Abstract
Purpose
To study the impact of achieving stringent complete response (sCR), an increasingly attainable goal, after autologous stem-cell transplantation (ASCT) in patients with multiple myeloma (MM).
Patients and Methods
Maximal response rates were determined in 445 consecutive patients who underwent ASCT within 12 months of diagnosis of MM. The patients achieving varying degrees of complete response (CR) are the focus of our study.
Results
One hundred and nine patients (25%) achieved sCR after ASCT. The median overall survival (OS) rate from the time of transplantation for patients attaining sCR was not reached (NR), in contrast to those patients achieving conventional complete response (CR; n = 37; OS, 81 months) or near CR (nCR; n = 91; OS, 60 months; P < .001). Five-year OS rates were 80%, 53%, and 47% for sCR, CR, and nCR, respectively. The median time to progression (TTP) from ASCT of patients achieving sCR was significantly longer (50 months) than TTP of patients achieving CR or nCR (20 months and 19 months, respectively). On multivariable analysis, post-ASCT response of sCR was an independent prognostic factor for survival (hazard ratio, 0.44; 95% CI, 0.25 to 0.80; versus CR; P = .008), in addition to proliferation rate, pre-ASCT cytogenetics, and performance status. OS rates of patients attaining sCR continued to remain superior at 2-year landmark (median, NR v 70 months for conventional CR group; P = .007).
Conclusion
Improved long-term outcome is seen after ASCT with achievement of sCR when compared with lesser degrees of responses. Myeloma trials reporting the response rates should identify patients achieving sCR and CR separately, owing to markedly disparate outcomes of the two categories.
INTRODUCTION
While multiple myeloma (MM) remains largely an incurable disease, the concept of operational cure, first proposed in patients who had sustained complete response (CR) beyond 10 years after high-dose therapy (HDT) with stem-cell support, is increasingly gaining ground.1–6 Deepening the magnitude of response to achieve operational cure in a proportion of MM patients, albeit small, is becoming an attainable goal with modern strategies using a combination of novel agents and autologous stem-cell transplantation (ASCT).7 However, owing to a paucity of clinical data, responses deeper than the traditionally defined CR have not, until recently, been considered more consequential.8,9 Strategies focused on achieving deep responses have been questioned as achieving CR has not consistently translated into improved overall survival (OS).4,7,10–17 Moreover, substantial heterogeneity exists in the definitions of response-related end points for patients with MM.2,8,12 In clinical trials, the subset of patients achieving near-complete response (nCR), characterized by patients with less than 5% bone marrow plasma cells (BMPCs) and monoclonal protein detectable by immunofixation only, is often grouped with those who achieve standard CR (absence of monoclonal protein by electrophoresis and immunofixation along with less than 5% BMPCs),18 based on an unsubstantiated assumption that the survival outcomes of patients in the two response categories are similar.
The development of International Myeloma Working Group (IMWG) uniform criteria for response is an endeavor to eliminate ambiguities in the response assessment and make cross-trial comparisons of efficacy simpler.6 The IMWG has created a newer, more rigorous response category of stringent complete response (sCR) because the current therapeutic strategies frequently lead to deeper responses. However, this category requires validation in clinical studies. We evaluated the impact of specific subcategories of CR (stringent CR, standard CR, and near CR/immunofixation-positive CR) and lesser degrees of responses on outcomes as measured by the time to progression (TTP) and OS of patients with MM undergoing early ASCT (ie, within 12 months of diagnosis) in the era of novel agents.
PATIENTS AND METHODS
Four hundred and forty-five prospectively followed patients with MM who underwent ASCT between September 2002 and December 2008 were assessed for their maximal response rates after ASCT. The start date was chosen to coincide with the routine availability of free light chain (FLC) assay for post-ASCT response assessment at our institution. The data were frozen for analyses in May 2012. The study was approved by the Mayo Clinic institutional review board and was performed in accordance with the Declaration of Helsinki.
The induction regimens used before ASCT varied. All patients had access to similar salvage therapies on relapse. Only responses after the first ASCT or planned tandem transplantations were considered. Maintenance therapy was generally not used after ASCT. Individual patient data of serum and urine protein electrophoresis, immunofixation, serum FLC assay, and bone marrow (BM) aspiration and biopsy obtained 60 days or later after ASCT were abstracted to determine the best response. Serum FLC assay (FREELITE, The Binding Site Ltd, Birmingham, United Kingdom) was performed by immunonephelometry using a commercial reagent set of polyclonal antibodies. The assay quantitated κ and λ free light chains, and a κ/λ ratio was calculated. FLC ratios (rFLC) outside the 0.26 to 1.65 range were considered abnormal.19 Immunohistochemical studies with antibodies to κ and λ immunoglobulin light chains were performed on paraffin sections of the BM biopsy specimens. Clonality of plasma cells was additionally confirmed with the previously described slide-based plasma cell labeling index (PCLI) method.20
Response categories were determined in accordance with the IMWG uniform response criteria. A very good partial response (VGPR) was defined as 90% or greater reduction in serum M-component plus a 24-hour urine M-component of less than 100 mg.6 Patients with less than 5% BMPCs and unmeasurable M-proteins by electrophoresis but persistent serum and/or urine immunofixation were categorized separately as nCR, though it should be emphasized that this response category has been incorporated into the larger VGPR category of the IMWG response criteria.
