Abstract
Human beings constantly engage in attributing causal explanations to one’s own and to others’ actions, and theory-of-mind (ToM) is critical in making such inferences. Although children learn causal attribution early in development, children with autism spectrum disorders (ASDs) are known to have impairments in the development of intentional causality. This functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) study investigated the neural correlates of physical and intentional causal attribution in people with ASDs. In the fMRI scanner, 15 adolescents and adults with ASDs and 15 age- and IQ-matched typically developing peers made causal judgments about comic strips presented randomly in an event-related design. All participants showed robust activation in bilateral posterior superior temporal sulcus at the temporo-parietal junction (TPJ) in response to intentional causality. Participants with ASDs showed lower activation in TPJ, right inferior frontal gyrus and left premotor cortex. Significantly weaker functional connectivity was also found in the ASD group between TPJ and motor areas during intentional causality. DTI data revealed significantly reduced fractional anisotropy in ASD participants in white matter underlying the temporal lobe. In addition to underscoring the role of TPJ in ToM, this study found an interaction between motor simulation and mentalizing systems in intentional causal attribution and its possible discord in autism.
Keywords: functional MRI, theory-of-mind, intentional causality, physical causality, causal attribution, diffusion tensor imaging, fractional anisotropy, functional connectivity, autism
Human beings are adept at making inferences about other minds through social cues such as facial expressions, gestures and body posture. Such inferences are our own theories about what goes on in others’ minds. Thus, theory-of-mind (ToM) is the ability to attribute mental states to oneself and to others (Premack and Woodruff, 1978). ToM helps us successfully navigate the interpersonal world by making common sense explanations of behavior. Deficits in ToM may hamper social interaction and may play a key role in effecting abnormal social behaviors in people with autism spectrum disorders (ASDs) (Baron-Cohen, Leslie and Frith, 1985; Perner et al., 1989; Reed and Peterson, 1990; Leekam and Perner, 1991; Baron-Cohen, 1995; Baron-Cohen et al., 1996; Swettenham et al., 1996; Williams et al., 2001; Senju et al., 2009). Behavioral studies have illustrated that individuals with autism are characterized by long developmental delays in acquiring mentalizing skills (Happe, 1994; Kiln, 2000; Baron-Cohen et al., 2001; Roeyers et al., 2001) and that their ToM impairment is independent of task complexity or lower overall abilities (Perner et al., 1989; Sodian and Frith, 1992; Leslie and Thaiss, 1992).
Understanding causal relations between events may be vital in interpreting the physical as well as the interpersonal world. While the former is mediated by laws of physics, the latter by social rules. In other words, physical causal attributions are based on folk physics, and intentional causal attributions are based on folk psychology. Behavioral studies of mechanical and intentional causal explanations of events suggest individuals with ASDs may have a relatively intact or superior development in the understanding of physical causality, but may lag behind in understanding intentional causality (Baron-Cohen et al., 1986; Baron-Cohen, 1995; Jolliffe and Baron-Cohen, 1997; Frith, 2003). They have enhanced reasoning abilities about physical events and prefer to use physical causality when reasoning about events (Binnie and Williams, 2003). In sum, evidence from behavioral studies points to a dichotomy between physical and intentional causal attribution ability in individuals with ASDs.
Although causal attribution has been studied widely using behavioral measures, its neural bases are relatively under-examined in ASD. In a positron emission tomography study using comic strip vignettes, Brunet et al. (2000) found that typically developing individuals activated right middle, medial and inferior prefrontal, and middle and superior temporal areas during intentional causal attribution. This stimuli set has been used in a few other studies finding activation in posterior cingulate cortex for representing intentions (Walter et al., 2004), in medial prefrontal cortex (MPFC), temporo-parietal junction (TPJ) and the temporal poles for empathy and ToM tasks (Vollm et al., 2006) and in TPJ, precuneus and anterior paracingulate cortex for intentions (Ciaramidaro et al., 2007). These findings underscore the role of frontal, medial and temporo-parietal structures in intentional causal attribution. While several brain areas have been implicated in processing ToM, MPFC and the posterior superior temporal sulcus (pSTS) at the TPJ have received more attention than any other (Fletcher et al., 1995; Brunet et al., 2000; Castelli et al., 2000; Gallagher et al., 2000; Vogeley et al., 2001; Castelli et al., 2002; Ruby and Decety, 2003; Saxe and Kanwisher, 2003; den Ouden et al., 2005; Kana et al., 2009). However, the specific role of these regions in ToM processing is a topic of debate. One proposal suggested that TPJ helps provide the cues for mentalizing, and the MPFC is involved in reasoning about the mental states (Gallagher and Frith, 2003). In contrast, Saxe and Kanwisher (2003) argued that the TPJ assembles the ToM cues and processes them, with no necessary role for the MPFC. This view is also supported by a case study involving extensive damage to the MPFC with no obvious deficits in ToM (Bird et al., 2004). In ASD, decreased response in MPFC and TPJ, as well as weaker connectivity between them, has been reported by previous studies (Castelli et al., 2002; Kana et al., 2009; Lombardo et al., 2011). If MPFC and TPJ can be conceptualized as the nodes of a ToM system, then that system may be functionally disrupted or altered in autism. This study aims to examine the role of these nodes and their integration in intentional attribution in ASD.
A different perspective on the cognitive and neural mechanism that mediates ToM pertains to a process of simulation (Gallese and Goldman, 1998; Goldman, 1998; Avikainen et al., 2002; Gazzola et al.,2006, 2007). This view, the simulation theory of mindreading, suggests that others’ actions are understood by ‘putting ourselves in their shoes’. At the neural level, this may be accomplished by a mirror mechanism, through activation of the ventral premotor cortex (PMv), specifically at the inferior frontal gyrus (IFG) and the inferior parietal lobule (IPL) (Hari et al., 1998; Rizzolatti and Fadiga, 1998; Gallese et al., 2004; Gazzola et al.,2006, 2007; Grafton and Hamilton, 2007). After an action is simulated in the mirror neuron system (MNS), the information is then passed along to the core ToM regions (e.g. MPFC, TPJ) for making appropriate inference about the intention behind the action (Keysers and Gazzola, 2007; Uddin et al., 2007; de Lange et al., 2008; Rizzolatti and Sinigaglia, 2008). The simulation view of ToM may also explain the deficits in mindreading in ASD from two aspects: (i) behavioral evidence of imitation deficits in ASD (Rogers, 1999; Williams et al., 2001) and (ii) anatomical and functional abnormalities associated with the MNS in ASD (Dapretto et al., 2006; Hadjikhani et al., 2006; Williams et al., 2006; Fletcher et al., 2010). It should also be noted, however, that there is some evidence suggesting a robust MNS function in ASD (Dinstein et al., 2010; Marsh and Hamilton, 2011). Therefore, a second aim of our study is to investigate the role of MNS in causal attribution and the integrity of the MNS in individuals with ASDs.
