Abstract
We used event-related potentials (ERPs) to tap the temporal dynamics of first impressions based on face appearance. Participants were asked to evaluate briefly presented faces for trustworthiness and political choice. Behaviorally, participants were better at discriminating faces that were pre-rated as untrustworthy. The ERP results showed that the P100 component was enhanced for untrustworthy faces, consistently with the view that signals of potential threat are given precedence in neural processing. The enhanced ERP responses to untrustworthy faces persisted throughout the processing sequence and the amplitude of early posterior negativity (EPN), and subsequent late positive potential (LPP) was increased with respect to trustworthy faces which, in contrast, elicited an enhanced positivity around 150 ms on frontal sites. These ERP patterns were found specifically for the trustworthiness evaluation and not for the political decision task. Political decision yielded an increase in the N170 amplitude, reflecting a more demanding and taxing structural encoding. Similar ERP responses, as previously reported in the literature for facial expressions processing, were found throughout the entire time course specifically elicited by faces explicitly judged as untrustworthy. One possibility might be that evolution has provided the brain with a ‘special toolkit’ for trust evaluation that is fast and triggers ERPs related to emotional processing.
Keywords: face perception, emotion, trustworthiness, event-related potentials
INTRODUCTION
Should I trust this person? This question jumps out automatically every time we encounter a stranger, especially when we have to rely on our first automatic impression. These impressions might be considered as a spontaneous, bottom–up valence evaluation of faces based on the extraction of particular facial cues.
A growing body of research indicates that people make reliable decisions about whether to trust and cooperate with someone only on the basis of facial appearance (Zebrowitz and Montepare, 2005; 2008; Oosterhof and Todorov, 2008). Therefore, trustworthiness appears to be an essential social tool that can provide information about whether to approach or avoid another individual.
Interestingly, several studies have shown that only minimal information is needed to form impressions from just seeing faces (Todorov and Uleman, 2004), or to make trait judgments such as trustworthiness, competence and aggressiveness (Willis and Todorov, 2006). This social evaluation process may occur unintentionally and very rapidly, since a very short exposure to faces might be sufficient (Bar et al., 2006; Willis and Todorov, 2006).
Even for decisions mainly based on objective and critical evaluation, we may be influenced by facial appearance. Surprisingly, an example of inferences that people make based often only on facial appearance is represented by the choice of leader in political voting. Voting is a domain in which individuals should make decisions by integrating multiple sources of information. In this type of social inference, top–down evaluation is probably triggered by cognitive strategies, expectations and political knowledge. However, these judgments may often be influenced by a rapid evaluation that occurs without conscious processing (Spezio et al., 2008). A number of recent studies have shown that rapid judgments about the personality traits of political candidates, based only on their facial appearance, predict electoral success well above chance (Todorov et al., 2005; Ballew and Todorov, 2007).
It is not yet clear why people make appearance-based trait inferences, given that these inferences often do not result in accurate social judgments (Olivola and Todorov, 2010a,b). One possibility might be that such inferences are based on cues that have an important adaptive role (Zebrowitz and Montepare, 2005, 2008; Todorov et al., 2008a, b; Said et al., 2011). As a matter of fact, the most basic decision that an organism must reach is to determine whether another organism is a friend or foe, prey or predator. Therefore, one possible interpretation is that evolution has provided the human primate with a ‘special toolkit’ for trust evaluation that is both fast and automatic.
An important contribution to this issue comes from computer modeling work, suggesting that inferences of trustworthiness derive from the similarity of emotionally neutral faces to expressions of happiness and anger (Oosterhof and Todorov, 2008; Said et al., 2009; Engell et al., 2010). A face that resembles a happy face is perceived as trustworthy, whereas one that resembles an angry face is perceived as untrustworthy (Said et al., 2009).
The amygdala has been consistently identified as one of the regions specifically involved in trustworthiness evaluation (Adolphs et al., 1998; Adolphs, 2002; Winston et al., 2002; Engell et al., 2007) and more broadly in face–valence evaluation (Todorov and Engell, 2008). The amygdala response increases as the perceived trustworthiness of faces decreases (Winston et al., 2002; Engell et al., 2007; Todorov et al., 2008a; Said et al., 2009). Interestingly, stronger activation in the amygdala was found during simulated voting judgments for candidates for whom participants chose to vote (Rule et al., 2010). In contrast, for images of losing candidates a greater activation was found in the insula and ventral anterior cingulate (Spezio et al., 2008). On the basis of these new intriguing findings on face evaluation, it becomes crucial to understand the neural underpinnings of face decisions.
To our knowledge, almost all studies on social face evaluation are based on functional magnetic resonance imaging (fMRI) measures, which are characterized by relatively slow hemodynamic brain responses. Thus, fMRI studies need to be complemented with methods that provide insights into the temporal sequences of face decisions, such as event-related brain potentials (ERP) which, given their high temporal resolution, might be particularly suitable to pinpoint the timing of processes leading to emotional and social judgments (Bartholow, 2010).
There are only a few studies that used ERPs to study the temporal dynamics of trustworthiness evaluation. One of the most relevant is the study by Rudoy and Paller (2007) that showed that perceptual information influence trustworthiness assessments well before relevant information has been retrieved from memory. Importantly, they found an early frontal correlate for faces evaluated as trustworthy that is thought to reflect the processing of facial expressions, as suggested previously (Eimer and Holmes, 2007).
On the light of the above findings, the aim of the present study was to assess the temporal sequence of ERPs elicited by two different social evaluation tasks based on facial appearance and first impressions. To this end, electroencephalographic (EEG) activity was recorded while participants performed a direct trustworthiness evaluation of briefly presented faces that varied along the trustworthiness dimension (Oosterhof and Todorov, 2008; Todorov et al., 2008a) and a political election task (‘would you vote for this political candidate?’), in which attention was not drawn on the concept of trust. Trust evaluation might be considered as an immediate evaluation, while political evaluation might be considered as a more reasoned and deliberative assessment. The key differences between these two tasks are principally that during a political vote, trustworthiness is not overtly requested and, more importantly, that evaluating a face for trustworthiness or choosing a political candidate has a substantially different emotional and adaptive value. From an evolutionary perspective, the primacy of trust is evident: understanding another person’s intent is more important to survival than whether will be a good or bad politician. Moreover, the political decision task might be influenced by stereotypes, political knowledge and social context (Little et al., 2007). Thus, direct trustworthiness assessment might be characterized by rapid, automatic, ‘intuitive preferences that come to mind quickly and without much reflection’ (Kahneman, 2003), while political evaluation requires more controlled processes.
Furthermore, people believe that competence is one of the most important qualities for a politician (McGraw, 2003; Todorov et al., 2005). Competence reflects traits that are related to perceived ability, including intelligence, skill, creativity and efficacy, while trust judgments depend on perceived warmth and honesty and determine approach–avoidance tendencies (Fiske et al., 2007).
In sum, although both evaluations are fundamental to social perception and cognition, trust judgments seem to be of primary relevance and crucial for survival (Fiske et al., 2007).
