Abstract
Patients with ESRD have high rates of depression, which is associated with diminished quality of life and survival. We determined whether individual cognitive behavioral therapy (CBT) reduces depression in hemodialysis patients with elevated depressive affect in a randomized crossover trial. Of 65 participants enrolled from two dialysis centers in New York, 59 completed the study and were assigned to the treatment-first group (n=33) or the wait-list control group (n=26). In the intervention phase, CBT was administered chairside during dialysis treatments for 3 months; participants were assessed 3 and 6 months after randomization. Compared with the wait-list group, the treatment-first group achieved significantly larger reductions in Beck Depression Inventory II (self-reported, P=0.03) and Hamilton Depression Rating Scale (clinician-reported, P<0.001) scores after intervention. Mean scores for the treatment-first group did not change significantly at the 3-month follow-up. Among participants with depression diagnosed at baseline, 89% in the treatment-first group were not depressed at the end of treatment compared with 38% in the wait-list group (Fisher’s exact test, P=0.01). Furthermore, the treatment-first group experienced greater improvements in quality of life, assessed with the Kidney Disease Quality of Life Short Form (P=0.04), and interdialytic weight gain (P=0.002) than the wait-list group, although no effect on compliance was evident at follow-up. In summary, CBT led to significant improvements in depression, quality of life, and prescription compliance in this trial, and studies should be undertaken to assess the long-term effects of CBT on morbidity and mortality in patients with ESRD.
Depression is a common and serious comorbid condition in ESRD patients, with prevalence rates estimated between 20% and 44%.1–10 Depression in ESRD patients has received much scientific attention and has been associated with lower quality of life (QOL),11–13 decreased adherence to the dialysis presciption,14–16 greater medical comorbidity, and decreased survival.17–21 It is unclear whether depression has a direct causal role in poor outcomes associated with ESRD or if depression is rather a marker of increased disease comorbidity and illness severity. The revised 2010 American Psychiatric Association guidelines highlight the roles of psychotherapy, particularly cognitive behavioral therapy (CBT), and selective reuptake inhibitors (SRIs) as the treatments of choice for patients with nonpsychotic major depression.22 However, despite clear treatment guidelines and the substantial human and economic burden of depression in ESRD, controlled intervention trials have been very limited in this population. One study compared 14 ESRD patients treated with the selective serotonin reuptake inhibitor (SSRI), fluoxetine, with those given a placebo.23 The intervention had a limited effect on depressive symptomatology. In studies of peritoneal dialysis patients, Wuerth et al. found that depressive symptoms were markedly ameliorated in patients who completed a 12-week open course of treatment with sertraline, bupropion, or nefazodone, despite low rates of compliance overall.24,25 Research conducted in Korea found depression scores to be significantly reduced in ESRD patients treated with fluoxetine compared with patients receiving no depression treatment.26
Our pilot uncontrolled study provided encouraging evidence that individual CBT is an efficacious, acceptable, and practical treatment option for hemodialysis-treated ESRD patients with depressive symptoms.27 One recent randomized trial of group CBT was conducted in Brazil,28 in which 85 depressed hemodialysis-treated ESRD patients were randomized to receive standard care or group CBT for 12 weeks. Both the intervention and control groups demonstrated significant improvement in depression scores measured by self-report and clinician-administered measures. The intervention arm exhibited more improvement in depressive affect and a commensurate improvement in QOL compared with the control group. From our pilot trials, it seems unlikely that a group intervention could be successfully implemented in the United States, because the coordination of patient schedules and transportation services to accommodate additional time at the dialysis center may be too difficult or unacceptable. To date, no randomized controlled trial using chairside CBT has been undertaken.
The overall aim of this study was to test the efficacy of an individual chairside cognitive behavioral intervention administered during hemodialysis, targeting depressive symptoms at two inner city hemodialysis units. The primary goal of the study was to determine whether a modified CBT intervention could significantly reduce clinician-rated and subjective depression scores compared with an intervention wait-list control condition. A secondary goal of the project was to determine whether the intervention had positive effects on QOL and fluid compliance, which have been shown to be associated with survival.29,30
Results
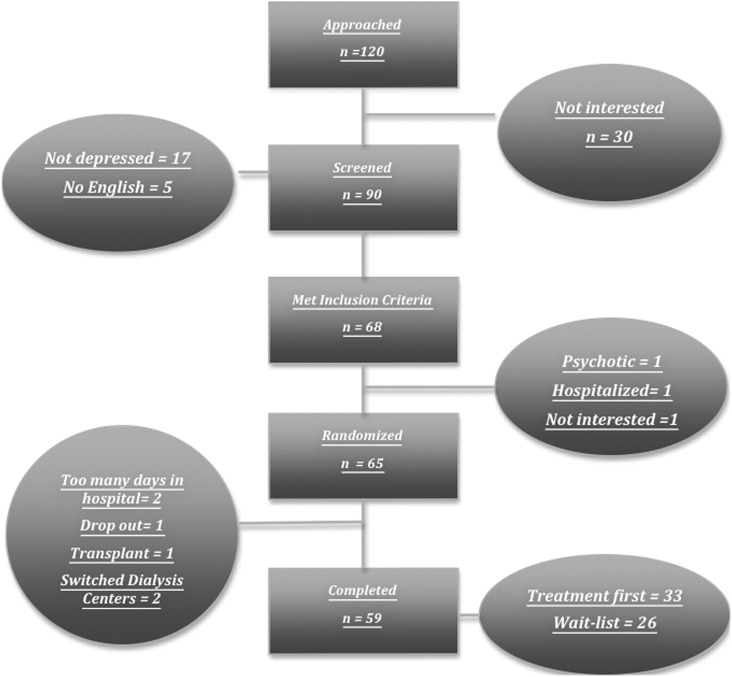
We approached 120 hemodialysis patients, 90 of which agreed to be screened for study inclusion. Sixty-five participants enrolled in the trial, with 59 completing all assessments (Figure 1). Only one participant dropped out of the trial volitionally. Two others exceeded the study timeframe due to frequent hospitalizations and a third received a kidney transplant. Two other participants switched dialysis centers during the study period. Baseline differences between those assigned to the treatment-first group and those assigned to the wait-list control group were evaluated (Table 1). There were no significant between-group differences in demographic, medical, or psychologic variables (P>0.05, all cases).
Figure 1.
Participant flow. This figure highlights the number of participants who were approached, screened, included, randomized, and who completed the intervention.