This study examined causal attribution in ASD using functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI). We investigated the functional and anatomical integrity (brain activation, functional connectivity and white matter integrity) of the MPFC–TPJ system (ToM system) as well as the IPL–IFG/vPMC simulation system (MNS). Using this approach, we aim to characterize the neural circuitry underlying ToM in general and its role in ASD. Considering previous findings of the role of TPJ in mentalizing tasks, we predict TPJ to be the primary locus of brain response to ToM. In addition, we hypothesize that the MNS may play a critical role in this task, and participants with ASD may exhibit altered connectivity among the nodes of ToM and MNS networks. As this study combines fMRI and DTI evidence of cortical connectivity in ASD, it provides a unique and novel opportunity to examine connectivity at functional and anatomical levels.
MATERIALS AND METHODS
Participants
Fifteen adolescents and young adults with high-functioning ASD (mean age: 21.14 years) and 15 age- and IQ-matched individuals with typical development (TD) (mean age: 22.18 years) participated in this fMRI study (see Table 1 for demographic information). There were 4 participants in our ASD group who were younger than 18 years and 11 participants who were 19 years and older. The TD group had 3 participants who were 18 years and younger and 12 participants who were 19 years and older. All participants were required to have an IQ of 80 or above measured by the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). The participants with ASD were recruited from the University of Alabama ASD Clinic and surrounding service providers. They had received a previous diagnosis of an ASD based on Autism Diagnostic Interview (ADI-R; Lord et al., 1994) symptoms and Autism Diagnostic Observation Schedule (Lord et al., 2000). Eight of the 15 ASD participants in this study had received a diagnosis of Asperger’s disorder. As ADI-R diagnoses are based on symptoms across the lifetime, current ASD symptoms were assessed using the Social Responsiveness Scale (SRS; Constantino, 2002). For the ASD group, the average SRS score was 80.5. As some participants were older than 18 years (the SRS is normed for children from 4 to 18 years of age), raw scores rather than t scores were reported. Nevertheless, the average raw score of this sample was consistent with a t-score of 65, which is within the mild to moderate range of autism symptom severity. The TD participants were recruited through newspaper advertisements and through the University of Alabama at Birmingham’s Psychology 101 course subject pool. They were screened through a parent-report (for participants younger than 18 years) or self-report history questionnaire to rule out neurological disorders, such as ASD, ADHD or Tourette’s disorder that could potentially confound the results.
Table 1.
Demographic information
| Autism |
Control |
Group difference |
||||||
|---|---|---|---|---|---|---|---|---|
|
N = 15 |
N = 15 |
|||||||
| Mean | Range | s.d. | Mean | Range | s.d. | t-value | P-value | |
| Age | 21.14 | 16–29 | 0.99 | 22.28 | 16–34 | 1.08 | 0.77 | 0.44 |
| Verbal IQ | 104.80 | 74–139 | 5.02 | 113.93 | 102–127 | 2.20 | 1.66 | 0.11 |
| Performance IQ | 107.70 | 73–129 | 4.33 | 107.20 | 89–124 | 2.48 | 0.11 | 0.92 |
| Full-scale IQ | 106.93 | 80–140 | 4.84 | 112.00 | 96–128 | 2.24 | 0.94 | 0.35 |
| Mind in the eyes | 19.07 | 15–24 | 0.70 | 21.60 | 18–24 | 0.55 | 2.84 | 0.01 |
| SRS raw total | 80.53 | 25–128 | 9.88 | |||||
Experimental paradigm
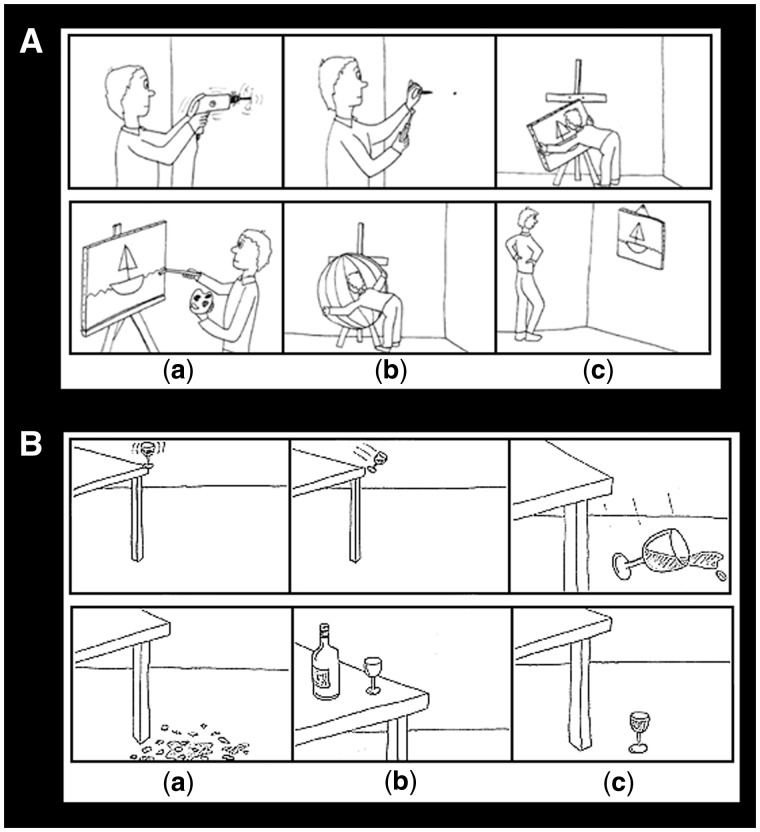
The stimuli consisted of a series of black and white comic strip vignettes (adapted from Brunet et al., 2000) depicting scenarios that demand either a physical causal attribution or an intentional causal attribution (see Figure 1 for a sample vignette from each condition) to arrive at a logical ending. The first part of the vignette was presented for 5 s and the participants’ task was to choose a logical ending to the story from the three choices in the second panel presented for 6 s. The entire vignette remained on the screen for a total of 11 s. The experiment was designed in an event-related format through the stimulus presentation software E-prime 1.2 (Psychology Software Tools, Pittsburgh, PA, USA), which recorded the reaction time (RT) and the performance accuracy data from participants. An Integrated Functional Imaging System (Invivo Corporation, Orlando, FL, USA) interface was used to present the visual stimuli onto a screen behind the participant while in the scanner. Participants made their responses on a fiber optic button response system and the participants indicated the correct answer choice (A, B or C) by a button press. Participants viewed a total of 11 physical cartoons and 11 intention cartoons. There were also five epochs of fixation baseline lasting 24 s each. Experimental trials were presented in random order (determined by using research randomizer) with fixation epochs dispersed equally across time. Before fMRI scan, each participant practiced the task on a laptop computer. The cartoon items used in the practice were different from that used in the MRI scanner.
