Abstract
Purpose
Multiple myeloma is a hematologic malignancy originating from clonal plasma cells. Despite effective therapies, outcomes are highly variable suggesting marked disease heterogeneity. The role of functional imaging for therapeutic management of myeloma, such as positron emission tomography with 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG-PET), remains to be determined. Although some studies already suggested a prognostic value of 18F-FDG-PET, more specific tracers addressing hallmarks of myeloma biology, e.g. paraprotein biosynthesis, are needed. This study evaluated the amino acid tracers L-methyl-[11C]-methionine (11C-MET) and [18F]-fluoroethyl-L-tyrosine (18F-Fet) for their potential to image myeloma and to characterize tumor heterogeneity.
Experimental Design
To study the utility of 11C-MET, 18F-Fet and 18F-FDG for myeloma imaging, time activity curves were compared in various human myeloma cell lines (INA-6, MM1.S, OPM-2) and correlated to cell-biological characteristics, such as marker gene expression and immunoglobulin levels. Likewise, patient-derived CD138+ plasma cells were characterized regarding uptake and biomedical features.
Results
Using myeloma cell lines and patient-derived CD138+ plasma cells, we found that the relative uptake of 11C-MET exceeds that of 18F-FDG 1.5- to 5-fold and that of 18F-Fet 7- to 20-fold. Importantly, 11C-MET uptake significantly differed between cell types associated with worse prognosis (e.g. t(4;14) in OPM-2 cells) and indolent ones and correlated with intracellular immunoglobulin light chain and cell surface CD138 and CXCR4 levels. Direct comparison of radiotracer uptake in primary samples further validated the superiority of 11C-MET.
Conclusion
These data suggest that 11C-MET might be a versatile biomarker for myeloma superior to routine functional imaging with 18F-FDG regarding diagnosis, risk stratification, prognosis and discrimination of tumor subtypes.
Introduction
Multiple myeloma (MM), classified as a post-germinal center Non-Hodgkin`s lymphoma, is a hematological neoplasm originating from plasma cells. MM accounts for approximately 1% of all cancers and around 10% of hematological malignancies [1,2]. Despite recent advent of new therapeutics enabling more durable partial or complete remissions, almost all patients eventually relapse and die from their disease. A critical question remains whether - not yet clearly defined - subgroups of patients can benefit from more aggressive therapies. Due to high inter- and intra-patient tumor heterogeneity, identification of molecular lesions driving myeloma in individual patients is essential for the development of novel therapeutic algorithms [3-5]. Besides planar x-ray, the role of imaging for therapeutic management of MM and risk stratification remains to be determined. Several studies have demonstrated the usefulness of positron emission tomography (PET) using the radiolabeled glucose analog 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG) for diagnosis, staging and prognostication, leading to implementation into the revised Salmon/Durie staging system (Salmon/Durie PLUS) [6-10].
However, 18F-FDG PET has limited sensitivity and specificity: glucose uptake in inflammatory lesions can lead to false positive findings; the generally low metabolic activity of MM might account for false negative results, especially in case of diffuse bone marrow involvement [11]. MM is characterized by excess production of aberrant immunoglobulins (M-protein). Therefore, radiotracers addressing paraprotein biosynthesis and/or amino acid transport might serve as surrogate markers reflecting metabolic activity of the disease and, hence, prove useful for assessing response to therapy and prognosis in individual patients.
This study aimed at evaluating the amino acid tracers L-methyl-[11C]-methionine (11C-MET) and [18F]-fluoroethyl-L-tyrosine (18F-FET) for their potential to characterize MM lesions non-invasively. Time activity curves of 11C-MET, 18F-FET and 18F-FDG were compared in various human myeloma cell lines and correlated to hallmarks of MM biology, including levels of immunoglobulin (Ig) light chains, proliferation rate, as well as CD138 and CXCR4 expression. In a more physiological model, primary CD138+-plasma cells were analyzed regarding retention of imaging biomarkers. Uptake patterns were correlated to biomedical features of individual patient samples. Our data suggest that 11C-MET represents a versatile imaging biomarker for MM with the potential to specifically detect MM lesions using PET and to discriminate tumor subtypes.
Materials and Methods
Ethics statement
All experiments involving human material were approved by the ethics committee of the University Wuerzburg (#192/12). Bone marrow biopsies from patients diagnosed with MM were taken after obtaining informed written consent from each patient.
Cell culture
The human myeloma cell line INA-6 [12] was a gift from the Dept. of Hematology, University Hospital Wuerzburg. OPM-2 (DSMZ no. ACC50) cells were purchased from the German Collection of Microorganisms and Cell Culture (DSMZ, Braunschweig, Germany) and MM.1S (ATCC no. CRL-2974) were obtained from LGC Standards (Wesel, Germany). Cell lines were cultured in Roswell Park Memorial Institute Medium 1640 (supplemented with 10% FCS, 2mM L-glutamine, 1mM sodium pyruvate, 100 U/mL penicilline and 100 µg/mL streptomycine; all media and supplements: Invitrogen, Darmstadt, Germany) at 37 °C in a 5% CO2, humidified atmosphere. Additionally, 2.7 ng/mL hrIL-6 (Miltenyi, Bergisch-Gladbach, Germany) were added to cultures of INA-6 cells. Cell line identity was confirmed at the DSMZ (July 2013) by testing for the expression of eight different short tandem repeat loci according to the guidelines for authentication of human cell lines and, additionally, by examining for presence of rodent mitochondrial DNA sequences. Regular testing of cell cultures using the Venor GeM Mycoplasma Detection Kit (Sigma-Aldrich, Taufkirchen, Germany) ensured absence of contamination with mycoplasma.
