Abstract
Background
A number of studies have examined the association between the polymorphisms of the low-density lipoprotein receptor-related protein 5 gene (LRP5), but previous results have been inconclusive. Thus we performed a meta-analysis of studies on the association between the LRP5 polymorphisms and bone mineral density (BMD) to assess their pooled effects.
Methods
Published literature from PubMed, EMBASE and ISI web of science were searched for eligible publications. Weighted mean difference (WMD) and 95% confidence interval (CI) was calculated using fixed- or random-effects model.
Results
A total of 19 studies with 25773 subjects were considered in this meta-analysis. Of them, 17 examined the association between the A1330V polymorphism and BMD, 8 were focused on the V667M polymorphism, and 2 analyzed the Q89R polymorphism. Individuals with the A1330V AA genotype showed significantly higher BMD than those with the AV/VV genotypes [at lumbar spine (LS): WMD = 0.02g/cm2, 95% CI = 0.01-0.03, P < 10-4; at femur neck (FN): WMD = 0.01g/cm2, 95% CI = 0.00-0.02, P = 0.01] or VV genotype (at LS: WMD = 0.02g/cm2, 95% CI = 0.01-0.04, P = 0.01). Significant associations were also detected in the analysis for V667M (VV vs. VM/MM: WMD at LS = 0.02g/cm2, 95% CI = 0.02-0.03, P < 10-5; WMD at FN = 0.01g/cm2, 95% CI = 0.01-0.02, P = 0.0002). As for Q89R, subjects with the QQ genotype tended to have higher BMD than those with the QR/RR genotypes at FN (WMD = 0.03g/cm2, 95% CI = 0.01-0.05, P = 0.005).
Conclusion
This meta-analysis demonstrated that the LRP5 polymorphisms may be modestly associated with BMD of LS and FN.
Introduction
Osteoporosis is a common disease characterized by low bone mass, microarchitectural deterioration of bone tissue and enhanced bone fragility which leads to an increased incidence of fracture. It is now well established that genetic factors play a major role in regulating bone mineral density (BMD) [1]. Association studies have been used to identify genetic variants that are associated with BMD and fractures [2–6]. Recently, genome-wide association studies (GWAS) have been successful in further identifying several common variants that are significantly associated with BMD and with fracture risk [7–10].
The low-density lipoprotein receptor (LDLR)-related protein 5 (LRP5) gene is one of the candidate genes that have been implicated in BMD. As a member of the LDLR family, this gene contains 23 exons that span more than 100 kb and encodes a single-pass transmembrane protein of 1614 amino acids [11]. LRP5 cooperates with members of the frizzled family of seven-pass transmembrane receptors to bind Wnt proteins, and forms a functional ligand-receptor complex that activates the Wnt-β-catenin pathway [12–14]. This Wnt-β-catenin pathway is one of the key pathways to affect osteoblast development. It has been reported that loss-of-function mutations of the LRP5 gene cause osteoporosis-pseudoglioma (OPPG), an autosomal recessive disease characterized by low bone mass and childhood fractures [15], whereas the LRP5 G171V mutation is associated with autosomal dominant high bone mass (HBM) traits [16–18]. Transgenic and knock-out mouse models mimic the human phenotypes of high and low bone mass, respectively [19]. In addition to these mutations, a number of polymorphisms have been described in the LRP5 gene. Among them, two coding polymorphisms A1330V (rs3736228) and V667M (rs4988321) were studied most, and were suggested to be associated with BMD [6,20–24]. Another missense variant is Q89R (rs41494349) located in exon 2, which has been reported to be significantly associated with BMD among Asian population [25,26].
Although many studies have investigated the relationship between these three LRP5 polymorphisms and BMD, published results have been conflicting. This may be due to that individual studies based on restricted sample sizes lack sufficient statistical power to detect effects of interest. Therefore, we performed the present meta-analysis to clarify the association of the LRP5 polymorphisms (A1330V, V667M and Q89R) with BMD.
Materials and Methods
Literature search strategy
The literature included in our analysis was selected from PubMed, EMBASE and ISI web of science using combinations of the following keywords: low-density lipoprotein receptor-related protein 5 gene (LRP5), polymorphism, A1330V (rs3736228), V667M (rs4988321), Q89R (rs41494349) and bone mineral density (BMD). Genetic association studies published before the end of March 1, 2013 on the association between BMD and polymorphisms in the LRP5 gene were retrieved, and their references were checked to identify other relevant publications. The publication language was restricted to English.
Eligible studies and data extraction
Eligible studies had to meet all of the following criteria: (1) were published in peer-reviewed journals and were independent studies using original data; (2) investigated the effect of the LRP5 polymorphisms on BMD; (3) provided sufficient data for calculation of weighted mean difference (WMD) with its 95 % confidence interval (CI) and P value; (4) described the genotyping method, equipment, and protocols used or provided reference to them. For each study, the following data were extracted independently by two authors: first author’s surname, year of publication, age, gender, ethnicity, study site, genotyping method, total number of subjects, skeletal sites evaluated for BMD, and genotype and BMD data. The results were compared and disagreements were discussed and resolved with consensus. Studies with different gender, age and ethnic groups were considered as individual studies for our analyses.
Statistical analysis
We pooled eligible studies according to the site of BMD measurement and performed analyses at the lumbar spine (LS), total hip (HT), femoral neck (FN) and trochanter, respectively. The main analyses addressed differences in BMD between genotypes: AA versus AV/VV or AA versus VV for A1330V; VV versus VM/MM for Val667Met; QQ versus QR/RR for Q89R. We calculated the weighted mean difference (WMD) based on the actual BMD values reported in the included studies. Cochran’s Chi square-based Q statistic test was performed to assess possible heterogeneity between the individual studies [27]. If heterogeneity existed (P < 0.05) across studies, the random effects model was used [28]; otherwise, the fixed effect model was adopted [29]. We also performed subgroup analyses based on ethnicity (Caucasian and Asian) and gender (women and men).
Sensitivity analyses were performed to assess the stability of the results, namely, each study in the meta-analysis was deleted each time to reflect the influence of the individual dataset to the overall OR. Publication bias was assessed by Egger’s test [30] and funnel-plot analysis. All P values are two-sided, and P < 0.05 was considered statistically significant. All the statistical analyses were performed by Review Manager v.5.
