Abstract
Background
Patients with preeclampsia are at risk for cardiovascular disease. Changes in cardiac function are subtle in preeclampsia and are difficult to quantify with conventional imaging. Strain measurements using speckle-tracking echocardiography have been used to sensitively quantify abnormalities in other disease settings.
Methods and Results
We evaluated the feasibility and sensitivity of strain imaging using speckle-tracking echocardiography in women with preeclampsia. Forty-seven women were enrolled in this pilot study and 39 were analyzed: 11 with preeclampsia, 17 without a hypertensive disorder and 11 with nonproteinuric hypertension. Echocardiographic ejection fraction and global peak longitudinal, radial and circumferential strain were measured.
Longitudinal strain was significantly worsened in women with preeclampsia compared to women without a hypertensive disorder (P=0.0009). Similar results were observed for radial strain (P=0.007) and circumferential strain (P=0.04). Women with preeclampsia also had significantly worsened longitudinal (P=0.04), radial (P=0.01) and circumferential (P=0.002) strain compared with women with nonproteinuric hypertension. Women with preeclampsia did not have a significantly different ejection fraction compared with women without a hypertensive disorder (P=0.52) and women with nonproteinuric hypertension (P=0.44).
Conclusions
Myocardial strain imaging using speckle tracking is more sensitive than left ventricular ejection fraction to detect differences in left ventricular systolic function in women with and without preeclampsia.
Keywords: preeclampsia, echocardiography, speckle tracking
Preeclampsia is a common hypertensive disorder of pregnancy. It is associated with both immediate, as well as long-term postpartum morbidity and mortality due to cardiac-related issues.1, 2 Even in clinically asymptomatic patients, subtle echocardiographic changes in left ventricular function have been observed in preeclampsia.3 Of the conventional echocardiographic indices, ejection fraction remains relatively preserved until later in the course of the disease process, making it less useful as a screening tool to follow patients over time.3 For this reason, the current assessment of pregnancy-related changes in myocardial function is based on either two-dimensional linear and volumetric chamber quantifications or Doppler indices of diastolic function.4 The availability of more sensitive and sophisticated non-invasive techniques may enhance our understanding of global ventricular function in the women with preeclampsia.
Speckle tracking is a recently developed echocardiographic technique that analyzes the degree of myocardial deformation, known as strain, throughout the cardiac cycle. Speckle tracking, is obtained, by an automated measurement of the distance between speckles, in a specific ventricular segment in a two-dimensional echocardiographic image. Speckles are created by the irregular reflection of ultrasound that can be tracked throughout the cardiac cycle. Because, it is based on tracking the course of a speckle of the image over time in relation to its original location it is angle-independent and is less prone to operator-related measurement errors. Speckle tracking allows for the measurement of longitudinal, radial and circumferential strain and these have been used to prognosticate changes in left ventricular function and geometry.5–8
Strain is a parameter representing deformation of an object, relative to its original shape, and is expressed as a percentage change from the original dimension. Strain using speckle tracking is calculated by assessing the differences in distance and velocity of the speckle during the cardiac cycle. Positive values reflect lengthening, negative values reflect contraction. Cardiac Myofibrils can be oriented in the radial, circumferential and longitudinal plane, giving them a helical nature. In contrast with left ventricular ejection fraction, which is a measure of global function, strain with speckle tracking measures both regional and global function and also identifies the myocyte group that is affected. Moreover, the calculation for left ventricular ejection fraction includes geometric assumptions that speckle tracking does not.
In this study we examined changes in myocardial strain as measured by speckle-tracking echocardiography in women with preeclampsia, women with nonproteinuric hypertension and women without a hypertensive disorder. We hypothesized that global left systolic strain measures would prove more sensitive than conventional left ventricular ejection fraction in detecting early changes in systolic left ventricular function manifesting as subclinical disease prior to overt progression.
Methods
Assembly of the Cohort
The institutional review board of Beth Israel Deaconess Medical Center in Boston, MA approved this study. Eligible women were enrolled from November 2009 through May 2012 after providing written informed consent. Women at least 18 years of age with a singleton pregnancy of at least 24 weeks and less than 41 weeks and with a diagnosis of preeclampsia, nonproteinuric hypertension or without any hypertensive disorder of pregnancy were eligible. Exclusion criteria included pre-existing cardiovascular disease, pulmonary disease and non-gestational diabetes mellitus. Participants were recruited upon admission to labor and delivery, the antepartum floor, or during a routine prenatal visit. All clinical data was abstracted from medical records.
The diagnoses of preeclampsia, including the distinction between mild and severe preeclampsia, and nonproteinuric hypertension were based on the National High Blood Pressure Education Program Working Group definition, also endorsed by the American Congress of Obstetricians and Gynecologists (ACOG).9 Mild preeclampsia was defined as ≥140 mm Hg systolic or ≥90 mm Hg diastolic with proteinuria (>300 mg/24 hours) in a previously normotensive woman after 20 weeks of gestation. Severe preeclampsia was defined by severe hypertension (≥160 mm Hg systolic and ≥110 mm Hg diastolic on 2 occasions 6 hours apart) and proteinuria (≥5 g/24 hrs) with or without evidence of end organ damage (such as HELLP (hemolysis, elevated liver enzymes and low platelets) syndrome, oliguria (<500 ml in 24 hours), pulmonary edema, seizures and fetal growth restriction).