Patients with disappearance of any soft tissue plasmacytomas and BMPCs of less than 5% with negative immunofixation studies were considered to be in standard CR. Patients achieving CR for whom the involved FLC reduced sufficiently to normalize the rFLC (κ/λ range, 0.26 to 1.65) in the absence of monoclonal BMPCs as assessed by immunohistochemistry or immunofluorescence were considered to have achieved sCR. The categories of partial remission (PR), stable disease (SD), and progressive disease (PD) were also used in accordance with the IMWG criteria.6 Survival curves were plotted by the Kaplan-Meier method and the differences were compared by log-rank tests.21
The following prognostic factors were evaluated in a univariable analysis: age, performance status, serum creatinine, lactate dehydrogenase, β2-microglobulin, cytogenetics, pre- and post-transplantation response status, and PCLI. Factors significantly prognostic for OS in the univariate model (P ≤ .05) were studied in a multivariable analysis using a Cox proportional hazards model.
TTP was defined as the time from ASCT to disease progression; deaths as a result of causes other than disease progression were censored.22,23 OS was defined as the time from ASCT to death from any cause or last follow-up. We performed landmark analysis for response categories of sCR and CR at 2 years to ensure that all the patients achieving at least a CR had sufficient time to reach the response level being studied. Patients were categorized as having sustained sCR if the duration of sCR was at least 6 months. The statistical analysis was performed using JMP 9 software (SAS Institute, Cary, NC).
RESULTS
The baseline characteristics of the patients are listed in Table 1. Two hundred and eighteen patients (49%) were alive at the time of analysis. The median time to ASCT was 6 months (range, 1.5 to 12 months) from diagnosis. The median estimated follow-up of patients was 77 months from ASCT (95% CI, 73 to 82 months). Only a few patients achieving less than VGPR underwent a second transplantation (n = 29) or received maintenance therapies (n = 12).
Table 1.
Patient Characteristics
| Parameter | No. of Patients* | % | Median | Range |
|---|---|---|---|---|
| Age at transplantation, years | 445 | 59 | 29-76 | |
| ≥ 65 | 25 | |||
| Creatinine at transplantation, mg/dL | 445 | 1 | 0.4-10.1 | |
| PCLI at transplantation, % | 442 | 0 | 0-11 | |
| Patients with PCLI ≥ 3% | 7 | |||
| LDH, U/L | 431 | 189 | 158-230† | |
| Patients with abnormal LDH (> 222 U/L) | 29 | |||
| CRP, mg/L | 425 | 0.4 | 0.3-1.3† | |
| Serum β2-microglobulin, μg/mL | 442 | 2.5 | 1.1-57 | |
| Serum creatinine, mg/dL | 1 | 0.4-10.1 | ||
| ≥ 2 | 7 | |||
| Durie-Salmon stage at diagnosis | 435 | |||
| 1 | 0 | |||
| 2a | 34 | |||
| 2b | 3 | |||
| 3a | 57 | |||
| 3b | 6 | |||
| Cytogenetics | 441 | |||
| Abnormal cytogenetics | 15 | |||
| Patients with bone disease | 445 | 85 | ||
| ECOG performance status < 2 | 441 | 91 | ||
| Time from diagnosis to ASCT, months | 445 | 6 | 1.5-12 | |
| Pretransplantation response | 445 | |||
| sCR | 7 | 2 | ||
| CR | 19 | 4 | ||
| nCR | 29 | 7 | ||
| VGPR‡ | 51 | 11 | ||
| PR | 207 | 47 | ||
| SD | 96 | 22 | ||
| PD | 36 | 8 |
Abbreviations: ASCT, autologous stem-cell transplantation; CR, complete response; CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; nCR, near complete response; PCLI, plasma cell labeling index; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease; VGPR, very good partial remission.
Total No. of patients for whom data were available at transplantation.
Interquartile range.
Excluding patients in nCR.