Results
Characteristics of studies
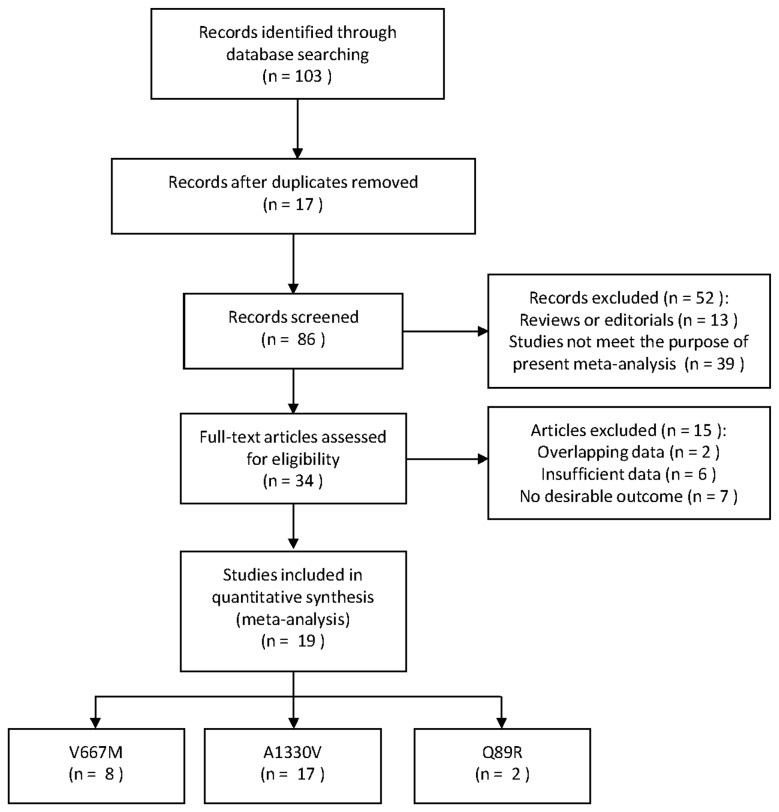
The literature search yielded 103 references. Study selection process is shown in Figure 1 and Table S1. A total of 19 eligible studies [20-26,31-42] were finally included with 25773 subjects (15871 women and 9902 men). For the A1330V polymorphism, 17 studies were available, including a total of 23837 subjects. For the V667M polymorphism, 8 studies involved a total of 14985 Caucasian subjects. For the Q89R polymorphism, 2 studies with a total of 1069 subjects were included, both of which were conducted in Asia. The detailed characteristics of all the studies and the main results in this meta-analysis are shown in Tables 1, 2, 3 and 4.
Figure 1. Summary of search strategy and result.
Table 1. Characteristics of individual studies included in the meta-analysis.
| Author | Year | Gender | Country | Ethnicity | Age (mean ± SD) | Genotyping method | Number | BMD | SNP |
|---|---|---|---|---|---|---|---|---|---|
| Brixen[21] | 2007 | Men | Denmark | Caucasian | 20-30 | TaqMan/Acyclo Prime-FP SNP Detection kits | 783 | LS, TH | Ala1330Val |
| Ezura[23] | 2007 | Women | Japan | Asian | 64.6±10.8 | Sd-PCR/Invader assay/TaqMan | 387 | LS | A1330V |
| Giroux[24] | 2007 | Women | Canada | Caucasian | 25-91 | Allele-specific PCR | 5144 | LS, FN | Val667Met, Ala1330Val |
| Giroux[31] | 2008 | Women | Canada | Caucasian | 18-58 | Allele-specific PCR | 1382 | LS, FN | Val667Met |
| Grundberg [32] | 2008 | Men | Sweden | Caucasian | Sweden 74.4±3.2; Hong Kong 72.4±5.0;GOOD 18.9±0.56 | TaqMan | 6082 | LS, FN | Val667Met, Ala1330Val |
| Jiang[33] | 2010 | Men and women | China | Asian | 48.9±12.5 | TaqMan | 425 | LS, TH | A1330V |
| Koh[25] | 2004 | Men | Korea | Asian | 25.6±3.7 | PCR-RFLP | 219 | LS, FN, trochanter | Q89R, A1330V |
| Koller[34] | 2005 | Women | USA | Caucasian | 33.3±7.0 | Fluorescent allele-specific PCR/MALDI-TOF | 1301 | LS, FN | A1330V |
| Kruk[35] | 2009 | Men | UK | Caucasian | 59.7±10.7 | PCR-Sequencing | 249 | LS, TH, FN, trochanter | Val667Met, Ala1330Val |
| Markatseli [36] | 2011 | Women | Greece | Caucasian | 56.8±4.9 | TaqMan | 221 | LS | Val667Met, Ala1330Val |
| Massart[37] | 2012 | Women | Italy | Caucasian | 20-50 | PCR-RFLP | 570 | LS, TH, FN | Val667Met, Ala1330Val |
| Mencej- Bedrac[38] | 2009 | Men and women | Slovenia | Caucasian | 62.3±9.5 | PCR-RFLP | 625 | LS, FN | A1330V |
| Meurs[22] | 2006 | Men and women | Netherlands | Caucasian | >50 | PCR-SBE | 5373 | LS, FN | A1330V |
| Mizuguchi [39] | 2004 | Women | Japan | Asian | 54.2±12.4 | PCR-Sequencing/TaqMan | 254 | LS | A1330V |
| Riancho[42] | 2011 | Women | Spain | Caucasian | 51-90 | Mass-array/ TaqMan | 873 | FN | A1330V |
| Saarinen[20] | 2007 | Men | Finland | Caucasian | 18-21 | PCR-Sequencing | 235 | LS, TH, FN, trochanter | A1330V |
| Stathopoulou[40] | 2010 | Women | Greece | Caucasian | >50 | iPLEX Gold assay | 554 | LS, TH, FN, trochanter | Val667Met |
| Yu[41] | 2010 | Men | China | Asian | 30.6±6.3 | PCR-RFLP | 422 | LS, TH, FN, trochanter | A1330V |
| Zhang[26] | 2005 | Women | China | Asian | 60.1±6.3 | PCR-RFLP | 647 | LS, FN, trochanter | Q89R, A1330V |
BMD: bone mineral density; TH: total hip;LS: lumbar spine; FN: femur neck.
