Abstract
During development, layer 2/3 neurons in the neocortex extend their axons horizontally, within the same layers, and stop growing at appropriate locations to form branches and synaptic connections. Firing and synaptic activity are thought to be involved in this process, but how neuronal activity regulates axonal growth is not clear. Here, we studied axonal growth of layer 2/3 neurons by exciting cell bodies or axonal processes in organotypic slice cultures of the rat cortex. For neuronal stimulation and morphological observation, plasmids encoding channelrhodopsin-2 (ChR2) and DsRed were coelectroporated into a small number of layer 2/3 cells. Firing activity induced by photostimulation (475 nm) was confirmed by whole-cell patch recording. Axonal growth was observed by time-lapse confocal microscopy, using a different excitation wavelength (560 nm), at 10–20-min intervals for several hours. During the first week in vitro, when spontaneous neuronal activity is low, DsRed- and ChR2-expressing axons grew at a constant rate. When high-frequency photostimulation (4 or 10 Hz) for 1 min was applied to the soma or axon, most axons paused in their growth. In contrast, lower-frequency stimulation did not elicit this pause behavior. Moreover, in the presence of tetrodotoxin, even high-frequency stimulation did not cause axonal growth to pause. These results indicate that increasing firing activity during development suppresses axon growth, suggesting the importance of neuronal activity for the formation of horizontal connections.
Introduction
Neocortical circuits are known to be established not only by genetic mechanisms but also by environmental factors such as sensory-evoked neuronal activity. Among the best examples of this formation process are the projections from the thalamic nuclei to the cortices [1]. Intrinsic cortical circuits are also thought to be established based on genetic and activity-dependent mechanisms. Axon collaterals from layer 2/3 pyramidal neurons, called horizontal axons, connect cells within the same layer, and these connections provide a substrate for lateral interactions across cortical columns [2]–[8]. In the visual cortex of higher mammals, the horizontal connections primarily link cortical cells in columns having the same orientation [9]–[15]. Horizontal connections have also been found in other cortical areas, and are the basis of information processing in the neocortex [16]–[23].
During development, horizontal axons start to grow in all directions from the cell body, and then display further elongation and initial branch formation at later periods [24], [25]. Ultimately, only specific locations undergo robust and complex branch formation, whereas other regions show selective retraction of inappropriate connections. This developmental scheme is similar among different mammalian species, albeit with some differences in the length of maturation period [24]–[28].
Neuronal activity is essential for the formation of horizontal connections, since the organization of these connections is disrupted by sensory deprivation and by blockade of action potentials [29]–[32]. Furthermore, our previous in vitro studies have demonstrated that firing and synaptic activity promote horizontal axon branching [33], [34]. Nevertheless, how electrical activity contributes to the regulation of horizontal axon growth remains unknown.
To address this issue, here we studied axon growth of layer 2/3 cells in organotypic cortical slice culture by manipulating firing activity using an optogenetic technique [35]–[38]. Light-gated algal channel channelrhodopsin-2 (ChR2) enables neuronal activity to be generated in a cell-specific way with high temporal resolution and is therefore an excellent tool to study the complex mechanism of cortical network formation. The results revealed that horizontally elongating axons pause in their growth after high-frequency stimulation, suggesting that developing firing activity contributes to circuit formation by regulating axonal growth.
Materials and Methods
Animals and Ethics Statement
Sprague-Dawley rats were used for this study. All experiments were performed according to guidelines laid down by the animal welfare committees of Osaka University and the Japanese Neuroscience Society. The protocol was approved by the Committee on the Ethics of Animal Experiments of Osaka University (Permit Number: FBS 07–037).
Organotypic Slice Culture
To observe horizontal axons, we used a previously described organotypic slice culture system [39], [40]. In this culture, cortical circuits including horizontal connections are reproduced with the cortical laminar structure preserved [33]. In brief, the occipital region of the cortex was dissected from postnatal day 0 (P0) or P1 rats. The slices were placed on a membrane filter (MilliCell-CM Low Height PICMORG-50, Millipore, Billerica, MA) coated with rat tail collagen. The culture medium consisted of a 1∶1 mixture of Dulbecco’s modified essential medium and Ham’s F12 (Life Technologies, Carlsbad, CA) with several supplements including insulin and transferrin [40]. The cultures were maintained at 37°C in an environment of humidified 95% air and 5% CO2.
Plasmids
A plasmid containing hChR2(H134R)-EYFP was obtained from Dr. Karl Deisseroth, Stanford University [41], and the coding region was placed in pCAGGS vector (a generous gift from Dr. Naofumi Uesaka, University of Tokyo) [42]. The plasmid was purified using a MaxiPrep kit (PureLink, Life Technologies, Carlsbad, CA) according to the supplier’s protocol, and dissolved in Hanks’ solution at appropriate concentrations. pCAGGS-DsRed and pCAGGS-EYFP plasmids were prepared in a similar way.
Local Cell Electroporation
Local electroporation with glass microelectrodes was performed after 1–2 days in culture [33]. In brief, pCAGGS-DsRed (2–3 µg/µl) or a mixture of pCAGGS-hChR2 (H134R)-EYFP (3 µg/µl) and pCAGGS-DsRed (2 µg/µl) was pressure-ejected with a glass micro capillary (70-µm diameter) onto the surface of the slice. In some cases, pCAGGS-EYFP (2 µg/µl) was used. Electrical pulses (10 trains of 200 square pulses of 1-msec duration, 200 Hz, 400–500 µA) were then applied with another glass microelectrode (200-µm diameter).
