Abstract
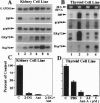
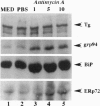
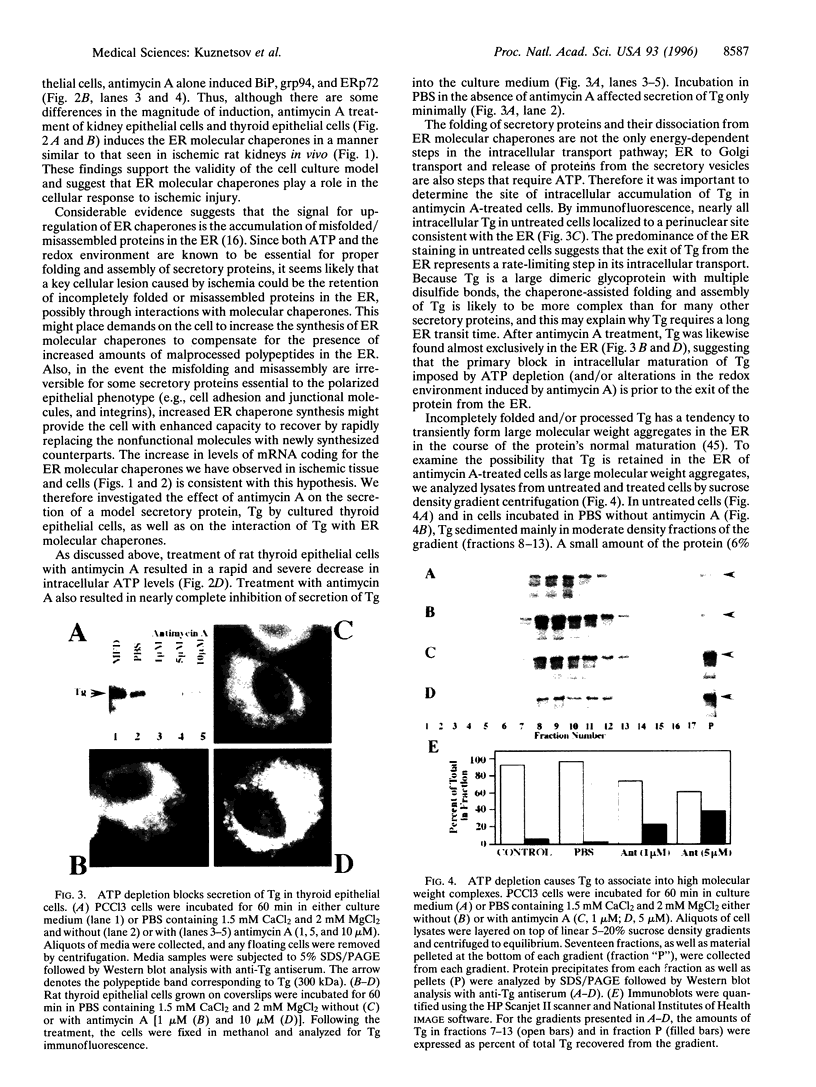
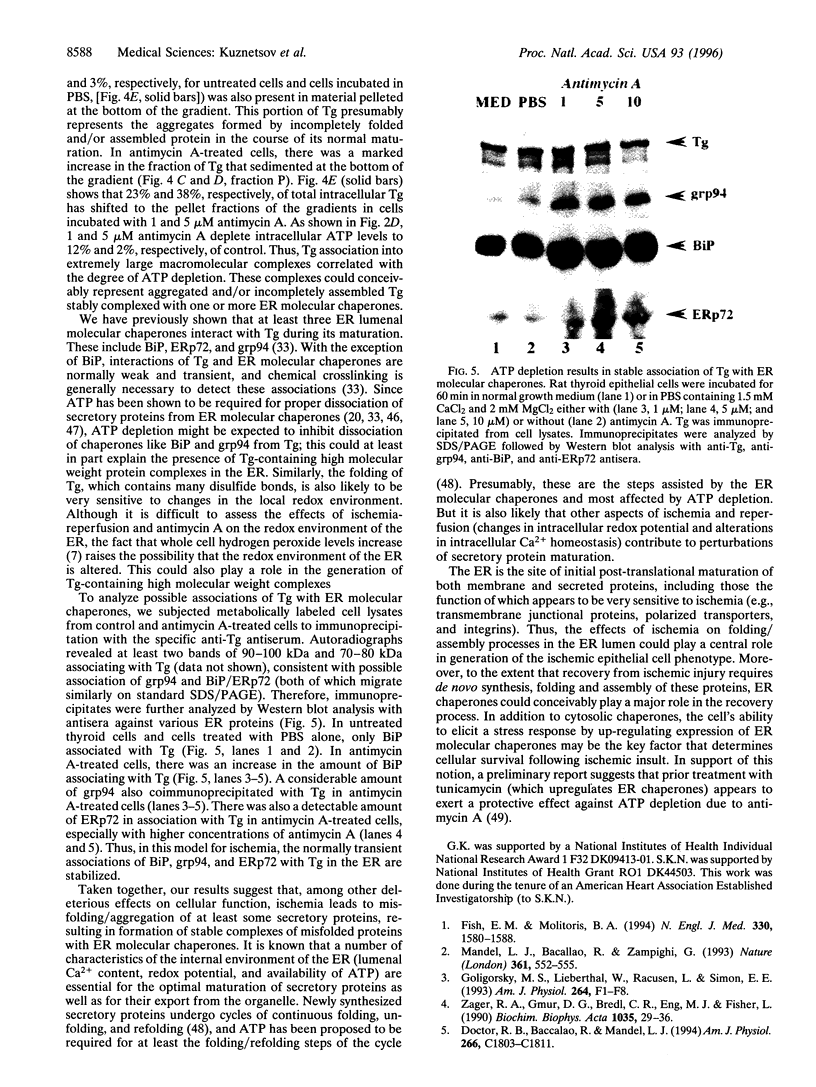
The effects of ischemia on the maturation of secretory proteins are not well understood. Among several events that occur during ischemia-reperfusion are a rapid and extensive decrease in ATP levels and an alteration of cellular oxidative state. Since the normal folding and assembly of secretory proteins are mediated by endoplasmic reticulum (ER) molecular chaperones, the function of which depends on ATP and maintenance of an appropriate redox environment, ischemia might be expected to perturb folding of secretory proteins. In this study, whole animal and cultured cell models for the epithelial ischemic state were used to examine this possibility. After acute kidney ischemia, marked increases in the mRNA levels of the ER chaperones glucose-regulated protein (grp)78/immunoglobulin-binding protein (BiP), grp94, and ER protein (ERp)72 were noted. Likewise, when cellular ATP was depleted to less than 10% of control with antimycin A, mRNA levels of BiP, ERp72, and grp94 were increased in kidney and thyroid epithelial cell culture models. Since the signal for the up-regulation of these stress proteins is believed to be the accumulation of misfolded/misassembled secretory proteins in the ER, their induction after ischemia in vivo and antimycin treatment of cultured cells suggests that maturation of secretory proteins in the ER lumen might indeed be perturbed. To analyze the effects of antimycin A on the maturation of secretory proteins, we studied the fate of thyroglobulin (Tg), a large oligomeric secretory glycoprotein, the folding and assembly of which seems to require a variety of ER chaperones. Treatment of cultured thyroid epithelial cells with antimycin A greatly inhibited ( > 90%) the secretion of Tg. Sucrose density gradient analysis revealed that in antimycin A-treated cells Tg associates into large macromolecular complexes which, by immunofluorescence, appeared to localize to the ER. Furthermore, coimmunoprecipitation studies after antimycin A treatment demonstrated that Tg stably associates with BiP, grp94, and ERp72. Together, our results suggest that a key cellular lesion in ischemia is the misfolding of secretory proteins as they transit the ER, and this leads not only to increased expression of ER chaperones but also to their stable association with and the subsequent retention of at least some misfolded secretory proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Tashjian A. H., Jr Ionomycin acts as an ionophore to release TRH-regulated Ca2+ stores from GH4C1 cells. Am J Physiol. 1986 Dec;251(6 Pt 1):C887–C891. doi: 10.1152/ajpcell.1986.251.6.C887. [DOI] [PubMed] [Google Scholar]