Isolation of CD138+-plasma cells
CD138+-plasma cells were isolated from bone marrow aspirates of 19 patients diagnosed with MM by Ficoll density gradient centrifugation (density 1.007; Sigma-Aldrich, Taufkirchen, Germany) and positive selection using CD138+-micro beads and MACS technology (Miltenyi, Bergisch-Gladbach, Germany) after obtaining informed written consent. Purity of isolated cells was controlled by flow cytometry using an anti-hCD138+-APC antibody (Miltenyi, Bergisch-Gladbach, Germany). Isolated cells were diluted in PBS to a defined concentration and directly analyzed in uptake experiments.
Flow cytometric analyses
Single cell suspensions were stained with fluorochrome conjugated antibodies against hCD138+-APC (Syndecan; clone B-B4) or hCXCR4-PE (hCD184; clone 12G5; Miltenyi, Bergisch-Gladbach, Germany) and analyzed with a BD FACSCalibur flow cytometer using the BD CellQuest software (Beckton Dickinson, Heidelberg, Germany). Intracellular staining of immunoglobulin kappa and lambda light chains was performed using anti-hIg kappa light chain-APC (clone IS11-24D5) and anti-hIg lambda light chain-FITC (clone IS7-24C7) antibodies with the Inside Stain Kit from Miltenyi (Bergisch-Gladbach, Germany) according to the manufacturer's instructions.
Cell proliferation assay
Cells were seeded at a density of 1*105 cells per well in a 96-well plate in triplicates, grown for 48 h and were subsequently fixed with 70% ethanol. After overnight storage at 4 °C, cells were washed and stained with rabbit-anti-hKi67-FITC antibody (clone SP6; abcam, Camebridge, UK) according to the manufacturer's instructions. Geometric mean fluorescent activity (GeoMean) of samples was quantified with a BD FACSCalibur flow cytometer using the BD CellQuest software (Beckton Dickinson, Heidelberg, Germany) and corrected for background staining.
Synthesis of 18F-FDG, 18F-FET and 11C-MET
Radiopharmaceuticals were produced in house with a 16 MeV Cyclotron (GE PETtrace 6; GE Healthcare, Milwaukee, USA). 18F-FDG was synthesized using GE FASTlab methodology according to the manufacturer‘s instructions. 18F-FET was synthesized on a GE TRACERlab FX-FN as previously described by Bourdier et al. [13]. 11C-MET was synthesized on a GE TRACERlab FX-C Pro by on-column 11C-methylation of L-homocysteine with 11CH3I according to the procedures described by Kniess [14] and Gomzina and co-workers [15]. Before use, radiochemicals were analyzed by HPLC for radiochemical identity and purity.
Cellular uptake experiments
Sub-confluent cell cultures were harvested and adjusted to a concentration of 400.000 cells/ 500 µL PBS per sample. Radioactive substances were diluted to 1*106 counts per minute (cpm)/ 50 µL PBS. After addition of 1*106 cpm, samples were incubated for various times up to 120 min at 37 °C. Tracer uptake was stopped by incubation on ice, followed by washing twice with PBS to remove residual radioactivity. Intracellular radioactivity was quantified using a semi-automated gamma-counter (Wallac 1480-Wizard, Perkin Elmer, Rodgau, Germany). All samples were measured in triplicates. Background activity- and decay-corrected data were expressed counts per minute (cpm) per 1000 cells.
Statistical analysis
Statistical significance was assessed using Kruskal-Wallis-testing and posthoc analysis. A p-value of <0.05 was considered to be statistically significant. Analysis of correlation was done according to Pearson.
Results
Hallmarks of MM biology in myeloma cell lines
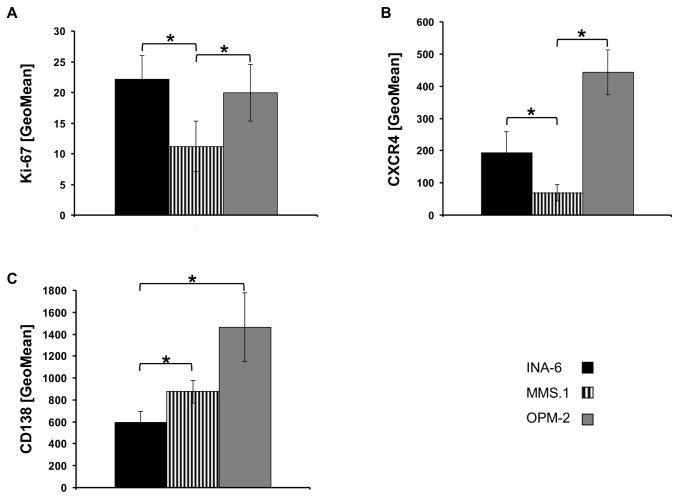
To reflect MM heterogeneity, MM cell lines with different clinical and cell-biological characteristics were selected (table 1). Cell lines were analyzed regarding hallmarks of MM pathology, such as proliferation rate, cell surface expression of CD138 and of CXCR4. The proliferative capacity, as assessed by flow cytometric Ki67-staining, differed significantly (p <0.05) between MM1.S versus OPM-2 and INA-6 cells, with the latter two growing roughly 2.5-times faster (Figure 1A). CXCR4, a homing factor for myeloma cells, was most abundant on OPM-2 cells; in contrast, INA-6 expressed only half as much CXCR4 and MM1.S cells approximately seven times less (Figure 1B). Quantification of the adhesion molecule CD138 revealed high cell surface levels on OPM-2 cells and markedly lower expression on MM1.S and INA-6 (Figure 1C).
