Abstract
Previous neuroimaging studies on empathy have not clearly identified neural systems that support the three components of empathy: affective congruence, perspective-taking, and prosocial motivation. These limitations stem from a focus on a single emotion per study, minimal variation in amount of social context provided, and lack of prosocial motivation assessment. In the current investigation, 32 participants completed a functional magnetic resonance imaging session assessing empathic responses to individuals experiencing painful, anxious, and happy events that varied in valence and amount of social context provided. They also completed a 14-day experience sampling survey that assessed real-world helping behaviors. The results demonstrate that empathy for positive and negative emotions selectively activates regions associated with positive and negative affect, respectively. In addition, the mirror system was more active during empathy for context-independent events (pain), whereas the mentalizing system was more active during empathy for context-dependent events (anxiety, happiness). Finally, the septal area, previously linked to prosocial motivation, was the only region that was commonly activated across empathy for pain, anxiety, and happiness. Septal activity during each of these empathic experiences was predictive of daily helping. These findings suggest that empathy has multiple input pathways, produces affect-congruent activations, and results in septally mediated prosocial motivation.
Keywords: empathy, prosocial behavior, septal area
INTRODUCTION
Human beings are intensely social creatures who have a need to belong and connect with others (Baumeister and Leary, 1995). Empathy helps create and maintain these social bonds by enabling people to comprehend, share and respond appropriately to others’ emotional states (Decety and Jackson, 2004). The neural bases of empathy have been a major topic of investigation for the past decade, yet the linkages between major models of empathy and the neural instantiation of the model components have been limited. Multiple models of empathy point to three major components: congruent affect with the target, perspective-taking and prosocial motivation to help the target (Batson, 1991; Zaki and Ochsner, 2012). The limbic system [i.e. amygdala, dorsal anterior cingulate cortex (dACC), anterior insula (AI) and ventral striatum (VS)], the putative mirror neuron system [i.e. intraparietal lobule (IPL), posterior inferior frontal gyrus (pIFG) and dorsal premotor cortex] and the mentalizing network [i.e. dorsomedial and medial prefrontal cortex (DMPFC and MPFC), precuneus, posterior cingulate, temporoparietal junction (TPJ), posterior superior temporal sulcus and temporal poles] have been associated with empathic processing (Singer et al., 2004; Lieberman, 2007; Mobbs et al., 2009; Lamm et al., 2011; Bruneau et al., 2012; Rameson et al., 2012; Spunt and Lieberman, 2012a, b). However, prior studies have not been able to tease apart their distinct contributions and relationship to each component of empathy. These limitations in prior research stem from three issues: focus on a single emotion per study, no variation in the amount of social context provided, and no measurement of the consequences of prosocial motivation (e.g. actual helping behavior).
Most notably, studies typically include targets experiencing only a single emotion, usually pain. A recent meta-analysis found that of 40 neuroimaging studies of empathy, 30 focused on empathy for pain (Fan et al., 2011). At first glance, meta-analyses appear to confirm the view that dACC and AI support core aspects of empathy for the emotions of others (Fan et al., 2011; Lamm et al., 2011). However, it is possible that these results reflect the undue influence of empathy for pain studies. Given that the dACC and AI are centrally involved in the personal experience of pain and distress (Davis, 2000; Peyron et al., 2000; Rainville, 2002; Eisenberger et al., 2003; Shackman et al., 2011), these regions may be specific to affective congruence with a target experiencing pain, rather than being activated more broadly during all empathic experiences.
Comparing empathy for positive and negative experiences (i.e. happiness, anxiety, and pain) within the same study allows us to identify which neural systems support affective congruence for different emotions. In contrast to the large number of neuroimaging studies focusing on empathy for negative emotions, only a handful of studies have examined empathy for positive emotions (Jabbi et al., 2007; Mobbs et al., 2009). For example, one study (Jabbi et al., 2007) examined responses to observing positive and negative gustatory experiences; however, their analyses were limited to testing for common responses in a small region of fronto-insular cortex. Another recent study examined empathy for positive emotions in a social context (i.e. watching game show contestants win money), but did not include any conditions measuring empathy for negative emotions. Furthermore, empathy for positive emotions did not activate the dACC or AI (Mobbs et al., 2009) and instead activated ventromedial prefrontal cortex (VMPFC).
A second limitation of prior work is that studies have not varied the amount of social context provided to help understand the experience of a target. Sometimes it is remarkably easy to step into someone else’s shoes and understand what he/she is experiencing. For example, when we see someone accidentally cut his/her hand with a knife (Jackson et al., 2005; Singer et al., 2006; Hein et al., 2010), no additional context is required to share the target’s pain. However, if we see someone who looks distressed (e.g. anguished facial expression), it may be difficult to fully empathize with that person if we do not know why he/she is distressed. In other words, there is no context to help us understand the other’s mental state. In the first case, when observed behavior is intrinsically meaningful, we might expect the mirror system to play a role in producing self-other resonance. In the latter case, when context is needed to interpret observed behavior, the mentalizing system might be critical for doing the work to actively put oneself in another’s shoes (Spunt and Lieberman, 2012a). A recent meta-analysis by Lamm et al. (2011) provides support for this idea. Across 32 empathy for pain studies, studies that used pictures that clearly showed why targets were in pain (e.g. due to a needle injection)—likely requiring no additional context to understand their pain—increased mirror system activity. In contrast, studies that used a symbolic cue to indicate the target was in pain led to more mentalizing system activity. Nevertheless, no study has ever directly manipulated the amount of social context that is provided about an emotional event that a participant is empathizing with.
The current investigation addressed the first two limitations of prior studies by presenting three kinds of empathy targets: targets experiencing pain, anxiety or happiness. By including targets experiencing both positive and negative emotions, we aimed to identify neural regions that are primarily involved in affective congruence with a particular emotion. Based on prior work, we expected that affective processing regions typically activated during the first-hand experience of pain and anxiety—dACC, AI and amygdala—would also be selectively recruited during empathy for pain and anxiety (Davis, 2000; Peyron et al., 2000; Paulus and Stein, 2006; Lamm et al., 2011). Furthermore, neural regions activated during the first-hand experience of positive affect—VS and VMPFC—would also be selectively recruited during empathy for happiness (Knutson et al., 2001; O'Doherty, 2004; Mobbs et al., 2009; Haber and Knutson, 2010; Kim et al., 2011). Furthermore, by asking participants to empathize with stimuli that do not require context to be understood (i.e. visible pain events) or stimuli that require more context to be understood (i.e. anxiety and happiness), the differential involvement of the mirror and mentalizing systems could be examined.
Finally, we addressed the third limitation of past studies by measuring real-world prosocial behavior. Despite the fact that most models of empathy posit prosocial motivation to be the dominant functional consequence of empathy, only a few neuroimaging studies have examined how neural activity during empathy relates to actual helping behavior (Hein et al., 2010; Rameson et al., 2012). In the current investigation, participants completed a two-week daily diary in which they reported on daily episodes of helping behavior. Given that prosocial motivation is considered a critical component of empathy, we hypothesized that helping behavior would be linked to brain regions commonly activated across all target emotions.