Response and Survival Outcomes
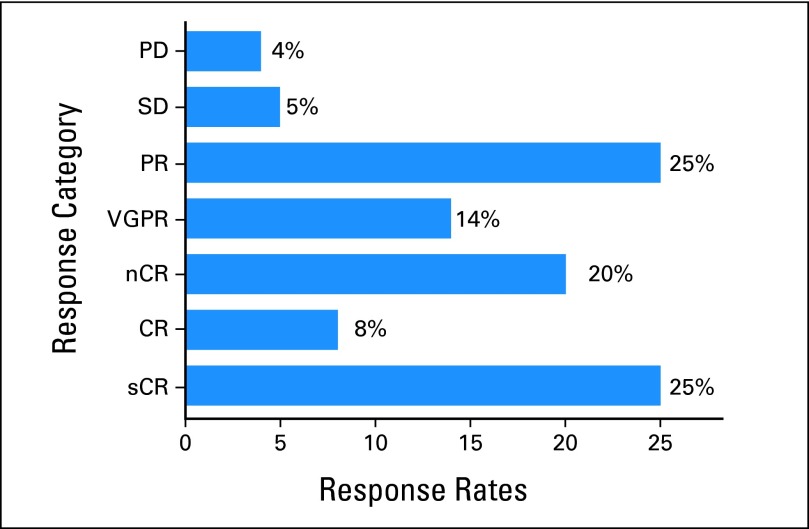
One hundred and forty-six patients (33%) achieved a response of CR or better after ASCT, including 109 patients (24%) who achieved sCR. An additional 91 patients met the criteria for nCR. Eighteen patients (4%) experienced disease progression despite ASCT (Fig 1). The TTP of patients who achieved CR or less after transplantation is shown by response categories in Figure 2A. No difference in TTP is noted among patients achieving responses ranging between SD and VGPR (including nCR). The median TTP is significantly longer (Fig 2B) for patients achieving sCR compared with CR/nCR.
Fig 1.
Best responses for 445 patients after autologous stem-cell transplantation. The response categories achieved by the patients were stringent complete response (sCR; n = 109), complete response (CR; n = 37), near complete response (nCR; n = 91), very good partial response (VGPR [excluding nCR]; n = 60), partial response (PR; n = 109), stable disease (SD; n = 21), and progressive disease (PD; n = 18). The sum of percentages is slightly greater than 100 because of rounding.
Fig 2.
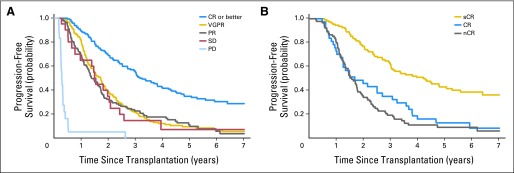
(A) The median time to progression (TTP) of patients achieving at least a complete response (CR; n = 146; 33%) is 39 months (95% CI, 33 to 48) compared with 20 (95% CI, 17 to 22), 17 (95% CI, 14 to 21), and 18 (95% CI, 9 to 25) months, respectively, for those achieving very good partial response (VGPR; including near complete response [nCR] as per International Myeloma Working Group definition; n = 151; 34%), partial response (PR; n = 109; 24%), and stable disease (SD; n = 21; 5%). Patients with progressive disease (PD; n = 18; 4%) had a short median TTP of 3 months (95% CI, 2.7 to 4). (B) Median TTP of patients achieving stringent complete response (sCR; n = 109) is 50 months (4.2 years; 95% CI, 36 to 63 months) compared with 20 months (1.7 years; 95% CI,15 to 36) and 19 months (1.6 years; 95% CI, 16 to 22 months) for groups attaining CR (n = 37) and nCR (n = 91), respectively (P < .001).
The median OS of the entire cohort from the time of diagnosis was 83 months (95% CI, 70 to 93). The median OS from diagnosis for patients achieving sCR was not reached (NR; 95% CI, NR to NR) versus 66 months for the rest of the cohort (95% CI, 60 to 80; P < .001).
The median OS from the time of transplantation of patients achieving at least a CR was 109 months (95% CI, 94 to NR) compared with 64 months (95% CI, 53 to 78), 59 months (95% CI, 50 to 86), and 56 months (95% CI, 40 to NR), respectively, for patients achieving VGPR (n = 145; 33%), PR (n = 109; 24%), and SD (n = 21; 5%). Patients with PD survived for a median of 9 months after ASCT (Fig 3A). We then examined patients with varying categories of CR, and found that patients with sCR have a significantly better outcome (median OS, NR; 5-year OS, 80%). However, as with the TTP, no difference in OS is evident between patients achieving CR (n = 37; 8%) and nCR (immunofixation-positive CR; n = 91; 20%; Fig 3B). The estimated 5-year OS is 80% (95% CI, 72 to 87), 53% (95% CI, 38 to 67), and 47% (95% CI, 37 to 57) for patients achieving sCR, CR, and nCR, respectively.