Table 1. Characteristics of MM-cell lines reflect tumor heterogeneity.
| cell line | INA-6 | MM1.S | OPM-2 |
|---|---|---|---|
| reference | Burger (1994) | ARCC CRL-2974 | DSMZ ACC50 |
| species | human | human | human |
| diagnosis | MM | MM | MM |
| Ig | IgG κ | IgA λ | IgG λ |
| growth | suspension | partially adherent | suspension |
| misc. | IL-6 dependent | dexamethasone sensitive | t(4;14) hypertriploid |
Figure 1. Hallmarks of MM-biology in MM-cell lines.
(A) Proliferation rate. Cells were stained with anti-hKi67 FITC antibody and geometric mean fluorescent intensity (GeoMean) was quantified by FACS. All samples were analyzed in duplicates and background corrected (n=4). Cell surface expression of CXCR4 (B) and CD138+ (C) was analyzed by FACS. Cells were stained with an anti- hCXCR4-PE or anti- hCD138-APC antibody in duplicate, background-corrected and GeoMean was quantified (n=5). Columns represent mean values and error bars the standard deviation. Asterisk indicate statistically significant differences (p <0.05).
Intracellular immunoglobulin light chain levels
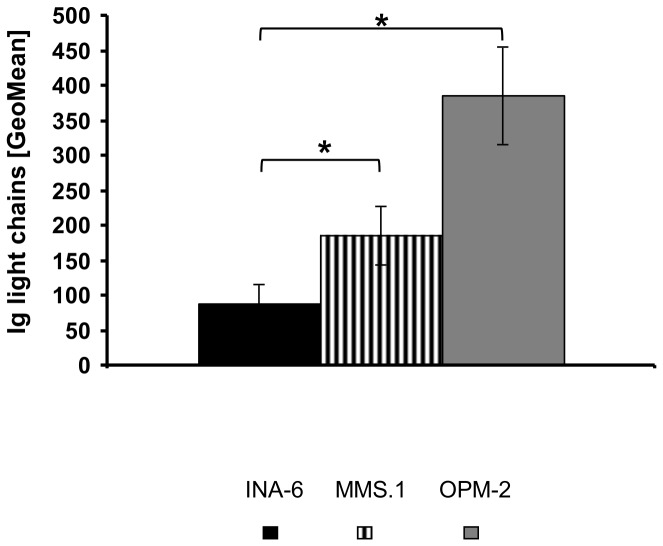
As MM is characterized by excess production of aberrant immunoglobulins, intracellular levels of kappa and lambda light chains were evaluated. In agreement with their origin (table 1), INA-6 cells stained positive for Ig kappa light chains, while all other cell lines produced Ig lambda light chains. Flow cytometric quantification demonstrated varying intracellular abundance of the respective light chains with increasing levels from INA-6 to MM1.S and OPM-2 cells (1 : 2 : 4; Figure 2).
Figure 2. Immunoglobulin κ/λ light chain levels.
Intracellular levels of either λ- (MM1.S, OPM-2) or κ- (INA-6) immunoglobulin light chains were determined by FACS analysis (GeoMean) using anti-Ig λ-FITC- and anti-Ig κ-APC antibodies. Background-corrected means ± standard deviation are shown (n=7). Asterisk indicate statistically significant differences (p <0.05).
Uptake of 11C-MET and 18F-FET by MM cell lines in comparison to 18F-FDG
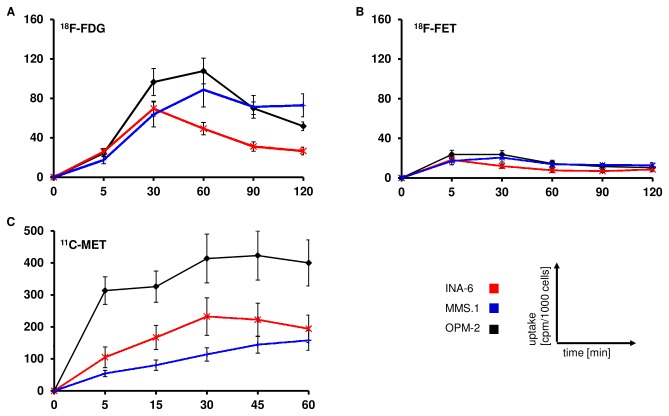
18F-FDG-PET is of value for the detection of MM-lesions, but radiotracers addressing the characteristic paraprotein biosynthesis might be more appropriate to reflect metabolic activity of the disease. Maximum uptake of 18F-FDG approximated 70-100 cpm/1000 cells in all cell lines and was reached after 30 min (INA-6) or 60 min (OPM-2, MM.1S), respectively. Thereafter, slightly decreasing radiotracer retention was observed (Figure 3A).
Figure 3. Uptake of 11C-MET and 18F-FET by MM-cell lines in comparison to 18F-FDG.
Intracellular radioactivity following incubation with 18F-FDG (A), 18F-FET (B) or 11C-MET (C) was quantified using a gamma-counter. Relative uptake of background- and decay-corrected triplicate-samples was expressed as cpm per 1000 cells (mean ± sem; n=5).