Table 1.
Baseline comparisons of study arms
| Participant Data | Total Sample (n=65) | Treatment Group (n=38) | Wait-List Group (n=27) | Inferential Statistic | ||
|---|---|---|---|---|---|---|
| γ2 | t | P | ||||
| Demographic | ||||||
| Female | 72.7 | 71.2 | 73.4 | 0.07 | >0.05 | |
| Self-identified race (black) | 93.9 | 96.9 | 93.3 | 6.1 | >0.05 | |
| US born | 27.3 | 39.4 | 16.7 | 3.13 | >0.05 | |
| Median household income ($) | 12,000–15,000 | 12,000–15,000 | 12,000–15,000 | |||
| Education (yr) | 11.2 (3.4) | 11.6 (2.9) | 10.9 (3.5) | 1.73 | >0.05 | |
| Married/cohabiting | 74.2 | 78.8 | 70.0 | 5.1 | >0.05 | |
| Employed (at least part-time) | 16.6 | 12.3 | 22.8 | 4.8 | >0.05 | |
| Previous transplant | 15.4 | 19.4 | 10.8 | 0.75 | >0.05 | |
| Dialysis vintage (mo) | 50.6 (31) | 54.8 (34) | 43.1 (24) | 1.45 | >0.05 | |
| Medical comorbiditya | 93.9 | 90.9 | 96.7 | 1.4 | >0.05 | |
| Medical information | ||||||
| Diabetes | 30.3 | 24.6 | 35.8 | 2.4 | >0.05 | |
| Hypertension | 68.2 | 73.4 | 63.7 | 5.8 | >0.05 | |
| URR | 71.2 (11) | 70.3 (13) | 71.9 (8) | 0.46 | >0.05 | |
| Serum albumin (g/dl) | 4.0 (0.36) | 4.0 (0.14) | 3.9 (0.45) | 0.85 | >0.05 | |
| Creatinine (mg/dl) | 11.4 (43.8) | 11.5 (4.2) | 11.3 (2.7) | 0.18 | >0.05 | |
| BUN (mg/dl) | 68.0 (20.1) | 63.3 (19.1) | 70.6 (19.8) | 1.3 | >0.05 | |
| Calcium Phosphate Product | 54.8 (17.5) | 51.6 (13.2) | 57.2 (18.2) | 0.93 | >0.05 | |
| Mini-Mental Status Examination score | 27.6 (2.3) | 27.8 (2.2) | 27.1 (2.5) | 1.2 | >0.05 | |
| IDWG (%Δkg per day) | 3.9 (2.2) | 4.1 (2.1) | 3.7 (2.1) | 0.94 | >0.05 | |
| Mental health information | ||||||
| Any psychiatric diagnosis (SCID Axis I) | 68.2 | 71.2 | 65.3 | 1.9 | >0.05 | |
| Major depression (SCID) | 48.5 | 54.5 | 42.2 | 2.7 | >0.05 | |
| Personality disorders (SCID-II) | 50.8 | 48.5 | 51.4 | 1.7 | >0.05 | |
| Self-reported depression (BDI-II) | 23.3 (9.6) | 25.3 (9.3) | 21.4 (8.9) | 1.4 | >0.05 | |
| Clinician-assessed depression (HAM-D) | 15.2 (6.4) | 15.0 (6.2) | 13.5 (5.0) | 1.8 | >0.05 | |
| QOL (KDQOL) | 101.6 (26.0) | 99.5 (29.5) | 104.8 (24.1) | 0.65 | >0.05 | |
Data are presented as the mean (SD) or percentage unless otherwise indicated.
At least one of the following conditions in addition to ESRD: diabetes mellitus, hypertension, HIV infection, chronic obstructive pulmonary disease, peripheral vascular disease, neoplasm, myocardial infarction, cerebrovascular disease, or coronary artery disease
In this study, 93.9% of participants were black and 27.3% were born in the United States. Participants had been treated with dialysis for a mean of 50.3 months (SD 31.2). The sample’s pretreatment mean scores placed participants in the moderately depressed range, as measured by both clinician-administered measures (Hamilton Depression Rating Scale [HAM-D]; mean 15.2 [SD 6.4]) and self-report mean depression scores (Beck Depression Inventory II [BDI-II]; mean 23.3 [SD 9.6]). Structured Clinical Interview for DSM Disorders (SCID) score agreement was high between the raters. Although there was some minor disagreement about particular symptoms (approximately 3% of symptoms reviewed), there were no instances of disagreement about the presence or absence of the depression diagnosis. Only two participants were being treated with antidepressants. They both were taking stable doses that had not been modified for several years. No one initiated SSRIs during the study and there were no changes in dosage for the two participants who were prescribed antidepressants (one in the treatment-first condition, one in the delayed condition).
Our results showed that 48.5% of the sample met criteria for a current diagnosis of major depression according to contemporary Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) standards pretreatment. In addition, 68.2% of the sample had at least one primary mental health diagnosis, and 50.0% of the sample had at least one personality disorder.
One of the advantages of conducting the trial chairside was that there were few missing data. We had complete data except for the QOL measure, which was incomplete for three participants at time 2 and five participants at time 3.
Primary Outcome: Depression
BDI-II
There was a significant treatment effect for the BDI self-report measure. The model-estimated mean change score during treatment was −11.7 points (SD 1.5; P<0.001) (raw mean change from 24.7 [SD 9.8] to 11.7 [SD 9.8]) among those receiving treatment first, and −4.8 points (SD 1.4; P<0.001) for those receiving treatment after completing the wait-list (raw mean change from 14.5 [SD 8.5] to 9.1 [SD 6.5]) (Table 2). There was also significant mean change in BDI score in the untreated group during the wait-list period (−6.7 points [SD 1.7]; P<0.001) (raw mean change from 21.9 [SD 8.9] to 14.5 [SD 8.5]). The magnitude of BDI improvement was significantly greater in the intervention group than it was in patients in the intervention wait-list condition (P=0.03). In addition, despite improving during the wait-list phase of the intervention, those participants showed further significant improvement after receiving the intervention (P=0.03).
Table 2.