- Bacallao R., Garfinkel A., Monke S., Zampighi G., Mandel L. J. ATP depletion: a novel method to study junctional properties in epithelial tissues. I. Rearrangement of the actin cytoskeleton. J Cell Sci. 1994 Dec;107(Pt 12):3301–3313. doi: 10.1242/jcs.107.12.3301. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Brenner M. B., Thomas D. Y., Williams D. B. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994 Mar;19(3):124–128. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Blond-Elguindi S., Fourie A. M., Sambrook J. F., Gething M. J. Peptide-dependent stimulation of the ATPase activity of the molecular chaperone BiP is the result of conversion of oligomers to active monomers. J Biol Chem. 1993 Jun 15;268(17):12730–12735. [PubMed] [Google Scholar]
- Borkan S. C., Emami A., Schwartz J. H. Heat stress protein-associated cytoprotection of inner medullary collecting duct cells from rat kidney. Am J Physiol. 1993 Sep;265(3 Pt 2):F333–F341. doi: 10.1152/ajprenal.1993.265.3.F333. [DOI] [PubMed] [Google Scholar]
- Braakman I., Helenius J., Helenius A. Role of ATP and disulphide bonds during protein folding in the endoplasmic reticulum. Nature. 1992 Mar 19;356(6366):260–262. doi: 10.1038/356260a0. [DOI] [PubMed] [Google Scholar]
- Bush K. T., Hendrickson B. A., Nigam S. K. Induction of the FK506-binding protein, FKBP13, under conditions which misfold proteins in the endoplasmic reticulum. Biochem J. 1994 Nov 1;303(Pt 3):705–708. doi: 10.1042/bj3030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang H. L., Terlecky S. R., Plant C. P., Dice J. F. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989 Oct 20;246(4928):382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- Clairmont C. A., De Maio A., Hirschberg C. B. Translocation of ATP into the lumen of rough endoplasmic reticulum-derived vesicles and its binding to luminal proteins including BiP (GRP 78) and GRP 94. J Biol Chem. 1992 Feb 25;267(6):3983–3990. [PubMed] [Google Scholar]
- Cox J. S., Shamu C. E., Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993 Jun 18;73(6):1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- Doctor R. B., Bacallao R., Mandel L. J. Method for recovering ATP content and mitochondrial function after chemical anoxia in renal cell cultures. Am J Physiol. 1994 Jun;266(6 Pt 1):C1803–C1811. doi: 10.1152/ajpcell.1994.266.6.C1803. [DOI] [PubMed] [Google Scholar]
- Donnelly T. J., Sievers R. E., Vissern F. L., Welch W. J., Wolfe C. L. Heat shock protein induction in rat hearts. A role for improved myocardial salvage after ischemia and reperfusion? Circulation. 1992 Feb;85(2):769–778. doi: 10.1161/01.cir.85.2.769. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Wasley L. C., Kaufman R. J. Protein dissociation from GRP78 and secretion are blocked by depletion of cellular ATP levels. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7429–7432. doi: 10.1073/pnas.87.19.7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Fish E. M., Molitoris B. A. Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med. 1994 Jun 2;330(22):1580–1588. doi: 10.1056/NEJM199406023302207. [DOI] [PubMed] [Google Scholar]
- Freedman R. B., Hawkins H. C., McLaughlin S. H. Protein disulfide-isomerase. Methods Enzymol. 1995;251:397–406. doi: 10.1016/0076-6879(95)51143-1. [DOI] [PubMed] [Google Scholar]