OVERVIEW
In the current study, participants were scanned as they saw images of targets experiencing positive and negative emotional events embedded in social context (i.e. context-dependent) as well as targets experiencing pain (i.e. context-independent). The positive context-dependent experiences consisted of images of people looking happy along with contextual information (e.g. ‘this person just got engaged to the love of their life’). The negative context-dependent experiences consisted of images of people looking anxious along with contextual information (e.g. ‘this person is waiting to find out if they will get laid off’). The painful context-independent experiences consisted of images of people experiencing pain (e.g. hand getting slammed in a car door) with no written contextual information given. To ensure that participants were experiencing empathy, we explicitly instructed them to intentionally empathize with the people in the photos. Outside of the scanner, participants also completed a daily survey on their helping behaviors for 14 consecutive nights.
METHODS
Participants
Informed consent was obtained from 32 healthy, right-handed UCLA undergraduates (16 male, mean age = 19.9 years, s.d. = 1.4) who were told the purpose of the study was to learn how emotion is processed in the brain.
Functional magnetic resonance imaging (fMRI) task
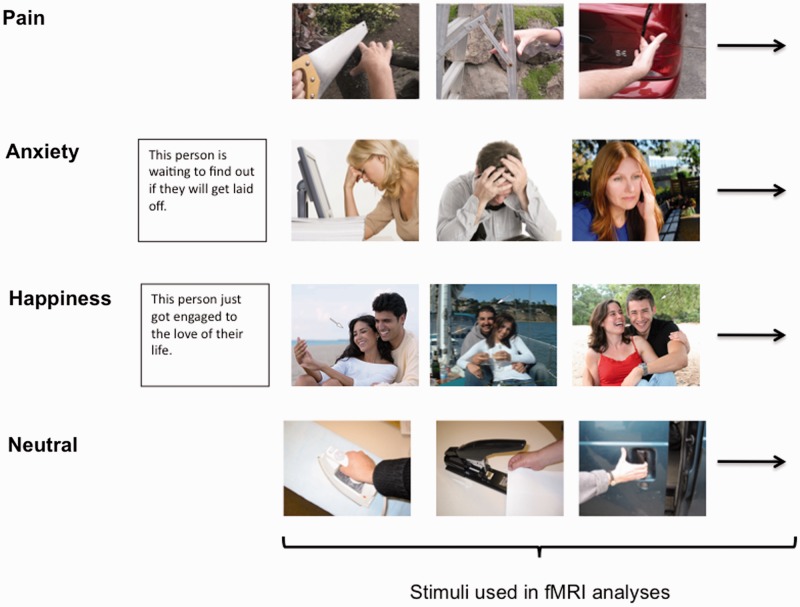
During the functional magnetic resonance imaging task, participants were asked to empathize with multiple blocks of photos depicting individuals experiencing pain, anxiety, and happiness (Figure 1). For these ‘empathy’ conditions, participants were instructed to empathize with the individuals in the photos and imagine how they felt in that situation. In addition, participants viewed neutral images of people performing everyday actions. These blocks were presented across four functional runs.
Fig. 1.
Examples of what participants saw for blocks of empathy for pain, anxiety, and happiness as well as blocks of neutral stimuli. Analyses focused on the time periods when pictures were shown. The time when contextual statements were shown prior to anxiety and happiness blocks were not included in the fMRI analyses.
Empathy for pain condition
The empathy for pain condition was adapted from Jackson et al. (2005) and used with their permission. Participants were asked to focus on how painful they thought it would be for the person in each situation and viewed pictures of people experiencing physical pain (e.g. hand slammed in car door). The empathy for pain condition consisted of two blocks with each block displaying 16 photos for 2 s, with 12 s rest periods separating blocks.
Empathy for anxiety and happiness conditions
The empathy for anxiety and happiness conditions were piloted and designed by the authors. Participants were told to take each target’s perspective and imagine how he/she felt about the situation and how it affected his/her life. Each block consisted of a contextual sentence describing a situation followed by six photos depicting different individuals in that situation. The empathy for anxiety and empathy for happiness conditions each consisted of three blocks. Sentences and photos were presented for 4 s each, with 12 s rest periods separating blocks.
Anxiety situations described events such as riding on a plane with dangerous turbulence, waiting to find out about getting laid off, and potentially not being able to pay rent. Happy situations included wining the lottery, getting engaged, and winning an important sporting event. An arrow indicated the target individual if a photo depicted multiple people. Participants were told photos depicted real events drawn from news stories, documentaries, and blogs. Within each block, half of the targets were male and half female. Images were selected from a larger pool in order to equate them on a number of features (i.e. arousal, valence, luminance and complexity). Subjective ratings of valence and arousal were made by 16 (8 male) undergraduate pilot judges.
Neutral condition
The neutral condition consisted of two blocks in which participants viewed photos of people performing everyday non-emotional actions (e.g. ironing and cutting vegetables; adapted from Jackson et al., 2005). Each block displayed 16 photos for 2 s, with 12 s rest periods separating blocks. These blocks served as a neutral condition to compare with ‘empathy’ conditions.
fMRI acquisition and data analysis
Scanning was performed on a Siemens Trio 3 T. Functional images were acquired using an EPI gradient-echo sequence (TR = 2000 ms, TE = 30 ms, 4 mm slice thickness/no gap, FOV = 19.2 cm, matrix = 64 × 64 and flip angle = 90°). A T2-weighted structural image was acquired coplanar with the functional images (TR = 5000 ms, TE = 34 ms, 4 mm slice thickness/no gap, FOV = 19.2 cm, matrix = 128 × 128, flip angle = 90°). All images were scalped using the Brain Extraction Tool of FSL (FMRIB Software Library; Oxford University, Oxford, UK) and realigned within runs using MCFLIRT. Images were then checked for residual motion and noise spikes using a custom automated diagnostic tool (thresholded at 2 mm motion or 2% global signal change from one image to the next). In SPM8 (Wellcome Department of Imaging Neuroscience, London), all functional and anatomical images were reoriented to set the origin to the anterior commissure and the horizontal (y) axis parallel to the AC-PC line. Furthermore, in SPM8, functional images were realigned within and between runs to correct for residual head motion and co-registered to the matched-bandwidth structural scan using a six-parameter rigid body transformation. The co-registered structural scan was then normalized into Montreal Neurological Institute (MNI) standard stereotactic space using the scalped ICBM152 template, and the resulting parameters were applied to all functional images. Finally, the normalized functional images were resliced into voxels of 3 mm3 and smoothed using an 8 mm full width at half maximum Gaussian kernel.
All single subject and group analyses were performed in SPM8. First-level effects were estimated using the general linear model and employing a canonical hemodynamic response function convolved with the experimental design. Low-frequency noise was removed using a high-pass filter. Group analyses were conducted using random-effects models to enable population inferences (Friston et al., 1999). To keep all conditions as well constrained and equivalent as possible, image presentation was modeled separately and used for all first-level contrasts. The contextual sentences in the empathy for anxiety and happiness conditions were modeled separately and were not included in the baseline condition.