Raw mean scores across assessment points for treatment completers (n=59)
| Variable | Group | Baseline | Phase 1 Condition | Assessment 2 | Phase 2 Condition | Assessment 3 |
|---|---|---|---|---|---|---|
| URR | Treatment (n=33) | 71.5 (12.6) | Treatment | 74.1 (6.2) | Follow-up | 73.1 (7.6) |
| Wait-list (n=26) | 71.8 (8.3) | Wait-list | 74.2 (6.8) | Delayed treatment | 74.0 (7.5) | |
| Serum albumin (g/dl) | Treatment | 4.0 (0.2) | Treatment | 4.1 (0.5) | Follow-up | 4.1 (0.4) |
| Wait-list | 3.9 (0.5) | Wait-list | 4.1 (0.5) | Delayed treatment | 4.1 (0.5) | |
| Serum creatinine concentration (mg/dl) | Treatment | 11.3 (3.8) | Treatment | 10.8 (3.0) | Follow-up | 10.9 (3.0) |
| Wait-list | 11.3 (2.7) | Wait-list | 10.5 (3.2) | Delayed treatment | 10.7 (2.2) | |
| IDWG (%Δkg per day) | Treatment | 4.0 (2.0) | Treatment | 2.8 (1.6) | Follow-up | 3.6 (1.8) |
| Wait-list | 3.6 (2.1) | Wait-list | 3.6 (2.0) | Delayed treatment | 2.5 (2.0) | |
| Major depression (SCID) | Treatment | 54 | Treatment | 5 | Follow-up | 10 |
| Wait-list | 33 | Wait-list | 31 | Delayed treatment | 4 | |
| Self-report depression (BDI) | Treatment | 24.7 (9.8) | Treatment | 11.7 (9.8) | Follow-up | 9.9 (8.5) |
| Wait-list | 21.9 (8.9) | Wait-list | 14.5 (8.5) | Delayed treatment | 9.1 (6.5) | |
| Clinician-assessed depression (HAM-D) | Treatment | 15.7 (6.8) | Treatment | 6.5 (6.8) | Follow-up | 6.7 (5.8) |
| Wait-list | 12.9 (5.3) | Wait-list | 10.9 (5.4) | Delayed treatment | 5.0 (4.3) | |
| QOL (KDQOL) | Treatment | 99.5 (27.9) | Treatment | 115.3 (25.5) | Follow-up | 118.3 (27.7) |
| Wait-list | 105.1 (23.7) | Wait-list | 110.6 (25.1) | Delayed treatment | 119.7 (24.7) |
Data are presented as the mean (SD) or percentage.
There was a significant sequence effect (F1,90=11.56; P=0.001), indicating that the treatment effect was greater for those treated first than those assigned to the wait-list, because the baseline was lower in the wait-list control group.
For those that received the treatment first, there was evidence of the persistence of the treatment effect in BDI scores because there was no significant mean change at the 3-month follow-up (−0.9 [SD 1.3]; P=0.50) (raw mean change from 11.7 [SD 9.8] to 9.9 [SD 8.5]).
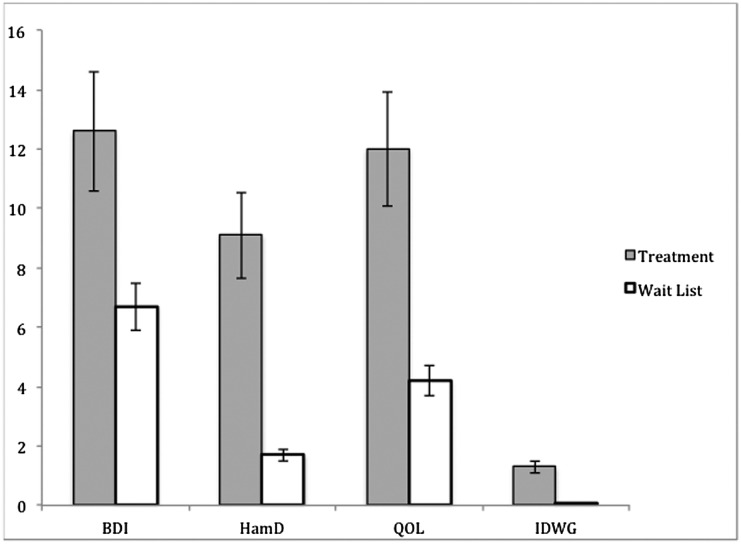
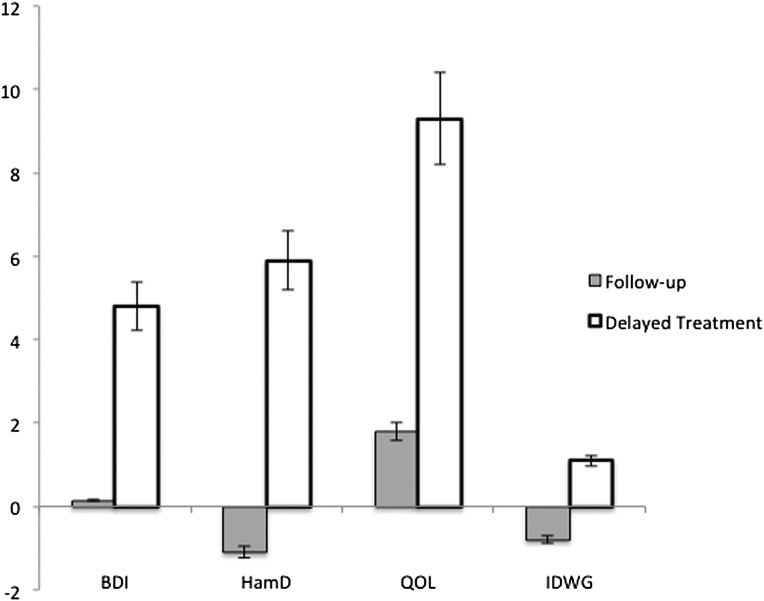
Figure 2 shows the model-estimated mean change scores of the outcome variables during the first phase of the study, and Figure 3 shows mean change scores during the second phase of the study.
Figure 2.
Model-adjusted mean change for the treatment and wait-list groups in phase 1. Treatment indicates group 1 participants, who received the intervention during this phase. Wait-list indicates group 2 participants, who received no intervention during this phase. Scales are presented so that the positive scores reflect improvement. The y-axis represents change scores for the individual measures.
Figure 3.
Model-adjusted mean change for the treatment and wait-list groups in phase 2. Follow-up indicates group 1 participants, who received no intervention during this phase. Delayed treatment indicates group 2 participants, who received the intervention during this phase. Scales are presented so that the positive scores reflect improvement. The y-axis represents change scores for the individual measures.