Fig 3.
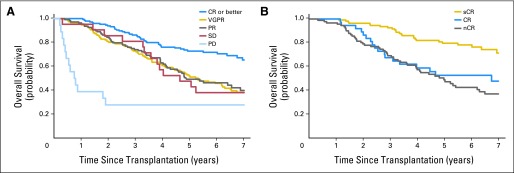
(A) Overall survival (OS) curves against response categories. The median OS of the patients achieving at least a complete response (CR; n = 146) was 109 months (9 years; 95% CI, 94 to not reached [NR]) compared with 64 months (5.3 years; 95% CI, 53 to 78 months), 59 months (4.9 years; 95% CI, 50 to 86 months), 56 months (4.6 years; 95% CI, 40 months to NR), respectively, for those achieving very good partial response (VGPR; n = 151), partial response (PR; n = 109), and stable disease (SD; n = 21). Patients with progressive disease (PD) survived for a median of 9 months (0.8 years; 95% CI, 5 to 88 months; P < .001). (B) OS of the patients achieving varying degrees of CR. Those with stringent complete response (sCR; n = 109) had a marked improvement in OS (median OS, NR; 95% CI, NR to NR) compared with the patients achieving CR (n = 37; median OS, 81 months; 95% CI, 37 to 109 months) or near complete response (nCR; n = 91; median OS, 60 months; 95% CI, 47 to 78). The 5-year OS is 80%, 53%, and 47%, for patients achieving sCR, CR, and nCR, respectively (P < .001).
Univariable and multivariable analyses for OS are listed in Table 2. Multivariable analysis was performed in 433 patients for whom pretransplantation data were available. Pretransplantation age, serum creatinine, and lactate dehydrogenase were not prognostic for OS on univariable analysis. Post-transplantation response status (sCR v other inferior response categories), pretransplantation PCLI, cytogenetics, and Eastern Cooperative Oncology Group performance status were independent prognostic factors for OS in the multivariable analysis (Table 2).
Table 2.
Univariate and Multivariate Analysis (Cox model) of Overall Survival
| Parameter | Univariate |
Multivariate (n = 433) |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Post-ASCT response | ||||||
| sCR v CR | 0.37 | 0.21 to 0.67 | .001 | 0.44 | 0.25 to 0.80 | .008 |
| sCR v nCR | 0.30 | 0.18 to 0.46 | < .001 | 0.35 | 0.22 to 0.56 | < .001 |
| sCR v VGPR* | 0.35 | 0.21 to 0.58 | < .001 | 0.42 | 0.25 to 0.70 | < .001 |
| sCR v PR | 0.34 | 0.21 to 0.53 | < .001 | 0.45 | 0.28 to 0.74 | .001 |
| sCR v SD | 0.30 | 0.16 to 0.60 | .001 | 0.33 | 0.16 to 0.67 | .003 |
| sCR v PD | 0.09 | 0.05 to 0.18 | < .001 | 0.14 | 0.06 to 0.28 | < .001 |
| Plasma cell labeling index, % | 5.02 | 3.29 to 7.36 | < .001 | 3.9 | 2.4 to 6.0 | < .001 |
| ≥ 3 v < 3 | ||||||
| β2-microglobulin, μg/mL | 1.5 | 1.09 to 1.95 | .01 | 1.08 | 0.78 to 149 | .63 |
| ≥ 3.5 v < 3.5 | ||||||
| Lactate dehydrogenase, u/L | 0.75 | 0.57 to 1.01 | .06 | |||
| Normal v abnormal | ||||||
| ECOG PS | ||||||
| > 1 v 0, 1 | 1.90 | 1.26 to 2.76 | .003 | 1.7 | 1.1 to 2.5 | .02 |
| Cytogenetics | ||||||
| Normal v abnormal | 0.48 | 0.35 to 0.67 | < .001 | 0.67 | 0.47 to 0.97 | .03 |
| Pre-ASCT response | 0.81 | 0.58 to 1.13 | .2 | |||
| PR v < PR | 0.57 | 0.44 to 0.74 | < .001 | |||
| Age, years | 0.99 | 0.73 to 1.33 | .95 | |||
| ≥ 65 v < 65 | ||||||
| Creatinine, mg/dL | 1.03 | 0.58 to 1.67 | .92 | |||
| ≥ 2 v 2 | ||||||
Abbreviations: ASCT, autologous stem-cell transplantation; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; nCR, near complete response; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
Excluding nCR.
Landmark analysis.