Whole-brain group-level analyses were performed using an uncorrected P value of <0.005 with a cluster threshold of 43 based on a Monte Carlo simulation in AFNI’s AlphaSim effectively producing a family-wise false discovery rate (FDR) of P = 0.05 (Lieberman and Cunningham, 2009). For visualization of results, group contrasts were overlaid on a surface representation of the MNI canonical brain using the SPM surfrend toolbox (http://spmsurfrend.sourceforge.net) and NeuroLens (http://www.neurolens.org/), as well as MRIcron (Rorden et al., 2007).
For region of interest (ROI) masks, anatomical ROIs were constructed in Wake Forest University Pickatlas Tool (Maldjian et al., 2003) using the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) as well as MarsBaR (http://marsbar.sourceforge.net). The septal area ROI was based on a microscopic atlas of the human brain (Mai et al., 2004) and was a box extending from x = ±6, between y = −2 and y = 0, and between z = 0 and z = 10. To create the bilateral VS ROI, we used the AAL VS bounded laterally at x = ±12, between y = 12 and y = 4, and between z = 0 and z = −12 (Mobbs et al., 2009). The bilateral amygdala ROI was taken directly from AAL. Based on a Monte Carlo simulation in AFNI’s Alphasim, we used a cluster threshold of 6 voxels for the combined mask of the septal area, VS and amygdala effectively producing a FDR of P = 0.05.
Daily helping checklist
For 14 evenings in a row, an e-mail was sent to each participant at 5 pm with a link to time-stamped online survey about helping behaviors (i.e. SurveyMonkey). Participants were instructed to complete the survey immediately before going to bed at night. Daily helping was measured with an 11-item checklist asking about helping strangers or acquaintances. They indicated whether they had performed each of the following actions by selecting yes or no: gave directions, delayed elevator, held open a door, made change, picked up a fallen object for someone, lent or gave money, let someone go ahead of you in line, helped a disabled or elderly person, lent an item of value (tool, clothes, car, etc.), helped with schoolwork, and asked someone if they needed help.
Items for this scale were selected based on the ratings of 17 pilot judges in an effort to select high-frequency items. Several items were adapted from the Self-Report Altruism Scale (Rushton et al., 1981). No measure of helping friends, family members or romantic partners was included because help given to close others and strangers differs in nature (Amato, 1990). In addition, we wanted to simulate previous studies linking empathy and helping behavior toward strangers (Batson et al., 1981; Batson, 1991, 1995, 2011). For all questions on the daily helping scales, a ‘no’ was coded as 0 because the event did not occur, whereas a ‘yes’ was coded as a 1. Scores for each day were computed by summing the responses to each of the 11 items. A mean daily score was then calculated by averaging the total score across the 14 days. Because this measure was a count of experiences, it was not appropriate to calculate alpha coefficients.
RESULTS
Daily helping descriptives
On average, participants completed 13.26 of 14 daily surveys (s.d. = 0.89). Overall, individuals reported doing at least one helpful thing for a stranger or acquaintance on 85% of the days. Averaging across the 14 days, individuals endorsed an average of 2.47 of 11 stranger helping items per day (s.d. = 1.39 items).
Neural correlates of empathy for pain, anxiety, and happiness
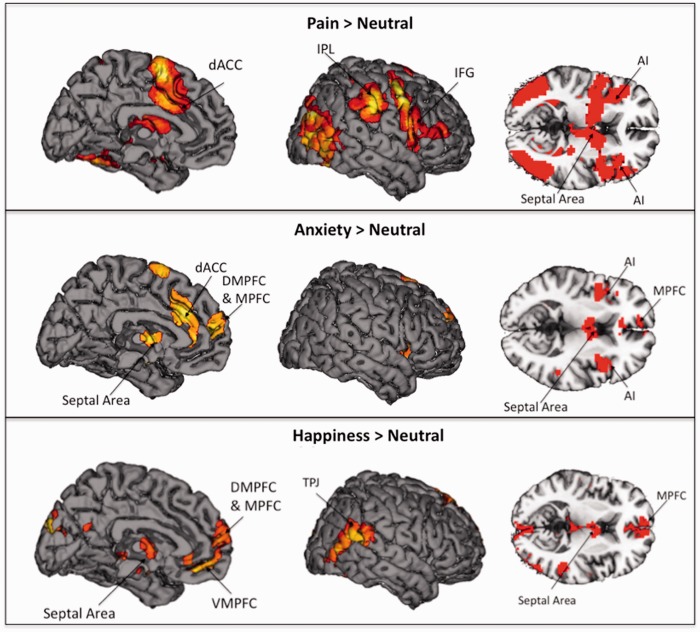
The first set of analyses aimed to examine neural activation during each of the empathy conditions compared with the neutral condition (i.e. Pain > Neutral; Anxiety > Neutral and Happiness > Neutral). As in previous research, empathy for pain increased activation in regions associated with negative affect, dACC and bilateral AI (Table 1; Figure 2, top panel). In addition, empathy for pain activated regions in the mirror system, including the IPL and pIFG (Spunt and Lieberman, 2012 b). The septal area, a region associated with caregiving and prosocial sentiments (Slotnick and Nigrosh, 1975; Stack et al., 2002; Krueger et al., 2007; Inagaki Eisenberger, 2011; Moll et al., 2011), also showed increased activation. No activations were present in the mentalizing system in this contrast.
Table 1.
Neural regions that were more active during empathy for pain, anxiety, and happiness compared with the neutral condition
| Region | BA | Hemisphere | Coordinates |
||||
|---|---|---|---|---|---|---|---|
| k | x | y | z | t | |||
| Pain > Neutral | |||||||
| Septal area | — | R | 4318a | 12 | 2 | 10 | 4.86 |
| Dorsal anterior cingulate cortex | 32/24 | R | 4318a | 3 | 17 | 28 | 7.29 |
| Anterior insula | 13 | R | 4318a | 42 | 5 | 1 | 5.38 |
| L | 4318a | −33 | 20 | 10 | 4.46 | ||
| Inferior frontal gyrus | 6/44 | R | 4318a | 42 | 11 | 22 | 5.47 |
| L | 4318a | −57 | 2 | 22 | 8.44 | ||
| Inferior parietal lobule/supramarginal gyrus | 40/2 | R | 416 | 57 | −37 | 37 | 3.93 |
| L | 820 | −39 | −40 | 43 | 5.56 | ||
| Supplementary motor area | 6 | L | 4318a | −9 | 2 | 61 | 7.60 |
| Postcentral gyrus | 40 | R | 69 | 27 | −46 | 55 | 4.30 |
| Putamen | — | R | 66 | 30 | −19 | −5 | 4.12 |
| Occipital lobe | 19 | R | 1666 | 45 | −70 | 1 | 8.34 |
| L | 1380 | −51 | −70 | 7 | 8.21 | ||
| Anxiety > Neutral | |||||||
| Septal area | — | R | 336b | 3 | −1 | 4 | 4.30 |
| Medial prefrontal cortex/dorsomedial prefrontal cortex | 10/9 | R | 202 | 6 | 56 | 22 | 4.39 |
| Dorsal anterior cingulate cortex | 32/24 | — | 521c | 0 | 29 | 25 | 4.52 |
| Anterior insula | 13 | R | 185 | 33 | 8 | 10 | 4.08 |
| L | 228 | −39 | 8 | −2 | 4.02 | ||
| Supplementary motor area | 6 | R | 521c | 6 | 11 | 67 | 4.18 |
| Amygdala/hippocampus | — | R | 336b | 18 | −4 | −8 | 5.88 |
| L | 336b | −18 | −10 | −14 | 4.96 | ||
| Happiness > Neutral | |||||||
| Septal area | — | R | 86 | 3 | −1 | 1 | 4.43 |
| Medial prefrontal cortex/dorsomedial prefrontal cortex | 10/9/8 | R | 473d | 6 | 59 | 13 | 5.94 |
| Dorsomedial prefrontal cortex | 8 | R | 87 | 9 | 44 | 55 | 4.51 |
| Precuneus | 31 | R | 43 | 3 | −58 | 25 | 4.02 |
| Temporoparietal junction | 40 | R | 625e | 54 | −43 | 19 | 6.16 |
| Temporal pole | 21/38 | L | 473d | −45 | 11 | −17 | 4.49 |
| Ventromedial prefrontal cortex | 11 | — | 473d | 0 | 47 | −14 | 5.83 |
| Hippocampus/amygdala | — | R | 235 | 21 | −10 | −11 | 5.77 |
| L | 67 | −18 | −10 | −14 | 5.30 | ||
| Occipital lobe | 19 | R | 625e | 42 | −55 | −14 | 6.98 |
| 18/19 | R | 286 | 6 | −94 | 7 | 4.36 | |
Threshold used was P < 0.005 and 43 voxel extent, which provides FDR corrected P < 0.05. BA refers to putative Brodmann’s area; L and R refer to left and right hemispheres; k refers to the cluster size (in voxels); x, y and z refer to MNI coordinates in the left–right, anterior–posterior and interior–superior dimensions, respectively; t refers to the t-score at those coordinates (local maxima).