Table 2. Meta-analysis of the A1330V polymorphism and BMD association.
| Site of BMD | LS |
TH |
||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup | No. of data sets | WMD [95% CI] | P value | I 2(P) | No. of data sets | WMD [95% CI] | P value | I 2(P) |
| Overall | ||||||||
| AA vs AV/VV | 11 | 0.02 [0.01,0.03] | <10-4 | 51% (0.02) | 2 | 0.03 [-0.07,0.14] | 0.53 | 91% (0.001) |
| AA vs VV | 11 | 0.02 [0.01,0.04] | 0.01 | 6% (0.39) | 7 | 0.00 [-0.05,0.05] | 1.00 | 67% (0.006) |
| Ethnicity | ||||||||
| Caucasian | 7 | 0.02 [0.01,0.03] | 0.001 | 67% (0.005) | 5 | -0.04 [-0.08,0.00] | 0.05 | 0% (0.42) |
| Asian | 4 | 0.01 [0.00,0.02] | 0.04 | 0% (0.68) | 2 | 0.07 [-0.01,0.15] | 0.07 | 64% (0.09) |
| Gender | ||||||||
| Women | 5 | 0.03 [0.01,0.04] | 0.0003 | 65% (0.02) | 3 | -0.06 [-0.13,0.01] | 0.11 | 0% (0.65) |
| Men | 6 | 0.01 [0.00,0.02] | 0.04 | 0% (0.47) | 3 | 0.00 [-0.07,0.06] | 0.89 | 69% (0.04) |
| Site of BMD | FN | Trochanter | ||||||
| Subgroup | No. of data sets | WMD [95% CI] | P value | I 2(P) | No. of data sets | WMD [95% CI] | P value | I 2(P) |
| Overall | ||||||||
| AA vs AV/VV | 8 | 0.01 [0.00,0.02] | 0.01 | 52% (0.04) | 3 | -0.00 [-0.01,0.02] | 0.59 | 83% (0.003) |
| AA vs VV | 9 | 0.00 [-0.01,0.02] | 0.67 | 24% (0.23) | 2 | -0.03 [-0.13,0.09] | 0.76 | 85% (0.009) |
| Ethnicity | ||||||||
| Caucasian | 6 | 0.01 [0.01,0.02] | 0.01 | 56% (0.04) | 2 | -0.01 [-0.03,0.01] | 0.47 | 90% (0.001) |
| Asian | 2 | 0.00 [-0.01,0.01] | 0.60 | 0% (0.36) | 1 | 0.01 [-0.01,0.03] | 0.22 | NA |
| Gender | ||||||||
| Women | 3 | 0.01 [0.01,0.02] | <10-4 | 0% (0.39) | 1 | 0.01 [-0.01,0.03] | 0.22 | NA |
| Men | 5 | 0.01 [-0.00,0.01] | 0.14 | 62% (0.03) | 2 | -0.01 [-0.03,0.01] | 0.47 | 90% (0.001) |
Table 3. Meta-analysis of the V667M polymorphism and BMD association.
| Site of BMD | LS |
TH |
||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup | No. of data sets | WMD [95% CI] | P value | I 2(P) | No. of data sets | WMD [95% CI] | P value | I 2(P) |
| Overall | ||||||||
| VV vs VM/MM | 10 | 0.02 [0.02,0.03] | <10-5 | 19% (0.27) | 5 | 0.01 [-0.01,0.02] | 0.43 | 23% (0.27) |
| Ethnicity | ||||||||
| Caucasian | 10 | 0.02 [0.02,0.03] | <10-5 | 19% (0.27) | 5 | 0.01 [-0.01,0.02] | 0.43 | 23% (0.27) |
| Gender | ||||||||
| Women | 7 | 0.03 [0.02,0.03] | <10-5 | 39% (0.13) | 4 | 0.01 [-0.01,0.03] | 0.32 | 37% (0.19) |
| Men | 3 | 0.02 [0.00,0.04] | 0.01 | 0% (0.61) | 1 | 0.00 [-0.04,0.3] | 0.87 | NA |
| Site of BMD | FN | Trochanter | ||||||
| Subgroup | No. of data sets | WMD [95% CI] | P value | I 2(P) | No. of data sets | WMD [95% CI] | P value | I 2(P) |
| Overall | ||||||||
| VV vs VM/MM | 9 | 0.01 [0.01,0.02] | 0.0002 | 42% (0.09) | 2 | 0.01 [-0.01,0.03] | 0.31 | 0% (0.34) |
| Ethnicity | ||||||||
| Caucasian | 9 | 0.01 [0.01,0.02] | 0.0002 | 42% (0.09) | 2 | 0.01 [-0.01,0.03] | 0.31 | 0% (0.34) |
| Gender | ||||||||
| Women | 6 | 0.02 [0.01,0.02] | <10-4 | 52% (0.06) | 1 | 0.02 [-0.01,0.05] | 0.16 | NA |
| Men | 3 | 0.00 [-0.01,0.02] | 0.58 | 0% (0.51) | 1 | -0.00 [-0.04,0.03] | 0.91 | NA |
Table 4. Meta-analysis of the Q89R polymorphism and BMD association.