Fig. 1.
A sample stimulus item from each experimental condition. (A) Top panel: intentional causality vignette; bottom panel: answer choices with (c) being the correct answer. (B) Top panel: physical causality vignette; bottom panel: answer choices with (a) being the correct answer.
Imaging parameters
Functional and structural MRI data were collected at the UAB Civitan International Research Center using a Siemens 3.0 Tesla Allegra head-only scanner (Siemens Medical Inc., Erlangen, Germany). For structural imaging, initial high-resolution T1-weighted scans were acquired using a 160-slice 3D MPRAGE volume scan with TR = 200 ms, TE = 3.34 ms, flip angle = 7°, field of view (FOV) = 25.6 cm, 256 × 256 matrix size and 1 mm slice thickness. For functional imaging, a single-shot, gradient-recalled, echo-planar pulse sequence was used for rapid image acquisition (TR = 1000 ms, TE = 30 ms, flip angle = 60°). Seventeen adjacent oblique axial slices were acquired in an interleaved sequence with 5 mm slice thickness, 1 mm slice gap, a 24 × 24 cm FOV and a 64 × 64 matrix, resulting in an in-plane resolution of 3.75 × 3.75 × 5 mm. Diffusion tensor images were also obtained for 21 of the 30 participants (8 ASD and 13 typically developing). Due to motion artifacts, and due to some participants declining to remain in the MRI scanner additional time for the DTI portion of the scan, we were not able to acquire DTI for all participants included in the fMRI analyses. The images were collected using a single-shot, spin-echo, echo-planar imaging (EPI) sequence. A diffusion-weighted, single-shot, spin-echo, EPI sequence was used (TR = 4400 ms, TE = 85 ms, bandwidth = 1860 Hz/voxel, FOV = 240 mm and matrix size = 128 × 128, resulting in an in-plane resolution of 1.87 × 1.87 × 3 mm). Thirty-two 3 mm thick slices were imaged (no slice gap) with no diffusion weighting (b = 0 s/mm2) and with diffusion weighting (b = 1000 s/mm2) gradients applied in 12 orthogonal directions. Twenty-four images of each slice by gradient direction combination were acquired and averaged to produce the final diffusion imaging data set for each participant.
Data analyses
Distribution of activation
The brain activation data were analyzed using Statistical Parametric Mapping (SPM8) software (Wellcome Department of Cognitive Neurology, London, UK). Images were corrected for slice acquisition timing, motion-corrected, normalized to the Montreal Neurological Institute (MNI) template, resampled to 2 × 2 × 2 mm voxels and smoothed with an 8 mm Gaussian kernel to decrease spatial noise. We performed statistical analysis on individual and group data using SPM8’s implementation of the general linear model (Friston et al., 1995). Within-group activation was analyzed for the ASD group, TD group and the whole group (ASD + TD) of participants. Activation was examined by modeling the 16 s following the onset of each cartoon to allow enough time for the hemodynamic response to rise and fall. Activation data were analyzed for all trials with separate regressors defined for intentional causality, physical causality and fixation baseline conditions. The within-group analyses used a cluster size of 80 mm3 determined by 10 000 Monte Carlo simulations at an uncorrected P-value of 0.001. According to Lieberman and Cunningham (2009), simulations can implicate cluster size thresholds that produce the best balance between Type I and Type II error. The between-group analyses used a cluster threshold of 40 contiguous voxels at an uncorrected P-value of 0.001.
Functional connectivity
Functional connectivity (the synchronization of brain activation across brain areas) was computed (separately for each participant) by correlating the average time course of signal intensity of all the activated voxels extracted from functionally defined regions of interest (ROIs). These ROIs were defined on the group activation map for the whole group (ASD + TD) for the contrast intention + physical vs fixation, so that it best represents the study. The functional connectivity described in this study is task-based, and the analysis was conducted using an in-house script and followed the method used in our previous studies (see Just et al., 2007; Kana et al., 2009). Because head motion can impact functional connectivity analyses (Satterthwaite et al., 2012; Van Dijk et al., 2012), we had a conservative threshold of 0.5 mm for head motion in any direction. In addition, we computed the mean head motion for each subject in each direction (measured as translation and rotation in millimeters for each brain volume in the x, y, z planes for the duration of the run) and used these values to compare head motion for the ASD and TD groups. A repeated measures analysis of variance (ANOVA) revealed that the two groups did not differ significantly on head motion [F(2,28) = 0.016, P = 0.901].
Eighteen ROIs were identified: supplementary motor area (SMA), left and right IFG (LIFG and RIFG), left and right PMv (LPMv and RPMv), left and right middle temporal gyrus (LMTG and RMTG), right superior temporal gyrus (RSTG), left and right IPL (LIPL and RIPL), left and right fusiform gyrus (LFFG and RFFG), left and right superior parietal lobule (LSPL and RSPL), left and right middle occipital gyrus (LMOG and RMOG) and left and right temporal parietal junction (LTPJ and RTPJ). A sphere was defined for each cluster (with a radius ranging from 8 to 12 mm; see Supplementary Table S1 for ROI coordinates) that best captured the cluster of activation in the contrast map for each group. The activation time course extracted for each participant over the activated voxels within the ROI originated from the normalized and smoothed images that were low-pass filtered and had the linear trend removed. After extracting the time course for the entire task, it was separated into each experimental condition. Correlation coefficients were calculated across the time courses from different ROIs. A Fisher’s r to z transformation was applied to the correlation coefficients for each participant before averaging and conducting the statistical comparison of conditions and groups using paired samples t-tests (without correction for multiple comparisons). After the preliminary analysis of connectivity among individual ROIs, five networks were constructed out of these ROIs to test network connectivity. These networks included motor (LPMv, RPMv, SMA), TPJ (LTPJ, RTPJ), right temporal (RMTG, RSTG), occipital (LMOG, RMOG), left parietal (LIPL, LSPL) and right parietal (RIPL, RSPL). The five networks for connectivity network analysis were primarily lobe/hemisphere-based. However, we called the frontal lobe regions (SMA, IFG and PMv) as motor network as it may be mediating motor simulation in this task. Thus, the networks include a motor network (centered in the frontal lobe) and other networks in temporal, occipital and parietal lobes.