HAM-D
Similar to the BDI, there was a highly significant treatment effect, with the model-estimated mean change score decreasing 9.1 points (SD 1.1; P<0.001) after treatment (raw mean change from 15.7 [SD 6.8] to 6.5 [SD 6.8]) for those receiving treatment first, and decreasing 5.9 points (SD 1.1; P<0.001) among those receiving treatment after being wait-listed (raw mean change from 10.9 [SD 5.4] to 5.0 [SD 4.3]). These findings indicate the intervention was successful at reducing HAM-D scores in both groups. There was no significant mean change in the untreated groups during the wait-list period (−1.9 points [SD 1.2]; P=0.17) (raw mean change from 12.9 [SD 5.3] to 10.9 [SD 5.4]). The difference in mean change score between treated and untreated groups was highly significant (P<0.001).
There was a significant sequence effect, because the effect size for those treated first was significantly more than that of those treated after completing the wait-list (F1,96=4.62; P=0.03).
As was the case for the BDI data, there was no significant mean change during the follow-up period for the treatment-first group (+0.3 [SD 1.0]; P=0.58) (raw mean change from 6.5 [SD 6.8] to 6.7 [SD 5.8]), indicating persistence of a treatment effect beyond the end of the treatment period.
SCID I
Among the 27 participants depressed at baseline, 17 of 19 (89.5%) treated participants were not depressed at the end of treatment compared with 3 of 8 (37.5%) participants in the wait-list condition (Fisher’s test, P=0.01). One initially depressed participant who reached remission at the end of treatment relapsed by the end of the 3-month post-treatment observation period.
Among participants depressed at the second assessment point, six of seven (85.7%) participants were not depressed after receiving the intervention compared with no change in the one still depressed participant who had already completed treatment in phase 1 (P=0.25).
QOL
There was a significant treatment effect for the intervention on QOL, measured by the Kidney Disease Quality of Life Scale (KDQOL). The model-estimated treatment effect was +12.0 points (SD 3.4; P=0.003) (raw mean score change from 99.5 [SD 27.9] to 115.3 [SD 25.5]) for participants treated first, and +11.3 points (SD 3.7; P=0.01) (raw mean change from 110.6 [SD 25.1] to 119.7 [SD 24.7]) for those treated after being on the wait-list. There was no significant mean change for the untreated group in the wait-list condition (+2.9 [SD 2.8]; P=0.14) (raw mean change from 105.1 [SD 23.7] to 110.6 [SD 25.1]). The difference in mean change score between treated and untreated groups was significant (P=0.04).
The difference between the effect size of those receiving treatment first or after completing the wait-list was not statistically significant (F1,72=0.17; P=0.68), suggesting that the timing of the intervention did not affect its efficacy. Pooled across both treatment phases, the estimated treatment effect was +11.7 points (SD 2.0).
For those that received the treatment first, there was evidence of the persistence of the treatment effect beyond the end of the treatment period, because there was no significant mean change (+1.4 [SD 2.5]; P=0.58) (raw mean change from 115.3 [SD 25.5] to 118.3 [SD 27.7]) in KDQOL score.
Fluid Compliance
There was a highly significant treatment effect for improving fluid compliance. The model-estimated mean change score during treatment was −1.3%Δkg/d (SD 0.3; P<0.001) (raw mean change from 4.0 [SD 2.0] to 2.8 [SD 1.6]) among those receiving treatment first and −1.1%Δkg/d (SD 0.3; P<0.001) (raw mean change from 3.6 [SD 2.0] to 2.5 [SD 2.0]) among those receiving treatment after coming off the wait-list. There was no significant change in fluid compliance during the wait-list phase (0.0%Δkg/d [SD 0.3]; P=0.95). The difference in mean change score between treated and untreated groups was significant (P=0.002).
The difference between the effect size of those receiving treatment first or after completing the wait-list was not statistically significant (F1,112=0.28; P=0.60), suggesting that the timing of the intervention did not affect its efficacy. Pooled across both treatment phases, the estimated treatment effect was −1.2 points (SD 0.2).
Despite the significant treatment effect, the increased compliance did not last. There was a significant mean change during the follow-up period compared with the improved compliance seen at the intervention’s completion (+0.6 Δkg/d [SD 0.3]; P=0.03) (raw mean change from 2.8 [SD 1.6] to 3.6 [SD 1.8]), indicating that participants quickly returned to their prior levels of nonadherence, losing much of the gains made during the intervention.
Comorbid Personality Disorders
Personality disorders were only measured at baseline because they are stable estimates of unchanging personality dimensions. Our results showed that 50.8% of the sample had at least one personality disorder, with paranoid personality disorder (16.9%) and avoidant personality disorder (13.6%) as the most prevalent. When the model was adjusted to account for the presence or absence of a personality disorder, there was no significant effect for BDI (t47.9=0.19; P=0.24), HAM-D outcomes (t40.5=−0.16; P=0.87), KDQOL (t32.2=0.41; P=0.68), or interdialytic weight gain (IDWG) (t48.52=0.41; P=0.68).
Sensitivity Analyses
A crude sensitivity analysis conducted on study outcomes (HAM-D, BDI-II, QOL) showed that the study results are robust. For individuals who dropped out of the study, we used their baseline scores as post-treatment scores. The observed differences in group means were minimal.
Discussion
Observational studies have linked increased depressive affect in ESRD patients to morbidity and mortality.17–21 We showed that a behavioral, nonpharmacologic intervention is effective at decreasing depressive affect as well as improving QOL and treatment adherence, measured as IDWG, in inner city ESRD patients. The intervention did not use medication, and had minimal side effects. We believe that the intervention can be easily generalized and operationalized to other dialysis units.
In this crossover randomized controlled trial, ESRD patients with elevated depressive affect were randomly assigned either to treatment first or to an intervention wait-list control. The intervention was 10 CBT sessions administered chairside during regular dialysis treatments, which was effective in reducing depression. Depression was measured in three different ways to capture its full presentation. There was substantial and significant improvement in clinician-administered measures of depression, in a self-report measure of depression, and in the proportion of patients with a depression diagnosis after intervention compared with controls. On the BDI self-report scale of depression, the mean score of the intervention group dropped from moderate depression to below the cut-off for clinical depression. Similarly, mean HAM-D scores were not only reduced, but also brought the intervention group below the clinical cut-off for remission. Despite participants indicating depression improvement during their wait-list phase, there was still continued significant improvement during the intervention phase. The remission rate for the first phase of the intervention was 90%, as assessed by the SCID. Taken together, these results highlight a marked effect of the depression intervention. The intervention was also successful at improving the secondary outcomes of QOL and fluid adherence.