If trustworthiness judgments are a good approximation of the general valence evaluation of faces (Todorov and Engell, 2008) and might be interpreted as an over-extension of the ability to read emotional expression (Todorov, 2008; Zebrowitz and Montepare, 2008), we might expect to find ERP responses similar to those found for facial expressions throughout the entire time course of processing. Several studies have shown that a rapid evaluation of the emotional and motivational significance of faces appears to emerge quite early at about 120 ms unfolding over a large temporal window (for reviews see Eimer and Holmes, 2007; Vuilleumier and Purtois, 2007).
To track the time course of trust and political vote decisions, early and later ERP components related to face and emotional processing were considered in the present study. Specifically, we focused on the early P100 component recorded over the posterior cortex that reflects attentional mechanisms (Hillyard et al., 1998) and was found to be influenced by facial expressions (Batty and Taylor, 2003; Eger et al., 2003; Williams et al., 2006; Rotshtein et al., 2010). In addition, an early effect might be found over frontal sites, as shown previously in response to facial emotions and trustworthy faces (Eimer and Holmes, 2007; Rudoy and Paller, 2009; Righi et al., 2012). Notably, a still open question is if face evaluation might affect the N170 that is considered the most widely used ERP marker of face perception, reflecting face structural encoding (Bentin et al., 1996). At subsequent latencies, we focused on an early posterior negativity (EPN), peaking on occipito-temporal locations around 250 ms, that has been found to be enhanced for threatening as compared to neutral faces (Shupp et al., 2004). Finally, beyond 300 ms, we considered the late positive potential (LPP), which reflects emotional and motivational processes (Schupp et al., 2000, 2004; Hajcak et al., 2007, 2009; Foti et al., 2009; Marzi and Viggiano, 2010) and might be sensitive to face evaluation. Furthermore, for the early and late components, specific analyses were conducted to explore mechanisms that might be considered stimulus-driven (influenced by face properties) and those related to congruent judgments for pre-rated trustworthy and untrustworthy faces.
Our predictions are as follows: similar effects, as those found for processing of emotional vs neutral faces, should be obtained by comparing the ERP responses to trustworthy and untrustworthy faces. The N170, that is thought to be ‘immune’ to emotional and social evaluation, might be influenced only by the nature of the task and by face properties. Furthermore, we might, in general, expect political evaluation to be similarly affected by appearance-based trait inferences as trustworthiness, but with a substantial difference in the emotional significance hence with different ERPs responses.
METHODS
Participants
Sixteen healthy adult volunteers participated in the study (seven females, nine males); their age ranged between 23 and 29 years (mean = 24.9). All participants were right-handed, as assessed by means of the Italian revised version of the Edinburgh Handedness Inventory (Viggiano et al., 2001). All participants had normal or corrected-to-normal vision. They reported taking no medication and had no history of neurological, ophthalmological or psychiatric disease. All participants gave informed written consent. The experimental methods were approved by the departmental ethics committee.
Stimuli
The stimuli were obtained from different sets of computer-generated faces developed by Oosterhof and Todorov (2008) and Todorov et al. (2008a). A total of 300 faces were selected from a pool of faces created and modified to represent extreme versions varying on the trust dimension. All faces had different identities and varied on three levels of trustworthiness (very trustworthy, very untrustworthy and neutral). The faces were generated using FaceGen Modeller 3.2 (Singular Inversions, 2007), according to the methods described in Oosterhof and Todorov (2008) who developed a computer model that manipulates faces to make them less or more trustworthy. All faces were male, bald, Caucasian, front facing and with direct gaze. For both the trustworthiness and the political decision task the stimuli presented were divided in three categories: very trustworthy, very untrustworthy and neutral faces.
Task and design
Participants sat in a comfortable chair in a dimly lit room facing a computer monitor at a distance of 57 cm. After filling out the informed consent form, the electrode cap was mounted and participants were given task instructions and practice trials. They were asked to minimize blinking and to maintain visual fixation on a small cross in the centre of the screen during task performance. Participants were not told that the faces presented were divided into different trustworthiness levels.
Testing consisted of two different evaluation tasks each including five blocks (with an additional short practice block at the beginning of each task) with 30 trials (divided in three trustworthiness levels). For all the participants the political election task was tested before the trustworthiness task. This order of testing, although it might represent a limitation, was justified by the necessity of the political decision task not being biased by a previous trustworthiness assessment. A 20 min rest separated the two tasks. In the first task (‘Political evaluation’) participants were asked to decide if they would cast their vote or not for the presented face. Responses were given on a 3-point scale: 1: ‘Yes, I would like to vote this face’, ‘I don’t know’ and 3: ‘No, I wouldn’t like to vote this face’. During the voting task, participants were not informed that the main thrust of the study was to study the processing and evaluation of trustworthiness.
In the second task (‘Trustworthiness evaluation’) overt processing of trustworthiness was investigated by having participants indicate whether the presented face looked trustworthy or not using a 3-point scale from 1: ‘Yes, I trust this face’, 2: ‘I don’t know’ and 3: ‘No, I don’t trust this face’. For both tasks participants had to respond by pressing one of three designated keys (counter-balanced across participants) on a standard keyboard with the index finger of the right hand.
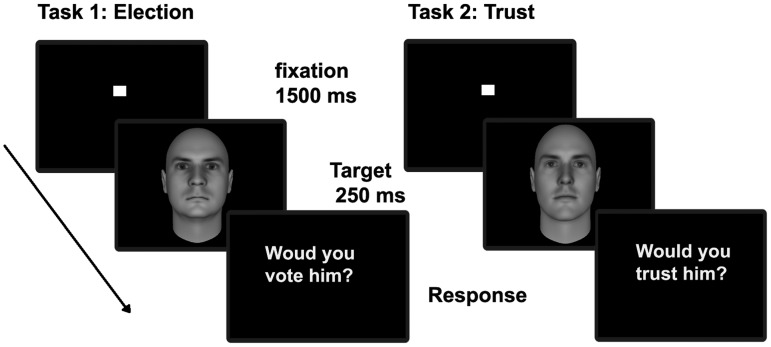
The order of the face stimuli was randomized within a sequence and across the two different tasks. To make sure that repetition effects could not interfere with the results each face was seen only once during the experiment. Each trial began with a fixation cross presented for 1500 ms, followed by the target face in the centre of the screen for 250 ms. A blank interval of 2800 ms separated the end of the rating period and the onset of the next face presentation. Participants could enter rating responses within 2000 ms from face onset. Figure 1 shows the experimental procedure.
Fig. 1.
Experimental design (face images were adapted from Oosterhof and Todorov 2008).
EEG data recording
The EEG was continuously recorded from 28 Ag/AgCl electrodes (F7, F3, Fz, F4, F8, FT7, FC3, FCz, FC4, FT8, T3, C3, Cz, C4, T4, TP7, Cp3, CPz, Cp4, TP8, T5, P3, Pz, P4, T6, O1, Oz, O2) with NeuroScan 4.3 and amplified using the SynAmps system. A common average reference and a forehead ground electrode were used. Vertical and horizontal electro-oculographic (EOG) activity was recorded with additional electrodes located above and below the left eye and outside the outer canthi of both eyes. For all electrodes the impedance was kept less than 5 kΩ. Electrical activity was amplified with a bandpass of 0.01–100 Hz and a sampling rate of 1000 Hz. In offline analysis the data were epoched into single sweep recordings from 200 ms before to 1000 ms after stimulus onset. Moreover each epoch was baseline corrected using the signal recorded during 200 ms that preceded the onset of the stimulus. All epochs with ocular artifacts greater than 40 μV were automatically rejected and in addition were visually scanned to find further artifacts. ERPs were then averaged separately for each experimental condition and low-pass filtered at 30 Hz (24 dB cut-off).