- Gabai V. L., Kabakov A. E. Rise in heat-shock protein level confers tolerance to energy deprivation. FEBS Lett. 1993 Aug 2;327(3):247–250. doi: 10.1016/0014-5793(93)80997-9. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Goligorsky M. S., Lieberthal W., Racusen L., Simon E. E. Integrin receptors in renal tubular epithelium: new insights into pathophysiology of acute renal failure. Am J Physiol. 1993 Jan;264(1 Pt 2):F1–F8. doi: 10.1152/ajprenal.1993.264.1.F1. [DOI] [PubMed] [Google Scholar]
- González-Flecha B., Cutrin J. C., Boveris A. Time course and mechanism of oxidative stress and tissue damage in rat liver subjected to in vivo ischemia-reperfusion. J Clin Invest. 1993 Feb;91(2):456–464. doi: 10.1172/JCI116223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman E. F., Saji M., Shimura Y., Lau J. T., Ashwell G. Thyrotropin regulation of sialic acid expression in rat thyroid cells. J Biol Chem. 1993 Feb 15;268(5):3604–3609. [PubMed] [Google Scholar]
- Helenius A. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol Biol Cell. 1994 Mar;5(3):253–265. doi: 10.1091/mbc.5.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida H., Kohmoto O., Bridge J. H., Barry W. H. Alterations in cation homeostasis in cultured chick ventricular cells during and after recovery from adenosine triphosphate depletion. J Clin Invest. 1988 Apr;81(4):1173–1181. doi: 10.1172/JCI113432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmazyn M., Mailer K., Currie R. W. Acquisition and decay of heat-shock-enhanced postischemic ventricular recovery. Am J Physiol. 1990 Aug;259(2 Pt 2):H424–H431. doi: 10.1152/ajpheart.1990.259.2.H424. [DOI] [PubMed] [Google Scholar]
- Kassenbrock C. K., Kelly R. B. Interaction of heavy chain binding protein (BiP/GRP78) with adenine nucleotides. EMBO J. 1989 May;8(5):1461–1467. doi: 10.1002/j.1460-2075.1989.tb03529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. S., Arvan P. Calnexin and BiP act as sequential molecular chaperones during thyroglobulin folding in the endoplasmic reticulum. J Cell Biol. 1995 Jan;128(1-2):29–38. doi: 10.1083/jcb.128.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. S., Bole D., Arvan P. Transient aggregation of nascent thyroglobulin in the endoplasmic reticulum: relationship to the molecular chaperone, BiP. J Cell Biol. 1992 Aug;118(3):541–549. doi: 10.1083/jcb.118.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov G., Chen L. B., Nigam S. K. Several endoplasmic reticulum stress proteins, including ERp72, interact with thyroglobulin during its maturation. J Biol Chem. 1994 Sep 16;269(37):22990–22995. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lovis C., Mach F., Donati Y. R., Bonventre J. V., Polla B. S. Heat shock proteins and the kidney. Ren Fail. 1994;16(2):179–192. doi: 10.3109/08860229409044859. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Bacallao R., Zampighi G. Uncoupling of the molecular 'fence' and paracellular 'gate' functions in epithelial tight junctions. Nature. 1993 Feb 11;361(6412):552–555. doi: 10.1038/361552a0. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Doctor R. B., Bacallao R. ATP depletion: a novel method to study junctional properties in epithelial tissues. II. Internalization of Na+,K(+)-ATPase and E-cadherin. J Cell Sci. 1994 Dec;107(Pt 12):3315–3324. doi: 10.1242/jcs.107.12.3315. [DOI] [PubMed] [Google Scholar]
