Abstract
Purpose
Frailty, a phenotype reported among 9.9% of individuals 65 years old and older (9.6% of women; 5.2% of men), has not been assessed among adult childhood cancer survivors (CCS). We estimated the prevalence of frailty and examined associations with morbidity and mortality.
Methods
Participants included 1,922 CCS at least 10 years from original cancer diagnosis (men, 50.3%; mean age, 33.6 ± 8.1 years) and a comparison population of 341 participants without cancer histories. Prefrailty and frailty were defined as two and ≥ three of the following conditions: low muscle mass, self-reported exhaustion, low energy expenditure, slow walking speed, and weakness. Morbidity was defined as grade 3 to 4 chronic conditions (Common Terminology Criteria for Adverse Events version 4.0). Fisher's exact tests were used to compare, by frailty status, percentages of those with morbidity. In a subset of 162 CCS who returned for a second visit, Poisson regression was used to evaluate associations between frailty and new onset morbidity. Cox proportional hazards regression was used to evaluate associations between frailty and death.
Results
The prevalence of prefrailty and frailty were 31.5% and 13.1% among women and 12.9% and 2.7% among men, respectively, with prevalence increasing with age. Frail CCS were more likely than nonfrail survivors to have a chronic condition (82.1% v 73.8%). In models adjusted for existing chronic conditions, baseline frailty was associated with risk of death (hazard ratio, 2.6; 95% CI, 1.2 to 6.2) and chronic condition onset (relative risk, 2.2; 95% CI, 1.2 to 4.2).
Conclusion
The prevalence of frailty among young adult CCS is similar to that among adults 65 years old and older, suggesting accelerated aging.
INTRODUCTION
Advances in treatment for childhood cancer have resulted in more than 360,000 childhood cancer survivors (CCS) in the United States, with nearly 250,000 survivors younger than 40 years.1 Treatment for childhood cancer is multimodal, and includes surgery, radiation, and combination chemotherapy, which may adversely affect normal tissues.2 Nearly two thirds of CCS have at least one chronic health condition 30 years after diagnosis.3 CCS are more likely than peers to be hospitalized for nonobstetrical reasons4 and have mortality rates more than eight times higher than age- and sex-matched peers.5 Even in the absence of overt organ-system disease, many young adult CCS report symptoms that interfere with daily life, including exercise-induced shortness of breath,6 fatigue,7 and reduced capacity to participate in physical activity,8 with survivors of childhood acute lymphoblastic leukemia, brain tumors, and Hodgkin's lymphoma demonstrating impaired fitness.9 These symptoms may be indicators of premature aging or frailty.
Frailty, characterized by a cluster of five measurements of physical states or abilities, is a phenotype most commonly described in older adults. The frailty phenotype identifies individuals who are highly vulnerable to adverse health outcomes, often precedes the onset of chronic disease, and is a predictor of early mortality.10,11
We hypothesized that frailty would be more prevalent among CCS than their non-CCS peers and would represent a predictive marker of subsequent poor health. To evaluate this hypothesis, we estimated the prevalence of frailty among a cohort of 1,922 adult CCS who underwent an extensive systematic clinical evaluation and examined associations with current and future morbidity and subsequent mortality.
METHODS
Study Population
Participants are members of the St Jude Lifetime cohort, an institutional review board–approved clinical study designed to evaluate health (including physical abilities) among CCS as they age. The study design has been described previously.12,13 Briefly, participants are CCS treated at St Jude Children's Research Hospital between 1962 and 2003, are at least 18 years old, and are at least 10 years from original cancer diagnosis. Treatment information and medical histories are abstracted from medical records and participants receive risk-based medical screening according to the Children's Oncology Group Long-Term Follow-Up Guidelines.14 This is augmented by a core assessment battery, which includes physical performance evaluations and five questionnaires that detail demographic, medical history, quality of life, and health-habit information. A population recruited as a comparison group for a separate investigation of physical function among survivors of childhood acute lymphoblastic leukemia was available to provide rates of frailty among individuals without childhood cancer histories. Comparison participants were recruited from families and friends of patients currently receiving treatment at St Jude Children's Research Hospital and completed the same assessments and questionnaires used to classify frailty among CCS.
Outcomes
The phenotypes of frailty and prefrailty were the primary outcomes. More detail about each item is included in Appendix Table A1 (online only). As originally defined by Fried et al,10 participants were defined as prefrail if they fulfilled two and frail if they fulfilled three or more of the following criteria.
Low lean muscle mass.
Dual x-ray absorptiometry and height were used to determine relative appendicular lean muscle mass, summing lean mass in arms and legs, and dividing by height in meters squared. Those with relative mass ≤ 1.5 standard deviations below age-, sex-, and race-specific values from the National Health and Nutrition Examination Study (NHANES) were classified as low lean muscle mass.15
Exhaustion.
Scores 1.3 standard deviations below the population mean of 50 on the vitality subscale of the Medical Outcomes Survey Short Form–36 (SF-36)16 were used to classify exhaustion.
Low energy expenditure.
Activity levels were captured with the physical activity questionnaire from National Health and Nutrition Examination Study.17 Type, frequency, intensity, and duration of activities were converted to kilocalories (kcal) per week. Men with less than 383 kcal/wk and women with less than 270 kcal/wk were classified with low activity.10
Slowness.
Participants were asked to walk at their usual pace for 15 feet. Women less than 159 cm tall and men less than 173 cm tall were classified as slow if they took ≥ 7 seconds to complete the distance. Women ≥ 159 cm tall and men ≥ 173 cm tall were classified as slow if they took ≥ 6 seconds to complete the distance.10
Weakness.
