. Thus, the diversity, and high number of TMQR in the present samples, together with the non-controlled use of quinolones, may explain the high levels of quinolone-resistance in this area.7,12
In conclusion, the mechanisms involved in quinolone resistance have been shown in a non-directly exposed vulnerable population, mostly breastfed infants, living in an area under high antibiotic pressure. A relatively high prevalence of different TMQR has been detected in this area, showing the real risk of a relatively easy selection of fluoroquinolone-resistant microorganisms.
Acknowledgments
Authors' contributions: MJP, JR designed the study. MJP, SM and CG performed the experiments MJP, SM, CG, LJV, TJO and JR gathered data and isolates. MJP, JR analysed the data. MJP and JR wrote the manuscript. All the authors read and approved the final manuscript. MJP is guarantor of the paper.
Acknowledgements: Thanks to Laura Puyol and Diana Barrios for their support in the laboratory management.
Funding: This work has been partially funded by Agencia Española de Cooperación Internacional al Desarrollo [grant numbers D/019499/08, D/024648/09 and D/030509/10 (JR, TJO)], the Fogarty International Center, National Institute of Health, USA [grant 1K01TW007405 (TJO)]. JR has a fellowship from the program I3, of the ISCIII [grant number: CES11/012].
Competing interests: None declared.
Ethical approval: This study was approved by the Ethical Review Board of the Instituto de Investigación Nutricional and by the Ethics Committee of the Universidad Peruana Cayetano Heredia
References
- 1.Okeke IN, Laxminarayan R, Bhutta ZA, et al. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5:481–93. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 2.Sharma M, Eriksson B, Marrone G, et al. Antibiotic prescribing in two private sector hospitals; one teaching and one non-teaching: A cross-sectional study in Ujjain, India. BMC Infect Dis. 2012;12:155. doi: 10.1186/1471-2334-12-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandy SJ, Thomas K, Antonisamy B, et al. Patterns of antibiotic use in the community and challenges of antibiotic surveillance cross-sectional study in Vellore, south India. J Antimicrob Chemother. 2013;68:229–36. doi: 10.1093/jac/dks355. [DOI] [PubMed] [Google Scholar]
- 4.Silveira de Castro M, Pilger D, Cardoso Ferreira MB, Kopittke L. Trends in antimicrobial utilization in a university hospital, 1990–1996. Rev Saúde Pública. 2002;36:553–8. doi: 10.1590/s0034-89102002000600003. [DOI] [PubMed] [Google Scholar]
- 5.García C, Horna G, Linares E, et al. Antimicrobial drug resistance in Peru. Emerg Infect Dis. 2012;18:520–1. doi: 10.3201/eid1803.100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gales AC, Castanheira M, Jones RN, Sander HS. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010) Diagn Microbiol Infect Dis. 2012;73:354–60. doi: 10.1016/j.diagmicrobio.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Pons MJ, Mosquito S, Ochoa TJ, et al. Niveles de resistencia a antimicrobianos, en especial a quinolonas, en cepas de Escherichia coli comensales en niños de la zona periurbana de Lima, Perú. Rev Peru Med Exp Salud Pública. 2012;29:82–6. doi: 10.1590/s1726-46342012000100012. [DOI] [PubMed] [Google Scholar]
- 8.Hooper DC. Emerging mechanisms of fluoroquinolone resistance. Emerg Infect Dis. 2001;7:337–41. doi: 10.3201/eid0702.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz J, Pons MJ, Gomes C. Transferable mechanisms of quinolone resistance. Int J Antimicrob Agents. 2012;40:196–203. doi: 10.1016/j.ijantimicag.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Martínez JM, Cano ME, Velasco C, et al. Plasmid-mediated quinolone resistance: An update. J Infect Chemother. 2011;17:149–82. doi: 10.1007/s10156-010-0120-2. [DOI] [PubMed] [Google Scholar]
- 11.Nys S, Okeke IN, Kariuki S, et al. Antibiotic resistance of faecal Escherichia coli from healthy volunteers from eight developing countries. J Antimicrob Chemother. 2004;54:952–5. doi: 10.1093/jac/dkh448. [DOI] [PubMed] [Google Scholar]
- 12.Ochoa TJ, Ruiz J, Molina M, et al. High frequency of antimicrobial drug resistance of diarrheagenic Escherichia coli in infants in Peru. Am J Trop Med Hyg. 2009;81:296–301. [PMC free article] [PubMed] [Google Scholar]