RESULTS
Behavioral data
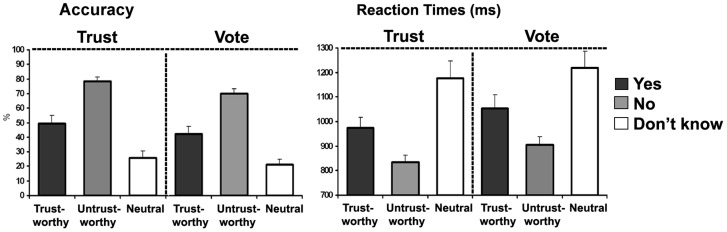
Although responses in this kind of experiment are inherently subjective, the ‘correctness’ of first impressions was inferred as a percentage of accuracy or ‘agreement’ in evaluation (Figure 2) with respect to the predetermined face categories (Oosterhof and Todorov, 2008). Therefore, percentage of ‘correct’ responses (e.g. ‘yes’ responses for trustworthy faces) was calculated with respect to the pre-selected faces categorized as very trustworthy, very untrustworthy or neutral. This was done also for the political evaluation task in order to find out if there might be consistency between political vote and trustworthiness (e.g. participants might prefer not to vote for faces that looked untrustworthy). Percentage of correct responses was submitted to an ANOVA factoring: Face Category (Untrustworthy, Trustworthy, Neutral) and Task (Trust, Vote).
Fig. 2.
Behavioral results. On the left: percentage of congruent responses (‘agreement’) to previously generated faces that were categorized as very trustworthy, very untrustworthy and neutral (Oosterhof and Todorov, 2008). On the right: mean RTs are shown for congruent responses.
The ANOVA showed a main effect of Face Category, F(1.7, 25.7) = 36.6, P < 0.001, with an overall greater percentage of agreement for negatively compared to positively evaluated faces. This finding was highly consistent, during the trust assessment, for faces that had been generated to look more untrustworthy; for untrustworthy faces there was also a higher percentage of negative responses in the political choice task.
Furthermore, reaction times (RTs) for correct response were submitted to an ANOVA, factoring Face Category and Task. Both main effects were significant: Face Category, F(2, 30) = 24.8, P < 0.01, and Task, F(1, 15) = 5.5, P < 0.04. The election vote task yielded longer RT compared to the trust task and for both tasks it took longer to respond ‘don’t know’ compared to ‘yes’ or ‘no’ responses, (P < 0.004; P < 0.001); moreover, faster RTs were found for negative with respect to positive evaluations, (P < 0.02). The behavioral results are shown in Figure 2.
ERP data
Subjective judgments
In order to investigate the neural correlates elicited by the participants’ subjective judgments, ERPs were constructed by separately averaging trials for the two responses-judgments (positive: ‘yes’ and negative: ‘no’), independently of the pre-rated face category, for each task trust and political evaluation (range of artifact free trials 38–55). For both tasks we did not include in the ERPs statistical analysis ‘Don’t know’ responses.
The ERP components of interest were selected on the basis of our primary predictions, of visual inspection of the components’ amplitude, and on the basis of previous studies of face processing and social-emotional evaluation (Schupp et al., 2000; Eimer and Holmes, 2007; Hajcak et al., 2007, 2009; Foti et al., 2009; Marzi and Viggiano, 2010).
The different ERP components of interest were quantified by computing and analyzing mean amplitude in specific time windows. The time windows were selected based on the topographical distribution and centered around the maximum amplitude of the ERP components. The P100 was quantified as average voltage over occipital recording sites (O1, OZ, O2) in the latency range 110–130 ms. The N170 was analyzed considering temporal electrodes (T5 and T6) between 130 and 220 ms. The early frontal positivity (EFP) was found of maximal amplitude over frontal and fronto-central sites (FZ and FCZ), between 130 and 220 ms. The EPN was observed and quantified as mean amplitude at occipito-temporal sites (O1, Oz, O2, T5 and T6) from 200 to 350 ms. Finally the LPP was largest at centro-parietal sites (CP3, CPz and CP4) between 300 and 500 ms. To test for statistical significance, separated repeated-measure ANOVAs were carried out for each component with: Task (Trust, Voting), Response (Positive, Negative) and Electrode (depending on the analysed component) as Factors.
For violations of the sphericity assumption the Greenhouse–Geisser correction was applied and adjusted degrees of freedom were used. Bonferroni’s correction was applied to all post hoc comparisons.
The first analysis was performed on the P100 component over occipital sites, to verify whether early attentional mechanisms would be influenced by the type of task or response. A significant interaction Task × Response × Electrode emerged, F(1.4, 22.2) = 6.5, P < 0.02, reflecting an enhanced positivity, during the trustworthiness task, for untrustworthy compared to trustworthy evaluated faces on electrode site O1, F(1, 15) = 8.6, P < 0.02. Moreover, faces judged as untrustworthy yielded enhanced amplitudes also compared to faces judged negatively, in the election task, on electrodes O1 and O2, F(1, 15) = 7.1, P < 0.02; F(1, 15) = 6.6, P < 0.03. No other effects were significant. Furthermore, on fronto-central sites (‘EFP’, with a maximal peak around 150 ms) an opposite pattern was found. The significant interaction Task × Response, F(1, 15) = 5.2, P < 0.04, showed that, for the trustworthiness task, faces judged as trustworthy elicited greater positivity relative to faces evaluated as untrustworthy, F(1, 15) = 8.1, P < 0.02. No further significant effects emerged.
To assess if social evaluation of faces might have an influence on the structural encoding processing, an analysis was performed on the mean amplitudes of the N170. As predicted, only the main effect of Task was significant, F(1, 15) = 37.2, P < 0.001, with an amplitude enhancement for the political vote compared to the trust evaluation. The main effect of Response did not reach significance, P = 0.08.
Consistent with our hypothesis, the EPN, i.e. a component that follows the N170 and is related to emotional processing, was influenced by the type of task and evaluation. The ANOVA showed a significant interaction between Task, Response and Electrode on occipital temporal sites, F(2.9, 43.6) = 5.5, P < 0.004, demonstrating that, on electrode T6, faces judged explicitly as untrustworthy elicited larger amplitude than faces judged as trustworthy, F(1, 15) = 5.0, P < 0.05. Moreover, negative judgments for the trust evaluation yielded enhanced amplitude compared to negative judgments in the election evaluation, F(1, 15) = 4.9, P < 0.05.
At the same latency on centro-parietal electrodes, a main effect of task emerged, F(1, 15) = 5.1, P < 0.05, with the trust evaluation eliciting enhanced positive amplitudes with respect to the political evaluation.