Survival analysis was repeated using a 2-year landmark for the response categories of stringent CR and standard CR. OS of patients surviving at least 2 years from ASCT and achieving sCR continued to remain superior compared with the standard CR group (Fig 4A).
Fig 4.
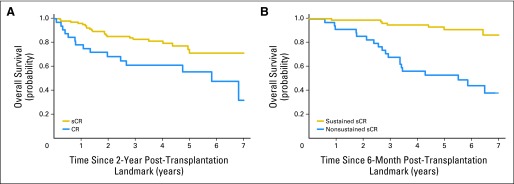
(A) Landmark analysis at 2 years studying survival outcomes of patients undergoing early autologous stem-cell transplantation (ASCT) and achieving stringent complete response (sCR; n = 105) versus complete response (CR; n = 32). Median overall survival (OS) was not reached for patients with sCR, but patients achieving CR had median OS of 70 months (5.8 years; P = .007). (B) Among patients receiving early transplantations who achieved sCR (n = 109), OS of patients with sustained sCR (n = 75) at 6 months from ASCT was not reached (7-year OS, 86%) versus median OS of 66 months or 5.5 years for those who had nonsustained-sCR (n = 34) after ASCT (7-year OS, 37%; P < .001).
Among the patients achieving sCR, OS of patients with sustained sCR at 6 months from ASCT was not reached (5-year OS, 91%; 7-year OS, 86%) versus those who had nonsustained-sCR who had a median OS of 66 months (5-year OS, 53%; 7-year OS, 37%; P < .001; Fig 4B).
DISCUSSION
With an increasing number of effective therapeutic options, the overarching debate about achieving cure versus control of MM has gained more relevance.2,4 The interest may be piqued, in part, because of a greater proportion of patients achieving deeper responses.24 CR was until recently considered an uncommon (< 10%) and elusive outcome of traditional therapies. Following the initial observations of HDT by McElwain and Powles25, a larger study by Cunningham et al26 demonstrated achievement of CR in a third of patients, but neither survival-benefit nor durability of response was observed. Stem-cell rescue in subsequent studies permitted use of higher doses of melphalan, leading to CR in up to 40% to 60% of the patients.27,28 However, such remissions have not been sustained in most studies, and an eventual relapse of MM suggested persistent disease. It is therefore logical to assume that the use of more sensitive immunohistochemical or flow cytometric techniques and FLC assay to detect clonality, or the absence thereof, would provide superior assessments of response. It is conceivable that the application of such techniques would improve the precision of the response evaluation as supported by our study results.
A post hoc analysis of the Velcade As Initial Standard Therapy in Multiple Myeloma (VISTA) trial of bortezomib plus melphalan-prednisone versus melphalan-prednisone demonstrated that achievement of CR in patients receiving the former therapy was associated with longer TTP (hazard ratio, 0.45; P = .004) and a longer treatment-free interval (hazard ratio, 0.39; P = .026) compared with achievement of VGPR, but it was unknown whether teasing apart CR further had prognostic implications.29
Baseline serum FLC, a useful determinant of prognosis in MM, aids in the detection of low tumor burden that is generally undetected by conventional tests. Normalization of rFLC suggests restoration of polyclonality and is a prerequisite for sCR.6 Despite the lack of clarity in outcomes of those achieving responses deeper than CR, the IMWG recommends serial FLC assessment for documenting sCR during therapy.6,19 Although FLC response alone at 2 months with conventional alkylator-based therapies was predictive of subsequent M-protein response on serum protein electrophoresis, it failed to predict outcomes, and data with novel agents are scarce.30 The benefits of transplantation in further cytoreduction have been studied extensively in clinical trials,31,32 and therefore we restricted our analysis to the maximal post-transplantation responses. We focused on the maximal response obtained rather than a snapshot of response assessment on day 100 after ASCT. This strategy potentially helped eliminate false-positive results owing to discordant rFLC (< 0.26 for κ and > 1.65 for λ MM subtypes), which in most cases normalized over time.
Several recent studies have demonstrated that achieving CR translates into better outcomes,33,34 but our mature data set goes a step further, underscoring the importance of clonality assessments during follow-up. Indeed, such assessments by immunohistochemistry, immunofluorescence, and FLC have become part of routine clinical practice at most transplantation centers. Our study validates the inclusion of sCR in the IMWG criteria by demonstrating a significant improvement in TTP and OS with achievement of sCR compared with CR after ASCT, and underscoring the additional prognostic significance of restoration of polyclonality (seen with sCR) beyond CR.