Levels of intracellular 18F-FET were significantly lower than those of 18F-FDG, with a maximum level of ~20 cpm/1000 cells (Figure 3B). Efflux of 18F-FET occurred rapidly. The highest retention was observed for 11C-MET and ranged between 144 cpm/1000cells for MM1.S cells (45 min), 232 cpm/1000cells for INA-6 (30 min) and 422 cpm/1000cells for OPM-2 cells (45 min). Already after 5 minutes post tracer application, relative uptake of 11C-MET exceeded maximal 18F-FDG retention drastically. Interestingly, 11C-MET levels discriminated two groups: methionine-uptake by OPM-2 cells was significantly higher than by INA-6 and MM.1S cells (Figure 3C).
Validation of 11C-MET, 18F-FET and 18F-FDG as surrogate markers of MM biology in CD138+-plasma cells
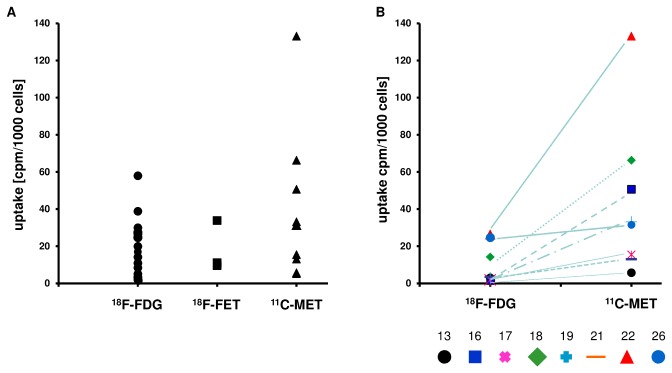
Next we set out to validate our findings using patient-derived MM cells (table 2). The strongly limited cell number in most samples only permitted single time point analyses. Whenever cell number allowed, cells isolated from one patient were split and one half was incubated for 60 min with either 11C-MET (patients no. 13, 16, 17, 18, 19, 21, 22, 26) or 18F-FET (patients no 7, 10, 11), whereas the second half was incubated with 18F-FDG for direct comparison between test and standard tracer. In agreement with the results in established cell lines, the amount of 18F-FET retained by primary MM-cells after 60 min tended to be less than that of 18F-FDG (Figure 4A). However, direct intra-sample comparison did not reveal clear differences between 18F-FET- and 18F-FDG-retention. Contrarily, primary MM cells had a markedly enhanced capacity to take up 11C-MET (Figure 4A). This latter finding was especially intriguing when directly comparing 18F-FDG and 11C-MET data (Figure 4B). Furthermore, higher 11C-MET retention in a sample tended to be accompanied by higher free immunoglobulin light chain levels (r = 0.509), but not by altered expression of Ki-67 (r= 0.033; Figure S1A+B). Together, these data underline the notion of 11C-MET being a promising marker for myeloma-imaging.
Table 2. Patient characteristics.
| Patient no. | age | sex | diagnosis | Ig | DS stage | initial diagnosis | cytogenetic alterations |
|---|---|---|---|---|---|---|---|
| 1 | 69 | ♀ | MM | κ light chains | IIIB | 06/2012 | del13q; t(4;14) |
| 2 | 61 | ♂ | MGUS | n.d. | n.d. | 2012 | n.d. |
| 3 | 73 | ♀ | MGUS | IgG κ | n.d. | n.d. | n.d. |
| 4 | 70 | ♀ | MM | IgA λ | II A | 01/2011 | n.d. |
| 5 | 80 | ♂ | MM | IgG κ | I | 07/2012 | n.d. |
| 6 | 41 | ♂ | MM | IgG κ | IIA | 12/2011 | hyperdiploid |
| 7 | 55 | ♂ | MM | IgG κ | n.d. | 08/2012 | normal |
| 9 | 71 | ♀ | MM | IgG κ | III A | 12/2011 | del13q |
| 10 | 62 | ♂ | MM | IgA λ | III A | n.d. | hyperdiploid |
| 11 | 64 | ♂ | MM | IgG κ | III A | 08/2012 | del13q |
| 12 | 62 | ♂ | MM | IgG κ | IIIA | 10/2012 | normal |
| 13 | 76 | ♂ | MM | IgG λ | III A | 10/2003 | normal |
| 14 | 64 | ♂ | MM | IgA κ | IA | 12/2002 | del13q |
| 15 | 73 | ♂ | MM | IgG κ | IIIA | 07/2006 | del13q; t(11;14) |
| 16 | 77 | ♂ | MM | λ light chains | n.d. | 06/2008 | n.d. |
| 17 | 65 | ♀ | MM | IgG λ | IIIB | 02/2009 | normal |
| 18 | 66 | ♂ | MM | IgG κ | IIA | 07/2006 | n.d. |
| 19 | 78 | ♂ | MM | IgG κ | IIA | 2006 | n.d. |
| 20 | 66 | ♀ | MM | IgG λ | IIIA | 1997 | del13q14; t(4;14) |
| 21 | 72 | ♂ | MM | IgG κ | IIIA | 04/1999 | n.d. |
| 22 | 53 | ♂ | MM | IgA λ | IIIB | 06/2007 | n.d. |
| 23 | 57 | ♀ | MM | IgG κ | IA | 06/2010 | del13q14; t(11;14) |
| 24 | 59 | ♂ | MM | IgG λ | IIIA | 04/2013 | t(11;14);t(14q32) tri13q14 |
| 25 | 73 | ♀ | MM | IgA κ | IIIA | 07/2013 | n.d. |
| 26 | 54 | ♀ | MM | IgG λ | II | 12/2008 | n.d. |
Figure 4. 11C-MET is superior to 18F-FET and 18F-FDG in CD138+-plasma cells.