Women in the nonproteinuric hypertension group had either gestational hypertension or chronic hypertension. Gestational hypertension was defined as BP ≥140/90 for the first time during pregnancy and no proteinuria; chronic hypertension was defined as BP ≥140/90 before pregnancy or diagnosed before 20 weeks of gestation. A maternal-fetal medicine specialist confirmed all diagnoses.
IVC dynamics are difficult to assess in the third trimester of pregnancy due to uterine enlargement coupled with IVC compression by the gravid uterus. We used fluid balance as a surrogate to assess volume status.
Echocardiography
Bedside transthoracic echocardiogram was performed using a Siemens X-300 (Mountainview, CA) machine and a P5-1 transducer by one of two expert echocardiographers who were blinded to the participant’s diagnosis. Images were obtained with the patient lying in the left lateral decubitus position and reported according to the American Society of Echocardiography guidelines.10 Images were stored in a cine loop format of three cardiac cycles of non-compressed data with associated electrocardiogram information. The sonographers performed a comprehensive examination, which included a complete two-dimensional and color flow-Doppler valvular assessment.
Ejection fraction was calculated using Simpson’s biplane disc method. Strain was analyzed using 2D speckle-tracking echocardiographic software (2D LV Analysis; TomTec Imaging Systems, Unterschleissheim, Germany. For evaluation of strain, the endocardial border in the left ventricle was traced, at end-systole in the appropriate imaging plane. The tracking quality of all images was assessed prior to analysis. Images where the endocardial tracking was considered inadequate in > 2 segments were excluded from strain measurements. Strain measurements were made from three consecutive cardiac cycles and then averaged in order to obtain peak systolic regional strain in the longitudinal, radial, and circumferential planes. Global peak systolic strain was calculated as an average of six regional values in the apical four-chamber and parasternal short-axis views at the mid-papillary level to measure longitudinal, radial, and circumferential strain, respectively.
To assess intraobserver and interobserver variability, 20 subjects were randomly selected and longitudinal, radial and circumferential strain measurements were repeated by the same investigator who performed all of the strain calculations for the study data (SS), and by one other investigator (FM). These investigators were blinded to any clinical characteristics of the patients and any previous measurements. Strain measurements that differed by more than 3% on an absolute scale were considered discrepant for assessing both intraobserver and interobserver variability. In cases of discrepancy, the reviewers reached consensus on the tracing and those strain measurements were used for analysis.
Statistical analysis
Descriptive statistics are presented as proportion or median and interquartile range, as appropriate. Comparisons between groups were made using the Chi-square or Fisher’s exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. The Spearman correlation coefficient was used to quantify intraobserver and interobserver variability. All analyses were performed using SAS 9.3 (SAS institute Inc., Cary, NC). All tests were two sided and P values <0.05 were considered statistically significant.
Results
We enrolled 47 women and 39 are included in this analysis. Of the eight women who were excluded, one was excluded because she had premature preterm rupture of membranes before the echocardiogram could be obtained and seven (six without preeclampsia and one with) had echocardiograms that could not be read due to poor image quality that was unrelated to any patient characteristics. The eight women who were excluded from the analysis due to lack of echocardiogram data were not different from women included in the analysis with respect to maternal age, BMI, history of pregnancy-induced hypertension, smoking, highest systolic pressure, highest diastolic pressure or gestational age at delivery (all P>0.12).
Eleven (28.2%) women were diagnosed with preeclampsia, 11 (28.2%) women were diagnosed with nonproteinuric hypertension and 17 (43.6%) did not have a hypertensive disorder of pregnancy; 3 (27.3%) of the women with preeclampsia were deemed severe and 8 (72.7%) mild. Neither the group without a hypertensive disorder nor the group with nonproteinuric hypertension differed from the group with preeclampsia with respect to maternal age, parity, race/ethnicity, smoking history and gestational age at the time of the echocardiogram (all P>0.08). As expected, women diagnosed with preeclampsia had higher systolic (P<0.0001) and diastolic blood pressure (P<0.0001) than women without any hypertensive disorder of pregnancy. Preeclamptic women also had higher pre-pregnancy body mass index (P=0.01) when compared to women without any hypertensive disorder of pregnancy. The groups with preeclampsia and nonproteinuric hypertension had similar systolic (P=0.74) and diastolic (0.87) blood pressures. Participant characteristics at the time of the echocardiogram, as well as delivery characteristics, are presented in Table 1. There was no significant difference between women with and without preeclampsia in the use of antihypertensive medication or fluid balance at the time of echocardiogram.