Multivariable analysis that incorporated other potential confounding factors, including pretransplantation response status, confirmed the independent high prognostic impact of achieving sCR post-transplantation for both OS (Table 2) and TTP (data not shown). However, our results require verification through prospective studies. Moreover, they cannot be extrapolated to the nontransplantation setting, though given the significant correlation of achieving CR with improved outcome, irrespective of age and ASCT eligibility in patients receiving novel agents, the results would probably be similar. Based on our findings, it is conceivable that obtaining responses of magnitude better than traditional CR in the early transplantation setting (provided a patient's quality of life is not compromised and the treatment-related toxicities are controlled) may alter the long-term disease course. However, it should be emphasized that only large randomized studies can ensure against biases, including the effect of unmeasured confounders.
It is equally important to emphasize that achieving sCR is but one marker of prognosis, a highly complex issue in MM. The interplay of several patient (host) and myeloma (disease biology) -related factors, including the durability of response, determine the outcome in individual patients. As such, our results highlighting the long-term benefits of maximal cytoreduction should be interpreted in context. A good proportion of our patients had received novel agents such as thalidomide (n = 149), lenalidomide (n = 103), and bortezomib (n = 32) for induction.
Notably, the definitions of sCR have somewhat differed in the studies conducted before the acceptance of the IMWG criteria.27,35 Although, our findings may seem to partly contradict a recent report in Journal of Clinical Oncology by Paiva et al,9 suggesting no difference between outcomes for patients with sCR and CR, the variation in the results are likely owing to the nonadherence in the latter study to the standard definition of the term sCR. In the Spanish study, sCR was defined as CR plus normal serum rFLC. The study did not mandate absence of clonality in the marrow as a prerequisite for achieving sCR.
The other important finding of equivalent survival outcomes in patients achieving lesser degrees of responses than CR merits further discussion. No survival difference is noted between patients achieving nCR and standard CR when those reaching sCR were categorized separately. Therefore, our study questions the prognostic value of further achieving immunofixation negativity after reaching nCR (ie, deepening response to standard CR) unless the rFLC normalizes and clonality disappears (ie, sCR is achieved). nCR and standard CR are two response categories merely separated by the detection of monoclonal protein on immunofixation, a test not infrequently riddled with subjective interpretation.36 Therefore, the comparable survival outcome of patients in these two categories in our study was not surprising. Indeed, sole reliance on this test has further come into question in a recent analysis of 295 patients on the GEM (Grupo Espanol de Mieloma) 2000 protocol.37 In this study, 31 patients who were immunofixation positive but minimal residual disease (MRD) negative by multiparameter flow cytometry had a significantly longer progression-free survival rate (65 v 37 months) compared with those who were immunofixation-negative but MRD-positive on day 100 after ASCT.37 To what extent the long half-life of some immunoglobulins leading to the persistence of small clonal bands on immunofixation after ASCT could account for this discrepancy is unclear. Indeed, small amounts of abnormal protein bands (monoclonal proteins and/or oligoclonal bands) seen after an ASCT could represent recovery of the immune system and may be unrelated to the original malignant clone. Given that a substantial proportion of the patients in our study with CR went on to attain sCR (75%), it is likely that the prognostic significance of CR seen in other studies so far is also primarily attributable to the proportion of patients with sCR rather than standard CR.
Use of second transplantation or maintenance therapies, albeit in a few patients who achieved less than a VGPR, could possibly have favorably affected the outcomes, making them comparable to those attaining VGPR/nCR. Patients with SD performed just as well as those attaining PR or VGPR, confirming the findings of a recent large Spanish study.28 A possible explanation could be related to the inherent disease biology of such patients with a monoclonal gammopathy of undetermined significance–like state that allows them a long progression-free interval and OS.4 A study from Arkansas found that a fraction of 10-year myeloma survivors had never attained CR and the gene expression profiles of such patients bore characteristics similar to those seen in monoclonal gammopathy of undetermined significance.38 Patients with PD failed to achieve cytoreduction despite HDT and, understandably, fared the worst.
Our analysis focuses on determination of survival outcome based on the best response obtained after ASCT, and particularly highlights the significance of reaching varying degrees of CR. The results indicate that sCR represents a deeper response state compared with conventional CR, translating to longer duration of response after stem-cell transplantation (ie, longer TTP) and improved OS. However, a criticism of the current response criteria is the reliance on random BM biopsies to ascertain the depth of response. Response assessment with sensitive imaging techniques such as magnetic resonance imaging or positron emission tomography (not factored in our cohort as well) is not universally advocated, despite unfavorable implications of persistent focal lesions beyond clinical CR, which can serve as potential sources for relapse.39
To overcome the inherent bias of studies comparing outcomes in responders and nonresponders (sCR v no sCR), we performed a landmark analysis of patients who survived at least 2 years after ASCT.40 In this analysis, patients achieving sCR have a markedly superior outcome compared with those achieving standard CR. However, among patients achieving sCR, those with sustained-sCR achieve the best outcome. We chose a 6-month cutoff merely to highlight the adverse implications of poorly sustained sCR.