a–eThese originate from the same larger cluster.
Fig. 2.
Neural regions that were more active during empathy for pain, anxiety, and happiness, each compared with the neutral condition.
Empathy for anxiety also elevated neural activation in the same negative affective regions as empathy for pain, the dACC and bilateral AI, along with the amygdala (Table 1; Figure 2, middle panel). In addition, empathy for anxiety engaged neural regions in the mentalizing system, including DMPFC and MPFC (Frith and Frith, 2006; Mitchell, 2009; Spunt and Lieberman, 2012b). As with empathy for pain, empathy for anxiety led to increased activity in septal area. No activations were present in the mirror system.
In contrast to empathy for pain and anxiety, empathy for happiness increased activation in a region that has been associated with positive affect and reward, the VMPFC (Table 1; Figure 2, bottom panel). Several regions associated with the mentalizing system also showed increased activation during empathy for happiness, including the MPFC, DMPFC, TPJ, precuneus and temporal pole. As with empathy for pain and anxiety, empathy for happiness elevated neural activity in the septal area.
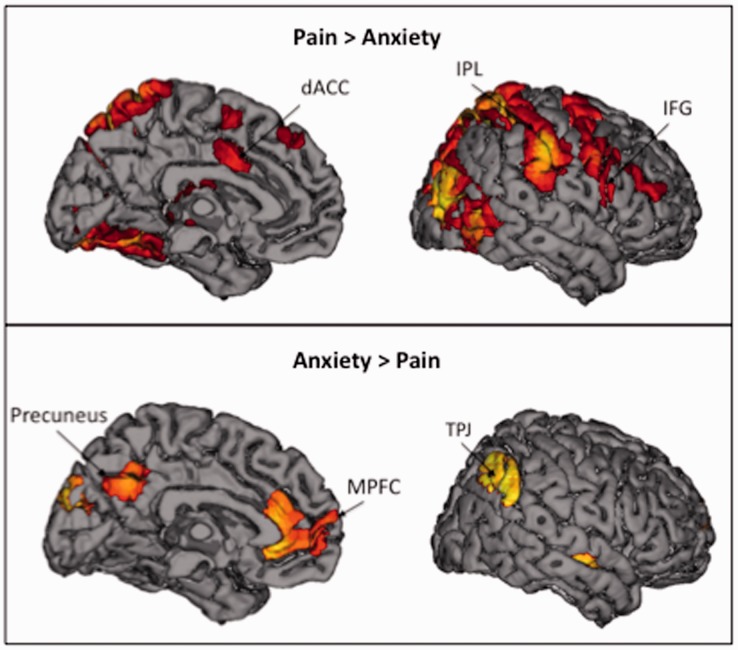
Differential activation during empathy for pain versus anxiety
In order to compare empathy for context-independent situations with context-dependent situations, we contrasted neural activity during empathy for pain and anxiety. Empathy for pain versus anxiety (i.e. Pain > Anxiety) showed increased bilateral activation in regions associated with the mirror system, such as IPL and pIFG (Table 2; Figure 3, top panel). Empathy for pain relative to anxiety also showed increased activation in dACC. The reverse contrast, Anxiety > Pain (Table 2; Figure 3, bottom panel), showed increased activation in the mentalizing system, including MPFC, TPJ and precuneus.
Table 2.
Neural regions that were more active during empathy for pain compared with empathy for anxiety or more active during empathy for anxiety compared with empathy for pain
| Region | BA | Hemisphere | Coordinates |
||||
|---|---|---|---|---|---|---|---|
| k | x | y | z | t | |||
| Pain > Anxiety | |||||||
| Dorsomedial prefrontal cortex | 8 | R | 57 | 6 | 35 | 49 | 4.67 |
| Dorsal anterior cingulate cortex | 24 | L | 134 | −6 | 5 | 32 | 5.10 |
| Inferior frontal gyrus | 44/6 | R | 1185 | 51 | 5 | 37 | 6.10 |
| L | 12 625a | −51 | 5 | 22 | 7.94 | ||
| Inferior parietal lobule/supramarginal gyrus | 40/39 | R | 12 625a | 57 | −22 | 46 | 8.64 |
| L | 12 625a | −51 | −31 | 49 | 10.56 | ||
| Posterior cingulate | 31 | L | 53 | −15 | −25 | 40 | 5.02 |
| Thalamus | — | R | 279 | 18 | −28 | 10 | 5.39 |
| Occipital lobe | 19/18 | R | 12 625a | 33 | −79 | 25 | 10.07 |
| L | 12 625a | −45 | −67 | −5 | 13.68 | ||
| Anxiety > Pain | |||||||
| Medial prefrontal cortex | 10/9 | — | 943b | 0 | 53 | 4 | 4.19 |
| Precuneus | 7/31 | R | 397c | 3 | −58 | 37 | 3.83 |
| Angular gyrus/inferior parietal lobule | 39/40 | R | 138 | 54 | −64 | 34 | 5.43 |
| L | 147 | −54 | −67 | 37 | 5.76 | ||
| Rostral anterior cingulate cortex | 32/24 | — | 943b | 0 | 29 | −5 | 4.64 |
| Middle temporal gyrus | 21 | R | 56 | 60 | −10 | −11 | 4.09 |
| Middle temporal gyrus/temporal pole | 21/38 | L | 77 | −45 | 8 | −17 | 3.82 |
| Precentral gyrus/postcentral gyrus | 3/4 | L | 78 | −42 | −25 | 67 | 6.20 |
| Occipital lobe | 18/19 | L | 397c | −3 | −91 | 28 | 4.82 |
Threshold used was P < 0.005 and 43 voxel extent, which provides FDR corrected P < 0.05. BA refers to putative Brodmann’s Area; L and R refer to left and right hemispheres; k refers to the cluster size (in voxels); x, y, and z refer to MNI coordinates in the left–right, anterior–posterior, and interior–superior dimensions, respectively; t refers to the t-score at those coordinates (local maxima).
a–cThese originate from the same larger cluster.