Sitting hand-grip strength (kg), with the forearm neutral and the elbow flexed 90 degrees, was measured using a hand-held dynamometer.18 Body mass index (BMI) –specific cut-points for strength10 were used to classify muscle weakness.
Other Variables
Chronic medical conditions, based on information obtained from medical records and clinical and laboratory examinations, were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, Version 4.03. Conditions were graded as mild (grade 1), moderate (grade 2), severe/disabling (grade 3), or life-threatening (grade 4). Vital status for participants and cause of death for those who died were obtained from the hospital cancer registry. Persons entered the mortality analysis on the date of their assessment and contributed at-risk time up to date of death or when censored on February 28, 2013. Sex, race, cancer diagnosis, age at diagnosis, age at assessment, time since diagnosis, BMI, smoking status, and alcohol use were considered along with radiation, chemotherapy, and surgery variables in models designed to identify factors associated with frailty phenotypes. Radiation (cranial [CRT], chest, abdominal/pelvic) and surgery (craniotomy, thoracotomy, laparotomy) were entered into models as dichotomous variables. Chemotherapy was modeled with two variables: length of exposure (years) and intensity (calculated by assigning numeric scores, 0 for no exposure, 1 for exposure below, or 2 for exposure above the median; summed for five classes of agents, yielding a total score from 0 to 10).
Statistics
Descriptive statistics were used to characterize the study population. Two sample t tests and χ2 statistics were calculated to compare participants with nonparticipants and sex-stratified percentages of prefrailty, frailty, and frailty components by age group and cancer type. Associations between demographic, treatment, behavioral characteristics, and having at least two frailty components were examined in logistic regression models. Fisher's exact tests were used to compare, by frailty status, the percentages of those participants with one, two, and two or more grade 3 to 4 chronic conditions at first visit. In a subset of 162 participants who had a second visit, Poisson regression, adjusted for sex and time between visits (median, 3.46 years; range, 1.03 to 4.97 years), was used to evaluate associations between frailty status at first visit and new onset of grade 3 to 4 chronic conditions at second visit. Cox proportional hazards regression, adjusted for chronic conditions, was used to evaluate associations between frailty status at initial visit and risk of death.
RESULTS
Participants
Frailty was evaluated among 1,922 participants (65%) recruited from 2,953 eligible cohort members (Table 1). Nonparticipants (n = 1,031) included 274 who declined, 473 nonresponders, and 72 participants lost to follow-up. An additional 212 individuals completed questionnaires but did not participate in medical or functional assessments (Fig 1). Nonparticipants were more likely than participants to be male and were less likely to be leukemia survivors, to have received CRT or chemotherapy, or to have undergone thoracotomy or limb-sparing surgery. Nonparticipants were older (mean age, 36.1 ± 8.5 years v 33.6 ± 8.1 years) and had survived longer (mean, 27.8 ± 8.0 years v 25.5 ± 7.7 years) than participants. Age at diagnosis did not differ between nonparticipants and participants. The distributions of age, sex, CRT, and chest radiation exposures did not differ between the 162 participants who returned for a second visit and the overall cohort. The comparison group included 166 women and 175 men, who were 18 to 50 years old (mean age, 29.0 ± 7.5 years) and younger than the survivor participants (mean, 33.6 ± 8.1 years; P < .001).