| Site of BMD | LS |
FN |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Subgroup | No. of data sets | WMD [95% CI] | P value | I 2(P) | No. of data sets | WMD [95% CI] |
P value | I 2(P) | |
| Overall |
|
||||||||
| QQ vs QR/RR | 2 | 0.00 [-0.02,0.03] | 0.89 | 0% (0.86) | 2 | 0.03 [0.01,0.05] |
0.005 | 72% (0.06) | |
| Ethnicity |
|
||||||||
| Asian | 2 | 0.00 [-0.02,0.03] | 0.89 | 0% (0.86) | 2 | 0.03 [0.01,0.05] |
0.005 | 72% (0.06) | |
| Gender | |||||||||
| Women | 1 | 0.00 [-0.03,0.03] | 0.83 | NA | 1 | 0.02 [-0.00,0.04] | 0.1 | NA | |
| Men | 1 | 0.00 [-0.05,0.04] | 0.93 | NA | 1 | 0.06 [0.02,0.10] | 0.003 | NA | |
| Site of BMD | Trochanter | ||||||||
| Subgroup | No. of data sets | WMD [95% CI] | P value | I 2(P) | |||||
| Overall | |||||||||
| QQ vs QR/RR | 2 | 0.02 [-0.00,0.03] | 0.10 | 23% (0.25) | |||||
| Ethnicity | |||||||||
| Caucasian | 2 | 0.02 [-0.00,0.03] | 0.10 | 23% (0.25) | |||||
| Gender | |||||||||
| Women | 1 | 0.01 [-0.01,0.03] | 0.26 | NA | |||||
| Men | 1 | 0.04 [-0.01,0.09] | 0.10 | NA | |||||
Association of the LRP5 A1330V polymorphism with BMD
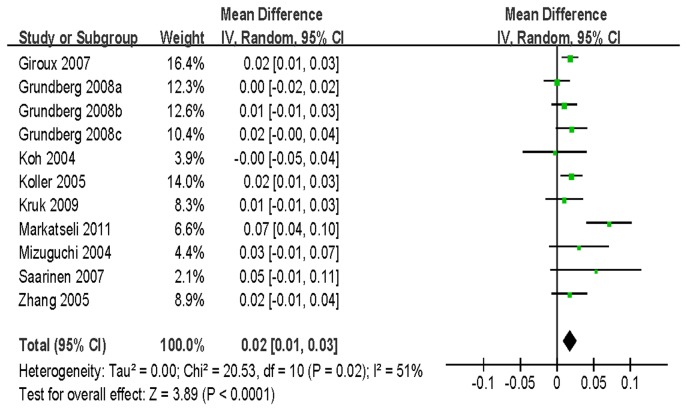
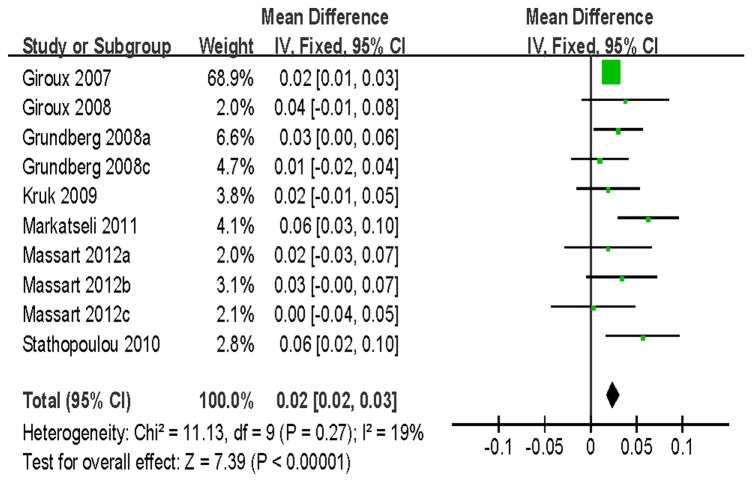
There were 17 studies analyzing LS BMD, 6 analyzing TH BMD, 12 analyzing FN BMD and 5 focused on trochanter BMD. In the pooled analyses, individuals with the AA genotype were found to have a statistically significant association with BMD compared to subjects with the AV/VV (at LS: WMD = 0.02 g/cm2, 95% CI = 0.01-0.03, P < 10-4; at FN: WMD = 0.01 g/cm2, 95% CI = 0.00-0.02, P = 0.01) (Figure 2 and Figure 3) or VV genotype (at LS: WMD = 0.02 g/cm2, 95% CI = 0.01-0.04, P = 0.01). Similar results were obtained when stratified by gender and ethnicity for AA versus AV/VV (Table 2). Interestingly, the association was not found in men [WMD = 0.01 g/cm2, 95% CI = (-0.00)-0.01, P = 0.14] or Asians [WMD = 0.00 g/cm2, 95% CI = (-0.01)-0.01, P = 0.60] in the analysis of FN BMD. We did not observe any significant association for BMD of TH and trochanter (Table 2).
Figure 2. WMD and 95% CI in LS BMD between A1330V AA and AV/VV genotypes.
Figure 3. WMD and 95% CI in FN BMD between A1330V AA and AV/VV genotypes.
Association of the LRP5 V667M polymorphism with BMD
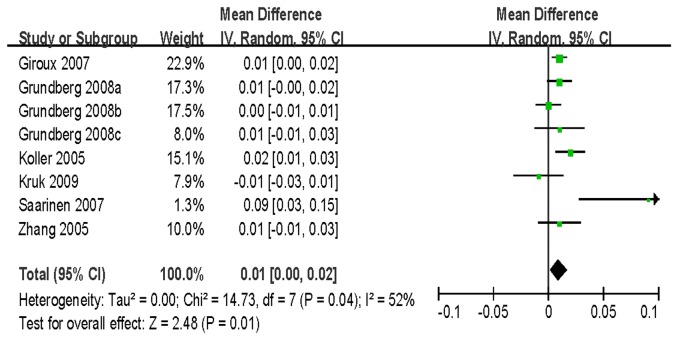
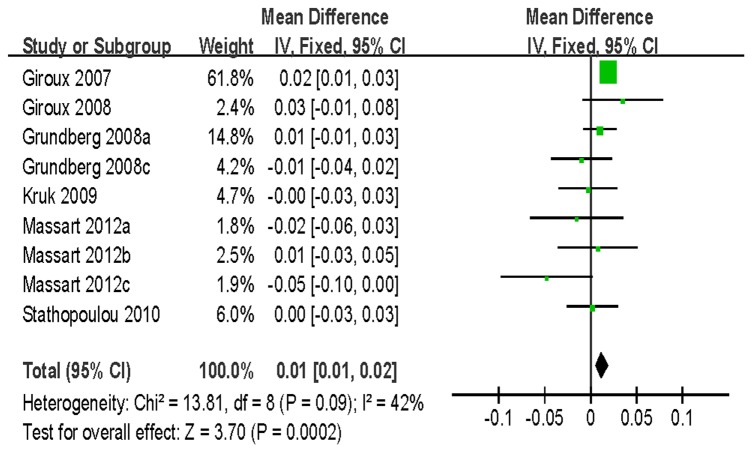
For BMD of LS, 7 studies with 8242 women and 4323 men were identified for the data analysis. Individuals with the VV genotype showed a significantly greater BMD than those with the VM/MM genotype (WMD = 0.02 g/cm2, 95% CI = 0.02–0.03, P < 10-5) without between-study heterogeneity (P = 0.27) (Figure 4). Similar significant associations were observed when women and men genotypes were analyzed separately (Table 3). For BMD of FN, a total of 10973 subjects based on 6 studies were identified for the data analysis. There was a significant association detected between the polymorphism and BMD (VV vs. VM/MM: WMD = 0.01 g/cm2, 95% CI = 0.01–0.02, P = 0.0002) (Figure 5). However, no association was found for men in the subgroup analysis stratified by gender [VV vs. VM/MM: WMD = 0.00 g/cm2, 95% CI = (-0.01)-0.02, P = 0.58]. Furthermore, our data showed no statistical evidence of significant association between this SNP and BMD of TH and trochanter (Table 3).