Later on during the time course of the ERP response, an ANOVA was performed on centro-parietal sites to investigate the effects on the LPP component. A significant effect of Task was found, F(1, 15) = 12.9, P < 0.004, and, more importantly, a significant interaction Task × Response × Electrode emerged, F(1.8, 27.4) = 3.8, P < 0.04. Post hoc analyses confirmed that the type of task reliably influenced the magnitude of the LPP with larger amplitudes for the trust compared to the election task; the interaction showed that on electrode CP4 untrustworthy faces yielded an enhanced positivity compared to trustworthy faces, F(1, 15) = 25.5, P < 0.001; while in the election task no significant differences emerged when comparing positive and negative evaluations. Finally, faces evaluated as untrustworthy elicited enhanced amplitude compared to faces that were negatively evaluated in the election task, F(1, 15) = 11.5, P < 0.005; this effect was evident on electrode CPZ, F(1, 15) = 7.5, P < 0.02. Grand averages for subjective responses or judgments are shown in Figure 3.
Fig. 3.
Grand averages for positive and negative judgments in the trust and election evaluations.
Stimulus-driven and congruent/incongruent judgments of trustworthiness
The results reported in the previous section concerned the ERPs elicited by the participants’ subjective judgments, independently of the pre-rated face category. To investigate whether and to what extent these evaluations might depend on inherent properties of the presented faces, additional analyses on pre-rated trustworthy and untrustworthy faces (Face-category), independently of behavioural judgments, were carried out for different time windows. These ANOVAs were conducted factoring Trustworthiness-Face-category (Untrustworthy, Trustworthy) and Electrode (two electrodes, depending on the considered ERP component).
Furthermore, separate ANOVAs were employed to analyze the trustworthiness effect when face judgments are congruent with the pre-rated face classification (e.g. untrustworthy faces judged congruently as untrustworthy). These specific analyses enable one to find out whether and when trustworthiness evaluations differ for congruent responses with the presented faces. The factors considered were: Trustworthiness-Face/judgments (trustworthy-yes and untrustworthy-no) and Electrode (two electrodes depending on the considered ERP component). All these analyses were restricted to specific components of interest. Only the significant effects found for the trustworthiness task are reported. Same latency windows and electrodes were considered as for the analysis on subjective judgments.
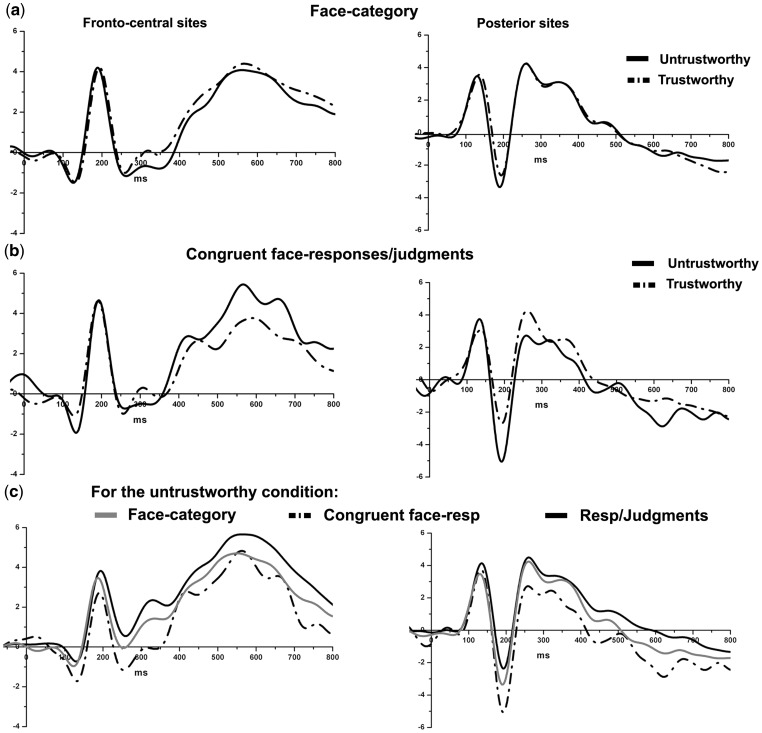
For the early P100 component no differences emerged from the ANOVA on Face-category, whereas for the ANOVA on congruent responses untrustworthy yielded an enhanced amplitude compared to trustworthy judgments, F(1, 15) = 8.9, P<0.01. Moreover, while the effect for Face-category was only close to significance (P = 0.07) on the N170 component, the ANOVA on congruent responses showed a significant difference with an enhanced negativity for untrustworthy compared to trustworthy congruent judgments, F(1, 15) = 5.8, P<0.04, see Figure 4a and b. This pattern might reflect the influence, at these early processing stages, of stimulus valence but only when congruent behavioral responses are given to pre-rated faces. From 400 to 600 ms, on electrodes CPZ and PZ, congruent responses to untrustworthy faces elicited greater amplitudes than congruent responses to trustworthy faces, F(1, 15) = 7.2, P<0.02; no significant differences emerged in the Face-category analysis. These findings suggest that the difference in trustworthiness on early and late ERP components requires a decisional process rather than being directly driven by the face stimuli. Figure 4c shows a comparison between grand averages waveforms for subjective judgments, face-category and congruent face judgments for the untrustworthy condition.
Fig. 4.
Trust evaluation: (a) grand averages for face categories (untrustworthy and trustworthy independently from judgments); (b) grand averages for congruent trustworthy and untrustworthy responses/judgments (e.g. pre-rated untrustworthy faces judged congruently as untrustworthy); (c) For the untrustworthy condition a comparison is shown between grand averages for: face-category, subjective judgments and congruent face-responses/judgments.
Finally, further analyses were performed to elucidate possible differences between congruent and incongruent judgments to pre-classified faces. For these purposes ANOVAs were performed factoring: Congruency (Congruent, Incongruent), Valence (Untrustworthy, Trustworthy) and Electrode (two electrodes—depending on the considered ERP component).
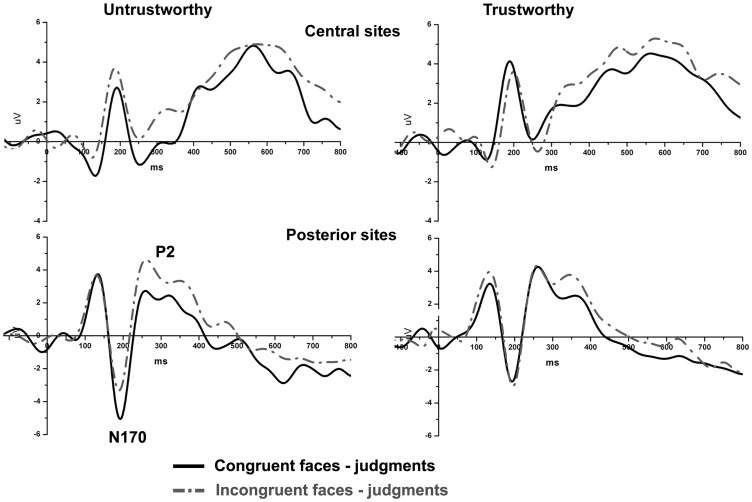
The analysis of the N170 showed an enhanced negativity for congruent compared to incongruent responses on T6 [significant interaction Congruency × Electrode, F(1, 15) = 6.9, P<0.02], see Figure 5. Later on, around 250 ms on temporal sites, an enhanced positivity was found for incongruent compared to congruent responses [reflected in the significant factor Congruency, F(1, 15) = 7.3, P<0.02]. No further significant effects were found.
