Abstract
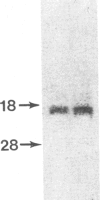
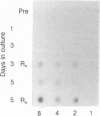
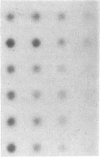
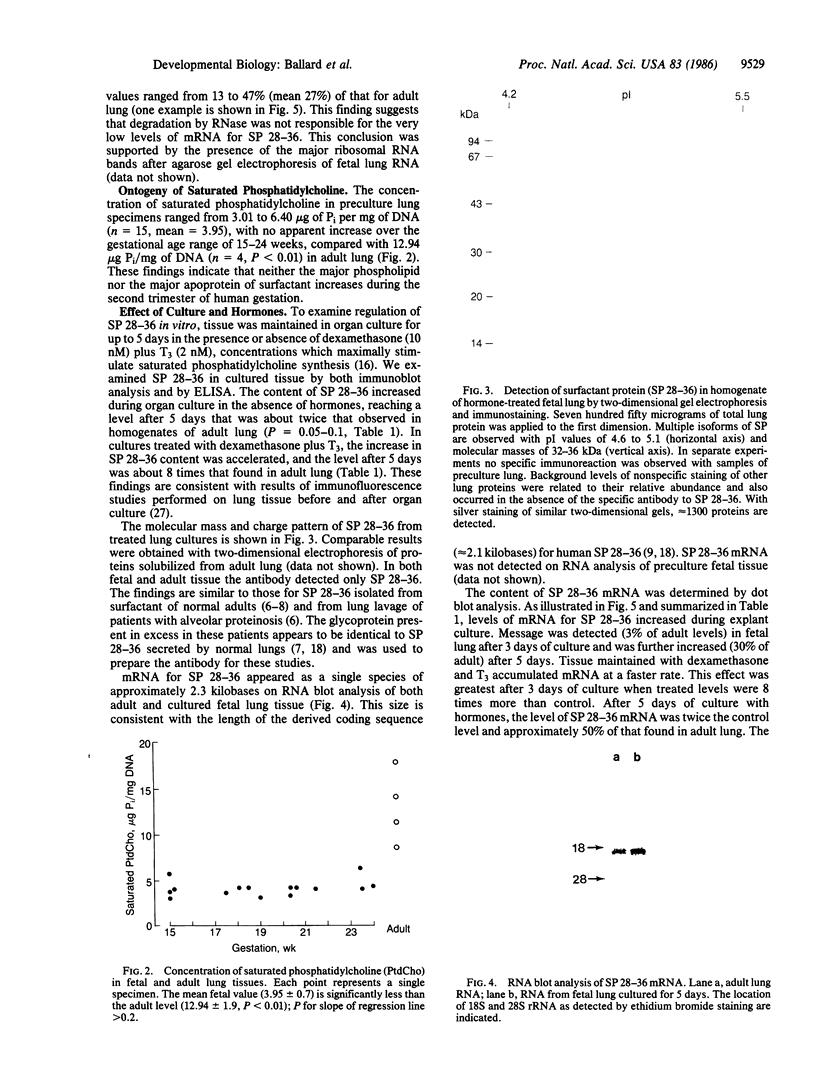
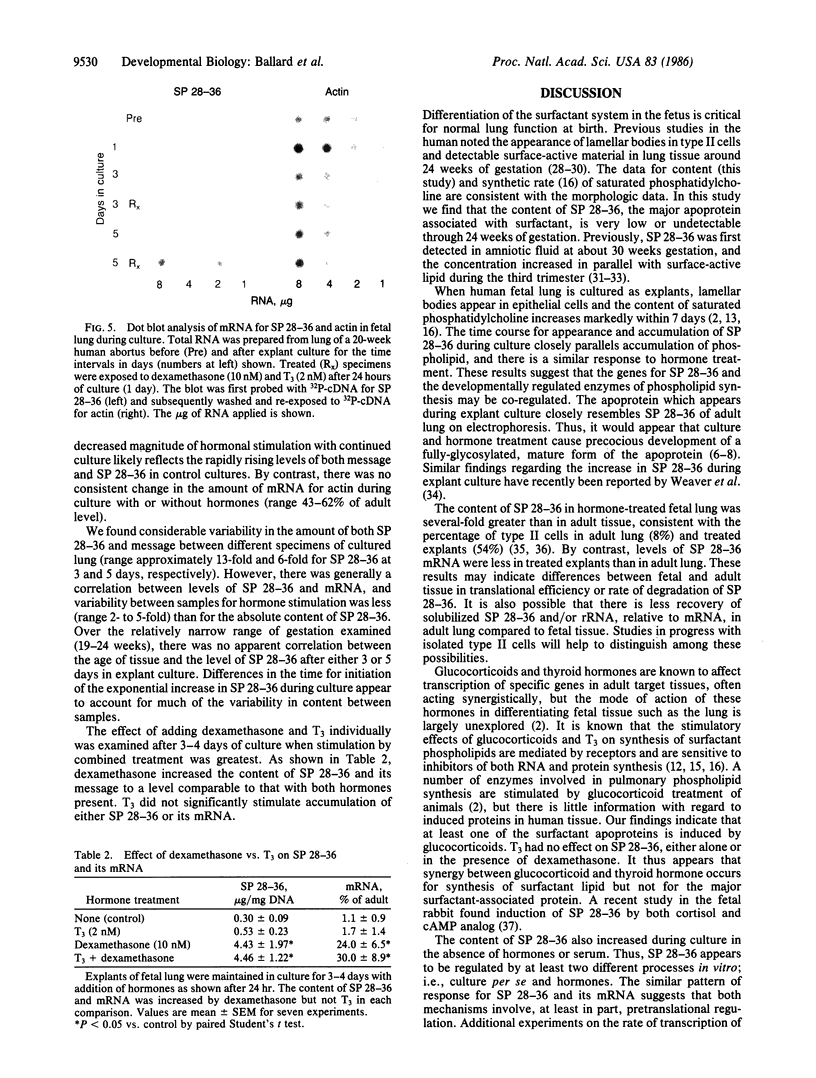
Pulmonary surfactant stabilizes lung alveoli, preventing respiratory failure and hyaline membrane disease in premature infants. In addition to lipids, surfactant contains apoproteins that are thought to be critical for normal surfactant function. We have examined the ontogeny and regulation of the major surfactant-associated protein of molecular mass 28-36 kDa (SP 28-36) in human fetal lung. SP 28-36 was not detected in tissue from second trimester abortuses by either immunoblot analysis or enzyme-linked immunosorbent assay (less than 0.02 microgram per mg of DNA). Levels of mRNA for SP 28-36, assayed by cDNA hybridization, were low or undetectable in all preculture specimens. The concentration of saturated phosphatidylcholine in lung tissue was 30% of the adult value with no apparent increase between 15 and 24 weeks gestation. SP 28-36 content increased during explant culture in the absence of serum and hormones, exceeding adult levels (3.2 +/- 1.0 micrograms per mg of DNA) after 5 days. In cultures treated with triiodothyronine (2 nM) and dexamethasone (10 nM), hormones that regulate phosphatidylcholine synthesis, the increase in SP 28-36 was accelerated (treated/control ratio was 7.1 and 3.4 at 3 and 5 days, respectively). Levels of mRNA for SP 28-36 also increased during culture and were stimulated by hormones (treated/control = 8.6 and 1.9 at 3 and 5 days, respectively). SP 28-36 and its mRNA increased similarly in the presence of dexamethasone alone, whereas triiodothyronine alone had no apparent effect. The molecular weight and charge pattern was similar for SP 28-36 of adult and cultured fetal tissue. These findings indicate that expression of the SP 28-36 gene is low during the second trimester, increases during explant culture, and is accelerated by glucocorticoid treatment.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERY M. E., MEAD J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959 May;97(5 Pt 1):517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- Ballard P. L., Ertsey R., Gonzales L. K., Liley H. G., Williams M. C. Isolation and characterization of differentiated alveolar type II cells from fetal human lung. Biochim Biophys Acta. 1986 Sep 4;883(2):335–344. doi: 10.1016/0304-4165(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Ballard P. L., Hovey M. L., Gonzales L. K. Thyroid hormone stimulation of phosphatidylcholine synthesis in cultured fetal rabbit lung. J Clin Invest. 1984 Sep;74(3):898–905. doi: 10.1172/JCI111507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPICHE M. A., GAUTIER A., HERNANDEZ E. I., REYMOND A. AN ELECTRON MICROSCOPE STUDY OF THE FETAL DEVELOPMENT OF HUMAN LUNG. Pediatrics. 1963 Dec;32:976–994. [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Gehr P., Bachofen M., Weibel E. R. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982 Jun;125(6):740–745. doi: 10.1164/arrd.1982.125.6.740. [DOI] [PubMed] [Google Scholar]