Table 1.
Characteristics of Participants and Nonparticipants
| Characteristic | Participants (n = 1,922) |
Nonparticipants (n = 1,031) |
Comparison Group (n = 341) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Sex | ||||||
| Female | 956 | 49.7 | 425 | 41.2* | 166 | 48.7 |
| Male | 966 | 50.3 | 606 | 58.8 | 175 | 51.3 |
| Age at assessment, years | ||||||
| 18-29 | 707 | 36.8 | 277 | 26.9* | 201 | 58.9* |
| 30-39 | 797 | 41.4 | 435 | 42.2 | 117 | 34.3 |
| 40-60.6† | 418 | 21.8 | 319 | 30.9 | 23 | 6.7 |
| Age at diagnosis, years | ||||||
| 0-4 | 747 | 38.9 | 377 | 36.6 | ||
| 5-9 | 463 | 24.1 | 261 | 25.3 | ||
| 10-14 | 414 | 21.5 | 226 | 21.9 | ||
| 15-28.6 | 298 | 15.5 | 167 | 16.2 | ||
| Time since diagnosis, years | ||||||
| 10-19 | 509 | 26.5 | 193 | 18.7* | ||
| 20-29 | 873 | 45.4 | 445 | 43.2 | ||
| 30-48.9 | 540 | 28.1 | 393 | 38.1 | ||
| Diagnosis | ||||||
| Leukemia | 841 | 43.8 | 395 | 38.3‡ | ||
| CNS tumor | 148 | 7.7 | 85 | 8.3 | ||
| Hodgkin's lymphoma | 253 | 13.2 | 120 | 11.6 | ||
| Non-Hodgkin's lymphoma | 110 | 5.7 | 91 | 8.8 | ||
| Neuroblastoma | 78 | 4.0 | 53 | 5.2 | ||
| Wilms tumor | 120 | 6.2 | 62 | 6.0 | ||
| Soft tissue sarcoma | 94 | 4.9 | 64 | 6.2 | ||
| Bone tumor | 136 | 7.1 | 72 | 7.0 | ||
| Retinoblastoma | 67 | 3.5 | 32 | 3.1 | ||
| Other solid tumor | 75 | 3.9 | 57 | 5.5 | ||
| Radiation | ||||||
| Chest | 580 | 30.2 | 293 | 28.4 | ||
| Abdomen/pelvis | 497 | 25.9 | 247 | 24.0 | ||
| Cranial | 674 | 35.1 | 315 | 30.6* | ||
| Other radiation | 123 | 6.4 | 73 | 7.1 | ||
| Chemotherapy | ||||||
| Any chemotherapy | 1,677 | 87.3 | 850 | 82.4* | ||
| Anthracyclines | 1,116 | 58.1 | 554 | 53.7§ | ||
| Alkylating agents | 1,184 | 61.6 | 594 | 57.6§ | ||
| Glucocorticoids | 1,009 | 54.0 | 478 | 46.4‡ | ||
| Platinum | 180 | 9.4 | 107 | 10.4 | ||
| Vinca-alkaloids | 1,480 | 77.0 | 722 | 70.0* | ||
| Methotrexate, IV/IT§ | 1,024 | 53.3 | 507 | 49.2§ | ||
| Surgery | ||||||
| Brain | 198 | 10.3 | 88 | 8.5 | ||
| Thoracotomy | 178 | 9.3 | 70 | 6.8§ | ||
| Laparotomy | 577 | 30.0 | 280 | 27.2 | ||
| Amputation | 60 | 3.1 | 25 | 2.4 | ||
| Limb sparing | 78 | 4.1 | 25 | 2.4§ | ||
| Body mass index, kg/m2¶ | ||||||
| < 18.5 | 68 | 3.5 | 9 | 2.6 | ||
| 18.5-24.9 | 604 | 31.4 | 135 | 40.0‡ | ||
| 25-29.9 | 552 | 29.0 | 93 | 27.2 | ||
| 30-39.9 | 539 | 28.1 | 83 | 24.3 | ||
| ≥ 40 | 155 | 8.1 | 21 | 6.2 | ||
| Smoking status | ||||||
| Never smoked | 1,240 | 64.5 | 209 | 61.3 | ||
| Former smoker | 230 | 12.0 | 36 | 10.6 | ||
| Current smoker | 435 | 22.6 | 96 | 28.2§ | ||
| Not reported | 17 | 0.9 | 0 | 0.0 | ||
| Heavy drinkers‖ | 66 | 3.5 | 19 | 5.6 | ||
Abbreviation: IV/IT, intravenous or intrathecal.
P < .001 (all comparisons are with survivor participants).
Of the patients who were 50-59 years old, 32 were men and 29 were women; one woman was 60 years old.
P < .01 (all comparisons are with survivor participants).
P < .05 (all comparisons are with survivor participants).
Adjusted for amputation.
Men: five or more drinks per day or 14 drinks per week; women: four or more drinks per day or seven drinks per week.
Fig 1.
CONSORT diagram.
Frailty