One of the primary barriers to appropriate depression care is the additional burden that an additional doctor’s appointment imposes. This study attempted to minimize this barrier, by offering the intervention during hemodialysis. We acknowledge that by reducing some of the effort required to see a mental health professional, we might be accessing participants who might not have been motivated enough to attend traditional mental health visits. In addition, there were relatively high rates of personality disorders (50.8%) in the sample. Despite this, attrition rates were modest. We believe that the success of the CBT intervention benefited by providing chairside CBT, a model easily generalizable to all US ESRD patients and potentially applicable to other medical populations that have reduced mobility. The presence of personality disorders did not affect the intervention‘s effectiveness. This is in particular contrast to medication trials, in which study adherence has generally been poor.31 We see this as an indication of psychotherapy as a viable depression intervention modality in ESRD patients.
The level of depression that was required to enter the study was minimal (≥10 on the BDI), although the sample average was in the moderate range. Despite a low threshold for inclusion, the study was still able to demonstrate significant improvement.
Although the rigor of the assessment and the intervention were strengths of the trial, the small sample size and demographics of the sample somewhat limit its generalizability. Furthermore, the current design does not preclude the possibility that participants responded to the increased attention, because there was no matched care control condition. However, there is strong evidence that CBT in other populations has demonstrated superiority over attention-matched controls.32 There was also modest participant attrition, particularly in those treated in phase 1. This could introduce some selection bias, and may be partly responsible for the observed phase effect. However, because there are profound health disparities in renal disease in the United States, with black patients being over-represented in hemodialysis treatment and under-represented in transplant recipients,33 the importance of this intervention to successfully treat this often scientifically neglected population should not be underestimated. Although the improvement was both clinically and statistically significant, it is interesting to note that the improvements in fluid adherence were not maintained through 3-month follow-up, in contrast to the mental health and QOL improvements. Perhaps the daily challenges associated with fluid restriction can be best met while in active treatment, but further exploration is warranted.
There are patient, physician, and systematic barriers to appropriate mental health care for medical patients.34 This study highlights how many of the barriers can be overcome. Participants did not need to make additional appointments or trips to see mental health professionals, and did not need to take medication to get substantial benefit. The study put almost no additional clinical burden on nephrologists, because they were apprised of patient progress but did not participate in the intervention. In addition, nephrologists were not asked to help decide on an appropriate psychotropic medication or to participate in dose titration, tasks that are often involved in starting a new antidepressant.35 Finally, this intervention may be implemented by well trained masters-level social workers, who are federally mandated in each dialysis unit, because it has been standardized. Future studies should attempt to identify the minimum therapist qualifications and time commitment required for the depression intervention to be efficacious.
The mechanisms that explain the relationship between depression and survival for ESRD patients have been posited, but there has been little empirical inquiry.1,2,6,7 In addition, it is unclear whether the negative association with survival would also then be reversed if the depression remits. The results of this trial highlight the effect of depression treatment on health behavior, particularly fluid adherence. Although the mechanistic steps between depression and mortality have not all been elucidated, the close relationship between depression and adherence has been supported in an efficacious intervention. These results demonstrate the decisive effect that depression treatment can have because once depressive affect was reduced, participants had better fluid adherence, at least temporarily. Certainly, larger-scale trials with longer follow-up periods are needed to substantiate these findings, but these data are the first indication that depression intervention may well affect survival.
Concise Methods
This crossover randomized clinical trial was conducted at two dialysis units in Brooklyn, New York. The study was approved by the Downstate Medical Center/Kings County Hospital Center Joint Institutional Review Board. Informed consent was obtained from all participants. Patients with ESRD receiving outpatient hemodialysis treatment aged >18 years were included. Inclusion criteria were ESRD treatment with hemodialysis for at least 6 months and elevated depressive affect (as evidenced on a self-report depression scale [BDI-II score >10]). Exclusion criteria were current hospitalization, altered mental status (Mini-Mental Status Examination score <23), psychosis, current substance abuse, current ongoing psychotherapy, a change in psychotropic medication in the last 6 months, or lack of English proficiency to participate in talk therapy. Patients with other comorbid psychiatric diagnoses or personality disorders were not excluded from the study. Participants received $40 for each of the three assessments completed.
Measures
All assessments were conducted by an independent assessor who was blind to the participant’s treatment condition and diagnostic history.
Depression
To accurately measure depression, three different depression measures were used: participant’s self-report, diagnosis of major depression, and clinician’s appraisal of depression severity.
BDI-II
The BDI-II is a 21-item self-report instrument for assessing depressive affect.36 Scores range from 0 to 63. Higher scores reflect the presence and severity of depressed mood. The BDI reflects cognitive-affective aspects (items regarding satisfaction with life and guilt) and somatic aspects (items regarding sleep disturbance and health concerns) of depression. Although BDI scores are not indicative of the full clinical syndrome of depression, they are a reliable and well validated self-report measure of depressive symptomatology in both clinical and nonclinical samples.37 The BDI has been used extensively in ESRD populations.12,14,18,21,38
SCID-I
The SCID is a semistructured interview administered by a clinician for making the major Axis I DSM-IV diagnoses.39 The modules for mood and anxiety disorders were included. The SCID guides the clinician in using a decision tree approach to determine psychiatric diagnoses. The SCID output provides a record of the current presence or absence of each of the disorders being considered. It has variable but acceptable reliability and validity and is regarded as the “gold standard” for determining psychiatric diagnoses.39–41
To ensure inter-rater reliability, all SCID interviews were audiotaped, and 15% of the interviews were reviewed for diagnostic accuracy.
HAM-D
The HAM-D is a 17-item screening instrument (range, 0–55) designed to measure the severity of illness in adults already diagnosed as having depression.42 The HAM-D is one of the most widely used instruments for measuring outcome in mood disorders.43 The HAM-D offers high validity and reliability in measuring response to treatment. It is effectively used in a variety of health areas, including renal disease.6 The HAM-D is administered by a clinician during a client interview and takes approximately 15–20 minutes to complete. A score >7 indicates clinically elevated depressive affect.