DTI analysis
The diffusion tensor images were analyzed using fMRIB Software Library (FSL). Preprocessing steps included skull stripping and eddy current correction. Transformation and intensity corrections from eddy current distortions were computed following the procedures outlined by Rohde et al. (2004). Diffusion tensors and fractional anisotropy (FA) values were then calculated by FSL’s Diffusion Toolbox. A voxel-wise analysis was completed in which FA values were compared between the ASD and TD participants to identify group differences. This analysis had no a priori assumptions and examined the white matter over the entire brain. The voxel-wise comparison was completed using tract-based spatial statistics (TBSS), which first aligned each participant’s diffusion image with the FMRIB58 FA template image using non-linear registration. A mean FA image was then created based on all participants’ scans including ASD and TD groups and a skeleton was produced from this image identifying all the major white matter tracts throughout the brain. After each realigned FA image is projected onto the FA skeleton, the voxels along this skeleton were compared between the two groups using unpaired t-tests to identify voxels in which FA values are significantly reduced. The mean FA values calculated for each region were also compared to the functional connectivity results.
RESULTS
Brain activation
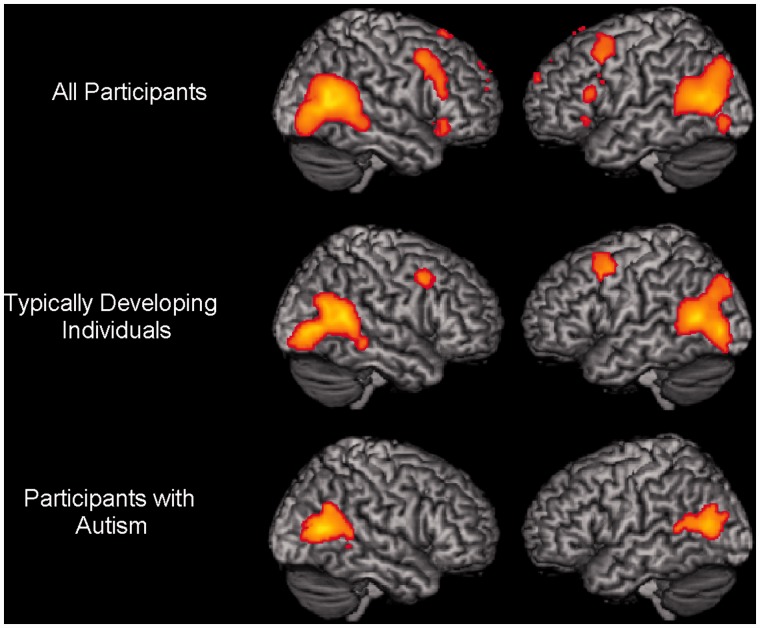
Direct contrast of intentional causality with physical causality (intention > physical) included separate within-group analysis (one-sample t-test) of the activation for the TD group, the ASD group and for all participants grouped together (whole group: ASD + TD). The results showed bilateral posterior STS at the TPJ as the primary center of significantly increased activation in all three groups (Figure 2; Supplementary Table S2A). This analysis also revealed a few other important results: the ASD group had a smaller cluster size (1488 voxels), relative to the TD group (2197 voxels), in the right pSTS/TPJ region. In addition to the pSTS/TPJ activation, the TD participants also recruited the precuneus, the ACC/SMA and bilateral ventral premotor cortex, especially the right IFG during intentional causal attribution, a pattern absent in participants with ASD. In the reverse contrast (physical > intention), we found bilateral activation of the IPL, the postcentral gyrus and the anterior part of STG in both TD and ASD groups (P < 0.001 uncorrected; k = 80 voxels) (see Supplementary Table S2B for a detailed list of activated areas).
Fig. 2.
Within-group brain activation patterns for the contrast intentional causality > physical causality in three different groups. Recruitment of posterior superior temporal sulci and TPJ in all participant groups. In addition, while the whole group and control participants recruited ventral premotor regions, it is missing in the autism group (P < 0.001 uncorrected; k = 80 voxels).
For between-group comparisons, the participants with ASD showed reduced activation, relative to TD participants, in bilateral IPL/angular gyrus, the RIFG, cuneus and the LPMv while making intentional causal attributions (intentional causality > physical causality) (P < 0.001 uncorrected for multiple comparisons; k = 40 voxels; Table 2). As our fMRI task involves visual evaluation of detailed cartoon strips, it is possible that participants with ASDs were biased by a local processing strategy widely reported in this population (Wang et al., 2007), focusing on smaller details of the cartoons rather than the meaningful whole. To examine this further, we conducted a simple regression using performance IQ measure as a covariate to determine activation in intentional causality >physical causality contrast in the ASD group. This analysis revealed no statistically significant clusters of activation in any region. This result suggests that our ASD participants’ assessment of the comic strip vignettes was not influenced by a local processing bias. Other regression analyses using Autism-Spectrum Quotient, ADI, SRS and FA values as covariates with our fMRI data did not yield any statistically significant findings.
Table 2.
Peak activation and cluster size for the contrast intentional vs physical for control > autism (P < 0.001 uncorrected for multiple comparisons; k = 40 voxels)
| Region | x | y | z | Cluster | t-value | P-value |
|---|---|---|---|---|---|---|
| Right angular gyrus | 32 | −52 | 42 | 90 | 3.76 | 0.000 |
| Right inferior frontal triangularis | 54 | 38 | 2 | 35 | 3.69 | 0.000 |
| Right cuneus | 22 | −76 | 46 | 83 | 3.46 | 0.001 |
| Left inferior parietal | −30 | −70 | 46 | 54 | 3.45 | 0.001 |
Functional connectivity
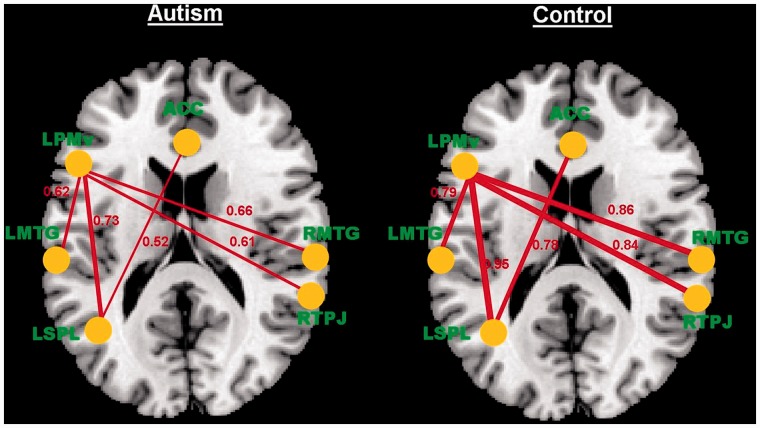
Functional connectivity analysis revealed significantly weaker connectivity in participants with ASD, relative to TD participants, in ToM-related areas and ventral premotor areas [LMTG:LPMv, t(28) = 2.158, P = 0.04; LPMv:LSPL, t(28) = 2.150, P = 0.04; LPMv:RMTG, t(28) = 2.109, P = 0.04; LPMv:RTPJ, t(28) = 2.313, P = 0.02; SMA:LSPL, t(28) = 2.578, P = 0.01] during intentional causal attribution (Figure 3). Most of these connections are between the ventral premotor cortex and temporal–parietal regions. In contrast, the ASD group showed stronger connectivity than the TD group only in the physical causality task. These connections include the following: LFFG:LMTG, t(28) = 2.64, P = 0.01; LFFG:RTPJ, t(28) = 2.11, P = 0.04; LMOG:LMTG, t(28) = 2.17, P = 0.03; LMTG:RFFG, t(28) = 3.17, P = 0.004 and LPMv:LSPL, t(28) = 2.30, P = 0.02. It should be noted that stronger connectivity in the ASD group was mostly between relatively posterior and more spatially proximal ROIs.