The prevalence of the frailty phenotype (≥ three components) was 2.7% among male participants and 13.1% among female participants (Table 2). Prefrailty (two components) was present among 12.9% of men and 31.5% of women. Among the comparison population, no participants fulfilled criteria for the frailty phenotype, but 4.6% of male participants and 7.8% of female participants were prefrail (two components). Among the survivors, the prevalence for frailty and prefrailty increased with age; the trend was more apparent in women. Low lean muscle mass was more prevalent among women (44.6%) than men (2.9%) between the ages of 18 to 39 years, with more pronounced differences in participants 40 years old and older. Self-reported exhaustion was present among 30% of women and 20.7% of men. Low energy expenditure was present in more than one third of CCS. Slow walking speed was uncommon. Among survivors ≥ 40 years old, muscle weakness was twice as common among women (14.7%) than men (6.8%).
Table 2.
Percentage of Cohort With Components of Frailty Phenotype by Age Group and Sex
| Component of Frailty Phenotype | Women |
Men |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) |
Age (years) |
|||||||||||||||
| All Ages |
18-29 |
30-39 |
≥ 40 |
All Ages |
18-29 |
30-39 |
≥ 40 |
|||||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Cancer survivors | ||||||||||||||||
| No. of patients | 956 | 100 | 350 | 100 | 408 | 100 | 198 | 100 | 966 | 100 | 357 | 100 | 389 | 100 | 220 | 100 |
| Low lean muscle mass | 519 | 54.4 | 107 | 30.6 | 230 | 56.4 | 182 | 91.9 | 40 | 4.1 | 13 | 3.6 | 9 | 2.3 | 18 | 8.2 |
| Self-reported exhaustion | 285 | 30.0 | 98 | 28.0 | 126 | 31.0 | 61 | 31.3 | 200 | 20.7 | 62 | 17.4 | 87 | 22.4 | 51 | 23.2 |
| Low energy expenditure | 469 | 49.1 | 165 | 47.1 | 203 | 49.8 | 101 | 51.0 | 391 | 40.5 | 118 | 33.1 | 168 | 43.2 | 105 | 47.7 |
| Slow walking speed | 26 | 2.7 | 8 | 2.3 | 10 | 2.5 | 8 | 4.0 | 20 | 2.1 | 9 | 2.5 | 5 | 1.3 | 6 | 2.7 |
| Weakness (handgrip) | 80 | 8.4 | 23 | 6.6 | 28 | 6.9 | 29 | 14.7 | 50 | 5.2 | 24 | 6.7 | 11 | 2.8 | 15 | 6.8 |
| Two components | 301 | 31.5 | 90 | 25.8 | 137 | 33.7 | 74 | 37.9 | 125 | 12.9 | 40 | 11.2 | 52 | 13.4 | 33 | 15.0 |
| ≥ Three components | 125 | 13.1 | 25 | 7.1 | 51 | 12.5 | 49 | 24.8 | 26 | 2.7 | 11 | 3.1 | 7 | 1.8 | 8 | 3.6 |
| Comparison group | ||||||||||||||||
| No. of patients | 166 | 100 | 94 | 100 | 60 | 100 | 12 | 100 | 175 | 100 | 107 | 100 | 57 | 100 | 11 | 100 |
| Low lean muscle mass | 3 | 1.8 | 2 | 2.1 | 1 | 1.7 | 0 | 0.0 | 5 | 2.9 | 3 | 2.8 | 2 | 3.5 | 0 | 0.0 |
| Self-reported exhaustion | 23 | 13.9 | 14 | 14.9 | 8 | 13.3 | 1 | 8.3 | 17 | 9.7 | 9 | 8.4 | 7 | 12.3 | 1 | 9.1 |
| Low energy expenditure | 55 | 33.1 | 30 | 31.9 | 22 | 36.7 | 3 | 25.0 | 41 | 23.4 | 21 | 19.6 | 17 | 29.8 | 3 | 27.3 |
| Slow walking speed | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Weakness (handgrip) | 1 | 0.6 | 1 | 1.1 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 |
| Two components | 13 | 7.8 | 9 | 9.6 | 4 | 6.7 | 0 | 0.0 | 8 | 4.6 | 2 | 1.9 | 5 | 8.8 | 1 | 9.1 |
| ≥ Three components | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
Frailty by Diagnostic Group
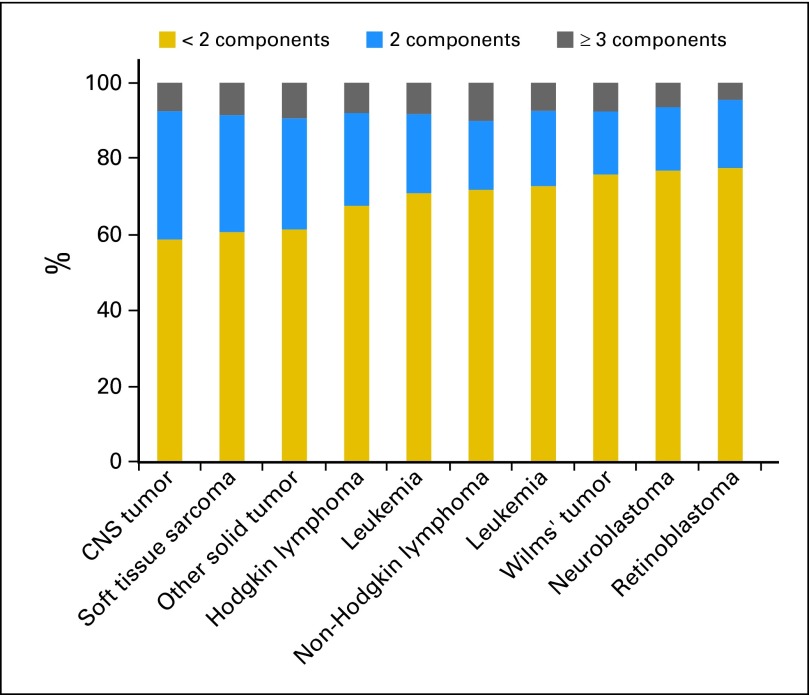
The combined prevalence of frailty and prefrailty was highest among CNS tumor (41.2%), soft tissue sarcoma (39.4%), and other solid tumor (38.7%) survivors (Fig 2). Approximately 30% of leukemia, lymphoma, and bone tumor survivors were prefrail or frail.
Fig 2.
Percentage of survivors with frailty (three or more components) and prefrailty (two components) phenotypes by diagnosis.
Factors associated with frailty.
Factors independently associated with a frailty phenotype (prefrail or frail) among men included CRT, abdominal/pelvic radiation, current smoking, and BMIs of less than 18.5 kg/m2, 30.0 to 39.9 kg/m2, or ≥ 40 kg/m2 (Table 3). Among women, increasing age and CRT were the only factors associated with having a frailty phenotype.
Table 3.