Figure 4. WMD and 95% CI in LS BMD between V667M VV and VM/MM genotypes.
Figure 5. WMD and 95% CI in FN BMD between V667M VV and VM/MM genotypes.
Association of the LRP5 Q89R polymorphism with BMD
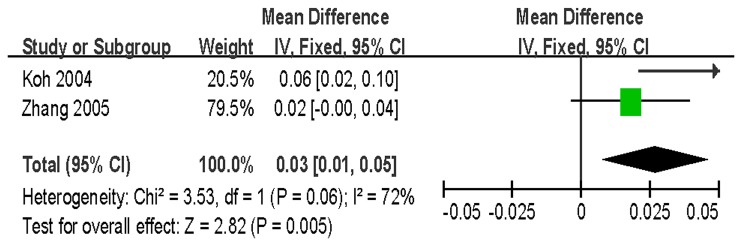
Two studies analyzed the relationship between Q89R and the BMD of LS, FN and trochanter among a total of 1069 Asian subjects. There was a significant association between the LRP5 Q89R polymorphism and FN BMD (QQ vs. QR/RR: WMD = 0.03 g/cm2, 95% CI = 0.01–0.05, P = 0.005) without between-study heterogeneity (P = 0.06) (Figure 6). When stratified by gender, significant association was found only in men (QQ vs. QR/RR: WMD = 0.06 g/cm2, 95% CI = 0.02–0.10, P = 0.003), but not in women (Table 4). The results of our meta-analyses for LS and trochanter BMD indicated no significant associations [LS: WMD = 0.00 g/cm2, 95% CI = (-0.02)-0.03, P = 0.89; trochanter: WMD = 0.02 g/cm2, 95% CI = (-0.00)-0.03, P = 0.10].
Figure 6. WMD and 95% CI in FN BMD between Q89R QQ and QR/RR genotypes.
Sensitivity analysis and potential publication bias
Sensitivity analysis was conducted by deleting each study at a time to reflect the influence of the individual dataset to the pooled WMDs, and the corresponding results were not qualitatively altered. The inverted funnel plots were symmetrical as shown in Figures S1-S5. Egger’s test was also performed to access the publication bias of the literatures, and no publication bias was detected for the associations of the LRP5 polymorphisms with BMD phenotypes (all P > 0.05).
Discussion
In this meta-analysis, we investigated the association of the LRP5 A1330V, V667M and Q89R polymorphisms with BMD. The LRP5 gene is a known locus associated with BMD in several genome-wide association studies (GWASs). Although early studies were carried out in Caucasians [6-8], the 11q13 locus (LRP5) was also reported to be associated with BMD in replication study in the East-Asians [43]. There were meta-analyses on the association between the LRP5 gene and BMD [44,45], however, both studies only examined the influence of the A1330V polymorphism on BMD. Moreover, limited studies were included in both meta-analyses. In the present study, more unbiased new articles with a larger sample size could provide new insight into the underlying relationship between the LRP5 gene and BMD. This is the most comprehensive meta-analysis focused on the associations of the A1330V, Val667Met and Q89R polymorphisms with BMD, which involved 19 studies with 25773 subjects in total. Our results suggested that the A1330V and Val667Met polymorphisms were related to LS and FN BMDs, and that the Q89R polymorphism was associated with FN BMD.
There have been several studies reported that the A1330V polymorphism is associated with BMD, and that individuals with the AV/VV or VV genotype have lower BMD than those with the AA genotype. Our data also supported a significant association between this SNP and BMD at LS and FN among Caucasian populations, which is consistent with previous GWAS studies [7]. However, we did not detect any association of this polymorphism with FN BMD among men or Asians. It has been observed that the relative distribution of the A1330V genotypes varied remarkably between genders as well as among different populations. For example, the AA genotype was 46%-73.5% in Asian women [23,25], however, much higher (73%) in Dutch women [22]. Among Caucasian populations, the AA genotype was detected in 56% of Dutch men [22], 91% of Finnish men [20], and 76% of Danish men [21]. In addition, there were statistically significant differences in A1330-allele frequencies with regard to gender in our study (P < 0.05). Possible explanation for the gender-related differences might be that sex-specific hormones (such as androgens and estrogens) were involved in the regulation of LRP5. LRP5, with its close homolog LRP6, functions as a cell membrane co-receptor for Wnt proteins in the canonical Wnt signaling pathway. Interestingly, a previous study reported the direct evidence of cross-talk between Wnt and estrogen signaling pathways via functional interaction between β-catenin and estrogen receptor (ER)-αin vivo [46]. The results from another more recent article also suggested the osteoporotic phenotype of ER knock-in (ERα−/NERKI) mice may involve the suppression of lymphoid enhancer factor-1 (Lef1)-mediated Wnt signaling [47]. Moreover, the study by Noh et al. [48] showed the Lef1 haploinsufficient (Lef1 +/-) mice display low bone mass phenotype in a gender- and age- specific manner. The findings of these functional studies, combined with our results, indicated that the effect of the LRP5 gene on BMD might be affected by gender.
For the analysis of V667M, our study consisted of 7 articles which were all using Caucasian subjects, while the analysis for the Q89R polymorphism included two studies both performed in Asian population. Significant association was observed between the V667M polymorphism and BMDs at LS and FN, which agreed with previous studies [21,24,31,32,36,40]. We also found that subjects with the Q89R QQ genotype tended to have high BMD than those with QR/RR genotypes. Our results appeared to be further supported by the positions at which these polymorphisms were situated in the LRP5 gene. LRP5 is a single pass membrane receptor whose extracellular protein contains four domains resembling a propeller with six blades containing YWTD spacer repeats followed by an epidermal growth factor (EGF)-like module, and an LDL receptor-like ligand-binding domain [49,50]. The Q89R polymorphism is located in exon 2, which encodes the first of four propellers, while V667M is localized at the top of the third propeller module. Although the precise function of each region is uncertain, the four propellers are of structural importance. It has been suggested that mutation in the first propeller region can alter the local hydrophobic environment, thus possibly affecting the interaction of LRP5 with other proteins [18]. In addition, the A1330V polymorphism is in exon 18 encoding the LDL receptor-like domain. It has been reported that a number of mutations in the LDL receptor propeller module can cause familial hypercholesterolemia [49], confirming that this domain is important for protein function. These findings provide evidence that all these polymorphisms might have the ability to alter the LRP5 activity, which in turn had an impact on BMD. But the question that arises is to what extent each SNP would exert influence on BMD.