Fig. 3.
Significantly weaker functional connectivity in participants with autism, relative to controls, in ToM-related areas and ventral premotor areas during intentional causal attribution.
As this analysis included several comparisons, it was followed up with a functional connectivity network analysis (based on the networks mentioned in the ‘Materials and methods’ section) to further examine the validity of our results. This analysis showed a significant difference between ASD and TD groups in connectivity between the ventral premotor and TPJ networks [t(28) = 2.11, P = 0.04], with the ASD group having significantly weaker functional connectivity than the TD group.
DTI results
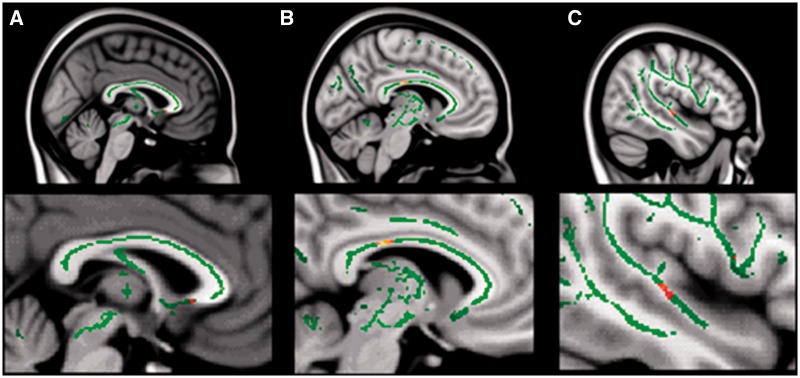
Voxel-wise analysis of the DTI data using TBSS identified three regions of significantly reduced FA values in our participants with ASD: the posterior midbody of the corpus callosum, the corona radiata and the white matter underlying the right middle/superior temporal lobe (Figure 4). After correcting for multiple comparisons, the only region that survived the correction was the white matter underlying the right temporal cortex. The mean FA in this area was significantly reduced in the ASD group when compared with the TD group [t(18) = 3.65, P = 0.0011]. It should be noted that the right temporal cortex was also the primary focus of our fMRI findings in this study. However, the mean FA of this region when correlated with the functional connectivity measures from the fMRI task did not yield any significant relationship.
Fig. 4.
Reduced FA in autism, relative to control participants, in three white matter regions: (A) rostrum of corpus callosum, (B) posterior midbody of corpus callosum and (C) temporal lobe. Green lines represent the white matter template created from all subjects using TBSS, and the red spots indicate the areas of significant reduction in FA in autism.
Behavioral data
To assess possible differences in performance accuracy and RT between the ASD and TD groups while making causal attribution, we conducted a 2 Group (ASD vs control) × 2 Condition (physical causality vs intentional causality) mixed ANOVA. This analysis showed a significant difference in accuracy rates between the conditions, F(1,28) = 68.1, P < 0.001, with all participants showing greater accuracy in physical than in intentional causal attribution. In addition, there was a significant difference between the ASD group (physical: mean = 92%, s.d. = 11%; intentional: mean = 61%, s.d. = 17%) and the TD group (physical: mean = 97%, s.d. = 5%; intentional: mean = 76%, s.d. = 18%) between conditions, F(1,28) = 5.57, P < 0.05, such that the ASD group made significantly more errors than the TD group while making intentional causal attribution. There was also a significant difference in RT between the two conditions, F(1,28) = 99.9, P < 0.001, with all participants being relatively quicker in responding to the physical cartoons as compared to the intentional cartoons. However, there was no statistically significant difference in RT between the TD group (physical: mean = 2862 ms, s.d. = 513; intentional: mean = 3894 ms, s.d. = 763) and the ASD group (physical: mean = 2902 ms, s.d. = 562; intentional: mean = 4009, s.d. = 650), F(1,28) = 0.14, P = 0.70.
DISCUSSION
The most pronounced effect in this study pertained to a significantly weaker functional connectivity between the ventral premotor cortex and the ToM network in participants with ASD, relative to TD participants, while engaged in intentional causal attribution. Brain activation results revealed that all participants recruited the pSTS/TPJ and the precuneus when making intentional attribution, while the TD participants additionally activated bilateral ACC, motor cortex and RIFG. Furthermore, DTI data collected from a subset of participants showed significantly reduced white matter integrity in ASD in the right middle/superior temporal cortex. Next, we discuss some of the main themes emerging from the results of this study.
The role of pSTS at the TPJ in ToM
Our finding of robust activation in pSTS at the TPJ in all three groups (ASD, TD and the whole group) during intentional causal attribution is consistent with the role of this region in tasks of ToM (Castelli et al., 2002; Saxe and Kanwisher, 2003; Saxe and Wexler, 2005; Perner et al., 2006; Saxe and Powell, 2006; Ciaramidaro et al., 2007; Gobbini et al., 2007; Kana et al., 2009; Young et al., 2010; Lombardo et al., 2011). In addition to its role in ToM, the TPJ has also been associated with tasks that involve perspective-taking (Ruby and Decety, 2003), empathy (Jackson et al., 2006; Lamm et al., 2007) and self-processing (Blanke and Arzy, 2005). Blanke et al. (2005) suggested that the TPJ is a necessary structure for conscious experience of the self and a necessary facet of the ability to mentalize about others. Therefore, this region may be vital in processing other’s beliefs and intentions, especially in relation to oneself.