Host and Treatment-Related Factors Associated With Two or More Components of the Frailty Phenotype
| Factor | Women (n = 956) |
Men (n = 966) |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients | Row (%)* | OR | 95% CI | No. of Patients | Row (%)* | OR | 95% CI | |
| Current age, years | ||||||||
| 18-29 | 350 | 32.0 | 1.0 | 357 | 14.3 | 1.0 | ||
| 30-39 | 408 | 46.1 | 1.7 | 1.2 to 2.4 | 389 | 15.2 | 1.2 | 0.8 to 2.0 |
| 40-67.3 | 198 | 62.1 | 2.8 | 1.8 to 4.3 | 220 | 18.6 | 1.2 | 0.7 to 2.2 |
| Age at diagnosis, years | ||||||||
| 0-4 | 376 | 40.2 | 1.0 | 371 | 14.3 | 1.0 | ||
| 5-9 | 228 | 43.4 | 1.1 | 0.7 to 1.5 | 235 | 17.5 | 1.3 | 0.8 to 2.2 |
| 10-14 | 206 | 48.1 | 1.2 | 0.8 to 1.8 | 208 | 13.0 | 0.8 | 0.4 to 1.4 |
| 15-28.6 | 146 | 52.7 | 1.3 | 0.8 to 2.1 | 152 | 19.7 | 1.7 | 0.9 to 3.1 |
| Cranial radiation | ||||||||
| No | 638 | 40.4 | 1.0 | 610 | 13.1 | 1.0 | ||
| Yes | 318 | 52.8 | 1.9 | 1.4 to 2.7 | 356 | 19.9 | 2.3 | 1.4 to 3.6 |
| Chest radiation | ||||||||
| No | 666 | 40.1 | 1.0 | 676 | 14.1 | 1.0 | ||
| Yes | 290 | 54.8 | 1.3 | 0.8 to 2.2 | 290 | 19.3 | 0.6 | 0.3 to 1.3 |
| Abdominal/pelvic radiation | ||||||||
| No | 703 | 40.7 | 1.0 | 722 | 13.3 | 1.0 | ||
| Yes | 253 | 55.3 | 1.2 | 0.7 to 2.0 | 244 | 22.5 | 2.6 | 1.2 to 5.4 |
| Chemotherapy intensity† | 1.0 | 0.9 to 1.1 | 1.0 | 0.9 to 1.1 | ||||
| Chemotherapy duration, years | 1.1 | 0.9 to 1.3 | 0.8 | 0.6 to 1.0 | ||||
| Smoking | ||||||||
| Never (referent) | 650 | 44.3 | 1.0 | 590 | 13.7 | 1.0 | ||
| Ever | 107 | 43.9 | 1.0 | 0.6 to 1.5 | 123 | 13.8 | 1.0 | 0.6 to 1.9 |
| Current | 195 | 44.6 | 1.1 | 0.7 to 1.5 | 240 | 20.0 | 2.1 | 1.3 to 3.3 |
| Heavy drinking‡ | ||||||||
| No | 908 | 44.3 | 1.0 | 890 | 15.7 | 1.0 | ||
| Yes | 18 | 44.4 | 0.9 | 0.3 to 2.6 | 48 | 8.3 | 0.5 | 0.2 to 1.5 |
| Body mass index, kg/m2§ | ||||||||
| < 18.5 | 43 | 53.5 | 1.4 | 0.7 to 2.7 | 25 | 64.0 | 12.4 | 4.9 to 31.9 |
| 18.5-24.9 | 339 | 46.6 | 1.0 | 265 | 12.5 | 1.0 | ||
| 25-29.9 | 225 | 42.2 | 0.7 | 0.5 to 1.0 | 330 | 10.3 | 0.9 | 0.5 to 1.6 |
| 30-39.9 | 252 | 43.3 | 0.7 | 0.5 to 1.0 | 287 | 18.8 | 1.8 | 1.1 to 3.2 |
| ≥ 40 | 96 | 41.7 | 0.7 | 0.4 to 1.1 | 59 | 23.7 | 2.9 | 1.4 to 6.4 |
Abbreviation: OR, odds ratio.
Percentage of those in each category with two or more components of the frailty phenotype.
Factor-based score (includes anthracyclines, alkylating agents, glucocorticoids, vinca-alkaloids, and intravenous or intrathecal methotrexate). Participants who were not exposed to a particular class of agent were assigned a 0 for that class, those below the median cumulative dose received a 1, and those at or above the median dose received a 2. Scores range from 0 (least intense) to10 (most intense).
Data for heavy drinking status are missing for 58 patients.
Adjusted for amputation.
Frailty and Chronic Health Conditions
Among the 151 cohort members with frailty, 82.1% had at least one, 53.6% had at least two, and 27.8% had at least three grade 3 to 4 chronic health conditions. In contrast, among participants who were nonfrail, 73.8% had at least one, 35.3% had at least two, and 13.1% had at least three grade 3 to 4 chronic conditions. Grade 3 to 4 chronic conditions were more common in respiratory, gastrointestinal, liver, genitourinary, neurologic, psychiatric, and second malignancy categories among participants who were frail than among those who were nonfrail (Appendix Table A2).
Progression of Frailty and Risk of Subsequent Chronic Conditions
Among 162 participants who returned for a second visit, frailty rates increased from 8.0% to 10.5%. Adjusting for sex and time between visits, frailty at first visit was associated with a relative risk of 2.2 (95% CI, 1.2 to 4.2) for a new grade 3 to 4 chronic condition at the follow-up visit.
Frailty and Mortality
Among the first 1,922 members of the cohort, there were 31 deaths after the first clinical assessment; 4.6% among participants with and 1.4% among those without the frailty phenotype. Causes of death included trauma (n = 1), second malignancies (n = 12), cardiac (n = 10), vascular (n = 2), respiratory (n = 3,) and multisystem organ failure (3). After adjusting for number of grade 3 to 4 chronic conditions in a proportional hazards model, risk for death among those who were frail was 2.6× greater than among those who were not frail (95% CI, 1.2 to 6.2; Table 4).
Table 4.
Risk for Death by Frailty Status
| Phenotype* | Total |
Women |
Men |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | Deaths (%) | HR† | 95% CI | No. of Patients | Deaths (%) | HR | 95% CI | No. of Patients | Deaths (%) | HR† | 95% CI | ||
| Frail | 151 | 4.6 | 2.6 | 1.2 to 6.2 | 125 | 3.2 | 1.9 | 0.6 to 3.0 | 26 | 11.5 | 6.0 | 4.6 to 7.3 | |
| Not frail | 1,771 | 1.4 | 831 | 1.3 | 940 | 1.4 | |||||||
Abbreviation: HR, hazard ratio.
Frail is defined as at least three from among low lean muscle mass, exhaustion, low energy expenditure, slowness, weakness. Not frail is defined as two or fewer from among low lean muscle mass, exhaustion, low energy expenditure, slowness, or weakness.