Morphological Time-lapse Observation of Growing Axons
At 3–10 days in vitro (DIV), a culture dish containing cortical slices was sealed with a coverslip and transferred to a microscope stage. During the 4–5-h observation, the slices were maintained at 37°C using a heating apparatus (Kokensha Engineering, Tokyo, Japan) and supplied with a 95% air and 5% CO2 humidified gas mixture [43]. The objective lens was coiled with a band-heater (Thermoplate, Tokai Hit, Shizuoka, Japan) to prevent condensation.
Morphological observation was performed using a confocal laser scanning microscope (Nikon C2, Nikon Instech, Tokyo, Japan) with a 10x objective lens (Nikon Plan Fluor, NA, 0.3) at 560-nm excitation (solid-state laser). After the cultures had been incubated for at least 1 h, images were acquired at 10–20-min intervals with 3–5-µm steps (3–4 optical sections). The observed axons were mostly traced back to cell bodies. Axons were followed for at least 30 min before photostimulation (see below). In both control and experimental conditions, 560-nm wavelength excitation light was used to observe growth cone behavior.
Photostimulation
A solid-state illuminator (475 nm peak wavelength; maximal power: 20 mW; Lumencor SPECTRA, Lumencor, Beaverton, OR) was used for photostimulation through the objective lens. The beam position was controlled manually through the microscope stage. The intensity of the light stimulus falling into the beam-focused area of a cortical slice was adjusted using a Lumencor Remote Control Accessory and measured with a light power meter (Laser Scan, Coherent, Santa Clara, CA). The duration and frequency of the light stimulus were controlled by applying square electrical pulses from a pulse generator (Master-8, AMPI, Jerusalem, Israel).
For time-lapse morphological experiments, blue light with the following pulse frequencies and duration was delivered: 0.1 Hz (duration: 200 msec, 60 pulses), 1 Hz (1 msec, 60 pulses), 4 Hz (200 msec, 240 pulses), and 10 Hz (1 msec, 600 pulses). After photostimulation, cortical cultures were observed for 1–2 h. At the end of the experiment, to verify that cells or axons were expressing sufficient levels of CAGGS-hChR2(H134R)-EYFP, blue light (475 nm) was applied to the slice and emitted fluorescence was detected.
Axonal growth was observed by applying light stimuli with different intensities to DsRed/ChR2-EYFP-cotransfected cells. We confirmed that a light intensity of 1.4 mW/mm2 was efficient in producing action potentials in the transfected cells (see below) without causing any photodynamic damage.
Patch-clamp Experiments
To confirm that the photostimulation induced action potentials in ChR2-expressing cells, whole-cell patch-clamp recording was carried out in slice cultures at room temperature. ChR2-EYFP-expressing cells were identified using an epifluorescence microscope (BX51W1, Olympus, Tokyo, Japan) equipped with a 40x water immersion objective (Olympus, NA, 0.8). The excitation light beam was introduced through the objective lens. The minimal light intensity for ChR2 excitation was determined by applying blue light of various intensities.
Resistance of patch pipettes was 7–8 MΩ when they were filled with an intracellular solution containing (in mM) 130 potassium methanesulfonate (CH3SO3K), 10 HEPES (C8H18N2O4S), 10 potassium chloride (KCl), 0.5 EGTA (C14H24N2O10), 0.1 spermine (C10H26N4), and 10 phosphocreatine (C4H10N3O5P) (pH 7.3, adjusted with KOH, 285–290 mOsm). The composition of standard bathing solution was (in mM) 124 sodium chloride (NaCl), 3 potassium chloride (KCl), 2 calcium chloride (CaCl2), 1.3 magnesium sulfate (MgSO4), 1.2 monopotassium phosphate (KH2PO4), 26 sodium hydrogen carbonate (NaHCO3), and 10 glucose (pH 7.4). The solution was bubbled with 95% O2 and 5% CO2.
The membrane voltage was recorded with a Multiclamp 700 A amplifier (Molecular Devices, Sunnyvale, CA), low-pass filtered at 5 kHZ, digitally sampled at 10–20 kHz, and monitored with pCLAMP 9.0 (Molecular Devices) and analyzed off-line using either Clampfit 9.0 or AxoScope 10.2 software.
Pharmacological Drug Application
To suppress firing activity, 1 µM TTX (Seikagaku-Kogyo, Tokyo, Japan) was added 1 h before the time-lapse experiment.
Image Processing and Statistical Analysis
Axon growth in the time-lapse study was measured using image processing software (ImageJ 1.45 s). Values are presented with ± standard error of the mean (SEM). Quantitative analysis (Student’s t-test) was performed using Microsoft Excel software.
Results
Axon Growth of Layer 2/3 Neurons in Organotypic Slice Cultures
Axonal growth of cortical cells was studied using an organotypic slice culture technique, which allowed us to perform gene manipulation and subsequent time-lapse observation easily. Furthermore, we could assess axon behavior in a cellular environment which is close to that in vivo, as cortical cell arrangement such as laminar structure is well preserved in this culture system [40].
A small number of upper layer cells were cotransfected with DsRed and ChR2-EYFP plasmids. ChR2-EYFP was used for optogenetic stimulation (475 nm), while DsRed was used to observe axonal growth under a different excitation light (560 nm) without activating ChR2. One day after electroporation, we detected strong and stable expression of DsRed/ChR2-EYFP in several cells. DsRed/ChR2-EYFP was highly expressed in the soma and axons, although the fluorescence intensity was slightly weaker in axons. After several days in culture, horizontally elongating axons were observed originating from pyramidal cells (Figure 1A), which is consistent with previous results [33], [34].