In the context of the modular theory of ToM, along with evidence from this study and other studies of ToM, the pSTS/TPJ may be the primary locus (module) for social attribution. When comparing the MNI coordinates of pSTS/TPJ in our study (62, −52, 16 for TD group, 54, −64, 12 for ASD group, 50, −62, 18 for the whole group) to those of others, our activation peaks were found to be similar to that in previous neuroimaging studies of ToM. The centroid of coordinates from eight previous studies of ToM was 58, −52, 22 (Castelli et al., 2002; Saxe and Kanwisher, 2003; Saxe and Wexler, 2005; Saxe and Powell, 2006; Mitchell, 2008; Kana et al., 2009; Scholz et al., 2009; Young et al., 2010). Although both TD and ASD participants in this study showed activation in pSTS/TPJ, their activation peaks differed slightly from each other (62, −52, 16 for TD group and 54, −64, 12 for ASD group). This difference in the location of activation for the TD and ASD groups is in line with the slight difference in the location of coordinates from a previous study on ToM in ASD (60, −40, 18 for TD group and 54, −46, 20 for ASD group; Kana et al., 2009).
Unlike some previous studies of ToM (Castelli et al., 2002; Frith and Frith, 2006; Kana et al., 2009), this study did not find a significant increase in activation or a group difference in activation in MPFC. This may be because of a few reasons: (i) by using cartoon stimuli, Bara et al. (2011) have found that the MPFC activates for communicative intentions (intentions involving a social partner), but not for private intentions (intentions involving a single character). In the original study by Brunet et al. (2000), although MPFC activation was found for the intention, their study utilized cartoons involving one or more characters. This study was restricted to cartoon strips depicting private intentions only, therefore, our absence of MPFC activation in TD participants is in line with the study by Bara and colleagues. (ii) MPFC may be activated in a large range of tasks, especially the ones that involve self-other reflections; so, it is possible that the MPFC is more generally involved in representing the self and self-monitoring (Uddin et al., 2007). (iii) It is possible that the participants in this study may be relying on a strategy focused on motor resonance and a mirror mechanism, perhaps pointing to a different neural route than that based on MPFC.
Involvement of a mirror mechanism in ToM
The ventral premotor activation and its connectivity with the TPJ during intentional causal attribution in this study may suggest a possible mirror mechanism mediating this process in TD participants (Gallese and Goldman, 1998; Avikainen et al., 2002; Grezes et al., 2004; Gazzola et al., 2006, 2007), perhaps in line with the simulation theory of mindreading (Goldman, 1998). The underlying neural mechanism for this mirroring process is most likely the MNS (Rizzolatti and Craighero, 2004; Agnew et al., 2007). The MNS, consisting of the IPL and the IFG, may thus be the network capable of processing information about the self and others to accomplish ToM. In particular, the IFG has been linked to filtering socially relevant stimuli (Oberman et al., 2007) and may be modulated by underlying intentions of others’ actions (Iacoboni et al., 2005). The MNS and the pSTS/TPJ response in TD participants for intentional causal attribution is in agreement with previous research, suggesting these networks may function as a team to determine others’ mental states (Keysers and Gazzola, 2007; Uddin et al., 2007; de Lange et al., 2008; Spunt and Lieberman, 2012). The participants with ASD in our study showed lower levels of activation, relative to TD participants, in IFG and IPL during intentional causal attribution. The finding that our participants with ASD recruited these regions to a lesser extent may be suggestive of their relatively less reliance on simulation in intentional causal attribution.
Underconnectivity between MNS and ToM systems in ASD
Significantly weaker functional connectivity was observed between ventral premotor and temporo-parietal ROIs in participants with ASD, relative to TD participants. This pattern was also reflected in the connections of ToM (pSTS and TPJ) and motor (SMA, precentral gyrus and IFG) networks in our participants with ASD. Although the finding of underconnectivity is in line with previous findings of lower functional connectivity between frontal and posterior brain regions in individuals with ASD (Just et al., 2004; Koshino et al., 2005; Villalobos et al., 2005; Kana et al., 2006; Just et al., 2007), the novel aspect of this study is that it provides evidence for the role of simulation in mentalizing. In addition, our findings are also supportive of the idea that information about intentionality may be processed by both the MNS and ToM regions (de Lange et al., 2008). Reduced connectivity between these networks may help explain why our participants with ASD had difficulty in processing the ToM-related information. Altered long distance connectivity may hinder the ASD participants from communicating between motor and ToM regions to complete the intentional task in the same manner as TD participants. To compensate for, they may have used a strategy similar to the one they used for physical causal attribution, but with less success. In participants with ASD, the frontal areas did not seem to coordinate with temporo-parietal regions to the same extent as the TD participants during intentional causal attribution. This provides additional evidence for the role of PMv/IFG (mirror mechanism) working with temporo-parietal (TPJ) areas for processing intentionality and its altered functioning in ASD.
Anatomical bases of underconnectivity in ASD
Of late, functional underconnectivity has been widely reported in people with ASD (see Kana and Just, 2011; Kana et al., 2011; Schipul et al., 2011 for reviews). However, its relation to anatomy is relatively less explored. The DTI data from a subset of participants in this study provided some evidence to the white matter abnormalities underlying the temporal cortex in participants with ASD. We found significantly reduced FA in the white matter underlying middle/superior temporal cortex in ASD. This is noteworthy given that pSTS/TPJ was consistently featured in our activation and functional connectivity results for the intention task. Such alterations in the white matter specialization as well as integration of this temporal lobe perhaps explaining why participants with ASD showed reduced functional connectivity between superior temporal cortex and other ROIs. This is also consistent with findings from previous DTI studies that showed decreased FA values bilaterally in the STS (Lee et al., 2007) and lower FA in STS, STG, TPJ and MTG (Barnea-Goraly et al., 2004) in ASD. It should also be noted that Fletcher et al. (2010) found white matter integrity of the arcuate fasciculus (AF) to be affected in ASD. The AF has projections to the STG, IFG and IPL, thus holding great importance for processing ToM which relies on these brain areas.
It should be noted that the area identified in the DTI analysis (with significantly lower FA) does not completely overlap the pSTS/TPJ area featured in the functional connectivity analysis in our study. Nevertheless, the FA may represent a white matter fiber bundle that spans across these regions. There is likely still a relationship between our anatomical finding and the functional abnormalities seen in ASD. The inferior longitudinal fasciculus (ILF) is the major white matter tract underlying the temporal lobe and stretches laterally across the temporal lobe and into visual areas (Catani et al., 2003; Wakana et al., 2004). It is possible that the reduced FA found in our DTI analysis may represent an aberrant projection of the ILF. This type of disconnect would certainly have an effect not only on the portions of temporal lobe stretching anterior to the ROI but also on the brain areas farther upstream (such as the pSTS we found in our fMRI results) and the ability to process information from the visual stream. It is also possible to have differences in the FA values in some white matter tracks without a corresponding difference in functional connectivity and vice versa. Although we did not find a correlation between white matter abnormalities and functional connectivity in either group, it may not be a coincidence that the alterations in functional and anatomical connectivity found in our study were centered in the temporal lobe. There could possibly be a problem with the connectivity, both functional and anatomical, in the temporal lobe as a whole. Future studies should further examine this relationship. Although the main strength of our study is in combining evidence at functional, white matter and connectional levels, it should be noted that the DTI sample size in our study, at least in one group, is relatively small. A larger sample could potentially allow for the detection of greater differences between groups within the white matter tracts.