KDQOL-SF
The KDQOL-SF assesses the QOL of patients with kidney disease using 43 disease-specific items, 36 generic items, and an overall health-ranking item.44 This totals 80 items with 12 disease-specific subscales and 8 generic subscales. It is a short form of the 134-item KDQOL. The KDQOL has been widely used in hemodialysis patients.11,13,28,45,46 The summary composite score is reported.
Personality Disorders
Personality disorders are a class of personality types and enduring behaviors associated with significant distress or disability, which deviate from social expectations particularly in relating to others. It has been suggested that depression treatment failures in CKD may be due to the incidence of a co-occurring personality disorder.24
SCID-II
The SCID-II is a semistructured interview designed to cover the 11 DSM-IV personality disorders (including personality disorder not otherwise specified) and the appendix categories of depressive personality disorder and passive-aggressive personality disorder. It is similar in style to the SCID-I and also has variable but acceptable reliability and validity.47
Laboratory Values
Information regarding medical history, laboratory, and treatment parameters,48 and interdialytic weight gain, as a measure of fluid compliance, were obtained from dialysis records. IDWG was calculated as the monthly average of the difference between the predialysis weight and the weight at the end of the previous dialysis session, divided by determined dry weight, expressed as a percentage of change in weight per day (%Δkg per day).29 Although hemodialysis treatment requires adherence to a complex medical prescription including dietary, fluid, medication and behavioral regimens, we have chosen to focus on IDWG as a measure of behavioral compliance because of its clinical relevance, precision of measurement, and known relationship to medical outcome.
Study Procedures
Patients were approached in the hemodialysis center and asked to undergo an assessment to determine eligibility for the clinical trial. Patients were approached consecutively until desired recruitment was reached. Once eligible participants completed the baseline assessment, they were randomly assigned to either the treatment-first condition or a wait-list control (Figure 4). The treatment group began the intervention within 1 week of completion of the assessment. The participants’ primary nephrologists were made aware of the results of the assessment, but were blinded to treatment condition.
Figure 4.
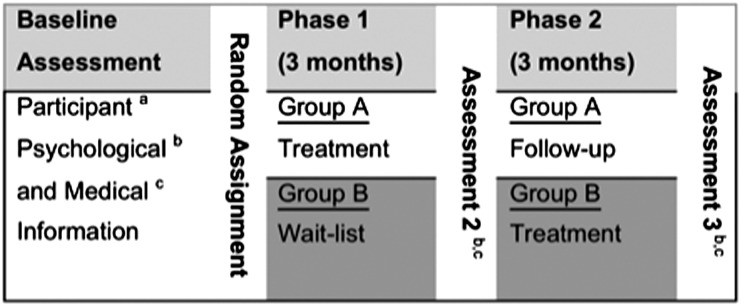
Crossover study design. aParticipant information includes personal, ethnic, and demographic information, as well as information about mental health, substance use history and treatment, and mental status. bPsychological information includes BDI-II, HAM-D, KDQOL, SCID-I, and SCID-II. cMedical information includes presence of diabetes mellitus, hypertension, urea reduction ratio, serum albumin concentration, BUN concentration, creatinine concentration, calcium phosphate product, and IDWG.
After 3 months, the treatment-first group completed the intervention and both groups were reassessed. The intervention wait-list group was then offered the intervention. After an additional 3 months, both groups had received the intervention and before and after measures for both groups, as well as 3-month follow-up for the first intervention group, were available.
Power Analyses
A power analysis was performed to determine the requisite study sample size. Using the methodology originally outlined by Sokal and Rohlf49 and the pilot data available from the 15 participants we had treated,27 it was determined that in a two-group design in which the difference of the BDI-II means is 10 and the SD is 8, 23 participants would be the minimum for each cell for a type I error rate of 0.05 and a power of 80%. In deciding on the target number of study participants to be recruited, we also accounted for attrition. Therefore, because the primary goal of this study was to compare participants who received the intervention to the wait-list control on measures of depression, we chose to recruit a conservative total of 30 participants in each condition, based on our estimated effect size and attrition rates.
Intervention
CBT was chosen as the intervention modality for several reasons. We believed that psychotherapeutic intervention would be the more appropriate intervention choice in contrast to pharmacotherapy because of the moderate nature of the depression severity seen in hemodialysis patients and its acceptability to the patients. CBT is the most studied effective psychotherapy treatment for moderate depression.22,50,51 CBT is a relatively short-term, focused psychotherapy for a wide range of psychologic problems including depression.52 The focus of CBT is on how one is thinking, behaving, and communicating today rather than on early childhood experiences, in contrast to traditional psychotherapy. The therapist assists the patient in identifying specific distortions (cognitive assessment) and biases in thinking and provides guidance on how to change this thinking. CBT teaches the patient to learn effective self-help skills that are used in homework assignments that help change the way the person currently thinks, feels, and behaves. CBT is action-oriented, practical, rational, and helps the patient gain independence and mastery in dealing with practical issues.52
We chose not to use SSRI treatment for depression for two reasons. First, there was reluctance on the part of the attending nephrologists to use SSRIs due to the paucity of controlled trials demonstrating safety and efficacy. Second, our pilot work revealed a reported reluctance from the patients to begin SRI treatment. People are aware that starting an SSRI often involves trial and error until the balance between antidepressant effect and side effects can be achieved. Patients often do not wish to stay on their medication long enough to give the SSRI an adequate trial, but instead stop the medication due to the side effects and multiple visits required.53
The intervention took place in individual format chairside, while the patients were being dialyzed. The protocol consisted of ten 60-minute weekly sessions spread over a maximum of 3 months. Standard CBT intervention for depression was modified and adapted for the particular challenges associated with being treated with hemodialysis. The primary modifications included the following: (1) psychoeducation emphasizing the difference between depression and medical illness; (2) an additional adherence component targeting compliance with the dialysis prescription; (3) adapting behavioral activation, a depression intervention strategy marked by the development of a hierarchy of reinforcing activities, to the medical limitations often imposed by ESRD; and (4) the identification of ESRD-specific cognitive distortions to be addressed in restructuring, a cognitive therapy technique in which maladaptive thought patterns are identified and challenged. See Table 3 for a detailed description of the intervention. The intervention was administered by the principal investigator, a doctoral-level psychologist, and doctoral-level trainees (authors N.V.H. and D.R.A.) under his supervision. The physical layout of the dialysis center and the level of ambient noise allowed for the maintenance of privacy during intervention sessions. The treatment manual is available by request from Dr. Cukor.