The Q89R and A1330V polymorphisms were in linkage disequilibrium in Korean and Chinese populations [25,26], but not among a European population [51]. Strong linkage disequilibrium was also observed between the SNPs V667M and A1330V [22,34,52]. In the study by van Meurs et al. [22], the haplotype analysis indicated that the A1330V polymorphism was probably driving the association with BMD, because two haplotypes containing the 1330V variant showed an association with low BMD and other bone-related endpoints, whereas the other haplotypes did not. It has been found that another SNP at the intron 17 (IVS17-1677C>A) in the LRP5 gene was related to total body BMD in Japanese subjects [53]. Further study by the same team showed that the IVS17-1677C>A SNP and A1330V were in strong linkage disequilibrium [23]. These data suggested a possibility that the A1330V polymorphism or other polymorphisms in linkage disequilibrium with it, which have not been identified yet, might be the main driving force behind the association with BMD. However, replications of this association in different ethnic populations as well as in vitro functional studies are warrant to validate the hypothesis.
In interpreting the results, some limitations of this meta-analysis should be addressed. First, our results were based on unadjusted estimates, while a more precise analysis should be conducted if all individual raw data were available, which would allow for the adjustment by other covariates including age, body height, body weight, and exercises habits. Second, the analysis for V667M was totally based on data of Caucasian subjects, while the analysis for Q89R was based on two studies performed in Asians. Notably, the V667M SNP was not polymorphic in the Hong Kong population [32], and the Q89R polymorphism was very rare in Caucasians [51,54]. Therefore, additional studies in different populations focused on other loci which are in LD with these variations are needed to further validate ethnic difference in their effects on BMD. Third, the effects of gene-gene or gene-environment interactions were not addressed in this meta-analysis. However, most studies did not provide the detailed information, which impeded us for further analysis.
In conclusion, the present study is the most comprehensive meta-analysis investigating the associations of the LRP5 polymorphisms with BMD. And our data demonstrated that the A1330V and V667M polymorphisms were significantly associated with BMDs at LS and FN, while the Q89R polymorphism was significantly associated with FN BMD. For future association studies, more accurate phenotype and genotype data, detailed individual information, larger sample size of different ethnic populations and standard statistical methods will be needed. Moreover, the interactions between gene-gene and gene-environment should also be evaluated.
Supporting Information
(DOC)
The process of study selection for the meta-analysis.
(DOCX)
Funnel plot for LS BMD between A1330V AA and AV/VV genotypes.
(TIF)
Funnel plot for FN BMD between A1330V AA and AV/VV genotypes.
(TIF)
Funnel plot for LS BMD between V667M VV and VM/MM genotypes.
(TIF)
Funnel plot for FN BMD between V667M VV and VM/MM genotypes.
(TIF)
Funnel plot for FN BMD between Q89R QQ and QR/RR genotypes.
(TIF)
Funding Statement
The authors have no support or funding to report.
References
- 1. Arden NK, Spector TD (1997) Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Miner Res 12: 2076-2081. doi: 10.1359/jbmr.1997.12.12.2076. PubMed: 9421240. [DOI] [PubMed] [Google Scholar]
- 2. Fang Y, van Meurs JB, d'Alesio A, Jhamai M, Zhao H et al. (2005) Promoter and 3'-untranslated-region haplotypes in the vitamin d receptor gene predispose to osteoporotic fracture: the rotterdam study. Am J Hum Genet 77: 807-823. doi: 10.1086/497438. PubMed: 16252240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Langdahl BL, Uitterlinden AG, Ralston SH, Trikalinos TA, Balcells S et al. (2008) Large-scale analysis of association between polymorphisms in the transforming growth factor beta 1 gene (TGFB1) and osteoporosis: the GENOMOS study. Bone 42: 969-981. doi: 10.1016/j.bone.2007.11.007. PubMed: 18284942. [DOI] [PubMed] [Google Scholar]
- 4. Mann V, Hobson EE, Li B, Stewart TL, Grant SF et al. (2001) A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest 107: 899-907. doi: 10.1172/JCI10347. PubMed: 11285309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uitterlinden AG, Burger H, Huang Q, Yue F, McGuigan FE et al. (1998) Relation of alleles of the collagen type Ialpha1 gene to bone density and the risk of osteoporotic fractures in postmenopausal women. N Engl J Med 338: 1016-1021. doi: 10.1056/NEJM199804093381502. PubMed: 9535665. [DOI] [PubMed] [Google Scholar]
- 6. van Meurs JB, Trikalinos TA, Ralston SH, Balcells S, Brandi ML et al. (2008) Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA 299: 1277-1290. doi: 10.1001/jama.299.11.1277. PubMed: 18349089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N et al. (2008) Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371: 1505-1512. doi: 10.1016/S0140-6736(08)60599-1. PubMed: 18455228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rivadeneira F, Styrkársdottir U, Estrada K, Halldórsson BV, Hsu YH et al. (2009) Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41: 1199-1206. doi: 10.1038/ng.446. PubMed: 19801982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB et al. (2008) Multiple genetic loci for bone mineral density and fractures. N Engl J Med 358: 2355-2365. doi: 10.1056/NEJMoa0801197. PubMed: 18445777. [DOI] [PubMed] [Google Scholar]
- 10. Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB et al. (2009) New sequence variants associated with bone mineral density. Nat Genet 41: 15-17. doi: 10.1038/ng.284. PubMed: 19079262. [DOI] [PubMed] [Google Scholar]