Table 1.
Participant characteristics
| Characteristic | Preeclampsia (n=11) |
No Hypertensive Disorder (n=17) |
P† | Nonproteinuric Hypertension (n=11) |
P‡ |
|---|---|---|---|---|---|
| Age (yrs) | 31.8 (26.0 – 34.9) | 29.0 (25.1–33.3) | 0.57 | 35.5 (27.7–38.8) | 0.19 |
| Gravidity | 2.0 (1.0 – 5.0) | 1.0 (1.0 – 2.0) | 0.05 | 1.0 (1.0–4.0) | 0.26 |
| Parity | 0.0 (0.0 – 2.0) | 0.0 (0.0 – 0.0) | 0.13 | 0.0 (0.0–1.0) | 0.77 |
| Pre-pregnancy body mass index (kg/m2) | 27.4 (24.0 – 32.9) | 22.5 (20.9–24.8) | 0.01 | 31.5 (29.2–39.5) | 0.03 |
| Race/ethnicity | 0.63 | 1.0 | |||
| White/Caucasian | 7 (63.6) | 8 (47.1) | 8 (72.7) | ||
| Black/African American | 2 (18.2) | 5 (29.4) | 2 (18.2) | ||
| Asian | 1 (9.1) | 3 (17.7) | 0 (0.0) | ||
| Other/Unknown | 1 (9.1) | 1 (5.9) | 1 (9.1) | ||
| History of pregnancy-induced hypertension | 3 (27.3) | 0 (0.0) | 0.05 | 0 (0.0) | 0.21 |
| Smoking | 0.34 | 0.64 | |||
| Never | 7 (63.6) | 15 (88.2) | 8 (72.7) | ||
| Past/quit before pregnancy | 2 (18.2) | 1 (5.9) | 3 (27.3) | ||
| Quit early pregnancy | 2 (18.2) | 1 (5.9) | 0 (0.0) | ||
| Gestational age at echocardiogram (wks) | 36.6 (32.7 – 37.4) | 38.0 (35.6 – 39.6) | 0.08 | 36.4 (33.4–38.1) | 0.86 |
| 24-hour urine protein (mg/24 hrs) | 570.0 (333.0– 896.0) | *N/A | _ _ | *N/A | _ _ |
| Blood pressure on day of echocardiogram | |||||
| Systolic blood pressure (mm Hg) | 138.0 (126.0–148.0) | 112.0 (107.0–120.0) | <0.0001 | 141.0 (130.0–147.0) | 0.74 |
| Diastolic blood pressure (mm Hg) | 87.0 (78.0–93.0) | 69.0 (64.0–72.0) | <0.0001 | 88.0 (77.0–90.0) | 0.87 |
| Mode of delivery | 0.02 | 0.48 | |||
| Vaginal delivery | 2 (18.2) | 11 (64.7) | 0 (0.0) | ||
| Cesarean delivery | 9 (81.8) | 6 (35.3) | 11 (100.0) | ||
| Birth weight (gm) | 2875 (1665– 3270) | 3270 (2845–3900) | 0.09 | 2990.0 (2560.0–3505.0) | 0.56 |
| Gestational age at delivery (wks) | 36.7 (32.9 – 37.4) | 39.3 (38.1 – 40.4) | 0.001 | 38.0 (36.7–39.1) | 0.06 |
Data are presented as n (%) or median (interquartile range).
Comparison is between women with preeclampsia and women without preeclampsia.
Comparison is between women with preeclampsia and women with nonproteinuric hypertension.
24-hour urine only available for patients with preeclampsia.