Fig. 3.
Neural regions that were more active during empathy for pain compared with empathy for anxiety, as well neural regions that were more active during empathy for anxiety compared with empathy for pain.
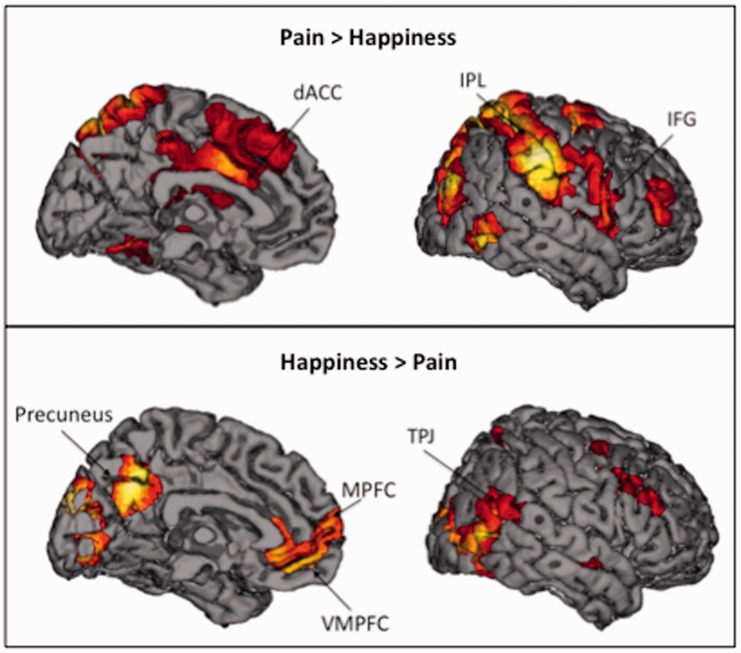
Differential activation during empathy for pain versus happiness
We next compared empathy for pain to empathy for happiness. Empathy for pain relative to happiness (i.e. Pain > Happiness) showed robust mirror system activity in bilateral IPL and pIFG (Table 3; Figure 4, top panel). Furthermore, empathy for pain increased activity in neural regions related to negative affect, dACC and bilateral AI, compared with empathy for happiness. Empathy for happiness compared with pain (i.e. Happiness > Pain) lead to elevated levels of neural activation in several regions associated with the mentalizing system, including MPFC, DMPFC, TPJ and precuneus (Table 3; Figure 4, bottom panel). In addition, empathy for happiness (versus pain) showed increased activation in VMPFC, a region previously associated with positive affect.
Table 3.
Neural regions that were more active during empathy for pain compared with empathy for happiness or more active during empathy for happiness compared with empathy for pain
| Region | BA | Hemisphere | Coordinates |
||||
|---|---|---|---|---|---|---|---|
| k | x | y | z | t | |||
| Pain > Happiness | |||||||
| Dorsal anterior cingulate cortex | 32/24 | L | 12 896a | −3 | 5 | 34 | 8.90 |
| Anterior inusla | 13 | R | 12 896a | 39 | 17 | −2 | 3.18 |
| L | 12 896a | −33 | 20 | 10 | 4.62 | ||
| Inferior frontal gyrus | 44 | R | 12 896a | 60 | 11 | 10 | 5.07 |
| L | 12 896a | −54 | 5 | 28 | 10.35 | ||
| Inferior parietal lobule/supramargingal gyrus | 40 | R | 12 896a | 36 | −37 | 46 | 7.92 |
| L | 12 896a | −51 | −31 | 49 | 12.41 | ||
| Supplementary motor area | 6 | — | 12 896a | 0 | 32 | 49 | 4.90 |
| Posterior insula | 13 | R | 12 896a | 39 | −10 | 1 | 5.48 |
| L | 12 896a | −42 | −4 | 10 | 9.58 | ||
| Superior parietal lobule | 7 | R | 12 896a | 21 | −67 | 46 | 10.10 |
| L | 12 896a | −18 | −64 | 61 | 9.54 | ||
| Dorsolateral prefrontal cortex | 46/9 | R | 12 896a | 39 | 47 | 19 | 4.72 |
| L | 12 896a | −39 | 32 | 37 | 5.88 | ||
| Middle frontal gryus/superior frontal gyrus | 6 | R | 533 | 24 | −4 | 52 | 8.00 |
| Middle temporal gyrus | 37 | R | 763b | 48 | −61 | −2 | 8.30 |
| Thalamus | — | L | 12 896a | −15 | −16 | 13 | 5.56 |
| Cerebellum/occipital lobe | 19 | R | 763b | 24 | −67 | −23 | 6.44 |
| Happiness > Pain | |||||||
| Medial prefrontal cortex/dorsomedial prefrontal cortex | 10/9 | R | 722c | 3 | 59 | 7 | 6.28 |
| Precuneus | 7/31 | R | 495 | 3 | −58 | 37 | 7.46 |
| Temporoparietal junction | 39/40 | R | 478 | 54 | −43 | 16 | 5.08 |
| Ventromedial prefrontal cortex | 11 | — | 722c | 0 | 38 | −14 | 6.52 |
| Middle temporal gyrus/temporal pole | 21/38 | R | 133 | 57 | −7 | −11 | 6.81 |
| L | 162 | −54 | −4 | −17 | 5.26 | ||
| Hippocampus/amygdala | — | R | 148 | 21 | −7 | −8 | 4.96 |
| Angular gyrus/inferior parietal lobule | 39 | L | 131 | −45 | −73 | 43 | 6.58 |
| Superior frontal gyrus | 8 | R | 155 | 15 | 41 | 52 | 4.92 |
| Cerebellum | — | R | 60 | 6 | −52 | 1 | 4.25 |
| Occipital lobe | 18/19 | — | 502 | 0 | −91 | 16 | 7.12 |
Threshold used was P < 0.005 and 43 voxel extent, which provides FDR corrected P < 0.05. BA refers to putative Brodmann’s Area; L and R refer to left and right hemispheres; k refers to the cluster size (in voxels); x, y and z refer to MNI coordinates in the left–right, anterior–posterior and interior–superior dimensions, respectively; t refers to the t-score at those coordinates (local maxima).
a–cThese originate from the same larger cluster.
Fig. 4.
Neural regions that were more active during empathy for pain compared with empathy for happiness, as well neural regions that were more active during empathy for happiness compared with empathy for pain.