- 11. Hey PJ, Twells RC, Phillips MS, Yusuke Nakagawa N, Brown SD et al. (1998) Cloning of a novel member of the low-density lipoprotein receptor family. Gene 216: 103-111. doi: 10.1016/S0378-1119(98)00311-4. PubMed: 9714764. [DOI] [PubMed] [Google Scholar]
- 12. Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC (2000) An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407: 535-538. doi: 10.1038/35035124. PubMed: 11029008. [DOI] [PubMed] [Google Scholar]
- 13. Tamai K, Semenov M, Kato Y, Spokony R, Liu C et al. (2000) LDL-receptor-related proteins in Wnt signal transduction. Nature 407: 530-535. doi: 10.1038/35035117. PubMed: 11029007. [DOI] [PubMed] [Google Scholar]
- 14. Wehrli M, Dougan ST, Caldwell K, O'Keefe L, Schwartz S et al. (2000) arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407: 527-530. doi: 10.1038/35035110. PubMed: 11029006. [DOI] [PubMed] [Google Scholar]
- 15. Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S et al. (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107: 513-523. doi: 10.1016/S0092-8674(01)00571-2. PubMed: 11719191. [DOI] [PubMed] [Google Scholar]
- 16. Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ et al. (2003) High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 18: 960-974. doi: 10.1359/jbmr.2003.18.6.960. PubMed: 12817748. [DOI] [PubMed] [Google Scholar]
- 17. Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A et al. (2002) High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346: 1513-1521. doi: 10.1056/NEJMoa013444. PubMed: 12015390. [DOI] [PubMed] [Google Scholar]
- 18. Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M et al. (2002) A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet 70: 11-19. doi: 10.1086/338450. PubMed: 11741193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kato M, Patel MS, Levasseur R, Lobov I, Chang BH et al. (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 157: 303-314. doi: 10.1083/jcb.200201089. PubMed: 11956231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saarinen A, Välimäki VV, Välimäki MJ, Löyttyniemi E, Auro K et al. (2007) The A1330V polymorphism of the low-density lipoprotein receptor-related protein 5 gene (LRP5) associates with low peak bone mass in young healthy men. Bone 40: 1006-1012. doi: 10.1016/j.bone.2006.11.010. PubMed: 17223614. [DOI] [PubMed] [Google Scholar]
- 21. Brixen K, Beckers S, Peeters A, Piters E, Balemans W et al. (2007) Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with peak bone mass in non-sedentary men: results from the Odense androgen study. Calcif Tissue Int 81: 421-429. doi: 10.1007/s00223-007-9088-z. PubMed: 18058054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Meurs JB, Rivadeneira F, Jhamai M, Hugens W, Hofman A et al. (2006) Common genetic variation of the low-density lipoprotein receptor-related protein 5 and 6 genes determines fracture risk in elderly white men. J Bone Miner Res 21: 141-150. PubMed: 16355283. [DOI] [PubMed] [Google Scholar]
- 23. Ezura Y, Nakajima T, Urano T, Sudo Y, Kajita M et al. (2007) Association of a single-nucleotide variation (A1330V) in the low-density lipoprotein receptor-related protein 5 gene (LRP5) with bone mineral density in adult Japanese women. Bone 40: 997-1005. doi: 10.1016/j.bone.2005.06.025. PubMed: 17306638. [DOI] [PubMed] [Google Scholar]
- 24. Giroux S, Elfassihi L, Cardinal G, Laflamme N, Rousseau F (2007) LRP5 coding polymorphisms influence the variation of peak bone mass in a normal population of French-Canadian women. Bone 40: 1299-1307. doi: 10.1016/j.bone.2007.01.004. PubMed: 17307038. [DOI] [PubMed] [Google Scholar]
- 25. Koh JM, Jung MH, Hong JS, Park HJ, Chang JS et al. (2004) Association between bone mineral density and LDL receptor-related protein 5 gene polymorphisms in young Korean men. J Korean Med Sci 19: 407-412. doi: 10.3346/jkms.2004.19.3.407. PubMed: 15201508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang ZL, Qin YJ, He JW, Huang QR, Li M et al. (2005) Association of polymorphisms in low-density lipoprotein receptor-related protein 5 gene with bone mineral density in postmenopausal Chinese women. Acta Pharmacol Sin 26: 1111-1116. doi: 10.1111/j.1745-7254.2005.00173.x. PubMed: 16115379. [DOI] [PubMed] [Google Scholar]
- 27. Davey Smith G, Egger M (1997) Meta-analyses of randomised controlled trials. Lancet 350: 1182. doi: 10.1016/S0140-6736(05)63833-0. PubMed: 9343537. [DOI] [PubMed] [Google Scholar]
- 28. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177-188. doi: 10.1016/0197-2456(86)90046-2. PubMed: 3802833. [DOI] [PubMed] [Google Scholar]
- 29. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719-748. PubMed: 13655060. [PubMed] [Google Scholar]
- 30. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629-634. doi: 10.1136/bmj.315.7109.629. PubMed: 9310563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giroux S, Elfassihi L, Cole DE, Rousseau F (2008) Replication of associations between LRP5 and ESRRA variants and bone density in premenopausal women. Osteoporos Int 19: 1769-1775. doi: 10.1007/s00198-008-0617-z. PubMed: 18418639. [DOI] [PubMed] [Google Scholar]
- 32. Grundberg E, Lau EM, Lorentzon M, Karlsson M, Holmberg A et al. (2008) Large-scale association study between two coding LRP5 gene polymorphisms and bone phenotypes and fractures in men. Osteoporos Int 19: 829-837. doi: 10.1007/s00198-007-0512-z. PubMed: 18026682. [DOI] [PubMed] [Google Scholar]
- 33. Jiang XY, Chen Y, Xu L, Li X, Cao FF et al. (2010) Association of LPR5 polymorphism with bone mass density and cholesterol level in population of Chinese Han. Exp Clin Endocrinol Diabetes 118: 388-391. doi: 10.1055/s-0029-1225613. PubMed: 20146170. [DOI] [PubMed] [Google Scholar]
- 34. Koller DL, Ichikawa S, Johnson ML, Lai D, Xuei X et al. (2005) Contribution of the LRP5 gene to normal variation in peak BMD in women. J Bone Miner Res 20: 75-80. doi: 10.1359/jbmr.2005.20.1.75. PubMed: 15619672. [DOI] [PubMed] [Google Scholar]