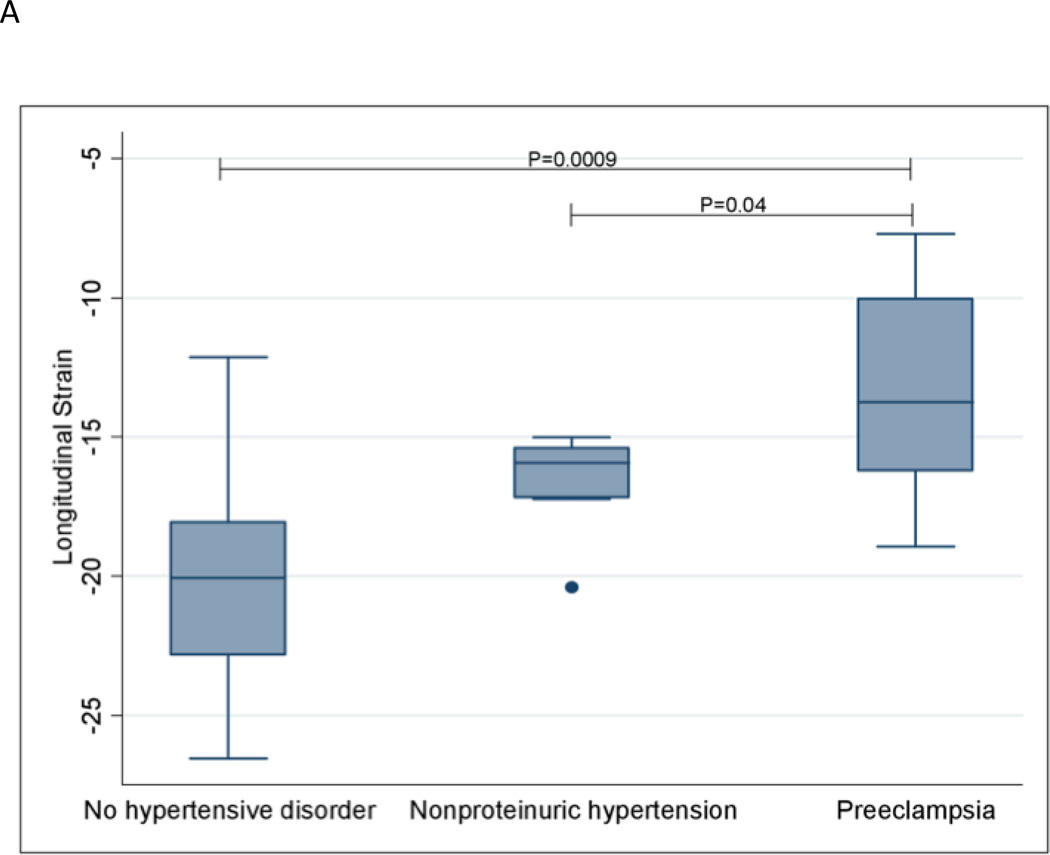
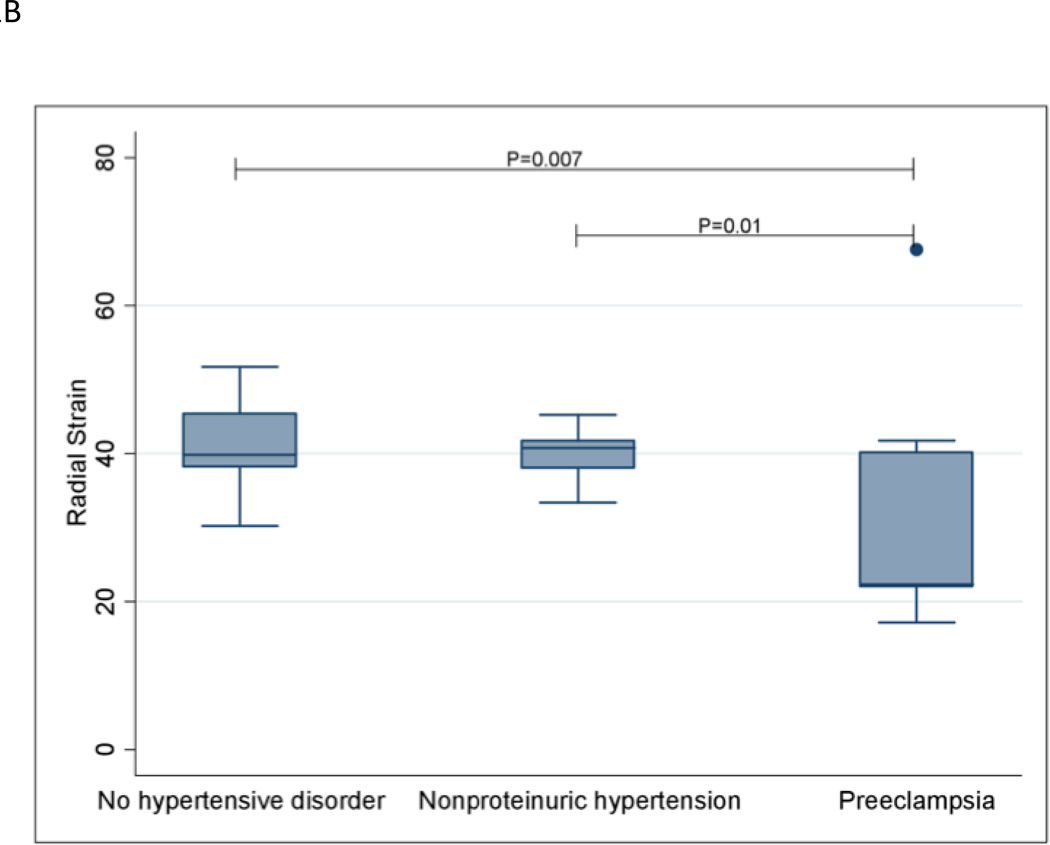
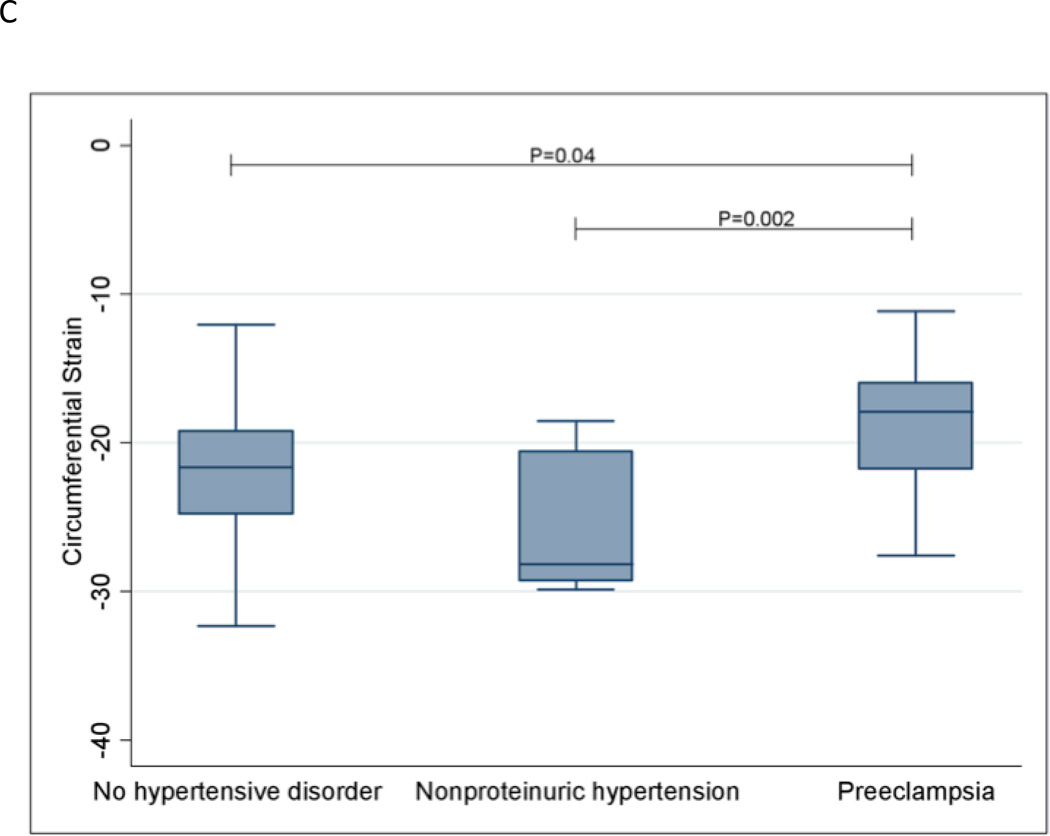
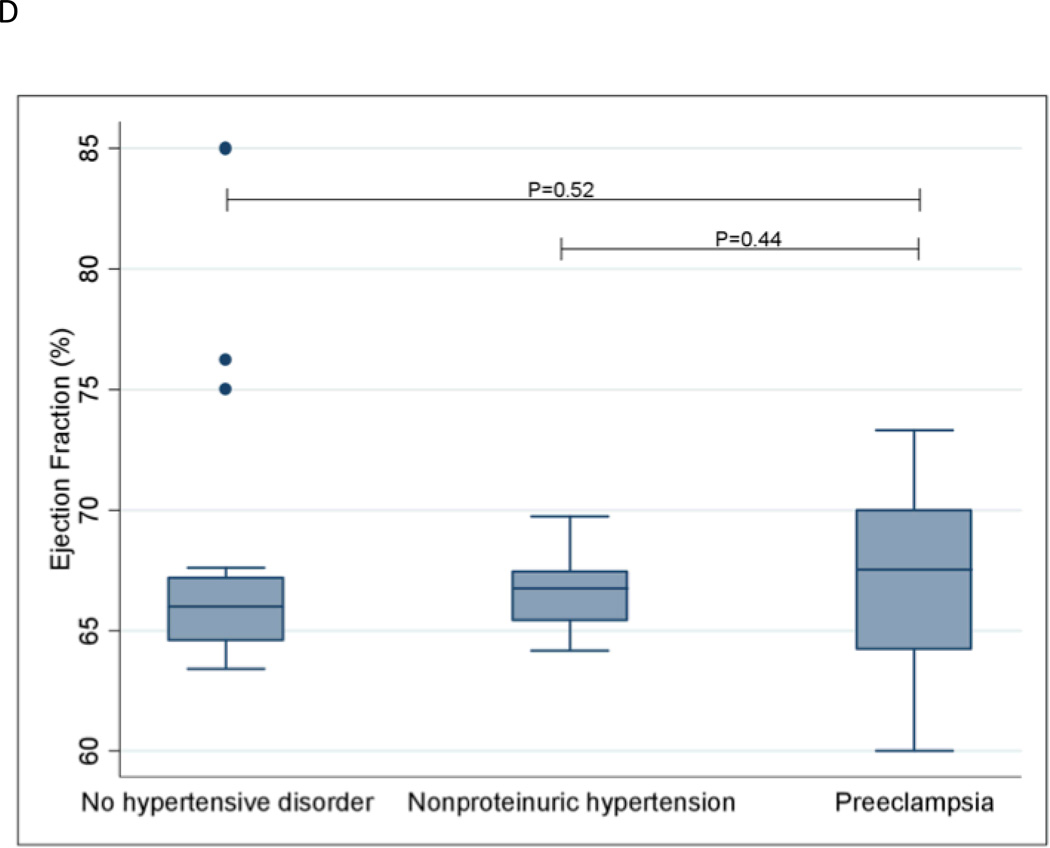
Longitudinal strain was significantly worsened in women with preeclampsia compared to women without a hypertensive disorder (P=0.0009). Similar results were observed for radial strain (P=0.007) and circumferential strain (P=0.04). Women with preeclampsia also had significantly worsened longitudinal (P=0.04), radial (P=0.01) and circumferential (P=0.002) strain compared with women with nonproteinuric hypertension. Strain measurements are shown in Figures 1A–1C. Women with preeclampsia did not have a significantly different ejection fraction compared with women without a hypertensive disorder (P=0.52) and women with nonproteinuric hypertension (P=0.44); see Figure 1D.