Differential activations during empathy for anxiety versus happiness
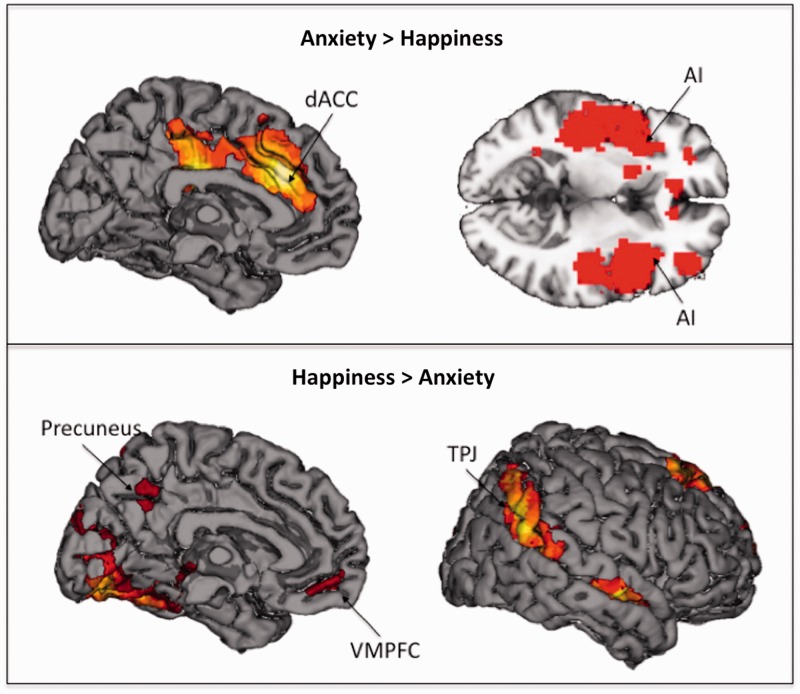
Empathizing with anxiety relative to happiness (i.e. Anxiety > Happy) was associated with dACC and AI (Table 4; Figure 5, top panel). Empathizing with happiness relative to anxiety (i.e. Happy > Anxiety) was associated with activity in VMPFC (see Table 4; Figure 5, bottom panel).
Table 4.
Neural regions that were more active during empathy for anxiety compared with empathy for happiness or more active during empathy for happiness compared with empathy for anxiety
| Region | BA | Hemisphere | Coordinates |
||||
|---|---|---|---|---|---|---|---|
| K | x | y | z | t | |||
| Anxiety > Happiness | |||||||
| Dorsal anterior cingulate cortex | 32/24 | L | 1201a | −3 | 29 | 31 | 5.87 |
| Anterior insula | 13 | R | 1372b | 39 | 14 | 4 | 5.23 |
| L | 1653c | −36 | 11 | −8 | 6.27 | ||
| Posterior cingulate | 31 | R | 1201a | 6 | −31 | 46 | 4.90 |
| Inferior parietal lobule/supramarginal gyrus | 40 | R | 1372b | 63 | −31 | 49 | 5.83 |
| L | 1653c | −42 | −1 | 10 | 6.28 | ||
| Posterior insula | 13 | R | 1372b | 42 | −22 | 7 | 4.34 |
| L | 1653c | −42 | −1 | 10 | 6.28 | ||
| Superior frontal gyurs/middle frontal gyrus | 10/9 | R | 363 | 21 | 47 | 28 | 4.87 |
| L | 376 | −36 | 35 | 37 | 4.93 | ||
| Happiness > Anxiety | |||||||
| Precuneus | 7 | — | 88 | 0 | −58 | 40 | 4.72 |
| Ventromedial prefrontal cortex | 11 | — | 66 | 0 | 50 | −11 | 4.84 |
| Dorsolateral prefrontal cortex | 9/46 | R | 392 | 54 | 23 | 37 | 4.28 |
| Middle temporal gyrus | 21 | R | 63 | 60 | −7 | −14 | 5.11 |
| L | 57 | −60 | −13 | −14 | 3.88 | ||
| Superior parietal lobule | 7 | L | 102 | −24 | −64 | 58 | 4.76 |
| Precentral gyrus | 9 | L | 83 | −39 | 5 | 34 | 4.19 |
| Occipital lobe | 18/19 | R | 5567d | 27 | −88 | 1 | 10.68 |
| L | 5567d | −30 | −82 | −5 | 12.74 | ||
Note. Threshold used was P < 0.005 and 43 voxel extent, which provides FDR corrected P < 0.05. BA refers to putative Brodmann’s Area; L and R refer to left and right hemispheres; k refers to the cluster size (in voxels); x, y and z refer to MNI coordinates in the left–right, anterior–posterior, and interior–superior dimensions, respectively; t refers to the t-score at those coordinates (local maxima).
a–dThese originate from the same larger cluster.
Fig. 5.
Neural regions that were more active during empathy for anxiety compared with empathy for happiness or more active during empathy for happiness compared with empathy for anxiety.
Common activations during empathy for pain, anxiety, and happiness
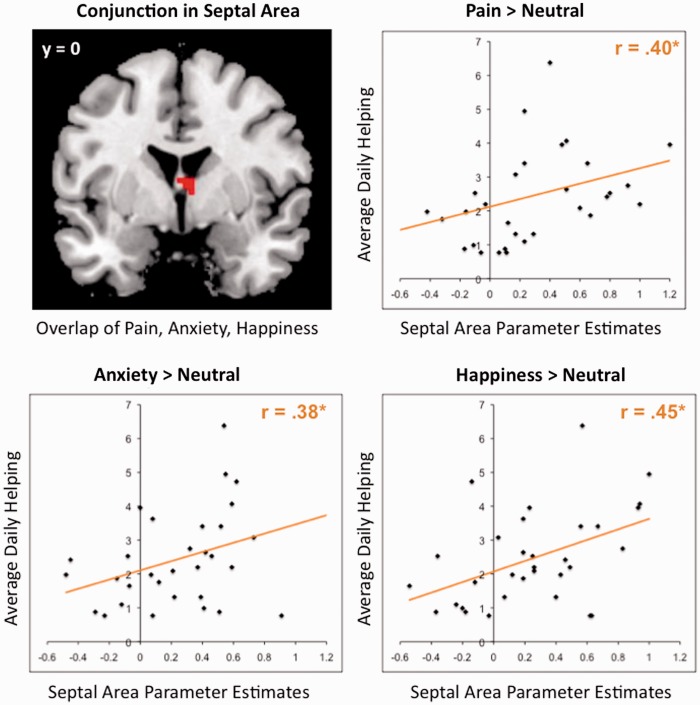
To determine whether there were core regions associated with diverse types of empathic experiences, we conducted two conjunction analyses (Nichols et al., 2005). The conjunction analyses identified regions active when empathizing with targets experiencing pain, anxiety, and happiness (i.e. conjunction of Pain > Neutral, Anxiety > Neutral and Happy > Neutral). One conjunction analysis focused on subcortical limbic regions (i.e. septal area, VS, and amygdala), and the second focused on the entire brain to identify common cortical regions. The whole-brain analysis did not identify regions commonly activated; however, the subcortical limbic mask identified one region that was commonly active across all three empathy conditions: the septal area (see Figure 6, top left quadrant).
Fig. 6.
The functional overlap in the septal area (six voxels) produced from the conjunction of each empathy condition compared with the neutral condition. No other region was present in the conjunction of each empathy condition. The scatterplots illustrate the correlation of average activity in the septal area for empathy for pain, anxiety and happiness with average daily helping. Each point represents a single participant.