- 35. Kruk M, Ralston SH, Albagha OM (2009) LRP5 Polymorphisms and response to risedronate treatment in osteoporotic men. Calcif Tissue Int 84: 171-179. doi: 10.1007/s00223-008-9207-5. PubMed: 19148563. [DOI] [PubMed] [Google Scholar]
- 36. Markatseli AE, Hatzi E, Bouba I, Georgiou I, Challa A et al. (2011) Association of the A1330V and V667M polymorphisms of LRP5 with bone mineral density in Greek peri- and postmenopausal women. Maturitas 70: 188-193. doi: 10.1016/j.maturitas.2011.07.016. PubMed: 21840657. [DOI] [PubMed] [Google Scholar]
- 37. Massart F, Marini F, Bianchi G, Minisola S, Luisetto G et al. (2013) Genetic predictors of skeletal outcomes in healthy fertile women: The Bonturno Study. Joint Bone Spine, 80: 414–9. PubMed: 23238007. [DOI] [PubMed] [Google Scholar]
- 38. Mencej-Bedrac S, Prezelj J, Kocjan T, Komadina R, Marc J (2009) Analysis of association of LRP5, LRP6, SOST, DKK1, and CTNNB1 genes with bone mineral density in a Slovenian population. Calcif Tissue Int 85: 501-506. doi: 10.1007/s00223-009-9306-y. PubMed: 19898734. [DOI] [PubMed] [Google Scholar]
- 39. Mizuguchi T, Furuta I, Watanabe Y, Tsukamoto K, Tomita H et al. (2004) LRP5, low-density-lipoprotein-receptor-related protein 5, is a determinant for bone mineral density. J Hum Genet 49: 80-86. doi: 10.1007/s10038-003-0111-6. PubMed: 14727154. [DOI] [PubMed] [Google Scholar]
- 40. Stathopoulou MG, Dedoussis GV, Trovas G, Katsalira A, Hammond N et al. (2010) Low-density lipoprotein receptor-related protein 5 polymorphisms are associated with bone mineral density in Greek postmenopausal women: an interaction with calcium intake. J Am Diet Assoc 110: 1078-1083. doi: 10.1016/j.jada.2010.04.007. PubMed: 20630166. [DOI] [PubMed] [Google Scholar]
- 41. Yu JB, Ke YH, He JW, Zhang H, Hu WW et al. (2010) No association between LRP5 gene polymorphisms and bone and obesity phenotypes in Chinese male-offspring nuclear families. Acta Pharmacol Sin 31: 1464-1469. doi: 10.1038/aps.2010.92. PubMed: 20953208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Riancho JA, Olmos JM, Pineda B, García-Ibarbia C, Pérez-Núñez MI et al. (2011) Wnt receptors, bone mass, and fractures: gene-wide association analysis of LRP5 and LRP6 polymorphisms with replication. Eur J Endocrinol 164: 123-131. doi: 10.1530/EJE-10-0582. PubMed: 20926594. [DOI] [PubMed] [Google Scholar]
- 43. Styrkarsdottir U, Halldorsson BV, Gudbjartsson DF, Tang NL, Koh JM et al. (2010) European bone mineral density loci are also associated with BMD in East-Asian populations. PLOS ONE 5: e13217. doi: 10.1371/journal.pone.0013217. PubMed: 20949110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG (2009) Association between the A1330V polymorphism of the low-density lipoprotein receptor-related protein 5 gene and bone mineral density: a meta-analysis. Rheumatol Int 29: 539-544. doi: 10.1007/s00296-008-0745-y. PubMed: 18932002. [DOI] [PubMed] [Google Scholar]
- 45. Tran BN, Nguyen ND, Eisman JA, Nguyen TV (2008) Association between LRP5 polymorphism and bone mineral density: a Bayesian meta-analysis. BMC Med Genet 9: 55. doi: 10.1186/1471-2156-9-55. PubMed: 18588671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kouzmenko AP, Takeyama K, Ito S, Furutani T, Sawatsubashi S et al. (2004) Wnt/beta-catenin and estrogen signaling converge in vivo. J Biol Chem 279: 40255-40258. doi: 10.1074/jbc.C400331200. PubMed: 15304487. [DOI] [PubMed] [Google Scholar]
- 47. Mödder UI, Rudnik V, Liu G, Khosla S, Monroe DG (2012) A DNA binding mutation in estrogen receptor-alpha leads to suppression of Wnt signaling via beta-catenin destabilization in osteoblasts. J Cell Biochem 113: 2248-2255. doi: 10.1002/jcb.24095. PubMed: 22573547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Noh T, Gabet Y, Cogan J, Shi Y, Tank A et al. (2009) Lef1 haploinsufficient mice display a low turnover and low bone mass phenotype in a gender- and age-specific manner. PLOS ONE 4: e5438. doi: 10.1371/journal.pone.0005438. PubMed: 19412553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jeon H, Meng W, Takagi J, Eck MJ, Springer TA et al. (2001) Implications for familial hypercholesterolemia from the structure of the LDL receptor YWTD-EGF domain pair. Nat Struct Biol 8: 499-504. doi: 10.1038/88556. PubMed: 11373616. [DOI] [PubMed] [Google Scholar]
- 50. Springer TA (1998) An extracellular beta-propeller module predicted in lipoprotein and scavenger receptors, tyrosine kinases, epidermal growth factor precursor, and extracellular matrix components. J Mol Biol 283: 837-862. doi: 10.1006/jmbi.1998.2115. PubMed: 9790844. [DOI] [PubMed] [Google Scholar]
- 51. Twells RC, Mein CA, Phillips MS, Hess JF, Veijola R et al. (2003) Haplotype structure, LD blocks, and uneven recombination within the LRP5 gene. Genome Res 13: 845-855. doi: 10.1101/gr.563703. PubMed: 12727905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferrari SL, Deutsch S, Choudhury U, Chevalley T, Bonjour JP et al. (2004) Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am J Hum Genet 74: 866-875. doi: 10.1086/420771. PubMed: 15077203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Urano T, Shiraki M, Ezura Y, Fujita M, Sekine E et al. (2004) Association of a single-nucleotide polymorphism in low-density lipoprotein receptor-related protein 5 gene with bone mineral density. J Bone Miner Metab 22: 341-345. PubMed: 15221492. [DOI] [PubMed] [Google Scholar]
- 54. Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Bénichou O et al. (2003) Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet 72: 763-771. doi: 10.1086/368277. PubMed: 12579474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
The process of study selection for the meta-analysis.
(DOCX)
Funnel plot for LS BMD between A1330V AA and AV/VV genotypes.
(TIF)
Funnel plot for FN BMD between A1330V AA and AV/VV genotypes.
(TIF)
Funnel plot for LS BMD between V667M VV and VM/MM genotypes.
(TIF)
Funnel plot for FN BMD between V667M VV and VM/MM genotypes.
(TIF)
Funnel plot for FN BMD between Q89R QQ and QR/RR genotypes.
(TIF)