HR from Cox proportional hazards model.
DISCUSSION
Frailty is an important, independent predictor of adverse health outcomes in aging populations,10,19 with both clinical and public health implications.20,21 To our knowledge, our study is the first to evaluate the prevalence and associated morbidity and mortality of frailty among a large population of CCS. The results of our assessment suggest that use of the frailty phenotype has strong potential to characterize CCS and aid understanding the heterogeneity of age- and treatment-related functional decline and chronic disease development.20 Moreover, there is potential clinical application for this phenotype as a screening tool to identify survivors at highest risk for subsequent adverse health outcomes.22,23
In the St Jude Lifetime cohort, 13.1% of women and 2.7% of men fulfilled criteria (≥ three of five) for frailty, with a mean age of 33 years. The overall prevalence, age trends, and sex differences for frailty in our CCS population are nearing those reported in cancer24,25 and noncancer10,19,26 cohorts of adults who are on average 30 years older. A recent meta-analysis of 44,894 persons at least 65 years old reported a weighted frailty prevalence of 9.9%. Across studies, frailty rates increase with advancing age; women are twice as likely as men to display this phenotype (9.6% v 5.2%).27 Sex differences in aging populations are likely the result of either earlier mortality among men, with selective survival among men who are nonfrail, or differential influences of hormones on body mass by sex.28 Our data suggest similar reasons for observed sex differences in frailty rates among CCS. In our cohort, frail male survivors were six times more likely to die than nonfrail male survivors, whereas frail female survivors were only twice as likely to die as nonfrail female survivors. Examination of treatment-related risk factors suggests a possible role for pituitary and/or gonadal hormone deficits.29 We found associations between CRT and frailty in both sexes and between abdominal/pelvic radiation and frailty in men. Lifestyle choices also affected frailty; but only among men, in whom smoking and body mass abnormalities were associated with the frailty phenotype.
Evaluating the utility of frailty as a phenotype within the context of a cross-sectional assessment of CCS is challenging. Clearly, the presence of serious chronic conditions can affect physical characteristics and abilities that determine levels of frailty. However, we were able to demonstrate in a subset of our population, followed longitudinally, that meeting criteria for frailty at an initial visit was associated with a risk of new onset grade 3 to 4 chronic conditions at a subsequent visit. We also found that frailty was an independent predictor of mortality among CCS even after accounting for chronic conditions. These data are consistent with data from older adult populations. In models adjusted for chronic conditions at baseline, Macklai et al19 reported increased odds (odds ratio, 1.77; 95% CI, 1.35 to 2.32) for worsening morbidity 2 years after a baseline assessment among frail versus nonfrail older adults, and Fried et al10 reported 3-year hazard ratios for first hospitalization and death of 2.23 (95% CI, 1.94 to 2.62) and 2.24 (95% CI, 1.51 to 3.33), respectively, among frail versus nonfrail older adults.
Even though we used similar criteria to define frailty, the phenotype in CCS seems to be somewhat different than described in other aging populations. Among CCS, low lean mass was common; muscle weakness was not. This is in contrast to studies among older adults that indicate loss of strength before loss of muscle mass.30 Among the elderly, age-related impairment of calcium-dependent excitation-contraction coupling necessary to produce muscle force31 seems to precede a multifactorial process (poor nutritional intake, oxidative stress, hormonal changes, and decreased physical activity) that precipitates myocyte apoptosis and loss of muscle mass.32,33 Children with cancer experience cancer-related muscle wasting during treatment,33 often compounded by poor nutritional intake, oxidative stress, hormonal disruption, and decreased physical activity during key periods of development. These factors may contribute to suboptimal lean mass, concomitant weakness, habitually low physical activity levels, diminished walking speed, and exhaustion in children with cancer. Full recovery and/or a return to normal muscle-growth trajectories and habitual physical activity may be inhibited by other late effects, including persistent inflammation, endocrine abnormalities, and physical disability.
A number of issues should be considered when interpreting results. First, our criteria for frailty differed slightly from those of Fried et al,10 who used unintentional weight loss of at least 10 pounds in the previous year to define loss of lean muscle mass, and two questions from the Center for Epidemiologic Studies Depression Questionnaire used to characterize exhaustion. Although evidence suggests that unintentional weight loss is associated with loss of lean mass (by dual x-ray absorptiometry)34 and that the Center for Epidemiologic Studies Depression questionnaire is correlated with the Medical Outcomes Survey Short Form–36,35 it is possible that our measures identified more or fewer persons with low lean mass or exhaustion than would have been identified using the original criteria. Second, although the phenotype appears to have utility, prospective assessments earlier in survivorship will be necessary to determine if frailty predicts risk for the onset of chronic conditions among those without pre-existing conditions, and to determine whether frailty predicts death in the absence of chronic conditions. Third, as nonparticipants were slightly less likely than participants to have received cranial radiation, thoracotomy, or limb-sparing surgery, returning participants may be sicker and perhaps more frail than nonparticipants. In addition, as our comparison group was not one-to-one or frequency-matched to our CCS population, we did not directly compare prefrailty and frailty rates between groups because of concerns about residual confounding by age. Fourth, because the subsample that returned for a second assessment was targeted for additional testing of treatment-related cardiac and/or cognitive outcomes, the association with frailty can only be generalized to populations at risk for these conditions. Finally, although the mortality analysis included all CCS who participated, it is important to note that the results were based on a relatively small number of deaths, and thus should be interpreted cautiously. Additional follow-up will be required to fully investigate the impact of the frailty phenotype. Because of the limited number of events following the baseline assessment of frailty, it was not possible to evaluate risks considering cancer diagnosis, treatment, and length of follow-up.