CD138+-plasma cells were incubated with either 18F-FDG, 18F-FET or 11C-MET for 60 min and intracellular radioactivity was quantified using a gamma-counter. Relative uptake of background- and decay-corrected samples was expressed as cpm per 1000 cells. Whenever possible, bone marrow samples were split and one half of the sample was incubated with 18F-FDG, the other with either 18F-FET (patients no 7, 10, 11) or 11C-MET (patients no. 13, 16, 17, 18, 19, 21, 22, 26). (A) 18F-FDG, 18F-FET and 11C-MET uptake by CD138+ PCs. Data from all samples analyzed are shown. (B) Direct comparison of 18F-FDG and 11C-MET uptake in split samples. Lines indicate corresponding samples from one patient.
Discussion
Despite limited sensitivity and specificity, whole body x-ray is still considered as standard imaging test for detecting bone disease. The role of functional imaging in this scenario has not been clearly defined yet [6,16]. There is a growing body of evidence though that molecular imaging techniques, such as dynamic contrast-enhanced magnetic resonance imaging (MRI) or PET/computed tomography (PET/CT), might prove beneficial for discriminating active lesions from indolent ones, for assessment of treatment response and for therapeutic management of MM [7,8,10,17-22]. 18F-FDG-PET/CT has even been described as an emerging modality for imaging patients with multiple myeloma by the International Myeloma Working Group (IMWG). However, the concept of increased glucose metabolism as a surrogate for myeloma viability is hampered by non-specific retention of 18F-FDG in inflammatory lesions and reduced sensitivity in diffuse bone marrow infiltration. Moreover, several functional imaging approaches might be needed to accurately reflect tumor heterogeneity in MM [6,11,18].
In this study assessing the utility of alternative, potentially more specific imaging biomarkers for PET imaging, we have demonstrated a significantly higher retention of the radiolabeled amino acid 11C-MET in biologically diverse myeloma cells. In established cell lines, uptake of 11C-MET exceeded maximal 18F-FDG retention already after short incubation time and reached an approximately 1.5- to 5-fold higher uptake as compared to 18F-FDG and other tracers studied. Our data suggest that PET using 11C-MET as surrogate marker for paraprotein biosynthesis and amino acid turnover may outperform the current practice of imaging MM glucose use. These findings were recapitulated in primary MM cells derived from patients, providing further evidence of the utility of the proposed approach for MM imaging.
Imaging paraprotein biosynthesis as read-out for viable myeloma lesions is supported by two recently published pilot clinical trials reporting an equal or even greater number of lesions in patients with plasma cell malignancies detected by 11C-MET-PET, as compared to 18F-FDG-PET [23,24]. Together, these encouraging results warrant larger prospective clinical trials to corroborate the initial findings and to further investigate the clinical value of 11C-MET-PET in non- or oligo-secretory myelomas as well as in the setting of dedifferentiated extramedullary disease. Furthermore, due to higher retention in myeloma cells, 11C-MET might prove useful for the detection of diffuse bone marrow involvement, a setting which is known as a weakness of 18F-FDG-PET imaging [16].
Importantly, in our study two distinct groups of cell lines could be discriminated on basis of 11C-MET retention: enhanced 11C-MET uptake tended to match with higher levels of intracellular immunoglobulin light chains, higher CD138 and CXCR4 expression on the cell surface and presence of cytogenetic aberrations associated with worse prognosis (t(4;14) in OPM-2). As immunoglobulin synthesis is a hallmark of MM, increased 11C-MET retention might thus be explained by at least partial incorporation into (para-) proteins, as has been shown for other tumor entities [25,26]. Molecules mediating the interaction between myeloma cells and bone marrow stromal cells, immunoglobulin levels and cytogenetic alterations are important determinants of myeloma pathology and serve as markers for disease activity and/or aggressiveness [27-31]. Based on this, the potential association of CD138, CXCR4 and intracellular immunoglobulins with 11C-MET uptake we found here, might allow for non-invasive risk stratification of the individual patient and response monitoring using imaging with PET/CT. Our data further suggest that relative 11C-MET uptake might be able to reflect myeloma tumor biology and, hence, might facilitate assessment of myeloma heterogeneity and discrimination of tumor subtypes.
The precise role of CD138 and CXCR4 in myeloma pathology and management remains to be determined though. With the introduction of very specific, targeted radiotracers, such as radiolabeled antibodies or artificial ligands (e.g. CXCR4 antagonists [32,33] or anti-CD138 antibodies [34,35]), these two factors present interesting targets for further research and potential theranostic applications [35-39]. As CXCR4 expression regulates myeloma cell homing and has very recently been linked to MM prognosis [40], this marker might further be useful for discriminating intra- and extramedullary MM lesions [41].