- Floros J., Phelps D. S., Taeusch H. W. Biosynthesis and in vitro translation of the major surfactant-associated protein from human lung. J Biol Chem. 1985 Jan 10;260(1):495–500. [PubMed] [Google Scholar]
- Floros J., Steinbrink R., Jacobs K., Phelps D., Kriz R., Recny M., Sultzman L., Jones S., Taeusch H. W., Frank H. A. Isolation and characterization of cDNA clones for the 35-kDa pulmonary surfactant-associated protein. J Biol Chem. 1986 Jul 5;261(19):9029–9033. [PubMed] [Google Scholar]
- Gonzales L. W., Ballard P. L., Ertsey R., Williams M. C. Glucocorticoids and thyroid hormones stimulate biochemical and morphological differentiation of human fetal lung in organ culture. J Clin Endocrinol Metab. 1986 Apr;62(4):678–691. doi: 10.1210/jcem-62-4-678. [DOI] [PubMed] [Google Scholar]
- Gross I., Ballard P. L., Ballard R. A., Jones C. T., Wilson C. M. Corticosteroid stimulation of phosphatidylcholine synthesis in cultured fetal rabbit lung: evidence for de novo protein synthesis mediated by glucocorticoid receptors. Endocrinology. 1983 Mar;112(3):829–837. doi: 10.1210/endo-112-3-829. [DOI] [PubMed] [Google Scholar]
- Gross I., Wilson C. M. Fetal lung in organ culture. IV. Supra-additive hormone interactions. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jun;52(6):1420–1425. doi: 10.1152/jappl.1982.52.6.1420. [DOI] [PubMed] [Google Scholar]
- Gross I., Wilson C. M. Fetal rat lung maturation: initiation and modulation. J Appl Physiol Respir Environ Exerc Physiol. 1983 Dec;55(6):1725–1732. doi: 10.1152/jappl.1983.55.6.1725. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage E. The morphological development of the pulmonary epithelium of human foetuses studied by light- and electron microscopy. Z Anat Entwicklungsgesch. 1973 Aug 30;140(3):271–279. doi: 10.1007/BF00525057. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Hamilton R. L., Jr Effects of a surfactant-associated protein and calcium ions on the structure and surface activity of lung surfactant lipids. Biochemistry. 1985 Jan 1;24(1):184–190. doi: 10.1021/bi00322a026. [DOI] [PubMed] [Google Scholar]
- Katyal S. L., Amenta J. S., Singh G., Silverman J. A. Deficient lung surfactant apoproteins in amniotic fluid with mature phospholipid profile from diabetic pregnancies. Am J Obstet Gynecol. 1984 Jan 1;148(1):48–53. doi: 10.1016/s0002-9378(84)80031-9. [DOI] [PubMed] [Google Scholar]
- Katyal S. L., Singh G. Analysis of pulmonary surfactant apoproteins by electrophoresis. Biochim Biophys Acta. 1981 Oct 28;670(3):323–331. doi: 10.1016/0005-2795(81)90104-5. [DOI] [PubMed] [Google Scholar]