Correlations between septal area and daily helping
In this last set of analyses, we examined whether septal area activation during empathy was a neural indicator of prosocial motivation. Specifically, we examined whether septal area activation predicted increased helping in daily life. To test this idea, we extracted parameter estimates from the functional cluster in the septal area identified in the masked conjunction analysis (see above) for each empathy condition compared with the neutral condition (i.e. Pain > Neutral, Anxiety > Neutral, and Happiness > Neutral). Then, average neural activity in the septal area for each contrast was correlated with mean daily helping (see Figure 6). Average neural activity in septal area for Pain > Neutral and mean daily helping were significantly correlated, r(30) = 0.40, P < 0.05. Average neural activity in septal area for Anxiety > Neutral and mean daily helping were also significantly correlated, r(30) = 0.38, P < 0.05. Finally, average neural activity in septal area for Happiness > Neutral and mean daily helping were significantly correlated as well, r(30) = 0.45, P < 0.05. These results suggest that septal area activation during a variety of empathic experiences consistently relates to daily helping and may be a neurocognitive mechanism supporting prosocial motivation.
DISCUSSION
Three patterns of results emerged from our data. First, regions associated with negative affect, dACC and AI, were active when empathizing with targets experiencing pain and anxiety, but not with happy targets. In contrast, VMPFC, a region associated with positive affect, was active when empathizing with happy targets, but not targets experiencing pain or anxiety. Second, the mirror system was more active during empathy for context-independent events (e.g. pain), whereas the mentalizing system was more active during empathy for context-dependent events (e.g. anxiety and happiness). Finally, the septal area was the only region that was commonly activated during empathy for pain, anxiety, and happiness. Moreover, septal area activity predicted helping behavior in daily life. Here, we discuss the results in terms of their implications for the three-component model of empathy.
Affective congruence
Numerous studies on the neuroscience of empathy have shown reliable activation of the dACC and AI. This has led to the impression that these two regions are core components of the human capacity for empathy, writ large. However, this interpretation is limited by the fact that the majority of these studies focused on pain, which produces strong dACC and AI during the first-hand experience of pain. From these studies alone, it has been unclear whether dACC and AI occur during empathy for pain because they are involved in empathy in general or because they are invoked when empathizing with this particular emotion.
The current results demonstrate that activation in dACC and AI seems to be specific to empathy for negative emotions and supports affective congruence with the target’s pain or anxiety. Notably, dACC and AI activation did not occur during empathy for positive emotion. Instead, empathy for happiness activated VMPFC, which has been identified in numerous studies on the first-hand experience of positive affect and pleasure (O'Doherty, 2004; Kringelbach, 2005; Berridge and Kringelbach, 2008; Haber and Knutson, 2010). Our findings support the notion of affective congruence, demonstrating that brain regions commonly associated with the first-hand experience of positive and negative affect are recruited during the act of empathizing with another’s positive or negative affect, respectively.
Generating a shared experience
Although the three-component model of empathy suggests that perspective-taking is critical for generating affective congruence, the current results suggest that perspective-taking is not the only route to shared emotional experiences. In line with emerging evidence in the literature, we suggest that there are two pathways to generating empathy (Lamm et al., 2011; Spunt and Lieberman, 2012a). In the current study, empathizing with emotional experiences that did not require contextual information in order to be understood (i.e. unambiguous physical pain events) engaged the mirror system, not the mentalizing system. Specifically, empathy for pain relative to empathy for anxiety and happiness showed robust activation in IPL and pIFG. In contrast, empathizing with emotional experiences that required contextual information in order to be understood (i.e. happy and anxious images) was associated with the mentalizing system, but not the mirror system. Specifically, DMPFC and MPFC were more active during empathy for anxiety and happiness compared with empathy for pain. This is consistent with previous neuroimaging work showing that empathy for experiences that require context to be understood (e.g. empathy for social rejection, social suffering, sadness, and positive and negative life events) draws on the mentalizing system, showing increased activation in DMPFC and MPFC (Zaki et al., 2009; Masten et al., 2011; Bruneau et al., 2012; Meyer et al., 2012; Rameson et al., 2012).
One limitation of the current study is that the emotion factor (pain, anxiety, happy) was not fully crossed with context (context given, context absent). In daily life, visible pain events are most often understood without additional context—seeing someone slam their hand in a car door is sufficient to understand the event. In contrast, when we observe someone looking happy or anxious, it is harder to empathize with that person in the absence of contextual information. For these reasons, we chose to provide context for the happy and anxious events, but not the painful events. Critically, the analyses all focused on just the period of time when the images were shown—not when the context was presented. In addition, the pictures for the painful events were more simplistic and unambiguous than the pictures for the happy and anxious events. Thus, additional visual context, rather than the contextual phrase, may account for increased activity in the mentalizing network during the happy and anxious events.
Although more rare in daily life, there are clearly cases in which painful events are better understood with context. For example, when a basketball player has fallen and is clearly in pain, it is difficult to empathize with his pain unless the prior play has been seen or described. Similarly, there are happy and anxious moments that do not need verbal context to be understood (e.g. a runner smiling as she breaks the tape at the finish line). In future studies, it will be important to include each of these types of events in order to disentangle the mirror and mentalizing systems’ contributions to empathy for different emotions, with and without context. Our hunch is that the need for context, rather than the emotion specifically, drives the relative contributions of the mirror and mentalizing systems to empathic experience.
Prosocial motivation
Despite the fact that most models of empathy consider prosocial motivation to be a key component of empathy, past studies have not identified a common neural region that is (1) activated across different empathic experiences and (2) associated with prosocial behavior. In the current study, we found that empathy for pain, anxiety, and happiness all activated septal area. Further, the functional overlap in septal area activation during empathy for each of these emotions predicted daily helping behavior. Although septal area is a largely unexamined region within social neuroscience (c.f. Krueger et al., 2007; Moll et al., 2011), animal research suggests that it plays a key role in maternal caregiving and prosocial motivation in mammals. Mice with septal lesions fail in all areas of maternal caregiving (Febo et al., 2005), and oxytocin receptors in septal area are associated with maternal responsiveness (Francis et al., 2000). One functional role of this region is to help new mothers treat their infants like kin rather than as to-be-avoided strangers (Francis et al., 2000). In neuroimaging studies on humans, septal area activation has been associated with a variety of prosocial emotions and behaviors, including unconditional trust (Krueger et al., 2007), prosocial sentiments (Moll et al., 2011), charitable donations (Moll et al., 2006), empathic concern and perspective-taking (Rankin et al., 2006), and giving support to loved ones (Inagaki and Eisenberger, 2011).
Thus, septal area activation during empathy may drive prosocial motivation and subsequent prosocial behavior. A series of behavioral studies has already demonstrated that empathic emotion is a source of altruistic motivation that predicts increased helping (Batson et al., 1981; Toi and Batson, 1982). Our results extend this finding by identifying the neural basis for prosocial motivation, which can be used to predict general patterns of prosocial behavior in real life (rather than in an experimental setting).
CONCLUSIONS
Our results suggest that there are several neurocognitive components involved in different aspects of empathy. First, limbic regions like dACC, AI and VMPFC are involved in affective congruence, supporting an emotional state that complements that of the target. Second, the mirror and mentalizing systems represent two pathways to sharing others’ emotions and are differentially engaged depending on the amount of context that is provided to understand another’s emotional experience. Finally, septal area appears to play a role in generating prosocial motivation during empathy more generally. In particular, the septal area may produce an other-focused, caregiving state of mind that motivates prosocial behavior.