Although our data suggest that more aggressive cells with a high uptake of 11C-Methionine feature a higher proliferation rate and higher levels of intracellular immunoglobulin light chains (OPM-2), the alternate hypothesis, that a reduction of immunoglobulin production is accompanied by enhanced proliferation in more aggressive myelomas, is plausible as well. Accordingly, we found a partial connection of immunoglobulin levels and 11C-MET uptake in patient-derived primary cells, but there was no statistically significant correlation. When comparing patients diagnosed with MGUS (patients no. 2, 3) to patients with aggressive symptomatic myeloma (translocation t(4;14); patients no. 1, 20), degree of bone marrow infiltration and Ki-67 index are lower in MGUS, but none of the other parameters described distinguishes between the asymptomatic precursor form and full-blown myeloma (table S1). Based on the data shown here this conflict cannot be unequivocally answered, particularly due to the limited sample size of our study. It also has to be considered that multiple myeloma is a very heterogenous disease. Attempts to stratify myeloma patients into risk groups have hardly been successful so far. Therefore it is conceivable that there simply is no general pattern characterizing a certain type of myeloma, but many different individual presentations in a longitudinal follow-up, underlining the need for individualized patient management.
It can be speculated that the minimal cell uptake of 18F-FET, as observed in our study, is due to its less efficient transport into cells caused by the 18F-linker. Furthermore, myeloma cells predominantly express the large amino acid transporter 1 (LAT1) and tyrosine preferentially enters cells via LAT2 [42]. Although the underlying pathophysiological mechanism remains unclear, 18F-FET does not seem to be a promising candidate biomarker in myeloma imaging.
In conclusion, 11C-MET might be superior to 18F-FDG regarding detection of active myeloma lesions. The higher sensitivity of 11C-MET could prove useful to overcome limitations of standard 18F-FDG-PET/CT including detection of minimal bone marrow infiltration, diffusely disseminated intramedullary disease and/or detection of myeloma cells with just marginally increased metabolism. The possibility of a connection between 11C-MET uptake and intracellular immunoglobulin light chain, CD138 and CXCR4 levels raises potential for patient risk stratification, response monitoring and treatment individualization.
Supporting Information
Free immunoglobulin light chain and Ki-67 expression in selected CD138+-plasma cell samples as a function of 11C-MET uptake. Levels of free immunoglobulin light chains in serum and percentage of Ki-67+ cells in bone marrow biopsies were obtained from routine diagnostic workup of selected patients (patients no. 13, 16, 17, 18, 19, 21, 22, 26). Correlation analysis according to Pearson of free immunoglobulin light chains (r = 0.509; A) or Ki-67 expression (r = 0.033; B) with 11C-MET uptake and of free immunoglobulin light chains and Ki-67 (r = 0.124; C) in CD138+-plasma cell samples is shown.
(DOCX)
Clinical presentation of MGUS vs. MM.
(DOCX)
Acknowledgments
We would like to thank Christa Albert for excellent technical assistance.
Funding Statement
This study was supported by the German Research Foundation (DFG) (105022/5-1 FUGG; http://www.dfg.de; funding was granted for establishment of a cyclotron and related laboratories) and the Open Access Publishing funding programme from the German Research Foundation (DFG) and the University of Wuerzburg. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Phekoo KJ, Schey SA, Richards MA, Bevan DH, Bell S et al. (2004) A population study to define the incidence and survival of multiple myeloma in a National Health Service Region in UK. Br J Haematol 127: 299-304. doi: 10.1111/j.1365-2141.2004.05207.x. PubMed: 15491289. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63: 11-30. doi: 10.3322/caac.21166. PubMed: 23335087. [DOI] [PubMed] [Google Scholar]
- 3. Kuehl WM, Bergsagel PL (2012) Molecular pathogenesis of multiple myeloma and its premalignant precursor. J Clin Invest 122: 3456-3463. doi: 10.1172/JCI61188. PubMed: 23023717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magrangeas F, Avet-Loiseau H, Gouraud W, Lodé L, Decaux O et al. (2013) Minor clone provides a reservoir for relapse in multiple myeloma. Leukemia 27: 473-481. doi: 10.1038/leu.2012.226. PubMed: 22874878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morgan GJ, Kaiser MF (2012) How to use new biology to guide therapy in multiple myeloma. Hematology Am Soc Hematol Educ Program; 2012: 342-349 [DOI] [PubMed] [Google Scholar]
- 6. Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M et al. (2009) International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia 23: 1545-1556. doi: 10.1038/leu.2009.89. PubMed: 19421229. [DOI] [PubMed] [Google Scholar]