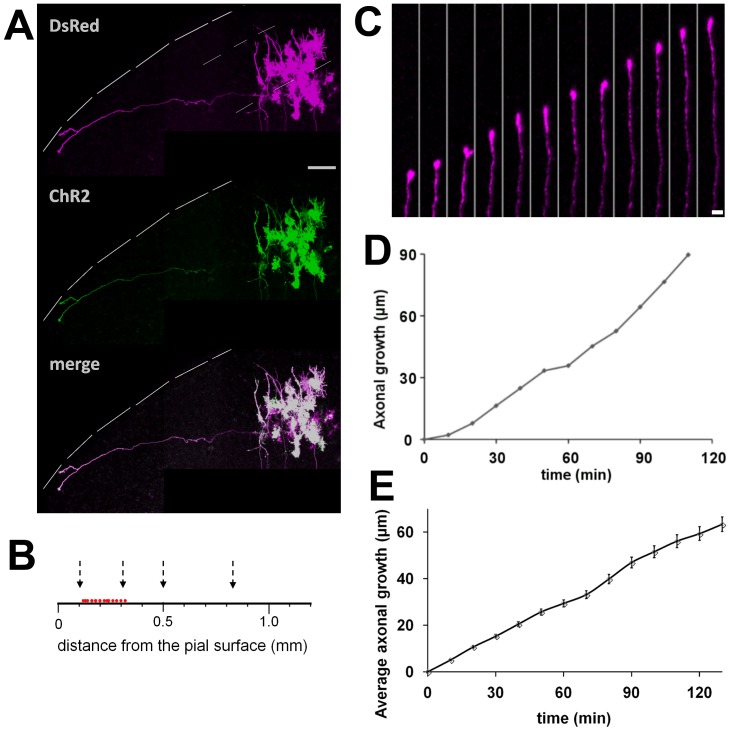
Figure 1. Horizontal axon growth in cortical slice culture.
A, DsRed and ChR2-EYFP plasmids were introduced into several layer 2/3 cells at 1 DIV, and labeled axons were observed by confocal microscopy (560 nm excitation) at 4 DIV. This excitation does not activate ChR2. Note that a horizontally elongating axon is clearly labeled. The interrupted lines indicate the pial surface and the presumed layer 2/3 borders. Scale bar, 100 µm. B, Laminar localizations of the cells projecting horizontal axons which were used for the time-lapse study. Red dots indicate the locations of the cell bodies. Arrows indicate the presumed borders of layer 1, 2/3, 4, 5 and 6. C, Growth of the horizontal axon revealed by time-lapse study. The axon was followed every 10 min. Scale bar, 5 µm. D, Growth curve of the horizontal axon shown in C. E, Average growth of horizontal axons observed in cortical slice cultures from 3 to 10 DIV (n = 32).
To examine their growth pattern, labeled axons were monitored every 10 min for several hours by time-lapse confocal microscopy with an excitation wavelength of 560 nm. Horizontally elongating axons from cells located at 100 to 300 µm (presumed layer 2/3) from the pial surface were selected for the observation (Figure 1B) [40]. We found that horizontally elongating axons grew continuously from 3 to 10 DIV, although the growth rates varied substantially among axons (Figure 1C, D, E). A quantitative analysis showed that the average growth rate was 29.9±1.7 µm/h (n = 32, Figure 1E), with 9.5 as minimal and 46.7 µm/h as maximal values.
Light-induced Action Potentials
Next, we studied the effect of firing activity on axon growth by applying photostimulation at around one week in culture, when firing activity scarcely takes place [33].
To confirm that photostimulation produces firing activity in ChR2-expressing cells, whole-cell patch-clamp recording was performed (Figure 2). Photostimulation with different durations (50 or 200 msec) was applied to the ChR2-expressing cells. Each 50-msec flash of light elicited firing (Figure 2C, upper). A longer pulse duration (200 msec) also resulted in a single action potential (Figure 2C, lower).
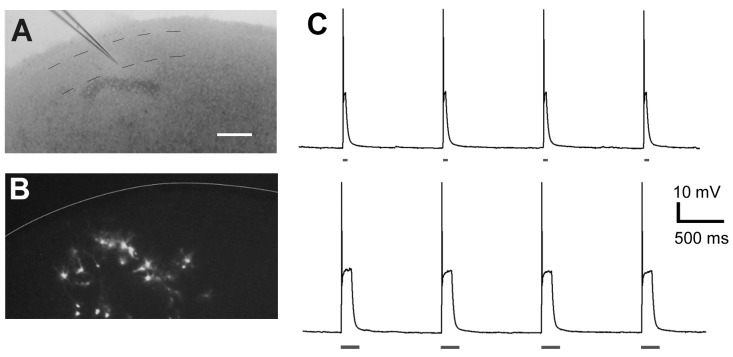
Figure 2. Electrophysiological responses of ChR2-expressing cortical neurons to photostimulation.
A and B, ChR2-expressing cells among layer 2/3 neurons were subjected to patch-clamp recording at 4 DIV. Interrupted lines in A indicate the presumed layer 2/3 borders. The white line in B indicates the pial surface. Bar, 200 µm. C, Each flash with 475-nm wavelength light (50-msec duration in the upper trace and 200-msec duration in the lower trace) elicited depolarization and a single action potential.
No spike response was detectable in non-transfected neurons, indicating that the irradiance alone does not elicit a response, which is consistent with previous results [36], [44], [45]. Thus, we confirmed that photoactivation efficiently generated firing activity specifically in ChR2-expressing neurons.
The Response of Horizontal Axons to High-frequency Photostimulation
Next, the behavior of horizontal axons was studied in response to photostimulation. The stimulation paradigm consisted of a 1-min photostimulation period with different frequencies (0.1, 1, 4 and 10 Hz).