Fig. 5.
Trust evaluation. Grand averages for congruent and incongruent face-judgment responses.
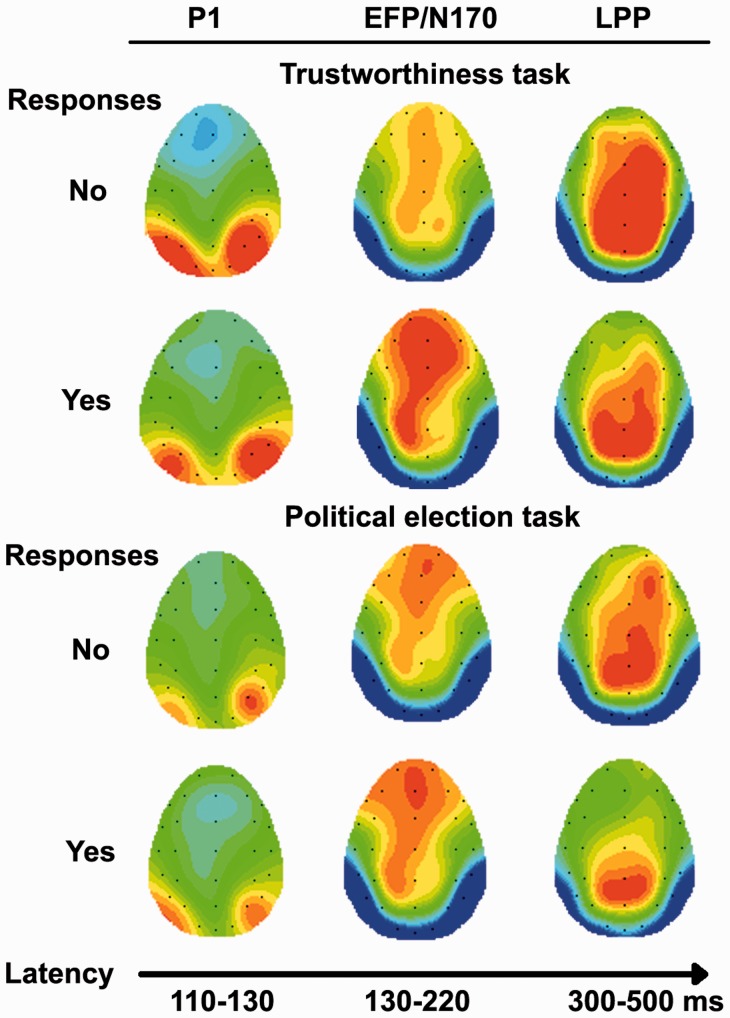
Topographical scalp maps
Figure 6 shows the topographical scalp maps for the trustworthiness and political election evaluations. An enhanced positivity for faces judged as untrustworthy (Trustworthiness task-Response: No) is visible on the P100 component, more evident for the trust with respect to the election task. The N170 component was sensitive to the task, with enhanced amplitudes for the political evaluation. Moreover, at the same latency as the N170, an enhanced early frontal positivity (EFP) is evident with a greater activation and a fronto-central distribution for faces judged as trustworthy (Trustworthiness task-Response: Yes). Finally, the LPP was enhanced for negative response in trust evaluation (Trustworthiness task-Response: No) with respect to the other conditions.
Fig. 6.
Scalp topographical maps for selected ERP components (P1, EFP, N170 and LPP) in response to positive (‘yes’) and negative (‘no’) evaluations (for both tasks).
Discussion
Right or wrong, people make very quick judgments about faces that determine how they feel about a person. Our brain is probably equipped with a special ‘toolkit’ for reading and evaluating first impressions about faces. The present study was aimed at tracking the time course of face evaluations for trustworthiness and political voting decisions. This topic is relevant because it addresses a fundamental question regarding when, that is, at what visual information processing stage, emotional and social ‘value’ is assigned to faces.
Behaviorally, a higher percentage of accuracy (agreement) in negative evaluation was found for faces that had been created to look more untrustworthy compared to the positive evaluation for trustworthy faces. In this regard, it has been suggested that people are remarkably efficient at making trustworthiness judgments from someone’s appearance with high reliability across individuals (Engell et al., 2007; Todorov et al., 2008a). Faces that were previously categorized as untrustworthy (Todorov et al., 2008a) yielded also greater percentage of negative responses in the election task (‘I won’t vote this person’). Negative facial traits might play a critical role in mediating the effects of appearance on voter decisions, an effect that may be of special importance when other information is absent (Spezio et al., 2008). The fact that both tasks showed similar percentage of congruent responses suggests that broadly similar processes underlie the different evaluations. The candidate’s appearance plays a key role in political choice (Todorov et al., 2005; Ballew and Todorov, 2007; Little et al., 2007; Spezio et al., 2008; Antonakis and Dalgas, 2009) and is based on specific facial cues that lead to consensus specifically for the negative evaluation. RT showed that it took longer to make the political vote evaluation with respect to the trust evaluation, indicating that the former task is more cognitively demanding and based on a more deliberative assessment.
To characterize the timing of these face evaluations, ERPs to subjective judgments for both tasks were compared. The ERP results showed, only for the trust evaluation task, an amplitude enhancement of the P100 component for faces evaluated as untrustworthy. These findings are consistent with our hypothesis that trustworthiness decisions are bottom–up, very rapid and automatic valence evaluation of faces in which attention is directed to particular face features. This amplitude enhancement of an early component that is related to low-level and face feature analysis probably reflects attentional mechanisms that were potentiated for emotionally relevant stimuli.
It is noteworthy to point out that ‘reading’ the face of conspecifics is an essential source of information for behaving appropriately and in this respect reading fast confers a marked advantage. This could be the reason why the responses to trust, in its negative dimension, emerged remarkably early in visual processing. These results suggest that a quick glimpse to emotionally relevant stimuli appears sufficient to tune the brain for the selective processing of emotional pictures (Schupp et al. 2004). Our observation on the P100 is consistent with the view that signals of potential danger are given precedence in neural processing (Williams et al., 2006) and that are processed by a mechanism for early automatic alerting to potential threat (Liddell et al., 2004, 2005; Williams et al., 2006).
The P100 amplitude has also been studied in research on facial expressions (Pizzagalli et al., 1999; Sato et al., 2001; Eimer and Holmes, 2002; Batty and Taylor, 2003; Eger et al., 2003; Pourtois et al., 2005). A modulation of early sensory responses has been found especially for fearful faces (Eimer and Holmes, 2002; Holmes et al., 2003; Pourtois et al., 2005).
These findings of greater attention during the trustworthiness task to more emotional or distinctive social stimuli may reflect an automatic vigilance effect in which attention is quickly and automatically drawn to stimuli with potential negative implications. This was not the case for negative responses in the political decision task for which the emotional meaning is probably less relevant. Importantly, the amygdala response was found to be more sensitive to differences at the negative that at the positive end of the trustworthiness dimension (Winston et al., 2002; Engell et al., 2007; Baron et al., 2011; Said et al., 2011). As to the role of amygdala, Todorov and Engell (2008) suggest that the valence evaluation of faces recruits a network of perceptual areas in temporal and occipital cortices whose response is modulated by the amygdala.