Overall, these results shed new light on past empathy research and add to an increasingly comprehensive neural model for empathy. The current findings suggest that the target’s specific emotions will stimulate congruent emotions in the observer, such as negative affect when experiencing empathy for negative emotions and positive affect when experiencing empathy for positive emotions. In addition, empathy may be induced by simply observing others’ emotional experiences, but at other times it may be necessary to actively take the target’s perspective in order to understand and connect with their emotions. Finally, empathy heightens our focus on and concern for others, regardless of what specific emotion the target is experiencing, and motivates us to behave prosocially.
However, it is still unclear how these different components of empathy interact and unfold in real-time (Zaki and Ochsner, 2012). For example, it may be that prosocial motivation heightens the affective response to the target or that the affective response to the target bolsters prosocial motivation. It is also unclear whether the mirror system and affective regions are activated simultaneously or sequentially. Furthermore, current theories about empathy might suggest that the mentalizing system should typically precede affective congruence when empathizing with context-dependent emotions. Thus, future studies will be able to explore these questions by examining the temporal sequencing of activation in neural regions associated with affective congruence (e.g. dACC, insula, VMPFC), mirroring or mentalizing (i.e. mirror system and mentalizing system), and prosocial motivation (i.e. septal area).
Acknowledgments
We are grateful to Naomi Eisenberger for her helpful feedback and Robert Spunt for his advice on statistical issues. Austin Grinberg and Kenny Casebere were instrumental in the data collection process. We also appreciate the support provided by the UCLA Brain Mapping Center. S.A.M. was supported by a graduate research fellowship from the National Science Foundation IGERT Interdisciplinary Relationship Science Program at the University of California, Los Angeles.
REFERENCES
- Amato PR. Personality and social network involvement as predictors of helping behavior in everyday life. Social Psychology Quarterly. 1990;53:31–43. [Google Scholar]
- Batson CD. The altruism question: Toward a social psychological answer. Hillsdale, NJ: Erlbaum Associates; 1991. [Google Scholar]
- Batson CD. Prosocial motivation: why do we help others. In: Tesser A, editor. Advanced Social Psychology. New York: McGraw-Hill; 1995. pp. 333–81. [Google Scholar]
- Batson CD. Altruism in humans. New York, NY: Oxford University Press; 2011. [Google Scholar]
- Batson CD, Duncan BD, Ackerman P, Buckley T, Birch K. Is empathic emotion a source of altruistic motivation? Journal of Personality and Social Psychology. 1981;40(2):290. [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199(3):457–80. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau EG, Pluta A, Saxe R. Distinct roles of the ‘Shared Pain' and ‘Theory of Mind' networks in processing others' emotional suffering. Neuropsychologia. 2012;50(2):219–31. doi: 10.1016/j.neuropsychologia.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Davis KD. The neural circuitry of pain as explored with functional MRI. Neurological Research. 2000;22(3):313–7. doi: 10.1080/01616412.2000.11740676. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behavorial and Cognitive Neuroscience Reviews. 2004;3(2):71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35(3):903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother–pup bonding during suckling. The Journal of Neuroscience. 2005;25(50):11637–44. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. Journal of Neuroendocrinology. 2000;12(12):1145–8. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Büchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10(4):385–96. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50(4):531–4. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Silani G, Preuschoff K, Batson CD, Singer T. Neural responses to ingroup and outgroup members' suffering predict individual differences in costly helping. Neuron. 2010;68(1):149–60. doi: 10.1016/j.neuron.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Eisenberger NI. Neural correlates of giving support to a loved one. Psychosomatic Medicine. 2012;74:3–7. doi: 10.1097/PSY.0b013e3182359335. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34(4):1744–53. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24(3):771–9. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O'Doherty JP. Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cerebral Cortex. 2011;21(4):769–76. doi: 10.1093/cercor/bhq145. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Krueger F, McCabe K, Moll J, et al. Neural correlates of trust. Proceedings of the National Academy of Sciences. 2007;104(50):20084–9. doi: 10.1073/pnas.0710103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54(3):2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual Review of Psychology. 2007;58:259–89. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4(4):423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. 2nd edn. Amsterdam: Elsevier Academic Press; 2004. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Masten CL, Morelli SA, Eisenberger NI. An fMRI investigation of empathy for ‘social pain' and subsequent prosocial behavior. Neuroimage. 2011;55(1):381–8. doi: 10.1016/j.neuroimage.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Meyer ML, Masten CL, Ma Y, et al. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Social Cognitive and Affective Neuroscience. 2012;8:446–54. doi: 10.1093/scan/nss019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP. Inferences about mental states. Philosophical Transantions of the Royal Society B Biological Sciences. 2009;364(1521):1309–16. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Meyer M, et al. A key role for similarity in vicarious reward. Science. 2009;324(5929):900. doi: 10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences. 2006;103(42):15623–8. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, et al. Impairment of prosocial sentiments is associated with frontopolar and septal damage in frontotemporal dementia. Neuroimage. 2011;54(2):1735–42. doi: 10.1016/j.neuroimage.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60(4):383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Clinical Neurophysiology. 2000;30(5):263–88. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Rainville P. Brain mechanisms of pain affect and pain modulation. Current Opinions in Neurobiology. 2002;12(2):195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Rameson LT, Morelli SA, Lieberman MD. The neural correlates of empathy: Experience, automaticity, and prosocial behavior. Journal of Cognitive Neuroscience. 2012;24(1):235–45. doi: 10.1162/jocn_a_00130. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129(Pt 11):2945–56. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. Journal of Cognitive Neuroscience. 2007;19(7):1081–8. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Chrisjohn RD, Fekken CG. The altruistic personality and the self-report altruism scale. Personality and Individual Differences. 1981;2(4):293–302. [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12(3):154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439(7075):466–9. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick BM, Nigrosh BJ. Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. Journal of Comparative & Physiological Psychology. 1975;88(1):118–27. doi: 10.1037/h0076200. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD. An integrative model of the neural systems supporting the comprehension of observed emotional behavior. Neuroimage. 2012 a;59(3):3050–9. doi: 10.1016/j.neuroimage.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD. Dissociating modality-specific and supramodal neural systems for action understanding. Journal of Neuroscience. 2012 b;32(10):3575–83. doi: 10.1523/JNEUROSCI.5715-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack EC, Balakrishnan R, Numan MJ, Numan M. A functional neuroanatomical investigation of the role of the medial preoptic area in neural circuits regulating maternal behavior. Behavioural Brain Research. 2002;131(1–2):17–36. doi: 10.1016/s0166-4328(01)00370-9. [DOI] [PubMed] [Google Scholar]
- Toi M, Batson CD. More evidence that empathy is a source of altruistic motivation. Journal of Personality and Social Psychology. 1982;43(2):281–92. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Zaki J, Ochsner K. The neuroscience of empathy: Progress, pitfalls and promise. Nature Neuroscience. 2012;15:675–80. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]
- Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proceedings of the National Academy of Sciences. 2009;106(27):11382–7. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]