Figure 1. Changes in echocardiogram parameters stratified by hypertensive diagnosis.
Global Longitudinal Strain
The extremes of the box represent the interquartile range; the bottom of the box represents the 25th percentile and top of the box represents the 75th percentile. The horizontal line across the box represents the median. The ends of the whiskers correspond to the lowest and highest values within 1.5 interquartile ranges below and above the interquartile range. The median (interquartile range) longitudinal strain measurements for the three groups are as follows: No hypertensive disorder—−20.1 (−22.8 – −18.1); Nonproteinuric hypertension—−15.9 (−17.2– −15.4); Preeclampsia—−13.7 (−16.2 – −10.0).
Global Radial Strain
The extremes of the box represent the interquartile range; the bottom of the box represents the 25th percentile and top of the box represents the 75th percentile. The horizontal line across the box represents the median. The ends of the whiskers correspond to the lowest and highest values within 1.5 interquartile ranges below and above the interquartile range. The median (interquartile range) radial strain measurements for the three groups are as follows: No hypertensive disorder—39.8 (38.3 – 45.4); Nonproteinuric hypertension—40.7 (38.1–41.8); Preeclampsia—22.4 (22.0–40.2).
Global Circumferential Strain
The extremes of the box represent the interquartile range; the bottom of the box represents the 25th percentile and top of the box represents the 75th percentile. The horizontal line across the box represents the median. The ends of the whiskers correspond to the lowest and highest values within 1.5 interquartile ranges below and above the interquartile range. The median (interquartile range) circumferential strain measurements for the three groups are as follows: No hypertensive disorder—−21.6 (−24.8 – −19.2); Nonproteinuric hypertension—−28.2 (−29.3 – −20.6); Preeclampsia—−17.9 (−21.8 – −16.0).
Ejection Fraction
The extremes of the box represent the interquartile range; the bottom of the box represents the 25th percentile and top of the box represents the 75th percentile. The horizontal line across the box represents the median. The ends of the whiskers correspond to the lowest and highest values within 1.5 interquartile ranges below and above the interquartile range. The median (interquartile range) ejection fractions for the three groups are as follows: No hypertensive disorder—66.0 (64.6–67.2); Nonproteinuric hypertension—66.7 (65.4–67.5); Preeclampsia—67.5 (64.2–70.0).
The intraobserver correlation coefficient was 0.94 for longitudinal strain, 0.87 for circumferential strain and 0.84 for radial strain (all P<0.001). The interobserver correlation coefficient was 0.92 for longitudinal strain, 0.82 for circumferential strain and 0.78 for radial strain (all P<0.001).
Discussion
The principal findings of this study suggest that myocardial dysfunction is present in women with preeclampsia even in the presence of a normal ejection fraction. We found significant decreases in radial, circumferential and longitudinal strain in women with preeclampsia compared with women without a hypertensive disorder, without a corresponding decrease in left ventricular ejection fraction. These significant decreases in strain are also seen when comparing women with preeclampsia and nonproteinuric hypertension, which suggests that hypertension alone is not causing the observed changes. While these measurements suggest that myocardial strain may help detect subclinical myocardial dysfunction, long-term follow-up studies are needed to determine whether strain can be used in decision making for therapeutic strategies.