The N170, that is commonly associated with face structural encoding (Bentin et al., 1996), does not appear to be specific for emotional processing (Holmes et al., 2003). The present findings showed that the N170 was not modulated by the emotional content of the faces but was influenced by the task performed. An amplitude enhancement of the N170 was found in response to the ‘political election judgment’ possibly reflecting a more complex structural encoding. Given the complexity of the voting evaluation it is reasonable to hypothesize that face encoding requires additional processing to access the mental representation of a plausible political candidate. The N170 enhancement therefore probably reflects the attentional modulation associated with a more demanding structural encoding.
Furthermore, we found that the enhanced positivity for untrustworthy faces, not only started early, but persisted throughout the processing sequence, such that the amplitude of the EPN, 220–400 ms post-stimulus, and subsequent LPP (around 500 ms post-stimulus) were increased. This was found for the explicit trustworthiness evaluation and not for the political decision task. Untrustworthy judged faces yielded enhanced negativity over temporo-occipital leads that started around 220 ms after stimulus onset resembling the effects found previously for the EPN (Shupp et al., 2000). This component has been found to be larger for threatening as compared to neutral faces (Shupp et al., 2004). Enhanced EPN amplitude in response to emotional stimuli has been similarly observed when viewing pictures of erotica, mutilation and threat as well as threatening and fearful faces (Schupp et al., 2000, 2004; Junghöfer et al., 2001; Leppänen et al., 2007). A very recent study (Dzhelyova et al., 2012) provided evidence that attribution of trustworthiness elicits an enhanced early EPN during explicit judgments, a result which was not found during a gender categorization task. Interestingly, Dzhelyova and coworkers (2012) found an amplitude enhancement of the EPN for untrustworthy male faces and trustworthy female faces. This might be interpreted as a more efficient processing for salient stimuli (Sato et al., 2001; Dzhelyova et al., 2012).
Faces evaluated as untrustworthy were found also to be subject to a continuing processing at later stages. Increased LPP amplitudes to untrustworthy faces indicated facilitated processing of untrustworthy compared to trustworthy faces, whereas no effects were found for the political election evaluation. Again, similar to the case for augmented EPN amplitudes, recent studies with emotional pictures observed enhanced LPP amplitudes (Schupp et al., 2000). A facilitated perceptual processing in these studies was particularly pronounced for stimuli of high evolutionary significance (Schupp et al., 2000). Importantly, in line with the present results, an ERP study, that investigated the neural correlates of trust, showed that untrustworthy faces elicited a more positive LPP than trustworthy faces, indicating an enhanced motivated attention (Yang et al., 2011). The right-lateralized effect, which emerged also for the EPN, is in line with studies that showed a primary role of the right hemisphere in face emotional recognition, particularly evident for stimuli with negative affective valence (Killgore et al., 2007; Bourne, 2011; Dzhelyova et al., 2012; Nijboer and Jellema, 2012; Meng et al., 2012).
In contrast to faces evaluated as untrustworthy, that influenced almost all ERPs pattern, faces judged as trustworthy had an effect restricted to an early time window, showing an enhanced positivity, with respect to untrustworthy faces, around 150 ms on frontal sites. This frontal effect, although with an earlier onset, is in line with the early frontal correlate of trustworthy faces found by Rudoy and Paller (2009). At this level of processing a representation of the face reward value might be ‘read out’. For this the ventromedial prefrontal cortex (VMPFC) seems particularly suitable in preference evaluation (Lieberman and Eisenberger, 2009). In this regard, it has been shown that fairness and cooperation activate the same hedonic regions of the brain as financial gain activating reward circuitry (Tabibnia and Lieberman, 2007; Tabibnia et al., 2008).
In the present study the ERPs responses were found to be strongly enhanced for the trust compared to the political election evaluation, probably reflecting the fact that emotional inferences formed on the basis of the observation of a face are more relevant for the trust decision. These findings suggest that face evaluation elicits multiple brain responses involved in attention, structural processing and decision making with an enhancement of the ERPs responses related to emotional processing when trustworthiness is overtly processed. Furthermore, it might be the case that in electoral decisions other processes, with a less emotional content and a more deliberative assessment, come into play. The current results also show that differential ERP patterns involved in the trust evaluation might be considered as an immediate assessment, while the political evaluation might be considered as a more reasoned and deliberative assessment. This is suggested by many studies showing that trustworthiness evaluation occurs automatically, driven spontaneously rather than by top–down processes (Winston et al., 2002; Willis and Todorov, 2006; Engell et al., 2007; Todorov et al., 2009; Dzhelyova et al., 2012). On the contrary, little is known about the processes involved in voting decisions but we might assume that in this decision facial evaluation might represent a sort of cognitive shortcut to facilitate evaluation characterized in a normal context by overloading information (Caprara et al., 1997; Little et al., 2007).
One question that remains open is if it is possible to tease apart the contribution of stimulus-driven responses (triggered by the pre-classified faces) and the subjective responses that were congruent with the pre-rated faces. This issue might have important implications for the dissociation between bottom–up and top–down mechanisms in emotional processing (Wright et al., 2008; Ochsner et al., 2009). When do bottom–up and stimulus-driven affective processes interact with top–down cognitive appraisal processes? To investigate this aspect, additional analyses enabled us to compare ERP responses to face categories (untrustworthy and trustworthy-independently from the subjects judgments) and ERP responses to congruent and incongruent judgments (e.g. untrustworthy faces judged as untrustworthy). In sum, differences in trustworthiness emerged at the level of the P100 and the N170 components related to the congruent explicit judgments and not only to the stimuli themselves. Thus, untrustworthy congruent face-judgments were enhanced for these components suggesting that the evaluation of trust is characterized by the extraction of specific salient facial cues, particularly evident for faces that were congruently judged as untrustworthy. At this level of processing, bottom–up and top–down processes are probably integrated to enhance the perceptual awareness of relevant features of the stimuli, as a function of a given task (Sato et al., 2001; Dzhelyova et al., 2012). Some particular structural features might be encoded while trustworthiness attributions are being made, for example brow ridge, chins shape and facial width (Stirrat and Perrett, 2010; Todorov et al., 2008a; Dzhelyova et al., 2012).
Later on, around 250 ms, incongruent judgments elicited enhanced amplitude compared to congruent judgments probably indexing the processing of incongruent structural face aspects. In this respect, the P200 has been linked to the analytical processing of spatial relations between facial features in individual faces (Latinus and Taylor, 2006) and the initiation of individual recognition mechanisms (Halit et al., 2000). Furthermore, on the LPP component, the differences in trustworthiness emerged for the subjective and congruent judgments probably reflecting the involvement of motivational and more cognitive evaluation. It is possible to interpret these findings considering that early components are sensitive to both task and stimulus-driven processes while later components are more sensitive to subjective judgments.