Young adult CCS have a higher than expected prevalence of frailty, suggesting that CCS may have accelerated aging. Additional research is warranted to examine the sex-specific biology and pathophysiology of this process and to translate this phenotype into a predictive model to identify CCS at risk for frailty so they can be targeted for formal assessment and intervention. With continued longitudinal follow-up of this cohort, assessment of the utility of a single element or subset of elements of frailty to predict poor outcomes is also important to improve its practicality as a tool in laboratory or clinical settings.
Supplementary Material
Appendix
Table A1.
Specific Criteria Used to Define Frailty in the Cardiovascular Health Study and the St Jude Lifetime Cohort Study
| Frailty Component | Cardiovascular Health Study Measure Criteria10 | St Jude Lifetime Cohort Criteria | Comments |
|---|---|---|---|
| Low lean muscle mass | Unintentional weight loss of ≥ 10 pounds in past year. | Lean muscle mass by DEXA ≤ 1.5 age and sex-specific SDS when compared with data from a national sample (NHANES).15 | Because obesity is prevalent among cancer survivors, lean mass was chosen over unintentional weight loss as a measure of muscle wasting, with more face validity in this younger population. In addition, loss of lean mass measured by DEXA is correlated with unintentional weight loss.34 |
| Self-reported exhaustion | Answered either a moderate amount of time or all of the time on either of the CEDS questions: I felt that everything I did was effort; and I could not get going. | Score ≤ 40 (1 SDS, based on a standard normal distribution, this represents approximately the lowest 6.7% of the general population) on the vitality subscale of the SF-36.16 | The vitality subscale of the SF-36 is specifically designed to measure vigor. The mental health subscale of the SF-36 and the CEDS depression scale are significantly correlated in adult cancer patients.34 |
| Low-energy expenditure | Expended < 383 Kcal/week (men) or < 270 Kcal/week (women) during leisure time physical activity (based on the short version of the Minnesota Leisure Time Activity Questionnaire). | Expended < 383 Kcal/week (men) or < 270 Kcal/week (women) during leisure time physical activity based on the NHANES Physical Activity Questionnaire.17 | Cut points are the same between studies. Type, duration, and frequency of physical activity from both questionnaires were converted to kcal/week based on the Compendium for Physical Activities.* |
| Slowness | Women < 159 cm tall and men < 173 cm tall were classified as slow if they took ≥ 7 seconds to walk 15 feet at their usual pace; and women ≥ 159 cm tall and men ≥ 173 cm tall were classified as slow if they took ≥ 6 seconds to walk 15 feet at their usual pace. | Measure and cut point are the same between studies. | |
| Weakness | Hand-grip strength stratified by body mass index and sex. | ||
| Men |
Women |
||
|---|---|---|---|
| BMI (kg/m2) | Cut Point (kg) | BMI (kg/m2) | Cut Point (kg) |
| ≤ 24 | ≤ 29 | ≤ 23 | ≤ 17 |
| 24.1 to 26 | ≤ 30 | 23.1 to 26 | ≤ 17.3 |
| 26.1 to 28 | ≤ 30 | 26.1 to 29 | ≤ 18 |
| > 28 | ≤ 32 | > 29 | ≤ 21 |
Abbreviations: BMI, body mass index; CEDS, Centers for Epidemiology Depression Scale; DEXA, dual x-ray absorptiometry; NHANES, National Health and Nutrition Examination Survey; SDS, standard deviation score; SF-36, Medical Outcomes Survey Short Form 36.
Ainsworth BE et al: Med Sci Sports Exerc 43:1575-1581, 2011.
Table A2.
Frailty Status by CTCAE v4.03 Grade 3 or Higher Chronic Condition
| Organ System With ≥ One Grade 3-4 Chronic Condition | Frailty Status (% of patients) |
P |
|||
|---|---|---|---|---|---|
| Pre-Frail (n = 426) | Frail (n = 151) | Non-Frail (n = 1,345) | Pre-Frail v Non-Frail | Frail v Non-Frail | |
| Heart | 8.4 | 6.0 | 4.8 | .004 | .52 |
| Vascular | 2.1 | 0.0 | 1.4 | .31 | .25 |
| Hematopoietic | 0.2 | 0.7 | 0.2 | .71 | .18 |
| Respiratory | 9.4 | 8.6 | 3.0 | < .001 | < .001 |
| Eyes, ears, nose, throat, larynx | 13.4 | 14.6 | 9.4 | .12 | .04 |
| Upper GI | 2.6 | 4.0 | 1.3 | .06 | .01 |
| Lower GI | 0.5 | 0.7 | 0.4 | .78 | .59 |
| Liver | 6.3 | 9.9 | 4.5 | .14 | .004 |
| Renal | 4.2 | 6.0 | 3.6 | .58 | .16 |
| Genitourinary | 4.7 | 7.3 | 1.4 | < .001 | < .001 |
| Musculoskeletal/integument | 3.5 | 2.0 | 3.1 | .68 | .44 |
| Neurologic | 14.1 | 13.9 | 5.7 | < .001 | < .001 |
| Endocrine/metabolic and breast | 39.2 | 37.8 | 44.8 | .04 | .10 |
| Psychiatric | 46.7 | 53.0 | 37.1 | < .001 | < .001 |
| Neoplasms | 8.0 | 13.9 | 6.0 | .15 | < .001 |
| No. of grade 3-4 conditions | |||||
| ≥ One | 80.3 | 82.1 | 73.8 | .01 | .03 |
| ≥ Two | 47.0 | 53.6 | 35.3 | < .001 | < .001 |
| ≥ Three or more | 22.5 | 27.8 | 13.1 | < .001 | < .001 |
Abbreviations: CTCAE v4.0, Common Toxicity Criteria Adverse Events, version 4.03; GI, gastrointestinal.