- 7. Durie BG (2006) The role of anatomic and functional staging in myeloma: description of Durie/Salmon plus staging system. Eur J Cancer 42: 1539-1543. doi: 10.1016/j.ejca.2005.11.037. PubMed: 16777405. [DOI] [PubMed] [Google Scholar]
- 8. Durie BG, Waxman AD, D'Agnolo A, Williams CM (2002) Whole-body (18)F-FDG PET identifies high-risk myeloma. J Nucl Med 43: 1457-1463. PubMed: 12411548. [PubMed] [Google Scholar]
- 9. Regelink JC, Minnema MC, Terpos E, Kamphuis MH, Raijmakers PG et al. (2013) Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: a systematic review. Br J Haematol 162: 50-61. doi: 10.1111/bjh.12346. PubMed: 23617231. [DOI] [PubMed] [Google Scholar]
- 10. Bartel TB, Haessler J, Brown TL, Shaughnessy JD Jr., van Rhee F et al. (2009) F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood 114: 2068-2076. doi: 10.1182/blood-2009-03-213280. PubMed: 19443657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Terpos E, Moulopoulos LA, Dimopoulos MA (2011) Advances in imaging and the management of myeloma bone disease. J Clin Oncol 29: 1907-1915. doi: 10.1200/JCO.2010.32.5449. PubMed: 21483016. [DOI] [PubMed] [Google Scholar]
- 12. Burger R, Trautmann U, Hansen-Hagge TE, Strobel G, Bartram C, Kalden JR, Gramatzki M (1994) Two new interleukin-6 dependent plasma cell lines carrying a chromosomal abnormality involving the IL-6 gene locus. Br J Haematol 87: 4212. [Google Scholar]
- 13. Bourdier T, Greguric I, Roselt P, Jackson T, Faragalla J et al. (2011) Fully automated one-pot radiosynthesis of O-(2-[18F]fluoroethyl)-L-tyrosine on the TracerLab FX(FN) module. Nucl Med Biol 38: 645-651. doi: 10.1016/j.nucmedbio.2011.01.001. PubMed: 21718939. [DOI] [PubMed] [Google Scholar]
- 14. Kniess T, Rode K, Wuest F (2008) Practical experiences with the synthesis of [11C] CH3I through gas phase iodination reaction using a TRACERlabFXC synthesis module. Appl Radiat Isot 66: 482-488. [DOI] [PubMed] [Google Scholar]
- 15. Gomzina NA, Kuznetsova OF (2011) L-[methyl-(11C)]-methionine of high enantiomeric purity production via on-line 11C-methylation of L-homocysteine thiolactone hydrochloride. Bioorg Khim 37: 216-222. PubMed: 21721254. [DOI] [PubMed] [Google Scholar]
- 16. Hillengass J, Landgren O (2013) Challenges and opportunities of novel imaging techniques in monoclonal plasma cell disorders: imaging "early myeloma". Leuk Lymphoma 54: 1355-1363. doi: 10.3109/10428194.2012.740559. PubMed: 23289361. [DOI] [PubMed] [Google Scholar]
- 17. Agarwal A, Chirindel A, Shah BA, Subramaniam RM (2013) Evolving role of FDG PET/CT in multiple myeloma imaging and management. AJR Am J Roentgenol 200: 884-890. doi: 10.2214/AJR.12.9653. PubMed: 23521465. [DOI] [PubMed] [Google Scholar]
- 18. Bannas P, Kröger N, Adam G, Derlin T (2013) Modern imaging techniques in patients with multiple myeloma. Rofo 185: 26-33. PubMed: 23196838. [DOI] [PubMed] [Google Scholar]
- 19. Hillner BE, Siegel BA, Shields AF, Liu D, Gareen IF et al. (2008) Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the national oncologic PET registry. J Nucl Med 49: 1928-1935. doi: 10.2967/jnumed.108.056713. PubMed: 18997054. [DOI] [PubMed] [Google Scholar]
- 20. Schirrmeister H, Buck AK, Bergmann L, Reske SN, Bommer M (2003) Positron emission tomography (PET) for staging of solitary plasmacytoma. Cancer Biother Radiopharm 18: 841-845. doi: 10.1089/108497803770418382. PubMed: 14629832. [DOI] [PubMed] [Google Scholar]
- 21. Shortt CP, Gleeson TG, Breen KA, McHugh J, O'Connell MJ et al. (2009) Whole-Body MRI versus PET in assessment of multiple myeloma disease activity. AJR Am J Roentgenol 192: 980-986. doi: 10.2214/AJR.08.1633. PubMed: 19304704. [DOI] [PubMed] [Google Scholar]
- 22. Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E et al. (2011) Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood 118: 5989-5995. doi: 10.1182/blood-2011-06-361386. PubMed: 21900189. [DOI] [PubMed] [Google Scholar]
- 23. Dankerl A, Liebisch P, Glatting G, Friesen C, Blumstein NM et al. (2007) Multiple Myeloma: Molecular Imaging with 11C-Methionine PET/CT--Initial Experience. Radiology 242: 498-508. doi: 10.1148/radiol.2422051980. PubMed: 17179397. [DOI] [PubMed] [Google Scholar]
- 24. Nakamoto Y, Kurihara K, Nishizawa M, Yamashita K, Nakatani K, et al. (2013) Clinical value of (1) (1)C-methionine PET/CT in patients with plasma cell malignancy: comparison with (1)(8)F-FDG PET/CT. Eur J Nucl Med Mol Imaging 40: 708-715 [DOI] [PubMed]
- 25. Ishiwata K, Vaalburg W, Elsinga PH, Paans AM, Woldring MG (1988) Comparison of L-[1-11C]methionine and L-methyl-[11C]methionine for measuring in vivo protein synthesis rates with PET. J Nucl Med 29: 1419-1427. PubMed: 3261334. [PubMed] [Google Scholar]
- 26. Jager PL, Vaalburg W, Pruim J, de Vries EG, Langen KJ et al. (2001) Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med 42: 432-445. PubMed: 11337520. [PubMed] [Google Scholar]
- 27. Dispenzieri A, Kyle R, Merlini G, Miguel JS, Ludwig H et al. (2009) International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia 23: 215-224. doi: 10.1038/leu.2008.307. PubMed: 19020545. [DOI] [PubMed] [Google Scholar]