CONCLUSIONS
This study provides a multilevel analysis of the alterations in connectivity in ASD during social cognition, with converging evidence from behavioral data, brain activation data and functional and anatomical connectivity measures. In addition to providing further evidence for the role of TPJ in ToM, this study finds preliminary evidence for a mirror mechanism mediating social attribution and a possible underfunctioning of it in people with ASD. The functional underconnectivity found in participants with ASD between the mirroring and mentalizing systems may be vital in understanding the deficits in social cognition in autism at the neural level. The white matter anomaly seen in participants with autism, strikingly in the right temporal cortex, also adds another domain in characterizing the brain functioning in ASD.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN Online.
Acknowledgments
This research was supported by the McNulty-Civitan Scientist Award and the CCTS Pilot Grant (5UL1 RR025777) to R.K. The authors would like to thank Eric Brunet for generously providing us with his stimulus set of cartoon strip vignettes. The authors would also like to thank Laura Klinger, Heather Wadsworth, Brittany Travers, Christopher Klein, Kathy Pearson and Elizabeth Blum for their help with different aspects of this study.
REFERENCES
- Agnew ZK, Bhakoo KK, Puri BK. The human mirror system: a motor resonance theory of mind-reading. Brain Research Reviews. 2007;54:286–93. doi: 10.1016/j.brainresrev.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Avikainen S, Forss N, Hari R. Modulated activation of the human SI and SII cortices during observation of hand actions. Neuroimage. 2002;15:640–6. doi: 10.1006/nimg.2001.1029. [DOI] [PubMed] [Google Scholar]
- Bara B, Ciaramidaro A, Walter H, Adenzato M. Intentional minds: a philosophical analysis of intention tested through fMRI experiments involving people with schizophrenia, people with autism, and healthy individuals. Frontiers in Human Neuroscience. 2011;5:7. doi: 10.3389/fnhum.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004;55:323–6. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. Cambridge: MIT Press/Bradford Books; 1995. [Google Scholar]
- Baron-Cohen S, Cox A, Baird G, Swettenham J, Drew A, Nightingale N, Morgan K, Charman T. Psychological markers of autism at 18 months of age in a large population. British Journal of Psychiatry. 1996;168:158–63. doi: 10.1192/bjp.168.2.158. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Mechanical, behavioral and intentional understanding of picture stories in autistic children. British Journal of Developmental Psychology. 1986;4:113–25. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “reading the mind in the eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry. 2001;42:241–51. [PubMed] [Google Scholar]
- Binnie L, Williams J. Intuitive psychology and physics among children with autism and typically developing children. Autism. 2003;7:173–93. doi: 10.1177/1362361303007002005. [DOI] [PubMed] [Google Scholar]
- Bird CM, Castelli F, Malik O, Frith U, Husain M. The impact of extensive medial frontal lobe damage on ‘theory of mind’ and cognition. Brain. 2004;127:914–28. doi: 10.1093/brain/awh108. [DOI] [PubMed] [Google Scholar]
- Blanke O, Arzy S. The out-of-body experience: disturbed self-processing at the temporo-parietal junction. Neuroscientist. 2005;11:16–24. doi: 10.1177/1073858404270885. [DOI] [PubMed] [Google Scholar]
- Blanke O, Mohr C, Michel CM, et al. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. Journal of Neuroscience. 2005;25:550–7. doi: 10.1523/JNEUROSCI.2612-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet E, Sarfati Y, Hardy-Baylé MC, Decety J. A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11:157–66. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(Pt 8):1839–49. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happé F, Frith U, Frith CD. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–25. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Ciaramidaro A, Adenzato M, Enrici I, et al. The intentional network: how the brain reads varieties of intentions. Neuropsychologia. 2007;45:3105–13. doi: 10.1016/j.neuropsychologia.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Constantino JN. The Social Responsiveness Scale. Los Angeles: Western Psychological Services; 2002. [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Current Biology. 2008;18:454–7. doi: 10.1016/j.cub.2008.02.057. [DOI] [PubMed] [Google Scholar]
- den Ouden HEM, Frith U, Frith C, Blakemore SJ. Thinking about intentions. Neuroimage. 2005;28:787–96. doi: 10.1016/j.neuroimage.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Humphreys K, Minshew N, Behrmann M, Heeger DJ. Normal movement selectivity in autism. Neuron. 2010;66:416–69. doi: 10.1016/j.neuron.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Happé F, Frith U, et al. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, et al. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage. 2010;51:1117–25. doi: 10.1016/j.neuroimage.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–4. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Frith U. Autism: Explaining the Enigma. Cambridge: Blackwell Publishing; 2003. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical Parametric Maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of “theory of mind”. Trends in Cognitive Science. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of “theory of mind” in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mindreading. Trends in Cognitive Science. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Science. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Aziz-Zadeh L, Keysers C. Empathy and the somatotopic auditory mirror system in human. Current Biology. 2006;16:1824–9. doi: 10.1016/j.cub.2006.07.072. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Rizzolatti G, Wicker B, Keysers C. The anthropomorphic brain: the mirror neuron system responds to human and robotic actions. Neuroimage. 2007;35:1674–84. doi: 10.1016/j.neuroimage.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: a comparison of theory of mind tasks. Journal of Cognitive Neuroscience. 2007;19:1803–14. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- Goldman A. The mentalizing folk. In: Sperber D, editor. Metarepresentation. London: Oxford; 1998. pp. 171–96. [Google Scholar]
- Grafton ST, Hamilton A. Evidence for a distributed hierarchy of action representation in the brain. Human Movement Science. 2007;26:590–616. doi: 10.1016/j.humov.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grezes J, Frith C, Passingham RE. Brain mechanisms for inferring deceit in the actions of others. Journal of Neuroscience. 2004;24:5500–5. doi: 10.1523/JNEUROSCI.0219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cerebral Cortex. 2006;16:1276–82. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- Happe FGE. An advanced test of theory of mind: understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. Journal of Autism and Developmental Disorders. 1994;24:129–54. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G. Activation of human primary motor cortex during action observation: a neuromagnetic study; Proceedings of the National Academy of Sciences United States of America; 1998. pp. 15061–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szackacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLoS Biology. 2005;3:529–35. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–61. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Jolliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? Journal of Child Psychology and Psychiatry. 1997;38:527–34. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951–61. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Just MA. Autism as a disorder of functional brain connectivity. In: Amaral DG, Geschwind D, Dawson G, editors. Handbook of Autism Spectrum Disorders. New York: Oxford University Press; 2011. pp. 981–9. [Google Scholar]