An integrated approach with post-transplantation consolidation and maintenance therapies could permit attainment of more profound response states, including molecular remission. Although a few studies have attempted to illustrate the prognostic benefits of obtaining responses of such magnitude through the use of allele-specific oligonucleotide polymerase chain reaction, the practical application of this time-consuming and patient-specific primer-requiring test is limited.8 Multiparameter flow cytometry immunophenotyping to assess MRD also lacks widespread application currently.37 In contrast, our findings have substantial immediate clinical relevance owing to utilization of routinely available laboratory techniques.
In conclusion, our study demonstrates that post-ASCT sCR is a realistic, attainable goal and a surrogate for improved survival, and as such, it should be desirable and reported as a separate category in all myeloma-related clinical trials.
Acknowledgment
We thank Shiv Lal Pandey, MBBS, and Utkarsh Painuly, MD, for their assistance with data collection.
Footnotes
Listen to the podcast by Dr Anderson at www.jco.org/podcasts
Supported in part by the Mayo Clinic Hematological Malignancies Program and Paul Calabresi K12 Award (Grant No. CA90628), by Grants No. CA 107476, CA 62242, CA100707, and CA 83724 from the National Cancer Institute, and by the Jabbs Foundation and the Henry J. Predolin Foundation.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Morie A. Gertz, The Binding Site Group Research Funding: Angela Dispenzieri, Celgene, Millenium Pharmaceuticals, Janssen Pharmaceuticals, Pfizer Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Prashant Kapoor, Shaji K. Kumar, S. Vincent Rajkumar, Morie A. Gertz
Collection and assembly of data: Prashant Kapoor, Shaji K. Kumar, Angela Dispenzieri, Morie A. Gertz
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Barlogie B, Tricot GJ, van Rhee F, et al. Long-term outcome results of the first tandem autotransplant trial for multiple myeloma. Br J of Haematol. 2006;135:158–164. doi: 10.1111/j.1365-2141.2006.06271.x. [DOI] [PubMed] [Google Scholar]
- 2.Hari P, Pasquini MC, Vesole DH. Cure of multiple myeloma: More hype, less reality. Bone Marrow Transplant. 2006;37:1–18. doi: 10.1038/sj.bmt.1705194. [DOI] [PubMed] [Google Scholar]
- 3.Mehta J, Singhal S. High-dose chemotherapy and autologous hematopoietic stem cell transplantation in myeloma patients under the age of 65 years. Bone Marrow Transplant. 2007;40:1101–1114. doi: 10.1038/sj.bmt.1705799. [DOI] [PubMed] [Google Scholar]
- 4.Rajkumar SV. Treatment of myeloma: Cure vs control. Mayo Clin Proc. 2008;83:1142–1145. doi: 10.4065/83.10.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sirohi B, Powles R. International myeloma grand round. Lancet Oncol. 2001;2:571–579. doi: 10.1016/s1470-2045(01)00491-0. [DOI] [PubMed] [Google Scholar]
- 6.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 7.Usmani SZ, Crowley J, Hoering A, et al. Improvement in long-term outcomes with successive Total Therapy trials for multiple myeloma: Are patients now being cured? Leukemia. 2013;27:226–232. doi: 10.1038/leu.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ladetto M, Pagliano G, Ferrero S, et al. Major tumor shrinking and persistent molecular remissions after consolidation with bortezomib, thalidomide, and dexamethasone in patients with autografted myeloma. J Clin Oncol. 2010;28:2077–2084. doi: 10.1200/JCO.2009.23.7172. [DOI] [PubMed] [Google Scholar]
- 9.Paiva B, Martinez-Lopez J, Vidriales MB, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol. 2011;29:1627–1633. doi: 10.1200/JCO.2010.33.1967. [DOI] [PubMed] [Google Scholar]
- 10.Alexanian R, Delasalle K, Wang M, et al. Curability of multiple myeloma. Bone Marrow Res. 2012;2012:916479. doi: 10.1155/2012/916479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavo M, Tosi P, Zamagni E, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–2441. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 12.Chanan-Khan AA, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol. 2010;28:2612–2624. doi: 10.1200/JCO.2009.25.4250. [DOI] [PubMed] [Google Scholar]
- 13.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): A randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 14.Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: Up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–3136. [PubMed] [Google Scholar]
- 15.Kumar SK, Dingli D, Lacy MQ, et al. Outcome after autologous stem cell transplantation for multiple myeloma in patients with preceding plasma cell disorders. Br J Haematol. 2008;141:205–211. doi: 10.1111/j.1365-2141.2008.07069.x. [DOI] [PubMed] [Google Scholar]