Admittedly, these results should be considered with caution due to the limited number of congruent trials, but we believe that this is an important issue to be addressed in future research by using a specific experimental design.
In conclusion, the present ERP results highlight the finding that emotional responses, triggered by face evaluation, appear both quite early and at longer latencies and differ as a function of the evaluated dimension (trust or vote). We interpret these findings by considering face evaluation as an overextension of the ability to read emotional expressions (Zebrowitz and Montepare, 2005, 2008; Oosterhof and Todorov, 2008; Said et al., 2009). Viewing people who look untrustworthy would produce emotional responses that might be used to make facial evaluations. It seems plausible that these emotional responses are triggered by facial cues that have adaptive significance. Taken together, the temporal profile of the ERP responses suggests that the evaluation of trust influences visual processing quite early and unfolds largely independently from structural encoding. The early effects found on the P100 component might suggest the involvement of attentional mechanisms with an alerting function (Williams et al., 2006). Moreover, the persistence of enhanced responses to ‘potential threat’ conveyed by an untrustworthy face may reflect emotional and motivational processes engaged during face evaluation.
Conflict of Interest
None declared.
REFERENCES
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–4. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Trust in the brain. Nature Neuroscience. 2002;5:192–3. doi: 10.1038/nn0302-192. [DOI] [PubMed] [Google Scholar]
- Antonakis J, Dalgas O. Predicting elections: child’s play. Science. 2009;323:1183. doi: 10.1126/science.1167748. [DOI] [PubMed] [Google Scholar]
- Ballew CC, Todorov A. Predicting political elections from rapid and unreflective face judgments. Proceedings of the National Academy of Sciences of United States of America. 2007;104:17948–53. doi: 10.1073/pnas.0705435104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Neta M, Linz H. Very first impressions. Emotion. 2006;6:269–78. doi: 10.1037/1528-3542.6.2.269. [DOI] [PubMed] [Google Scholar]
- Baron SG, Gobbini MI, Engell AD, Todorov A. Amygdala and dorsomedial prefrontal cortex responses to appearance-based and behavior-based person impressions. Social Cognitive and Affective Neuroscience. 2011;6:572–81. doi: 10.1093/scan/nsq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD. Event-related brain potentials and social cognition: On using physiological information to constrain social-cognitive theories. Social Cognition. 2010;28:723–747. [Google Scholar]
- Batty M, Taylor MJ. Early processing of the six basic facial emotional expressions. Cognitive Brain Research. 2003;17:613–20. doi: 10.1016/s0926-6410(03)00174-5. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–65. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne VJ. Examining the effects of inversion on lateralisation for processing facial emotion. Cortex. 2011;47:690–5. doi: 10.1016/j.cortex.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Dzhelyova M, Perrett DI, Jentzsch I. Temporal dynamics of trustworthiness perception. Brain Research. 2012;1435:81–90. doi: 10.1016/j.brainres.2011.11.043. [DOI] [PubMed] [Google Scholar]
- Caprara GV, Barbaranelli C, Zimbardo P. Politicians’ uniquely simple personalities. Nature. 1997;385:493. [Google Scholar]
- Eger E, Jedynak A, Iwaki T, Skrandies W. Rapid extraction of emotional expression: evidence from evoked potential fields during brief presentation of face stimuli. Neuropsychologia. 2003;41:808–17. doi: 10.1016/s0028-3932(02)00287-7. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A. An ERP study on the time course of emotional face processing. Neuroreport. 2002;13:427–31. doi: 10.1097/00001756-200203250-00013. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A. Event-related brain potential correlates of emotional face processing. Neuropsychologia. 2007;45:15–31. doi: 10.1016/j.neuropsychologia.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. Journal of Cognitive Neuroscience. 2007;19:1508–19. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Engell AD, Todorov A, Haxby JV. Common neural mechanisms for the evaluation of facial trustworthiness and emotional expressions as revealed by behavioral adaptation. Perception. 2010;39:931–41. doi: 10.1068/p6633. [DOI] [PubMed] [Google Scholar]
- Fiske ST, Cuddy AJC, Glick P. Universal dimensions of social cognition: warmth and competence. Trends in Cognitive Sciences. 2007;11:77–83. doi: 10.1016/j.tics.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–30. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Neural response to emotional pictures is unaffected by concurrent task difficulty: an event-related potential study. Behavioral Neuroscience. 2007;121:1156–62. doi: 10.1037/0735-7044.121.6.1156. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Motivated and controlled attention to emotion: time-course of the late positive potential. Clinical Neurophysiology. 2009;120:505–10. doi: 10.1016/j.clinph.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Halit H, de Haan M, Johnson MH. Modulation of event-related potentials by prototypical and atypical faces. Neuroreport. 2000;11:1871–75. doi: 10.1097/00001756-200006260-00014. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: Electrophysiological and neuroimaging evidence. Philosophical Transactions of the Royal Society: Biological Sciences. 1998;353:1257–70. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Vuilleumier P, Eimer M. The processing of emotional facial expression is gated by spatial attention: evidence from event-related brain potentials. Brain Research. Cognitive brain research. 2003;16:174–84. doi: 10.1016/s0926-6410(02)00268-9. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Bradley MM, Elbert TR, Lang PJ. Fleeting images: a new look at early emotion discrimination. Psychophysiology. 2001;38:175–8. [PubMed] [Google Scholar]
- Kahneman D. A perspective on judgment and choice: mapping bounded rationality. The American Psychologist. 2003;58:697–720. doi: 10.1037/0003-066X.58.9.697. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. The right-hemisphere and valence hypotheses: could they both be right (and sometimes left)? Social Cognitive and Affective Neuroscience. 2007;2:240–50. doi: 10.1093/scan/nsm020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latinus M, Taylor MJ. Face processing stages: impact of difficulty and the separation of effects. Brain Research. 2006;1123:179–87. doi: 10.1016/j.brainres.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Leppänen JM, Kauppinen PK, Peltola MJ, Hietanen JK. Differential electrocortical responses to increasing intensities of fearful and happy emotional expressions. Brain Research. 2007;1166:103–9. doi: 10.1016/j.brainres.2007.06.060. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, et al. A direct brainstem-amygdala-cortical ‘alarm’ system for subliminal signals of fear. NeuroImage. 2005;24:235–43. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Williams LM, Rathjen J, Shevrin H, Gordon E. A temporal dissociation of subliminal versus supraliminal fear perception: an event-related potential study. Journal of Cognitive Neuroscience. 2004;16:479–86. doi: 10.1162/089892904322926809. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI. Neuroscience. Pains and pleasures of social life. Science. 2009;323:890–1. doi: 10.1126/science.1170008. [DOI] [PubMed] [Google Scholar]
- Little AC, Burris RP, Jones BC, Craig Roberts S. Facial appearance affects voting decisions. Evolution and Human Behavior. 2007;28:18–27. [Google Scholar]
- Marzi T, Viggiano MP. When memory meets beauty. Biological Psychology. 2010;84:192–205. doi: 10.1016/j.biopsycho.2010.01.013. [DOI] [PubMed] [Google Scholar]