First, high-frequency photostimulation was applied to the cell bodies which were the origins of labeled axons, as ChR2 was strongly expressed in the soma (Figure 1A). Before photostimulation, all observed axons were confirmed to elongate at constant growth rates for 30–60 min (35.1±3.5 µm/h, n = 11). We found that most of them transiently stopped growing after high-frequency photostimulation (4 and 10 Hz) (Figure 3A–C). A few axons showed obvious retraction. To quantify this axonal behavior, growth rates were calculated after photostimulation. The average growth rate was 0.47±9.9 µm/h (n = 11) at 10 min after photostimulation, significantly slower than the average before stimulation (Student’s t-test, p<0.005). Some axons started to grow again 20 min after photostimulation, but the average growth rate was still significantly lower (19.0±5.5 µm/h, p<0.05). Axonal growth rate was fully restored at 30 min after photostimulation (34.2±5.6 µm/h, Figure 3D).
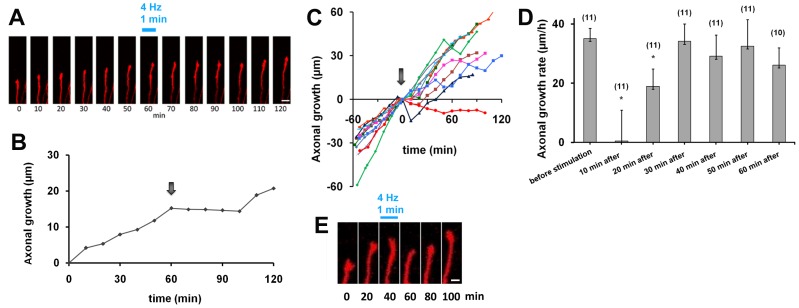
Figure 3. Horizontal axons exhibit pause behavior in response to high-frequency photostimulation directed at the soma of labeled neurons.
A, A horizontal axon from a cell cotransfected with DsRed and ChR2-EYFP was observed. Photostimulation (4 Hz, 1 min) with 475-nm excitation was applied to the cell body after a 60-min observation of axon growth at 560-nm excitation. After the photostimulation at 475-nm excitation, the axon was further observed at 560-nm excitation. Scale bar, 5 µm. B, Growth of the horizontal axon shown in A. The arrow indicates the time of photostimulation. C, Growth of individual axons before and after photostimulation. Despite differences in growth rates, most axons display pause behavior after high-frequency photostimulation (arrow), n = 11. D, Axonal growth rates before and after high-frequency stimulation. After high-frequency photostimulation, axonal growth rate decreased significantly and this decline persisted for 20 min (Student’s t-test, *p<0.005). E, Axonal tip morphology before and after photostimulation. Scale bar, 2 µm.
Based on the above quantitative analysis, each axon’s behavior was categorized into pause and non-pause behavior. Because the 46% reduction in growth rate at 20 min after photostimulation (Figure 3D) was statistically significant, axons which exhibited more than a 50% decrease at this time point were defined as displaying pause behavior. Consequently, 9 of the 11 axons examined showed pause behavior. At 30 min after photostimulation, most of these (7 of the 9 axons) displayed fully restored growth rates, although the remaining two axons still exhibited significantly lower growth rates at this time point. All growth cones grew at normal rates by 50 min after photostimulation. We also observed the morphology of the growth cone. All axons exhibited typical growth cones with lamellipodia structures before photostimulation, but immediately after photostimulation the sizes of the lamellipodia decreased (Figure 3E).
A second time-lapse study was carried out after applying photostimulation directly to the axon, in order to explore whether action potentials generated in axons are required for the pause behavior (Figure 4A, B). Most of the observed axons exhibited slight retraction in response to photostimulation (Figure 4C), and quantitative analysis showed a marked decrease in axonal growth rate after photostimulation. The average growth rates (n = 8) at 10, 20, and 30 min after photostimulation were −8.5±9.0 µm/h, −4.9±3.9 µm/h, and −3.1±5.1 µm/h, respectively, displaying a significant reduction compared with that before stimulation (17.9±2.1 µm/h, Student’s t-test, p<0.005). Axonal growth rate was restored by 60 min (19.2±6.6 µm/h) after stimulation.
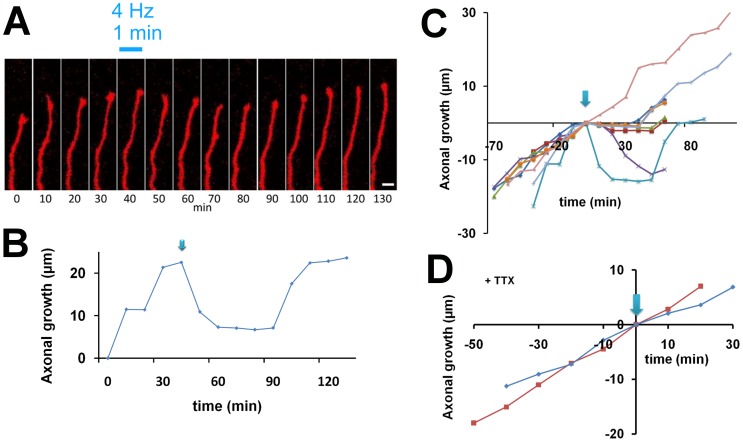
Figure 4. Horizontal axon growth before and after high-frequency stimulation to the axon.