- Kana RK, Libero LE, Moore MS. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Physics of Life Reviews. 2011;8:410–37. doi: 10.1016/j.plrev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–93. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Social Neuroscience. 2009;4:135–52. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends in Cognitive Science. 2007;11:194–6. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Kiln A. Attributing social meaning to ambiguous visual stimuli in higher-functioning autism and Asperger syndrome: the Social Attribution Task. Journal of Child Psychology and Psychiatry. 2000;41:831–46. [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–21. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neuroscience Letters. 2007;424:127–32. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Leekam S, Perner J. Does the autistic child have a metarepresentational deficit? Cognition. 1991;40:203–18. doi: 10.1016/0010-0277(91)90025-y. [DOI] [PubMed] [Google Scholar]
- Leslie AM, Thaiss L. Domain specificity in conceptual development: neuropsychological evidence from autism. Cognition. 1992;43:225–51. doi: 10.1016/0010-0277(92)90013-8. [DOI] [PubMed] [Google Scholar]
- Lieberman M, Cunningham W. Type I and Type II error concerns in fMRI research: re-balancing the scale. SCAN. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore E, MRC AIMS Consortium, Baron-Cohen S. Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. Neuroimage. 2011;56:1832–88. doi: 10.1016/j.neuroimage.2011.02.067. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communicative deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Marsh L, Hamilton A. Dissociation of mirroring and mentalising systems in autism. Neuroimage. 2011;56:1511–9. doi: 10.1016/j.neuroimage.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cerebral Cortex. 2008;18:262–71. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Pineda JA, Ramachandran VS. The human mirror neuron system: a link between action observation and social skills. Social Cognitive and Affective Neuroscience. 2007;2:62–6. doi: 10.1093/scan/nsl022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perner J, Aichhorn M, Kronbichler M, Staffen W, Ladurner G. Thinking of mental and other representations: the roles of left and right temporo-parietal junction. Social Neuroscience. 2006;1:245–58. doi: 10.1080/17470910600989896. [DOI] [PubMed] [Google Scholar]
- Perner J, Frith U, Leslie AM, Leekam SR. Exploration of the autistic child’s theory of mind: knowledge, belief, and communication. Child Development. 1989;60:688–700. [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behavioral and Brain Science. 1978;1:515–26. [Google Scholar]
- Reed T, Peterson C. A comparative study of autistic subjects’ performance at two levels of visual and cognitive perspective taking. Journal of Autism and Developmental Disorders. 1990;20:555–68. doi: 10.1007/BF02216060. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L. Grasping objects and grasping action meanings: the dual role of monkey rostroventral premotor cortex (area F5) Novartis Foundation Symposium. 1998;218:81–95. doi: 10.1002/9780470515563.ch6. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. Mirrors in the Brain: How Our Minds Share Actions and Emotions. Oxford: Oxford University Press; 2008. [Google Scholar]
- Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magnetic Resonance in Medicine. 2004;51:103–14. doi: 10.1002/mrm.10677. [DOI] [PubMed] [Google Scholar]
- Roeyers H, Buysse A, Ponnet K, Pichal B. Advancing advanced mind-reading tests: empathic accuracy in adults with a pervasive developmental disorder. Journal of Child Psychology and Psychiatry. 2001;42:271–8. [PubMed] [Google Scholar]
- Rogers SJ. An examination of the imitation deficit in autism. In: Nadel J, Butterworth G, editors. Imitation in Infancy. Cambridge: Cambridge University Press; 1999. pp. 254–83. [Google Scholar]
- Ruby P, Decety J. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. European Journal of Neuroscience. 2003;17:2475–80. doi: 10.1046/j.1460-9568.2003.02673.x. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T, Wolf D, Loughead J, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–32. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: fMRI investigations of theory of mind. Neuroimage. 2003;19:1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell L. It’s the thought that counts: specific brain regions for one component of Theory of Mind. Psychological Science. 2006;17:692–9. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–9. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Schipul SE, Keller TA, Just MA. Inter-regional brain communication and its disturbance in autism. Frontiers in Systems Neuroscience. 2011;5:1–11. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, Saxe R. Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS One. 2009;4:e4869. doi: 10.1371/journal.pone.0004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, Southgate V, White S, Frith U. Mindblind eyes: an absence of spontaneous theory of mind in Asperger syndrome. Science. 2009;325:883–5. doi: 10.1126/science.1176170. [DOI] [PubMed] [Google Scholar]
- Sodian B, Frith U. Deception and sabotage in autistic, retarded and normal children. Journal of Child Psychology and Psychiatry. 1992;33:591–605. doi: 10.1111/j.1469-7610.1992.tb00893.x. [DOI] [PubMed] [Google Scholar]
- Spunt R, Lieberman M. Dissociating modality-specific and supramodal neural systems for action understanding. Journal of Neuroscience. 2012;32:3575–83. doi: 10.1523/JNEUROSCI.5715-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swettenham J, Baron-Cohen S, Gomez JC, Walsh S. What’s inside a person’s head? Conceiving of the mind as a camera helps children with autism develop an alternative theory of mind. Cognitive Neuropsychiatry. 1996;1:73–88. doi: 10.1080/135468096396712. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Science. 2007;4:153–7. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Van Dijk K, Sabuncu M, Buckner R. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25:916–25. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, et al. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14:170–81. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Vollm BA, Taylor ANW, Richardson P, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–8. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Walter H, Adenzato M, Ciaromidaro A. Understanding intentions in social interaction: the role of the anterior paracingulate cortex. Journal of Cognitive Neuroscience. 2004;16:1854–63. doi: 10.1162/0898929042947838. [DOI] [PubMed] [Google Scholar]
- Wang L, Mottron L, Peng D, Berthiaume C, Dawson M. Local bias and local-to-global interference without global deficit: a robust finding in autism under various conditions of attention, exposure time, and visual angle. Cognitive Neuropsychology. 2007;24:550–74. doi: 10.1080/13546800701417096. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Williams JHG, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and ‘mirror neuron’ functioning in autistic spectrum disorder. Neuropsychologia. 2006;44:610–21. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Williams JH, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neuroscience and Biobehavioral Reviews. 2001;25:287–95. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Young L, Dodell-Feder D, Saxe R. What gets the attention of the temporo-parietal junction? An fMRI investigation of attention and theory of mind. Neuropsychologia. 2010;48:2658–64. doi: 10.1016/j.neuropsychologia.2010.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.