Table 3.
Intervention session content
| Session | Content of the Intervention Sessions | |
|---|---|---|
| 1–2 | Assessment | Assess patient’s motivation for change, goals for treatment, “stage of change”; evaluate need for patient to modify fluid intake, compliance with medical regimen |
| Psychoeducation | Highlight similarities/differences between depression and medical illness | |
| 3–6 | Behavioral intervention | Behavioral activation—increase participants’ enjoyable activities |
| Cognitive intervention | Train participants on the relationship between dysfunctional automatic thoughts and negative perceptions and outcomes | |
| 7–8 | Teach and practice healthy living (compliance) skills in session | |
| Increase positive social contacts—initiating contact, building support network | ||
| 9–10 | Plan for termination of therapy—identify which interventions were helpful and which were not, relapse prevention | |
Wait-List
Participants in the wait-list condition who scored >15 on the BDI or indicated any suicidal ideation had their primary nephrologist notified and informed of the patient’s condition and participation in this intervention trial. The nephrologists were encouraged to treat the patients according to usual standard of care, including formal psychologic or psychopharmacologic treatment. The wait-list control participants were reassessed by study personnel 3 months after randomization, and were then offered the intervention if they still met eligibility criteria (i.e., were not in current ongoing psychotherapy, or had a change in psychotropic medication in the last 6 months). Due to ethical considerations, both arms in this trial were offered the depression intervention. This approach also provided additional data on participants’ improvement during the intervention phase compared with an observational period.
Statistical Analyses
Baseline differences between groups were evaluated using t tests. Data were compared in each of the two chronological phases of the study, and for each continuous outcome (BDI, HAM-D, KDQOL, and IDWG). Baseline score was subtracted from outcome score to create a change score dependent variable. A mixed linear model was constructed, with fixed factors (phase, sequence [immediate intervention versus wait-list], and their interaction [treatment effect]). Such analyses allow estimation of treatment effect controlling for any phase or sequence effects. The unadjusted mean values and SDs are presented to aid interpretation. Inferential tests were only done on the mixed linear model. All available data were used, including early scores for participants who later dropped out. The intrapatient covariance matrix was modeled as unstructured. Satterthwaite adjustments were made to denominator degrees of freedom. Model residuals were inspected for skew and for outliers. SAS Release 9.2 (PROC GLIMMIX) was used for these analyses (SAS Institute, Cary, NC). The Fisher exact test (PROC FREQ) was used to explore group differences at each phase in terms of onset and remission of SCID-defined major depression. A P value ≤0.05 was considered significant, using two-tailed comparisons. Unadjusted results are reported as the means (SDs) or as percentages.
Disclosures
None.
Acknowledgments
We express our gratitude to the dialysis center staff and patients at both sites that enabled us to conduct our study. We particularly thank the nursing and social work staff, who were always encouraging and helpful. We also extend a special acknowledgment to Ms. Maria Siciliano, the renal dietician, who assisted us with the IDWG data.
The research reported in this publication was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (award number K23DK076980).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Cukor D, Cohen SD, Peterson RA, Kimmel PL: Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol 18: 3042–3055, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Kimmel PL: Psychosocial factors in dialysis patients. Kidney Int 59: 1599–1613, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein FO, Finkelstein SH: Depression in chronic dialysis patients: Assessment and treatment. Nephrol Dial Transplant 15: 1911–1913, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Hedayati SS, Bosworth HB, Kuchibhatla M, Kimmel PL, Szczech LA: The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int 69: 1662–1668, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Watnick S, Wang PL, Demadura T, Ganzini L: Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis 46: 919–924, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Cukor D, Peterson RA, Cohen SD, Kimmel PL: Depression in end-stage renal disease hemodialysis patients. Nat Clin Pract Nephrol 2: 678–687, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Kimmel PL: Depression in patients with chronic renal disease: What we know and what we need to know. J Psychosom Res 53: 951–956, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Kimmel PL, Peterson RA: Depression in end-stage renal disease patients treated with hemodialysis: Tools, correlates, outcomes, and needs. Semin Dial 18: 91–97, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Smith MD, Hong BA, Robson AM: Diagnosis of depression in patients with end-stage renal disease. Comparative analysis. Am J Med 79: 160–166, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Hinrichsen GA, Lieberman JA, Pollack S, Steinberg H: Depression in hemodialysis patients. Psychosomatics 30: 284–289, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Kimmel PL, Emont SL, Newmann JM, Danko H, Moss AH: ESRD patient quality of life: Symptoms, spiritual beliefs, psychosocial factors, and ethnicity. Am J Kidney Dis 42: 713–721, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Drayer RA, Piraino B, Reynolds CF, 3rd, Houck PR, Mazumdar S, Bernardini J, Shear MK, Rollman BL: Characteristics of depression in hemodialysis patients: Symptoms, quality of life and mortality risk. Gen Hosp Psychiatry 28: 306–312, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Cukor D, Coplan J, Brown C, Friedman S, Cromwell-Smith A, Peterson RA, Kimmel PL: Depression and anxiety in urban hemodialysis patients. Clin J Am Soc Nephrol 2: 484–490, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Cukor D, Rosenthal DS, Jindal RM, Brown CD, Kimmel PL: Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int 75: 1223–1229, 2009 [DOI] [PubMed] [Google Scholar]