Previous work has show that women with preeclampsia have an angiogenic imbalance with high circulating levels of antiangiogenic proteins such as soluble fms-like tyrosine kinase-1 (sFlt1) and soluble endoglin and low levels of proangiogenic proteins such as vascular endothelial growth factor (VEGF) and placenta growth factor (PlGF).11 We have recently shown that high levels of sFlt1 in women with preclampsia can cause cardiac dysfunction characterized by an abnormal myocardial performance index, a sensitive marker of diastolic dysfunction and that sFlt1 be pathogenic in women with peripartum cardiomyopathy12. The decrease in longitudinal strain may represent attenuation of early longitudinal muscle relaxation leading to elevation in filling pressures and diastolic dysfunction, while changes that we observed in radial and circumferential strain in the setting of a normal ejection fraction likely represent transmural subclinical dysfunction. The observed subclinical left ventricular dysfunction likely develops from biochemical perturbations, combined with an increased end systolic wall stress from an increased afterload, leading to subendocardial microvascular ischemia and fibrosis. A key effector of biochemical perturbations is likely sFlt1, which causes both systemic vasoconstriction and intense small vessel myocardial vasoconstriction. This would explain why the longitudinal strain is most affected given that it is a functional measurement of subendocardial longitudinally oriented myocardium. sFlt1 is not elevated in women with nonproteinuric gestational hypertension, consistent with our findings of different strain patterns among these women.13
It is interesting to note that the median longitudinal strain in women with nonproteinuric hypertension (−15.9), lies in the spectrum between women with preeclampsia (−13.7) and without a hypertensive disorder (−20.1). This observation is consistent with known decreases in longitudinal strain induced by hypertension and is suggestive of additional factors that lead strain decreases observed in preeclampsia. Values for longitudinal strain in women with nonproteinuric hypertension observed in this study are consistent with values obtained by other investigators. E.g. Kyoung-Im Cho et al, recently demonstrated a decreased mean longitudinal strain (−17.6± 2.95) in women with gestational hypertension.14
The increased median circumferential strain found in women with nonproteinuric hypertension (−28.2) compared with women without a hypertensive disorder (−21.6) likely represents a compensatory increase in circumferential fiber function in the setting of decreased longitudinal fiber function to preserve normal left ventricular systolic function.15
Our values for strain in healthy pregnant women are consistent with values observed in healthy volunteers. Normal mean values for longitudinal, radial and circumferential strain are −20.9 (±−2.4), 31.7(±7.6) and −27.3(±3.9), respectively.16 This supports our finding that the changes in strain in women with preeclampsia are due to the preeclampsia and not related to the pregnancy per se.
Reduced global longitudinal, circumferential and radial strain with preserved left ventricular ejection fraction has been previously reported in other disease settings. Subclinical left ventricular dysfunction detected by strain with normal ejection fraction has been identified in amyloidosis,17, 18 Behcet’s disease7and prediabetes.19 In hypertensive patients with heart failure and preserved ejection fraction aldosterone antagonism resulted in improved myocardial function as evidenced by increased strain. Strain increase was independent of blood pressure change.20 Despite many therapeutic advances, patients presenting with heart failure have an adverse prognosis.21 Early detection of left ventricular dysfunction with the institution of appropriate therapy may reduce the risk of future cardiovascular disease.22 Indeed, Stoodley et al. recently demonstrated that two-dimensional myocardial strain imaging immediately detects changes in left ventricular systolic function soon after anthracycline chemotherapy. The authors found significantly reduced longitudinal and radial systolic strain one week after chemotherapy despite no detectable changes in ejection fraction.23
To our knowledge this study is the first to employ speckle tracking to evaluate ventricular mechanics in women with preeclampsia. The strengths of this study include the uniformity of diagnosis of preeclampsia, blinding of sonographers, and the use of an angle-independent measurement that reduces inter- and intra-observer variability, which was evidenced by the high correlation coefficients both within and between sonographers.8
This study has several limitations. Although we observed statistically significant differences, the sample size was small. Thus, larger studies are needed to determine whether our findings can be replicated. Long-term follow up also is needed to determine the clinical relevance of the observed changes in strain. While the angle-independent nature of strain reduces inter- and intra-observer variability, variability in strain measurements is an inherent limitation of the technique. However, our measurements are similar to other published studies.
In summary we conclude that strain echocardiography using speckle tracking may help detect subclinical left ventricular dysfunction in women with preeclampsia. Whether detection of this early subclinical dysfunction has long-term implications and whether possibly modification of risk factors could lead to reduction in increased cardiovascular risk in women with preeclampsia needs to be evaluated in future studies.16
Acknowledgments
We thank Dawn McCullough, RN, and Saira Salahuddin, MD, PhD, for patient recruitment and data collection.