A, High-frequency photostimulation (4 Hz) elicited retraction of a representative growth cone. Scale bar, 5 µm. B, Growth of the horizontal axon shown in A. The arrow indicates the time of photostimulation. C, Growth of individual axons before and after photostimulation (arrow). D, Horizontal axon growth in the presence of TTX after high-frequency photostimulation. TTX (1 µM) was added to the culture medium 1 h prior to the observation. Photostimulation was applied at 4 Hz for 1 min to the cell body (n = 2).
These axons were classified into two groups using the definition of pause behavior described above (i.e., more than 50% reduction at 20 min after stimulation). Seven axons out of eight exhibited pause behavior, while one axon was not obviously affected by the high-frequency photostimulation (Figure 4C). This result suggests that action potentials generated in axons at high frequencies also cause the pause behavior.
Growth Cone Response in TTX-treated Culture
To further confirm that generation and propagation of action potentials are required for the pause behavior, the sodium channel blocker tetrodotoxin (TTX, 1 µM) was added to the culture medium one hour before the time-lapse experiment. Axons also grew normally, with growth cones, in blocker-containing medium. We found that in the presence of TTX, high-frequency photostimulation to the soma (4 Hz for 1 min) did not affect axonal growth rate (18.6±2.5 µm/h before stimulation, 21.8±6.8 µm/h at 10 min after stimulation and 14.8±3.3 µm/h at 20 min after stimulation, n = 2; Figure 4D). This result indicates that axons stop growing transiently due to firing activity elicited by photostimulation.
The Influence of Low-frequency Photostimulation on Axon Growth
We investigated the influence of frequency of firing activity on axon growth. Photostimulation for 1 min at 1 Hz produced a weak effect on axonal growth (Figure 5A). The axons tested were classified as displaying pause and non-pause behavior, based on their growth rates before and after photostimulation (see above). Half of the axons did not show any pause (n = 3), whereas the others paused in their growth for 20 min (n = 3; Figure 5A).
Figure 5. Axonal growth after low-frequency stimulation of the soma.
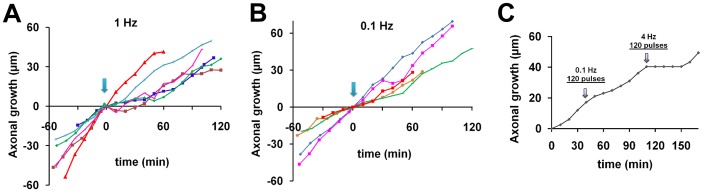
A, B, Photostimulation at 1(A) or 0.1 Hz (B) for 1 min was applied (arrows) during the time-lapse study. C, A total of 120 pulses was applied to a single axon at low frequency (0.1 Hz) and subsequently at high frequency (4 Hz). Pause behavior was induced by high-frequency stimulation but not by low-frequency stimulation, even though the number of pulses was the same in both cases.
Photostimulation for 1 min at 0.1 Hz did not result in any significant effect on the growth of any axon tested (Figure 5B). The growth rates were 23.5±7.2 µm/h, 34.7±9.6 µm/h and 38.4±5.3 µm/h at 10, 20, and 30 min after stimulation, respectively, and were not significantly different from that before stimulation (26.8±3.5 µm/h, n = 5; Figure 5B).
The total amount of firing activity may be critical for the pause behavior. To test this possibility, we increased the number of pulses while maintaining the frequency at 0.1 Hz. Even though the number of stimuli (120 pulses) was 20 times larger, axonal growth did not pause after the low-frequency stimulation. However, the same number of pulses applied subsequently to the same axon at high frequency (4 Hz, 120 pulses for 30 sec) elicited pause behavior for the next 40 min (Figure 5C). Therefore, the pause behavior depends on frequency rather than on the total amount of firing activity.
Axonal Responses to Different Frequencies of Photostimulation
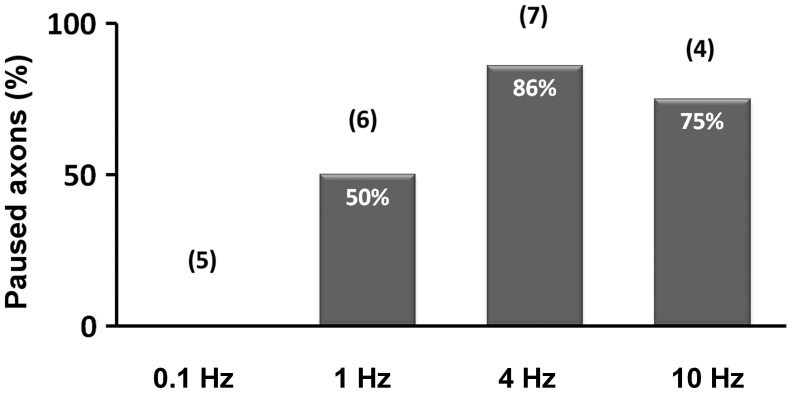
The relationship between frequency of photostimulation and axonal response is shown in Figure 6. Based on the above analysis, high-frequency photostimulation was highly effective for the pause behavior (86% for 4 Hz, n = 7, 75% for 10 Hz, n = 4), intermediate stimulation was partly effective (50% for 1 Hz, n = 6), but low-frequency stimulation (0.1 Hz) had no effect on axonal growth (n = 5).
Figure 6. Occurrence of pause behavior after photostimulation with different frequencies.
Histograms show the percentage of axons displaying pause behavior after photostimulation with different frequencies. The numbers in parentheses are the numbers of samples examined for each frequency.
Discussion
In the present study, we used optogenetic stimulation to investigate the influence of firing activity on cortical axon growth in vitro. The results demonstrate that horizontal axons grow at a constant rate after several days in culture, when spontaneous firing scarcely occurs, but exhibit pause behavior immediately after high-frequency firing is induced (see Figure 6). In the presence of TTX, photostimulation failed to elicit pause behavior. These results imply that high-frequency firing during development is involved in suppressing axon growth of layer 2/3 neurons and contributes to the formation of horizontal connections in the neocortex.