Footnotes
Supported by Grant No. CA 21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Kirsten K. Ness, Kevin R. Krull, Gregory T. Armstrong, Leslie L. Robison, Melissa M. Hudson
Administrative support: Kirsten K. Ness, Leslie L. Robison, Melissa M. Hudson
Provision of study materials or patients: Melissa M. Hudson
Collection and assembly of data: Kirsten K. Ness, Kevin R. Krull, Webb A. Smith, Kyla Shelton, Sabeen Ali, Leslie L. Robison, Melissa M. Hudson
Data analysis and interpretation: Kirsten K. Ness, Kevin R. Krull, Kendra E. Jones, Daniel A. Mulrooney, Gregory T. Armstrong, Daniel M. Green, Wassim Chemaitilly, Webb A. Smith, Carmen L. Wilson, Charles A. Sklar, Kyla Shelton, Deo Kumar Srivastava, Leslie L. Robison, Melissa M. Hudson
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al., editors. Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Review, 1975-2010. http://seer.cancer.gov/csr/1975_2010/ [Google Scholar]
- 2.Oeffinger KC, Hudson MM, Landier W. Survivorship: Childhood cancer survivors. Prim Care. 2009;36:743–780. doi: 10.1016/j.pop.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 4.Kurt BA, Nolan VG, Ness KK, et al. Hospitalization rates among survivors of childhood cancer in the Childhood Cancer Survivor Study cohort. Pediatr Blood Cancer. 2012;59:126–132. doi: 10.1002/pbc.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2002;95:2431–2441. doi: 10.1002/cncr.10978. [DOI] [PubMed] [Google Scholar]
- 7.Jóhannsdóttir IM, Hjermstad MJ, Moum T, et al. Increased prevalence of chronic fatigue among survivors of childhood cancers: A population-based study. Pediatr Blood Cancer. 2012;58:415–420. doi: 10.1002/pbc.23111. [DOI] [PubMed] [Google Scholar]
- 8.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2009;115:1984–1994. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman MC, Mulrooney DA, Steinberger J, et al. Deficits in physical function among young childhood cancer survivors. J Clin Oncol. 2013;31:2799–2805. doi: 10.1200/JCO.2012.47.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 11.Hogan DB, MacKnight C, Bergman H. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15(suppl 3):1–29. [PubMed] [Google Scholar]
- 12.Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: Results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2013;60:856–864. doi: 10.1002/pbc.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Children's Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. www.survivorshipguidelines.org.
- 15.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Physical Activity and Physical Fitness: PAQ 2007. http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/
- 18.Mathiowetz V, Kashman N, Volland G, et al. Grip and pinch strength: Normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74. [PubMed] [Google Scholar]
- 19.Macklai NS, Spagnoli J, Junod J, et al. Prospective association of the SHARE-operationalized frailty phenotype with adverse health outcomes: Evidence from 60+ community-dwelling Europeans living in 11 countries. BMC Geriatr. 2013;13:3. doi: 10.1186/1471-2318-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: An emerging research and clinical paradigm—Issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strawbridge WJ, Shema SJ, Balfour JL, et al. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci. 1998;53:S9–S16. doi: 10.1093/geronb/53b.1.s9. [DOI] [PubMed] [Google Scholar]
- 22.Muntinga ME, Hoogendijk EO, van Leeuwen KM, et al. Implementing the chronic care model for frail older adults in the Netherlands: Study protocol of ACT (frail older adults: Care in transition) BMC Geriatr. 2012;12:19. doi: 10.1186/1471-2318-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett JA, Winters-Stone KM, Dobek J, et al. Frailty in older breast cancer survivors: Age, prevalence, and associated factors. Oncol Nurs Forum. 2013;40:E126–E134. doi: 10.1188/13.ONF.E126-E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bylow K, Hemmerich J, Mohile SG, et al. Obese frailty, physical performance deficits, and falls in older men with biochemical recurrence of prostate cancer on androgen deprivation therapy: A case-control study. Urology. 2011;77:934–940. doi: 10.1016/j.urology.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santos-Eggimann B, Cuénoud P, Spagnoli J, et al. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64:675–681. doi: 10.1093/gerona/glp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: A systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 28.Walston J, Fried LP. Frailty and the older man. Med Clin North Am. 1999;83:1173–1194. doi: 10.1016/s0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong GT, Stovall M, Robison LL. Long-term effects of radiation exposure among adult survivors of childhood cancer: Results from the childhood cancer survivor study. Radiat Res. 2010;174:840–850. doi: 10.1667/RR1903.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell WK, Williams J, Atherton P, et al. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength: A quantitative review. Front Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersson DC, Betzenhauser MJ, Reiken S, et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41:1234–1238. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Sakuma K, Yamaguchi A. Sarcopenia and cachexia: The adaptations of negative regulators of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2012;3:77–94. doi: 10.1007/s13539-011-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JS, Visser M, Tylavsky FA, et al. Weight loss and regain and effects on body composition: The Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010;65:78–83. doi: 10.1093/gerona/glp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: Evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.