- Marber M. S., Mestril R., Chi S. H., Sayen M. R., Yellon D. M., Dillmann W. H. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995 Apr;95(4):1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarella R. A., Srinivasan M., Haugejorden S. M., Green M. ERp72, an abundant luminal endoplasmic reticulum protein, contains three copies of the active site sequences of protein disulfide isomerase. J Biol Chem. 1990 Jan 15;265(2):1094–1101. [PubMed] [Google Scholar]
- Nigam S. K., Goldberg A. L., Ho S., Rohde M. F., Bush K. T., Sherman MYu A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes Ca(2+)-binding proteins and members of the thioredoxin superfamily. J Biol Chem. 1994 Jan 21;269(3):1744–1749. [PubMed] [Google Scholar]
- Nigam S. K., Jin Y. J., Jin M. J., Bush K. T., Bierer B. E., Burakoff S. J. Localization of the FK506-binding protein, FKBP 13, to the lumen of the endoplasmic reticulum. Biochem J. 1993 Sep 1;294(Pt 2):511–515. doi: 10.1042/bj2940511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMACHANDRAN S., GOTTLIEB D. Mode of action of antibiotics. II. Specificity of action of antimycin A and ascosin. Biochim Biophys Acta. 1961 Oct 28;53:396–402. doi: 10.1016/0006-3002(61)90451-6. [DOI] [PubMed] [Google Scholar]
- Rowling P. J., Freedman R. B. Folding, assembly, and posttranslational modification of proteins within the lumen of the endoplasmic reticulum. Subcell Biochem. 1993;21:41–80. doi: 10.1007/978-1-4615-2912-5_3. [DOI] [PubMed] [Google Scholar]
- Sciandra J. J., Subjeck J. R., Hughes C. S. Induction of glucose-regulated proteins during anaerobic exposure and of heat-shock proteins after reoxygenation. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4843–4847. doi: 10.1073/pnas.81.15.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki C. K., Bonifacino J. S., Lin A. Y., Davis M. M., Klausner R. D. Regulating the retention of T-cell receptor alpha chain variants within the endoplasmic reticulum: Ca(2+)-dependent association with BiP. J Cell Biol. 1991 Jul;114(2):189–205. doi: 10.1083/jcb.114.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Why S. K., Mann A. S., Thulin G., Zhu X. H., Kashgarian M., Siegel N. J. Activation of heat-shock transcription factor by graded reductions in renal ATP, in vivo, in the rat. J Clin Invest. 1994 Oct;94(4):1518–1523. doi: 10.1172/JCI117492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992 Oct;72(4):1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Williams R. S., Thomas J. A., Fina M., German Z., Benjamin I. J. Human heat shock protein 70 (hsp70) protects murine cells from injury during metabolic stress. J Clin Invest. 1993 Jul;92(1):503–508. doi: 10.1172/JCI116594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wägar G. Effect of actinomycin D on TSH-induced stimulation of thyroidal protein synthesis in the rat. Acta Endocrinol (Copenh) 1974 Sep;77(1):64–70. [PubMed] [Google Scholar]
- Zager R. A., Gmur D. J., Bredl C. R., Eng M. J., Fisher L. Regional responses within the kidney to ischemia: assessment of adenine nucleotide and catabolite profiles. Biochim Biophys Acta. 1990 Jul 20;1035(1):29–36. doi: 10.1016/0304-4165(90)90169-w. [DOI] [PubMed] [Google Scholar]