Combining time-lapse observation of a fluorescent protein and ChR2-expressing axons with optogenetic stimulation is also shown to be a powerful method for investigating activity-dependent processes in the brain. Using this approach, we demonstrate that firing activity contributes to the pause behavior and acts as a negative regulator for horizontal axon growth.
Activity-dependence of Horizontal Axon Growth
As shown previously, spontaneous firing activity is almost absent in cortical slice culture during the first week in vitro, but begins to increase at around 10 DIV, reaching its peak at 14 DIV [33]. This temporal profile is compatible with the development of neuronal activity in vivo [46]–[48]. Taken together, the present findings suggest that horizontal axon growth is suppressed by increasing neuronal activity during cortical circuit formation.
The rapid response of the growth cone to high-frequency firing suggests that the pause behavior is cell-autonomous. Given that new branches tend to emerge during periods when axon growth has ceased, neuronal firing could strengthen the connectivity between cortical cells which are synchronously activated [43], [49]. As a consequence, only some of the horizontal axons originating from a layer 2/3 cell could form more extensive branches to make stronger connections with cortical cells in specific locations [24], [25], [32]. Consistent with this, our previous study has also shown that axonal branching of layer 2/3 cells is promoted by spontaneous activity and subsequent synaptic activity [33]. Overall, the present findings suggest that increasing spike activity during postnatal stages contributes to the establishment of horizontal connections by inhibiting axon growth and promoting branch formation.
However, it should be noted that an intrinsic mechanism also contributes to axon branching of layer 2/3 cells, because some aspects of their branching pattern are highly preserved irrespective of environmental conditions [49], [50].
The Efficiency of Neuronal Activity
Even after high-frequency stimulation (4 Hz and 10 Hz), not all of the tested axons showed pause behavior. When intermediate-frequency stimulation (1 Hz) was applied, only half of the examined axons paused growing. As there are subclasses among upper-layer pyramidal cells [23], [51], distinct populations may respond differentially to firing activity. Another possibility is that the history of neuronal activity may influence axonal responses, as a growth cone that has experienced electrical stimulation-induced collapse becomes insensitive to a further stimulation [52].
Possible Mechanisms of Growth Cone Pausing in Response to Neuronal Activity
Previous studies have shown that axon growth is arrested in peripheral and invertebrate neurons when the cells are electrically stimulated [53], [54]. Moreover, it has been demonstrated that the suppression of growth cone motility is attributable to a rise in Ca2+ concentration in the growth cones in the PNS [55]–[57]. Similarly, Ca2+ elevation may be essential for the pause behavior of horizontal axons in the cortex.
Is gene expression involved in the observed pause behavior? In the classical collapse assay, addition of the repulsive guidance molecule Sema-3A is known to induce immediate growth cone collapse, which lasts for 30–60 min [58]. The same phenomenon was observed even in axons that were isolated from the soma. This indicates that the effect is independent of gene expression. As axonal responses in the present study resemble Sema-3A-induced growth cone collapse in temporal terms, it is likely that the pause behavior is also independent of nuclear gene expression. However, local translation in axonal tips may be partly responsible for the pause behavior of cortical axons [59], [60].
It has also been shown that electrical activity alters growth cone responsiveness to environmental factors. For instance, light electrical stimulation of Xenopus motoneurons (10 action potentials at 2 Hz), which by itself does not induce any change in axonal outgrowth, is able to lower the sensitivity of growth cones to attractive guidance cues or to convert repulsive cues into attractive ones [61]. Thus, growth cone responses may be affected differentially by the strength of electrical activity. In vitro experiments in developing rat neocortical slices demonstrate the presence of spatiotemporally organized patterns of spontaneous cortical activity [62], [63]. It is likely that such unique firing patterns can also influence axon behavior [64], [65].
In summary, various intrinsic and extrinsic neuronal events including firing and synaptic activity probably contribute to the decision of an axon to advance, retract, pause, or turn. The present study indicates that firing activity is also an influential factor for axonal development in the mammalian neocortex, which is crucial for the formation of its columnar structure.
Acknowledgments
We thank Dr. Edward Ruthazer for helpful suggestions. We thank Dr. Ian Smith for critical reading of the manuscript. We also thank MEXT for supporting O.M. as a Research Student.