- McGraw KM. Political impressions: formation and management. In: Sears DO, Huddy L, Jervis R, editors. Oxford Handbook of Political Psychology. New York: Oxford, University Press; 2003. pp. 394–432. [Google Scholar]
- Meng M, Cherian T, Singal G, Sinha P. Lateralization of face processing in the human brain. Proceedings. Biological Sciences, The Royal Society. 2012;279:2052–61. doi: 10.1098/rspb.2011.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijboer TCW, Jellema T. Unequal impairment in the recognition of positive and negative emotions after right hemisphere lesions: a left hemisphere bias for happy faces. Journal of Neuropsychology. 2012;6:79–93. doi: 10.1111/j.1748-6653.2011.02007.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, Gabrieli JDE, Gross JJ. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychological Science. 2009;20:1322–31. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivola CY, Todorov A. Fooled by first impressions? Reexamining the diagnostic value of appearance-based inferences. Journal of Experimental Social Psychology. 2010a;46:315–24. [Google Scholar]
- Olivola CY, Todorov A. Elected in 100 milliseconds: appearance-based trait inferences and voting. Journal of Nonverbal Behavior. 2010b;34:83–110. [Google Scholar]
- Oosterhof NN, Todorov A. The functional basis of face evaluation. Proceedings of the National Academy of Sciences U S A. 2008;105:11087–92. doi: 10.1073/pnas.0805664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D, Regard M, Lehmann D. Rapid emotional face processing in the human right and left brain hemispheres: an ERP study. Neuroreport. 1999;10:2691–8. doi: 10.1097/00001756-199909090-00001. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Dan ES, Grandjean D, Sander D, Vuilleumier P. Enhanced extrastriate visual response to bandpass spatial frequency filtered fearful faces: time course and topographic evoked-potentials mapping. Human Brain Mapping. 2005;26:65–79. doi: 10.1002/hbm.20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi S, Marzi T, Toscani M, Baldassi S, Ottonello S, Viggiano MP. Fearful expressions enhance recognition memory: electrophysiological evidence. Acta Psychologica. 2012;139:7–18. doi: 10.1016/j.actpsy.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Richardson MP, Winston JS, Kiebel SJ, Vuilleumier P, Eimer M, Driver J, Dolan RJ. Amygdala damage affects event-related potentials for fearful faces at specific time windows. Human Brain Mapping. 2010;31:1089–105. doi: 10.1002/hbm.20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudoy JD, Paller KA. Who can you trust? Behavioral and neural differences between perceptual and memory-based influences. Frontiers in Human Neuroscience. 2009;3:16. doi: 10.3389/neuro.09.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule NO, Freeman JB, Moran JM, Gabrieli JDE, Adams RB, Ambady N. Voting behavior is reflected in amygdala response across cultures. Social Cognitive and Affective neuroscience. 2010;5:349–55. doi: 10.1093/scan/nsp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said CP, Sebe N, Todorov A. Structural resemblance to emotional expressions predicts evaluation of emotionally neutral faces. Emotion. 2009;9:260–4. doi: 10.1037/a0014681. [DOI] [PubMed] [Google Scholar]
- Said CP, Haxby JV, Todorov A. Brain systems for assessing the affective value of faces. Philosophical Transaction of the Royal Society of London B: Biological Sciences. 2011;366:1660–70. doi: 10.1098/rstb.2010.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Yoshikawa S, Matsumura M. Emotional expression boosts early visual processing of the face: ERP recording and its decomposition by independent component analysis. Neuroreport. 2001;12:709–14. doi: 10.1097/00001756-200103260-00019. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–61. [PubMed] [Google Scholar]
- Schupp HT, Ohman A, Junghofer M, Weike AI, Stockburger J, Hamm AO. The facilitated processing of threatening faces: an ERP analysis. Emotion. 2004;4:189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Rangel A, Alvarez RM, et al. A neural basis for the effect of candidate appearance on election outcomes. Social Cognitive and Affective Neuroscience. 2008;3:344–52. doi: 10.1093/scan/nsn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirrat M, Perrett DI. Valid facial cues to cooperation and trust: male facial width and trustworthiness. Psychological Science. 2010;21:349–354. doi: 10.1177/0956797610362647. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Lieberman MD. Fairness and cooperation are rewarding: evidence from social cognitive neuroscience. Annals of the New York Academy of Sciences. 2007;1118:90–101. doi: 10.1196/annals.1412.001. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Satpute AB, Lieberman MD. The sunny side of fairness: preference for fairness activates reward circuitry (and disregarding unfairness activates self-control circuitry) Psychological Science. 2008;19:339–47. doi: 10.1111/j.1467-9280.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- Todorov A. Evaluating faces on trustworthiness: an extension of systems for recognition of emotions signaling approach/avoidance behaviors. Annals of the New York Academy of Sciences. 2008;1124:208–24. doi: 10.1196/annals.1440.012. [DOI] [PubMed] [Google Scholar]
- Todorov A, Baron SG, Oosterhof NN. Evaluating face trustworthiness: a model based approach. Social Cognitive and Affective Neuroscience. 2008a;3:119–27. doi: 10.1093/scan/nsn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Engell AD. The role of the amygdala in implicit evaluation of emotionally neutral faces. Social Cognitive and Affective Neuroscience. 2008;3:303–12. doi: 10.1093/scan/nsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Mandisodza AN, Goren A, Hall CC. Inferences of competence from faces predict election outcomes. Science. 2005;308:1623–6. doi: 10.1126/science.1110589. [DOI] [PubMed] [Google Scholar]
- Todorov A, Said CP, Engell AD, Oosterhof NN. Understanding evaluation of faces on social dimensions. Trends in Cognitive Science. 2008b;12:455–60. doi: 10.1016/j.tics.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Todorov A, Uleman JS. The person reference process in spontaneous trait inferences. Journal of Personality and Social Psychology. 2004;87:482–93. doi: 10.1037/0022-3514.87.4.482. [DOI] [PubMed] [Google Scholar]
- Viggiano MP, Borelli P, Vannucci M, Rocchetti G. Hand preference in Italian students. Laterality. 2001;6:283–6. doi: 10.1080/713754412. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–94. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Williams LM, Palmer D, Liddell BJ, Song L, Gordon E. The ‘when’ and ‘where’ of perceiving signals of threat versus non-threat. Neuroimage. 2006;31:458–67. doi: 10.1016/j.neuroimage.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Willis J, Todorov A. First impressions: making up your mind after a 100-ms exposure to a face. Psychological Science. 2006;17:592–8. doi: 10.1111/j.1467-9280.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5:277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Wright P, Albarracin D, Brown RD, Li H, He G, Liu Y. Dissociated responses in the amygdala and orbitofrontal cortex to bottom-up and top-down components of emotional evaluation. NeuroImage. 2008;39:894–902. doi: 10.1016/j.neuroimage.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Qi S, Ding C, Song Y. An ERP study on the time course of facial trustworthiness appraisal. Neuroscience Letters. 2011;496:147–51. doi: 10.1016/j.neulet.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Montepare JM. Psychology. Appearance DOES matter. Science. 2005;308:1565–6. doi: 10.1126/science.1114170. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Montepare JM. Social psychological face perception: why appearance matters. Social and Personality Psychology Compass. 2008;2:1497–517. doi: 10.1111/j.1751-9004.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]