Sources of Funding
S.R. is supported by Harvard Diversity and Community Partnership Faculty Fellowship Award and K08HD068398-01A1 (NIH/NICHD). This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). DT is supported by the National Institutes of Health (NHLBI 1U01HL108712-01). The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: Prospective evidence from the child health and development studies cohort. Hypertension. 2010;56:166–171. doi: 10.1161/HYPERTENSIONAHA.110.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97:533–538. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 3.Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 2011;57:85–93. doi: 10.1161/HYPERTENSIONAHA.110.162321. [DOI] [PubMed] [Google Scholar]
- 4.Tyldum EV, Backe B, Stoylen A, Slordahl SA. Maternal left ventricular and endothelial functions in preeclampsia. Acta Obstet Gynecol Scand. 2012;91:566–573. doi: 10.1111/j.1600-0412.2011.01282.x. [DOI] [PubMed] [Google Scholar]
- 5.Dedobbeleer C, Rai M, Donal E, Pandolfo M, Unger P. Normal left ventricular ejection fraction and mass but subclinical myocardial dysfunction in patients with friedreich's ataxia. Eur J Echocardiogr. 2012;13:346–352. doi: 10.1093/ejechocard/jer267. [DOI] [PubMed] [Google Scholar]
- 6.Ng AC, Delgado V, Bertini M, Antoni ML, van Bommel RJ, van Rijnsoever EP, van der Kley F, Ewe SH, Witkowski T, Auger D, Nucifora G, Schuijf JD, Poldermans D, Leung DY, Schalij MJ, Bax JJ. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: A two-dimensional speckle tracking analysis. Eur Heart J. 2011;32:1542–1550. doi: 10.1093/eurheartj/ehr084. [DOI] [PubMed] [Google Scholar]
- 7.Yagmur J, Sener S, Acikgoz N, Cansel M, Ermis N, Karincaoglu Y, Tasolar H, Karakus Y, Pekdemir H, Ozdemir R. Subclinical left ventricular dysfunction in behcet's disease assessed by two-dimensional speckle tracking echocardiography. Eur J Echocardiogr. 2011;12:536–541. doi: 10.1093/ejechocard/jer088. [DOI] [PubMed] [Google Scholar]
- 8.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: Fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369. doi: 10.1016/j.echo.2010.02.015. quiz 453-355. [DOI] [PubMed] [Google Scholar]
- 9.Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: A report from the american society of echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european association of echocardiography, a branch of the european society of cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 12.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, Hess P, Farrell C, Koulisis N, Khankin EV, Burke SD, Tudorache I, Bauersachs J, del Monte F, Hilfiker-Kleiner D, Karumanchi SA, Arany Z. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–338. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, Calda P, Holzgreve W, Galindo A, Engels T, Denk B, Stepan H. The sflt-1/plgf ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58, e51–e58. doi: 10.1016/j.ajog.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 14.Cho KI, Kim SM, Shin MS, Kim EJ, Cho EJ, Seo HS, Shin SH, Yoon SJ, Choi JH. Impact of gestational hypertension on left ventricular function and geometric pattern. Circ J. 2011;75:1170–1176. doi: 10.1253/circj.cj-10-0763. [DOI] [PubMed] [Google Scholar]
- 15.Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Ara N, Oki T. Possible mechanisms of left ventricular torsion evaluated by cardioreparative effects of telmisartan in patients with hypertension. Eur J Echocardiogr. 2010;11:690–697. doi: 10.1093/ejechocard/jeq044. [DOI] [PubMed] [Google Scholar]
- 16.Biaggi P, Carasso S, Garceau P, Greutmann M, Gruner C, Tsang W, Rakowski H, Agmon Y, Woo A. Comparison of two different speckle tracking software systems: Does the method matter? Echocardiography. 2011;28:539–547. doi: 10.1111/j.1540-8175.2011.01386.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu D, Niemann M, Hu K, Herrmann S, Stork S, Knop S, Ertl G, Weidemann F. Echocardiographic evaluation of systolic and diastolic function in patients with cardiac amyloidosis. Am J Cardiol. 2011;108:591–598. doi: 10.1016/j.amjcard.2011.03.092. [DOI] [PubMed] [Google Scholar]
- 18.Porciani MC, Cappelli F, Perfetto F, Ciaccheri M, Castelli G, Ricceri I, Chiostri M, Franco B, Padeletti L. Rotational mechanics of the left ventricle in al amyloidosis. Echocardiography. 2010;27:1061–1068. doi: 10.1111/j.1540-8175.2010.01199.x. [DOI] [PubMed] [Google Scholar]
- 19.Ernande L, Rietzschel ER, Bergerot C, De Buyzere ML, Schnell F, Groisne L, Ovize M, Croisille P, Moulin P, Gillebert TC, Derumeaux G. Impaired myocardial radial function in asymptomatic patients with type 2 diabetes mellitus: A speckle-tracking imaging study. J Am Soc Echocardiogr. 2010;23:1266–1272. doi: 10.1016/j.echo.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110:558–565. doi: 10.1161/01.CIR.0000138680.89536.A9. [DOI] [PubMed] [Google Scholar]
- 21.Park D, McManus D, Darling C, Goldberg JH, Gore JM, Lessard D, Goldberg RJ. Recent trends in the characteristics and prognosis of patients hospitalized with acute heart failure. Clin Epidemiol. 2011;3:295–303. doi: 10.2147/CLEP.S25799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the acc/aha 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 23.Stoodley PW, Richards DA, Hui R, Boyd A, Harnett PR, Meikle SR, Clarke J, Thomas L. Two-dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. Eur J Echocardiogr. 2011;12:945–952. doi: 10.1093/ejechocard/jer187. [DOI] [PubMed] [Google Scholar]