- King R. J., Carmichael M. C., Horowitz P. M. Reassembly of lipid-protein complexes of pulmonary surfactant. Proposed mechanism of interaction. J Biol Chem. 1983 Sep 10;258(17):10672–10680. [PubMed] [Google Scholar]
- King R. J., Klass D. J., Gikas E. G., Clements J. A. Isolation of apoproteins from canine surface active material. Am J Physiol. 1973 Apr;224(4):788–795. doi: 10.1152/ajplegacy.1973.224.4.788. [DOI] [PubMed] [Google Scholar]
- King R. J., Ruch J., Gikas E. G., Platzker A. C., Creasy R. K. Appearance of paoproteins of pulmonary surfactant in human amniotic fluid. J Appl Physiol. 1975 Nov;39(5):735–741. doi: 10.1152/jappl.1975.39.5.735. [DOI] [PubMed] [Google Scholar]
- Kuroki Y., Takahashi H., Fukada Y., Mikawa M., Inagawa A., Fujimoto S., Akino T. Two-site "simultaneous" immunoassay with monoclonal antibodies for the determination of surfactant apoproteins in human amniotic fluid. Pediatr Res. 1985 Oct;19(10):1017–1020. doi: 10.1203/00006450-198510000-00013. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mendelson C. R., Johnston J. M., MacDonald P. C., Snyder J. M. Multihormonal regulation of surfactant synthesis by human fetal lung in vitro. J Clin Endocrinol Metab. 1981 Aug;53(2):307–317. doi: 10.1210/jcem-53-2-307. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Phelps D. S., Taeusch H. W., Jr, Benson B., Hawgood S. An electrophoretic and immunochemical characterization of human surfactant-associated proteins. Biochim Biophys Acta. 1984 Dec 7;791(2):226–238. doi: 10.1016/0167-4838(84)90013-x. [DOI] [PubMed] [Google Scholar]
- Setaro F., Morley C. G. A modified fluorometric method for the determination of microgram quantities of DNA from cell or tissue cultures. Anal Biochem. 1976 Mar;71(1):313–317. doi: 10.1016/0003-2697(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Sueishi K., Benson B. J. Isolation of a major apolipoprotein of canine and murine pulmonary surfactant. Biochemical and immunochemical characteristics. Biochim Biophys Acta. 1981 Sep 24;665(3):442–453. doi: 10.1016/0005-2760(81)90257-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. T., Damm D., Miller J., Spratt K., Schilling J., Hawgood S., Benson B., Cordell B. Isolation and characterization of the human pulmonary surfactant apoprotein gene. 1985 Sep 26-Oct 2Nature. 317(6035):361–363. doi: 10.1038/317361a0. [DOI] [PubMed] [Google Scholar]
- Whitsett J. A., Hull W., Ross G., Weaver T. Characteristics of human surfactant-associated glycoproteins A. Pediatr Res. 1985 May;19(5):501–508. doi: 10.1203/00006450-198505000-00018. [DOI] [PubMed] [Google Scholar]
- Wright J. R., Benson B. J., Williams M. C., Goerke J., Clements J. A. Protein composition of rabbit alveolar surfactant subfractions. Biochim Biophys Acta. 1984 Dec 21;791(3):320–332. doi: 10.1016/0167-4838(84)90343-1. [DOI] [PubMed] [Google Scholar]