Funding Statement
This study was supported by Grants-in-Aid for Scientific Research on Innovative Areas “Mesoscopic Neurocircuitry” (number 23115102) of the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) to N.Y., and for Scientific Research (B) (numbers 20300110 and 23300118) of the Japan Society for the Promotion of Science to NY. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lopez-Bendito G, Molnar Z (2003) Thalamocortical development: how are we going to get there? Nat Rev Neurosci 4: 276–289. [DOI] [PubMed] [Google Scholar]
- 2. Gilbert CD, Wiesel TN (1979) Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature 280: 120–125. [DOI] [PubMed] [Google Scholar]
- 3. Gilbert CD, Wiesel TN (1983) Clustered intrinsic connections in cat visual cortex. J Neurosci 3: 1116–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rockland KS, Lund JS, Humphrey AL (1982) Anatomical binding of intrinsic connections in striate cortex of tree shrews (Tupaia glis). J Comp Neurol 209: 41–58. [DOI] [PubMed] [Google Scholar]
- 5. Burkhalter A, Charles V (1990) Organization of local axon collaterals of efferent projection neurons in rat visual cortex. J Comp Neurol 302: 920–934. [DOI] [PubMed] [Google Scholar]
- 6. Lohmann H, Rorig B (1994) Long-range horizontal connections between supragranular pyramidal cells in the extrastriate visual cortex of the rat. J Comp Neurol 344: 543–558. [DOI] [PubMed] [Google Scholar]
- 7. McGuire BA, Gilbert CD, Rivlin PK, Wiesel TN (1991) Targets of horizontal connections in macaque primary visual cortex. J Comp Neurol 305: 370–392. [DOI] [PubMed] [Google Scholar]
- 8. Melchitzky DS, Sesack SR, Pucak ML, Lewis DA (1998) Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. J Comp Neurol 390: 211–224. [DOI] [PubMed] [Google Scholar]
- 9. Ts’o DY, Gilbert CD, Wiesel TN (1986) Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J Neurosci 6: 1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yabuta NH, Callaway EM (1998) Cytochrome-oxidase blobs and intrinsic horizontal connections of layer 2/3 pyramidal neurons in primate V1. Vis Neurosci 15: 1007–1027. [DOI] [PubMed] [Google Scholar]
- 11. Sincich LC, Blasdel GG (2001) Oriented axon projections in primary visual cortex of the monkey. J Neurosci 21: 4416–4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bosking WH, Zhang Y, Schofield B, Fitzpatrick D (1997) Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. J Neurosci 17: 2112–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malach R, Amir Y, Harel M, Grinvald A (1993) Relationship between intrinsic connections and functional architecture revealed by optical imaging and in vivo targeted biocytin injections in primate striate cortex. Proc Natl Acad Sci U S A 90: 10469–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stettler DD, Das A, Bennett J, Gilbert CD (2002) Lateral connectivity and contextual interactions in macaque primary visual cortex. Neuron 36: 739–750. [DOI] [PubMed] [Google Scholar]
- 15. Kisvarday ZF, Toth E, Rausch M, Eysel UT (1997) Orientation-specific relationship between populations of excitatory and inhibitory lateral connections in the visual cortex of the cat. Cereb Cortex 7: 605–618. [DOI] [PubMed] [Google Scholar]
- 16. Frostig RD, Xiong Y, Chen-Bee CH, Kvasnak E, Stehberg J (2008) Large-scale organization of rat sensorimotor cortex based on a motif of large activation spreads. J Neurosci 28: 13274–13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adesnik H, Scanziani M (2010) Lateral competition for cortical space by layer-specific horizontal circuits. Nature 464: 1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keller A, Asanuma H (1993) Synaptic relationships involving local axon collaterals of pyramidal neurons in the cat motor cortex. J Comp Neurol 336: 229–242. [DOI] [PubMed] [Google Scholar]
- 19. Malach R, Schirman TD, Harel M, Tootell RB, Malonek D (1997) Organization of intrinsic connections in owl monkey area MT. Cereb Cortex 7: 386–393. [DOI] [PubMed] [Google Scholar]
- 20. Matsubara JA, Phillips DP (1988) Intracortical connections and their physiological correlates in the primary auditory cortex (AI) of the cat. J Comp Neurol 268: 38–48. [DOI] [PubMed] [Google Scholar]
- 21. Read HL, Winer JA, Schreiner CE (2001) Modular organization of intrinsic connections associated with spectral tuning in cat auditory cortex. Proc Natl Acad Sci U S A 98: 8042–8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharma J, Angelucci A, Sur M (2000) Induction of visual orientation modules in auditory cortex. Nature 404: 841–847. [DOI] [PubMed] [Google Scholar]
- 23. Bruno RM, Hahn TT, Wallace DJ, de Kock CP, Sakmann B (2009) Sensory experience alters specific branches of individual corticocortical axons during development. J Neurosci 29: 3172–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Callaway EM, Katz LC (1990) Emergence and refinement of clustered horizontal connections in cat striate cortex. J Neurosci 10: 1134–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Durack JC, Katz LC (1996) Development of horizontal projections in layer 2/3 of ferret visual cortex. Cereb Cortex 6: 178–183. [DOI] [PubMed] [Google Scholar]
- 26. Tanigawa H, Wang Q, Fujita I (2005) Organization of horizontal axons in the inferior temporal cortex and primary visual cortex of the macaque monkey. Cereb Cortex 15: 1887–1899. [DOI] [PubMed] [Google Scholar]
- 27. Callaway EM (1998) Prenatal development of layer-specific local circuits in primary visual cortex of the macaque monkey. J Neurosci 18: 1505–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galuske RA, Singer W (1996) The origin and topography of long-range intrinsic projections in cat visual cortex: a developmental study. Cereb Cortex 6: 417–430. [DOI] [PubMed] [Google Scholar]
- 29. Callaway EM, Katz LC (1991) Effects of binocular deprivation on the development of clustered horizontal connections in cat striate cortex. Proc Natl Acad Sci U S A 88: 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lowel S, Singer W (1992) Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science 255: 209–212. [DOI] [PubMed] [Google Scholar]