- 16.Tricot G, Reiner M, Sawyer J, et al. A complete remission (CR) is not a prerequisite for prolonged survival after autotransplants for multiple myeloma. Blood. 2004:104. (abstr 926) [Google Scholar]
- 17.Dingli D, Pacheco JM, Nowakowski GS, et al. Relationship between depth of response and outcome in multiple myeloma. J Clin Oncol. 2007;25:4933–4937. doi: 10.1200/JCO.2007.11.7879. [DOI] [PubMed] [Google Scholar]
- 18.Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation: Myeloma Subcommittee of the EBMT—European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 19.Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215–224. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- 20.Greipp PR, Kumar S. Plasma cell labeling index. Methods Mol Med. 2005;113:25–35. doi: 10.1385/1-59259-916-8:25. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan E. MP: Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar SK, Lacy MQ, Dispenzieri A, et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer. 2012;118:1585–1592. doi: 10.1002/cncr.26422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gay F, Larocca A, Wijermans P, et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: Analysis of 1175 patients. Blood. 2011;117:3025–3031. doi: 10.1182/blood-2010-09-307645. [DOI] [PubMed] [Google Scholar]
- 25.McElwain TJ, Powles RL. High-dose intravenous melphalan for plasma-cell leukaemia and myeloma. Lancet. 1983;2:822–824. doi: 10.1016/s0140-6736(83)90739-0. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham D, Paz-Ares L, Milan S, et al. High-dose melphalan and autologous bone marrow transplantation as consolidation in previously untreated myeloma. J Clin Oncol. 1994;12:759–763. doi: 10.1200/JCO.1994.12.4.759. [DOI] [PubMed] [Google Scholar]
- 27.Barlogie B, van Rhee F, Shaughnessy JD, Jr, et al. Making progress in treating multiple myeloma with total therapies: Issue of complete remission and more. Leukemia. 2008;22:1633–1636. doi: 10.1038/leu.2008.40. [DOI] [PubMed] [Google Scholar]
- 28.Lahuerta JJ, Mateos MV, Martínez-López J, et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: Sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26:5775–5782. doi: 10.1200/JCO.2008.17.9721. [DOI] [PubMed] [Google Scholar]
- 29.Harousseau JL, Palumbo A, Richardson PG, et al. Superior outcomes associated with complete response in newly diagnosed multiple myeloma patients treated with nonintensive therapy: Analysis of the phase 3 VISTA study of bortezomib plus melphalan-prednisone versus melphalan-prednisone. Blood. 2010;116:3743–3750. doi: 10.1182/blood-2010-03-275800. [DOI] [PubMed] [Google Scholar]
- 30.Dispenzieri A, Zhang L, Katzmann JA, et al. Appraisal of immunoglobulin free light chain as a marker of response. Blood. 2008;111:4908–4915. doi: 10.1182/blood-2008-02-138602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: Results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28:4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 32.Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118:5752–5758. doi: 10.1182/blood-2011-05-355081. [DOI] [PubMed] [Google Scholar]
- 33.van de Velde HJ, Liu X, Chen G, et al. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92:1399–1406. doi: 10.3324/haematol.11534. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Lopez J, Blade J, Mateos MV, et al. Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. Blood. 2011;118:529–534. doi: 10.1182/blood-2011-01-332320. [DOI] [PubMed] [Google Scholar]
- 35.Barlogie B, Hall R, Zander A, et al. High-dose melphalan with autologous bone marrow transplantation for multiple myeloma. Blood. 1986;67:1298–1301. [PubMed] [Google Scholar]
- 36.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paiva B, Vidriales MB, Cerveró J, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112:4017–4023. doi: 10.1182/blood-2008-05-159624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhan F, Barlogie B, Arzoumanian V, et al. Gene-expression signature of benign monoclonal gammopathy evident in multiple myeloma is linked to good prognosis. Blood. 2007;109:1692–1700. doi: 10.1182/blood-2006-07-037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barlogie B, Anaissie E, Haessler J, et al. Complete remission sustained 3 years from treatment initiation is a powerful surrogate for extended survival in multiple myeloma. Cancer. 2008;113:355–359. doi: 10.1002/cncr.23546. [DOI] [PubMed] [Google Scholar]
- 40.Hoering A, Crowley J, Shaughnessy JD, Jr, et al. Complete remission in multiple myeloma examined as time-dependent variable in terms of both onset and duration in Total Therapy protocols. Blood. 2009;114:1299–1305. doi: 10.1182/blood-2009-03-211953. [DOI] [PMC free article] [PubMed] [Google Scholar]