- 15.DiMatteo MR, Lepper HS, Croghan TW: Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 160: 2101–2107, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Kimmel PL, Peterson RA, Weihs KL, Simmens SJ, Alleyne S, Cruz I, Veis JH: Psychosocial factors, behavioral compliance and survival in urban hemodialysis patients. Kidney Int 54: 245–254, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Kimmel PL, Peterson RA, Weihs KL, Simmens SJ, Alleyne S, Cruz I, Veis JH: Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int 57: 2093–2098, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Hedayati SS, Bosworth HB, Briley LP, Sloane RJ, Pieper CF, Kimmel PL, Szczech LA: Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int 74: 930–936, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Troidle L, Watnick S, Wuerth DB, Gorban-Brennan N, Kliger AS, Finkelstein FO: Depression and its association with peritonitis in long-term peritoneal dialysis patients. Am J Kidney Dis 42: 350–354, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Lopes AA, Bragg J, Young E, Goodkin D, Mapes D, Combe C, Piera L, Held P, Gillespie B, Port FK, Dialysis Outcomes and Practice Patterns Study (DOPPS) : Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int 62: 199–207, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal Asher D, Ver Halen N, Cukor D: Depression and nonadherence predict mortality in hemodialysis treated end-stage renal disease patients. Hemodial Int 16: 387–393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Psychiatric Association: Practice Guideline for the Treatment of Patients With Major Depressive Disorder, 3rd Ed., Washington, DC, American Psychiatric Association, 2010
- 23.Blumenfield M, Levy NB, Spinowitz B, Charytan C, Beasley CM, Jr, Dubey AK, Solomon RJ, Todd R, Goodman A, Bergstrom RF: Fluoxetine in depressed patients on dialysis. Int J Psychiatry Med 27: 71–80, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Wuerth D, Finkelstein SH, Finkelstein FO: The identification and treatment of depression in patients maintained on dialysis. Semin Dial 18: 142–146, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Wuerth D, Finkelstein SH, Kliger AS, Finkelstein FO: Chronic peritoneal dialysis patients diagnosed with clinical depression: Results of pharmacologic therapy. Semin Dial 16: 424–427, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Koo JR, Yoon JY, Joo MH, Lee HS, Oh JE, Kim SG, Seo JW, Lee YK, Kim HJ, Noh JW, Lee SK, Son BK: Treatment of depression and effect of antidepression treatment on nutritional status in chronic hemodialysis patients. Am J Med Sci 329: 1–5, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Cukor D: The hemodialysis center: A model for psychosocial intervention. Psychiatr Serv 58: 711–712, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Duarte PS, Miyazaki MC, Blay SL, Sesso R: Cognitive-behavioral group therapy is an effective treatment for major depression in hemodialysis patients. Kidney Int 76: 414–421, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Kimmel PL, Varela MP, Peterson RA, Weihs KL, Simmens SJ, Alleyne S, Amarashinge A, Mishkin GJ, Cruz I, Veis JH: Interdialytic weight gain and survival in hemodialysis patients: Effects of duration of ESRD and diabetes mellitus. Kidney Int 57: 1141–1151, 2000 [DOI] [PubMed] [Google Scholar]
- 30.López-Gómez JM, Villaverde M, Jofre R, Rodriguez-Benítez P, Pérez-García R: Interdialytic weight gain as a marker of blood pressure, nutrition, and survival in hemodialysis patients. Kidney Int Suppl (93): S63–S68, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Finkelstein FO, Watnick S, Finkelstein SH, Wuerth D: The treatment of depression in patients maintained on dialysis. J Psychosom Res 53: 957–960, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Dobson KS: A meta-analysis of the efficacy of cognitive therapy for depression. J Consult Clin Psychol 57: 414–419, 1989 [DOI] [PubMed] [Google Scholar]
- 33.U.S. Renal Data System : USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009 [Google Scholar]
- 34.Nutting PA, Rost K, Dickinson M, Werner JJ, Dickinson P, Smith JL, Gallovic B: Barriers to initiating depression treatment in primary care practice. J Gen Intern Med 17: 103–111, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimmel PL, Cohen SD, Peterson RA: Depression in patients with chronic renal disease: Where are we going? J Ren Nutr 18: 99–103, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Steer RA, Brown GK: Manual for the Beck Depression Inventory-II, San Antonio, TX, Psychological Corporation, 1996 [Google Scholar]
- 37.Beck AT, Steer RA, Garbin MG: Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev 8: 77–100, 1998 [Google Scholar]
- 38.Craven JL, Rodin GM, Littlefield C: The Beck Depression Inventory as a screening device for major depression in renal dialysis patients. Int J Psychiatry Med 18: 365–374, 1988 [DOI] [PubMed]
- 39.First MB, Spitzer RL, Gibbon M, Williams J: Structured Clinical Interview for DSM-IV Diagnoses, (SCID-I), Washington, D.C., American Psychiatric Press, Inc., 1997 [Google Scholar]
- 40.Shear MK, Greeno C, Kang J, Ludewig D, Frank E, Swartz HA, Hanekamp M: Diagnosis of nonpsychotic patients in community clinics. Am J Psychiatry 157: 581–587, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Lobbestael J, Leurgans M, Arntz A: Inter-rater reliability of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) and Axis II Disorders (SCID II). Clin Psychol Psychother 18: 75–79, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Hamilton MA: A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berrios GE, Bulbena A: The Hamilton Depression Scale and the numerical description of the symptoms of depression. In: The Hamilton Scales, edited by Bech P, Coppen A, Heidelberg, Springer Verlag, 1990, pp 80–92 [DOI] [PubMed] [Google Scholar]
- 44.Hays RD, Kallich JD, Mapes DL, Coons SJ, Amin N, Carter WB, Kamberg C: Kidney Disease Quality of Life Short Form (KDQOL-SF™), Version 1.3: A Manual for Use and Scoring, Santa Monica, CA, RAND, 1997 [Google Scholar]
- 45.Kurella M, Luan J, Yaffe K, Chertow GM: Validation of the Kidney Disease Quality of Life (KDQOL) cognitive function subscale. Kidney Int 66: 2361–2367, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Cukor D, Coplan J, Brown C, Peterson RA, Kimmel PL: Course of depression and anxiety diagnosis in patients treated with hemodialysis: A 16-month follow-up. Clin J Am Soc Nephrol 3: 1752–1758, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.First MB, Spitzer RL, Gibbon M, Williams J: Structured Clinical Interview for DSM-IV Personality Disorders, (SCID-II), Washington, DC, American Psychiatric Press Inc, 1997 [Google Scholar]
- 48.Kaveh K, Kimmel PL: Compliance in hemodialysis patients: Multidimensional measures in search of a gold standard. Am J Kidney Dis 37: 244–266, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Sokal R, Rohlf F: Biometry, 3rd Ed., New York, W.H. Freeman and Company, 1995 [Google Scholar]
- 50.Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H: Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 61: 34–41, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Whooley MA, Simon GE: Managing depression in medical outpatients. N Engl J Med 343: 1942–1950, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Beck AT: Depression: Causes and Treatment, Philadelphia, University of Pennsylvania Press, 2006 [Google Scholar]
- 53.Bull SA, Hunkeler EM, Lee JY, Rowland CR, Williamson TE, Schwab JR, Hurt SW: Discontinuing or switching selective serotonin-reuptake inhibitors. Ann Pharmacother 36: 578–584, 2002 [DOI] [PubMed] [Google Scholar]