- 31. Ruthazer ES, Stryker MP (1996) The role of activity in the development of long-range horizontal connections in area 17 of the ferret. J Neurosci 16: 7253–7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Katz LC, Shatz CJ (1996) Synaptic activity and the construction of cortical circuits. Science 274: 1133–1138. [DOI] [PubMed] [Google Scholar]
- 33. Uesaka N, Hirai S, Maruyama T, Ruthazer ES, Yamamoto N (2005) Activity dependence of cortical axon branch formation: a morphological and electrophysiological study using organotypic slice cultures. J Neurosci 25: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohnami S, Endo M, Hirai S, Uesaka N, Hatanaka Y, et al. (2008) Role of RhoA in activity-dependent cortical axon branching. J Neurosci 28: 9117–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang F, Wang LP, Boyden ES, Deisseroth K (2006) Channelrhodopsin-2 and optical control of excitable cells. Nat Methods 3: 785–792. [DOI] [PubMed] [Google Scholar]
- 36. Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, et al. (2005) Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol 15: 2279–2284. [DOI] [PubMed] [Google Scholar]
- 37. Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, et al. (2003) Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A 100: 13940–13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Petreanu L, Huber D, Sobczyk A, Svoboda K (2007) Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 10: 663–668. [DOI] [PubMed] [Google Scholar]
- 39. Yamamoto N, Kurotani T, Toyama K (1989) Neural connections between the lateral geniculate nucleus and visual cortex in vitro. Science 245: 192–194. [DOI] [PubMed] [Google Scholar]
- 40. Yamamoto N, Yamada K, Kurotani T, Toyama K (1992) Laminar specificity of extrinsic cortical connections studied in coculture preparations. Neuron 9: 217–228. [DOI] [PubMed] [Google Scholar]
- 41. Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268. [DOI] [PubMed] [Google Scholar]
- 42. Niwa H, Yamamura K, Miyazaki J (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108: 193–199. [DOI] [PubMed] [Google Scholar]
- 43. Yamamoto N, Higashi S, Toyama K (1997) Stop and branch behaviors of geniculocortical axons: a time-lapse study in organotypic cocultures. J Neurosci 17: 3653–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wyatt RM, Tring E, Trachtenberg JT (2012) Pattern and not magnitude of neural activity determines dendritic spine stability in awake mice. Nat Neurosci 15: 949–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nirenberg S, Pandarinath C (2012) Retinal prosthetic strategy with the capacity to restore normal vision. Proc Natl Acad Sci U S A 109: 15012–15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mirmiran M, Corner M (1982) Neuronal discharge patterns in the occipital cortex of developing rats during active and quiet sleep. Brain Res 255: 37–48. [DOI] [PubMed] [Google Scholar]
- 47. Flint AC, Maisch US, Kriegstein AR (1997) Postnatal development of low [Mg2+] oscillations in neocortex. J Neurophysiol 78: 1990–1996. [DOI] [PubMed] [Google Scholar]
- 48. Rochefort NL, Garaschuk O, Milos RI, Narushima M, Marandi N, et al. (2009) Sparsification of neuronal activity in the visual cortex at eye-opening. Proc Natl Acad Sci U S A 106: 15049–15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Szebenyi G, Callaway JL, Dent EW, Kalil K (1998) Interstitial branches develop from active regions of the axon demarcated by the primary growth cone during pausing behaviors. J Neurosci 18: 7930–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Katz LC (1991) Specificity in the Development of Vertical Connections in Cat Striate Cortex. Eur J Neurosci 3: 1–9. [DOI] [PubMed] [Google Scholar]
- 51. van Brederode JF, Foehring RC, Spain WJ (2000) Morphological and electrophysiological properties of atypically oriented layer 2 pyramidal cells of the juvenile rat neocortex. Neuroscience 101: 851–861. [DOI] [PubMed] [Google Scholar]
- 52. Diefenbach TJ, Guthrie PB, Kater SB (2000) Stimulus history alters behavioral responses of neuronal growth cones. J Neurosci 20: 1484–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cohan CS, Kater SB (1986) Suppression of neurite elongation and growth cone motility by electrical activity. Science 232: 1638–1640. [DOI] [PubMed] [Google Scholar]
- 54. Fields RD, Neale EA, Nelson PG (1990) Effects of patterned electrical activity on neurite outgrowth from mouse sensory neurons. J Neurosci 10: 2950–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cohan CS, Connor JA, Kater SB (1987) Electrically and chemically mediated increases in intracellular calcium in neuronal growth cones. J Neurosci 7: 3588–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gomez TM, Spitzer NC (1999) In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature 397: 350–355. [DOI] [PubMed] [Google Scholar]
- 57. Ibarretxe G, Perrais D, Jaskolski F, Vimeney A, Mulle C (2007) Fast regulation of axonal growth cone motility by electrical activity. J Neurosci 27: 7684–7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Campbell DS, Regan AG, Lopez JS, Tannahill D, Harris WA, et al. (2001) Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J Neurosci 21: 8538–8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Campbell DS, Holt CE (2001) Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32: 1013–1026. [DOI] [PubMed] [Google Scholar]
- 60. Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, et al. (2005) Local translation of RhoA regulates growth cone collapse. Nature 436: 1020–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ming G, Henley J, Tessier-Lavigne M, Song H, Poo M (2001) Electrical activity modulates growth cone guidance by diffusible factors. Neuron 29: 441–452. [DOI] [PubMed] [Google Scholar]
- 62. Yuste R, Peinado A, Katz LC (1992) Neuronal domains in developing neocortex. Science 257: 665–669. [DOI] [PubMed] [Google Scholar]
- 63. Schwartz TH, Rabinowitz D, Unni V, Kumar VS, Smetters DK, et al. (1998) Networks of coactive neurons in developing layer 1. Neuron 20: 541–552. [DOI] [PubMed] [Google Scholar]
- 64. Mire E, Mezzera C, Leyva-Diaz E, Paternain AV, Squarzoni P, et al. (2012) Spontaneous activity regulates Robo1 transcription to mediate a switch in thalamocortical axon growth. Nat Neurosci 15: 1134–1143. [DOI] [PubMed] [Google Scholar]
- 65. Hanson MG, Landmesser LT (2004) Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron 43: 687–701. [DOI] [PubMed] [Google Scholar]