- 28. Magrangeas F, Nasser V, Avet-Loiseau H, Loriod B, Decaux O et al. (2003) Gene expression profiling of multiple myeloma reveals molecular portraits in relation to the pathogenesis of the disease. Blood 101: 4998-5006. doi: 10.1182/blood-2002-11-3385. PubMed: 12623842. [DOI] [PubMed] [Google Scholar]
- 29. Reijmers RM, Spaargaren M, Pals ST (2013) Heparan sulfate proteoglycans in the control of B cell development and the pathogenesis of multiple myeloma. FEBS J 280: 2180-2193. doi: 10.1111/febs.12180. PubMed: 23419151. [DOI] [PubMed] [Google Scholar]
- 30. Iwama K, Chihara D, Tsuda K, Ugai T, Sugihara H et al. (2013) Normalization of free light chain kappa/lambda ratio is a robust prognostic indicator of favorable outcome in patients with multiple myeloma. Eur J Haematol 90: 134-141. doi: 10.1111/ejh.12050. PubMed: 23210517. [DOI] [PubMed] [Google Scholar]
- 31. Salamon J, Derlin T, Bannas P, Busch JD, Herrmann J et al. (2013) Evaluation of intratumoural heterogeneity on (1)(8)F-FDG PET/CT for characterization of peripheral nerve sheath tumours in neurofibromatosis type 1. Eur J Nucl Med Mol Imaging 40: 685-692. doi: 10.1007/s00259-012-2314-6. PubMed: 23232507. [DOI] [PubMed] [Google Scholar]
- 32. Kuhne MR, Mulvey T, Belanger B, Chen S, Pan C et al. (2013) BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin Cancer Res 19: 357-366. doi: 10.1158/1078-0432.CCR-12-2333. PubMed: 23213054. [DOI] [PubMed] [Google Scholar]
- 33. Harris SM, Davis JC, Snyder SE, Butch ER, Vavere AL et al. (2013) Evaluation of the Biodistribution of 11C-Methionine in Children and Young Adults. J Nucl Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Delcommenne M, Klingemann HG (2012) Detection and characterization of syndecan-1-associated heparan sulfate 6-O-sulfated motifs overexpressed in multiple myeloma cells using single chain antibody variable fragments. Hum Antibodies 21: 29-40. PubMed: 22885958. [DOI] [PubMed] [Google Scholar]
- 35. Rousseau C, Ferrer L, Supiot S, Bardiès M, Davodeau F et al. (2012) Dosimetry results suggest feasibility of radioimmunotherapy using anti-CD138 (B-B4) antibody in multiple myeloma patients. Tumour Biol 33: 679-688. doi: 10.1007/s13277-012-0362-y. PubMed: 22389160. [DOI] [PubMed] [Google Scholar]
- 36. Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F et al. (2009) CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 113: 4341-4351. doi: 10.1182/blood-2008-10-186668. PubMed: 19139079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diamond P, Labrinidis A, Martin SK, Farrugia AN, Gronthos S et al. (2009) Targeted disruption of the CXCL12/CXCR4 axis inhibits osteolysis in a murine model of myeloma-associated bone loss. J Bone Miner Res 24: 1150-1161. doi: 10.1359/jbmr.090210. PubMed: 19335218. [DOI] [PubMed] [Google Scholar]
- 38. Kim HY, Hwang JY, Kim SW, Lee HJ, Yun HJ et al. (2010) The CXCR4 Antagonist AMD3100 Has Dual Effects on Survival and Proliferation of Myeloma Cells In Vitro. Cancer Res Treat 42: 225-234. doi: 10.4143/crt.2010.42.4.225. PubMed: 21253325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Udi J, Schüler J, Wider D, Ihorst G, Catusse J et al. (2013) Potent in vitro and in vivo activity of sorafenib in multiple myeloma: induction of cell death, CD138-downregulation and inhibition of migration through actin depolymerization. Br J Haematol 161: 104-116. doi: 10.1111/bjh.12226. PubMed: 23384035. [DOI] [PubMed] [Google Scholar]
- 40. Finno CJ, Famula T, Aleman M, Higgins RJ, Madigan JE et al. (2013) Pedigree analysis and exclusion of alpha-tocopherol transfer protein (TTPA) as a candidate gene for neuroaxonal dystrophy in the American Quarter Horse. J Vet Intern Med 27: 177-185. doi: 10.1111/jvim.12015. PubMed: 23186252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stessman HA, Mansoor A, Zhan F, Janz S, Linden MA et al. (2013) Reduced CXCR4 expression is associated with extramedullary disease in a mouse model of myeloma and predicts poor survival in multiple myeloma patients treated with bortezomib. Leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Okubo S, Zhen HN, Kawai N, Nishiyama Y, Haba R et al. (2010) Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. J Neurooncol 99: 217-225. doi: 10.1007/s11060-010-0117-9. PubMed: 20091333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Free immunoglobulin light chain and Ki-67 expression in selected CD138+-plasma cell samples as a function of 11C-MET uptake. Levels of free immunoglobulin light chains in serum and percentage of Ki-67+ cells in bone marrow biopsies were obtained from routine diagnostic workup of selected patients (patients no. 13, 16, 17, 18, 19, 21, 22, 26). Correlation analysis according to Pearson of free immunoglobulin light chains (r = 0.509; A) or Ki-67 expression (r = 0.033; B) with 11C-MET uptake and of free immunoglobulin light chains and Ki-67 (r = 0.124; C) in CD138+-plasma cell samples is shown.
(DOCX)
Clinical presentation of MGUS vs. MM.
(DOCX)