- 13.Ochoa TJ, Ecker L, Barletta F, et al. Age-related susceptibility to infection with diarrheagenic Escherichia coli among infants from periurban areas in Lima, Perú. Clin Infect Dis. 2009;49:1694–1702. doi: 10.1086/648069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. Wayne, PA, USA: CLSI; 2011; Performance standards for antimicrobial susceptibility testing. Twenty-first Informational Supplement M100-S21.2011. [Google Scholar]
- 15.Sáenz Y, Ruiz J, Zarazaga M, et al. Effect of efflux pump inhibitor Phe-Arg-β-naphthylamide on the MIC values of quinolones, tetracycline and chloramphenicol in Escherichia coli isolates of different origin. J Antimicrob Chemother. 2002;53:544–5. doi: 10.1093/jac/dkh117. [DOI] [PubMed] [Google Scholar]
- 16.Vila J, Ruiz J, Goñi P, Jimenez de Anta MT. Detection of mutations in parC in quinolone-resistant clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1996;40:491–3. doi: 10.1128/aac.40.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park CH, Robicsek A, Jacoby GA, et al. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob Agents Chemother. 2006;50:3953–5. doi: 10.1128/AAC.00915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cattoir V, Poirel L, Rotimi V, et al. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J Antimicrob Chemother. 2007;60:394–7. doi: 10.1093/jac/dkm204. [DOI] [PubMed] [Google Scholar]
- 19.Yamane K, Wachino JI, Suzuki S, Arakawa Y. Plasmid-mediated qepA gene among Escherichia coli clinical isolates from Japan. Antimicrob Agents Chemother. 2008;52:1564–6. doi: 10.1128/AAC.01137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HB, Wang M, Park CH, et al. OqxAB encoding a multidrug efflux pump in human clinical isolates of Enterobacteriaceae. Antimicrob Agents Chemother. 2009;53:3582–4. doi: 10.1128/AAC.01574-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;45:55–8. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vila J, Marcos MA, Jimenez de Anta MT. A comparative study of different PCR-based DNA fingerprinting techniques for typing of the Acinetobacter calcoaceticus- A. baumannii complex. J Med Microbiol. 1996;44:482–9. doi: 10.1099/00222615-44-6-482. [DOI] [PubMed] [Google Scholar]
- 23.Murray TS, Baltimore RS. Pediatric uses of fluoroquinolone antibiotics. Pediatr Ann. 2007;36:336–42. doi: 10.3928/0090-4481-20070601-09. [DOI] [PubMed] [Google Scholar]
- 24.Ecker L, Ochoa TJ, Vargas M, et al. Factors affecting caregivers' use of antibiotics available without a prescription in Peru. Pediatrics. 2013;131 doi: 10.1542/peds.2012-1970. e1771–9. [DOI] [PubMed] [Google Scholar]
- 25.Llanos-Zavalaga LF, Mayca J, Contreras C. Características de la prescripción en los consultorios de medicina del hospital Cayetano Heredia de Lima, Perú. Rev Esp Salud Pública. 2002;76:207–14. [PubMed] [Google Scholar]
- 26.Wirtz VJ, Dreser A, Gonzales R. Trends in antibiotic utilization in eight Latin American countries, 1997–2007. Rev Panam Salud Pública. 2010;27:219–25. doi: 10.1590/s1020-49892010000300009. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz L, Pons MJ, Gomes C, et al. High antimicrobial-resistance levels of Escherichia coli isolated from meat of several markets in Lima, Peru. Trop Med Int Health. 2013;18(Suppl 1) 173. [Google Scholar]
- 28.Ruiz J. Mechanisms of resistance to quinolones: Target alterations, decreased accumulation and DNA gyrase protection. J Antimicrob Chemother. 2003;51:1109–17. doi: 10.1093/jac/dkg222. [DOI] [PubMed] [Google Scholar]
- 29.Poirel L, Cattoir V, Nordmann P. Plasmid-mediated quinolone resistance; interactions between human, animal, and environmental ecologies. Front Microbiol. 2012;3:24. doi: 10.3389/fmicb.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribera A, Ruiz J, Jimenez de Anta MT, Vila J. Effect of an efflux pump inhibitor on the MIC of nalidixic acid for Acinetobacter baumannii and Stenotrophomonas maltophilia clinical isolates. J Antimicrob Chemother. 2002;49:697–8. doi: 10.1093/jac/49.4.697. [DOI] [PubMed] [Google Scholar]
- 31.Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev. 2009;22:664–89. doi: 10.1128/CMR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pons MJ, Gomes C, Ruiz J. QnrVC, a new transferable Qnr-like family. Enferm Infecc Microbiol Clin. 2013;31:191–2. doi: 10.1016/j.eimc.2012.09.008. [DOI] [PubMed] [Google Scholar]


