Abstract
In adult patients with CKD, hypertension is linked to the development of left ventricular hypertrophy, but whether this association exists in children with CKD has not been determined conclusively. To assess the relationship between BP and left ventricular hypertrophy, we prospectively analyzed data from the Chronic Kidney Disease in Children cohort. In total, 478 subjects were enrolled, and 435, 321, and 142 subjects remained enrolled at years 1, 3, and 5, respectively. Echocardiograms were obtained 1 year after study entry and then every 2 years; BP was measured annually. A linear mixed model was used to assess the effect of BP on left ventricular mass index, which was measured at three different visits, and a mixed logistic model was used to assess left ventricular hypertrophy. These models were part of a joint longitudinal and survival model to adjust for informative dropout. Predictors of left ventricular mass index included systolic BP, anemia, and use of antihypertensive medications other than angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Predictors of left ventricular hypertrophy included systolic BP, female sex, anemia, and use of other antihypertensive medications. Over 4 years, the adjusted prevalence of left ventricular hypertrophy decreased from 15.3% to 12.6% in a systolic BP model and from 15.1% to 12.6% in a diastolic BP model. These results indicate that a decline in BP may predict a decline in left ventricular hypertrophy in children with CKD and suggest additional factors that warrant additional investigation as predictors of left ventricular hypertrophy in these patients.
Left ventricular hypertrophy (LVH) occurs frequently in children with CKD, with a reported prevalence of 17%–49%.1–5 Some data in children with CKD suggest that the development of LVH is associated with other cardiac abnormalities, such as impaired systolic function.6–9 Thus, understanding the factors that contribute to LVH in children with CKD may lead to interventions to reduce future cardiovascular risk.
In adults with CKD, hypertension has clearly been linked to the development of LVH.10–14 Reduction in LVH by antihypertensive treatment is associated with improved outcome and decreased risk of cardiovascular morbidity and mortality.10–12 However, the link between elevated BP and LVH in children with CKD remains controversial.1–3,15
To better understand the relationships between BP and LVH in children with CKD, we analyzed BP and echocardiographic data from children enrolled in the Chronic Kidney Disease in Children (CKiD) cohort, a prospective observational study of children with mild to moderate CKD. The objectives of this study were to (1) analyze the longitudinal effects of casual BP on left ventricular mass index (LVMI) and (2) determine whether casual BP is associated with the development and/or regression of LVH in children with CKD.
Results
Cohort Characteristics
Of 586 children in the CKiD cohort, 478 children had an echocardiogram (ECHO) at one or more of the study visits (visits 2, 4, and 6). Of these children, 63.8% had an ECHO at two or more visits for a total of 898 observations. Demographic and clinical characteristics of the subject population are summarized in Table 1. The data are summarized overall and by visit, because not all children had an ECHO at all three visits. The first ECHO occurred at either visit 2 or 4 for all but three children. At visit 2 (V2; 1 year into the study), median age was 12 years, with median duration of CKD of 7.4 years. Sex distribution showed a 2:3 female to male ratio; African American to non-African American ratio was 1:4. Approximately one fifth of children were reported to have a low birth weight; 13% of children were born prematurely. Participants tended to be short with preserved weight; 16% of participants were obese. Most children had stage 3 CKD (GFR between 30 and 60 ml/min per 1.73 m2), with 80% being of nonglomerular origin.
Table 1.
Characteristics of study population
| Characteristic | Median (IQR) or N (%) at V2 (n=435) | Median (IQR) or N (%) at V4 (n=321) | Median (IQR) or N (%) at V6 (n=142) | Median (IQR) or N (%) All Person-Visits (n=898) |
|---|---|---|---|---|
| LVMI | 33.6 (27.7–39.4) | 31.5 (26.7–36.5) | 28.6 (24.0–33.5) | 32.0 (26.6–37.8) |
| LVH | 68 (16) | 43 (13) | 16 (11) | 127 (14) |
| Age at ECHO (yr) | 12 (8–15) | 13 (10–17) | 14 (11–18) | 13 (9–16) |
| Female sexa | 176 (40) | 125 (39) | 53 (37) | 189 (40) |
| African-American racea | 90 (21) | 58 (18) | 29 (20) | 103 (22) |
| Weight percentileb | 44.1 (19.6–77.1) | 48.4 (20.7–76.8) | 50.9 (18.8–78.3) | 46.9 (19.6–77.2) |
| Height percentileb | 24.7 (8.1–52.7) | 27.1 (8.8–50.8) | 29.1 (11.8–52.6) | 26.0 (8.8–52.5) |
| BMI percentileb | 61.1 (35.4–85.5) | 61.3 (32.7–87.2) | 61.9 (31.9–85.9) | 61.3 (34.3–86.2) |
| BMI>95th (obese)b | 68 (16) | 45 (14) | 17 (12) | 130 (14) |
| Low birth weighta | 79 (19) | 57 (19) | 22 (16) | 84 (18) |
| Preterm birth<36 wka | 54 (13) | 39 (13) | 13 (9) | 59 (13) |
| Duration of CKD | 7.4 (4.0–11.2) | 9.4 (6.3–12.7) | 10.9 (8.5–14.2) | 8.6 (5.6–12.5) |
| Glomerular CKDa | 87 (20) | 52 (16) | 21 (15) | 97 (20) |
| Iohexol GFR | 43.8 (32.7–56.9) | 45.6 (32.3–61.4) | 41.1 (30.2–55.2) | 43.8 (32.1–58.0) |
| SBP z scoreb,c | 0.4 (−0.3–1.0) | 0.0 (−0.6–0.7) | 0.1 (−0.5–0.7) | 0.2 (−0.4–0.8) |
| DBP z scoreb,c | 0.5 (−0.1–1.1) | 0.2 (−0.3–0.8) | 0.4 (−0.4–0.9) | 0.4 (−0.2–0.9) |
| Antihypertensive medications | 283 (65) | 210 (65) | 98 (69) | 591 (66) |
| ACEI/ARB | 237 (54) | 177 (55) | 86 (61) | 86 (61) |
| Calcium channel blocker | 63 (14) | 45 (14) | 25 (18) | 133 (15) |
| Other | 13 (3) | 9 (3) | 2 (1) | 24 (3) |
| ≥2 medications | 30 (7) | 21 (7) | 15 (11) | 66 (7) |
| Hypoalbuminemia | 5 (1) | 0 (0.0) | 0 (0.0) | 5 (1) |
| Nephrotic proteinuriad | 53 (13) | 49 (16) | 25 (19) | 127 (15) |
| Hemoglobin | 12.6 (11.7–13.6) | 12.7 (11.6–13.8) | 12.6 (11.4–13.8) | 12.7 (11.6–13.7) |
| Anemia | 132 (31) | 100 (31) | 50 (37) | 50 (37) |
| wrCRPe | 0.3 (0.1–1.8) | 0.2 (0.0–1.3) | 0.3 (0.2–1.9) | 0.3 (0.1–1.6) |
| Albumin-corrected calcium | 9.4 (9.1–9.6) | 9.2 (9.0–9.5) | 9.1 (8.8–9.3) | 9.3 (9.0–9.5) |
| Phosphate | 4.7 (4.2–5.2) | 4.5 (4.0–5.1) | 4.4 (3.9–5.0) | 4.6 (4.0–5.1) |
| Calcium × phosphate | 44.0 (39.1–48.8) | 42.0 (37.2–47.0) | 39.6 (35.1–45.0) | 42.6 (37.7–47.7) |
IQR, interquartile range; BMI, body mass index; wrCRP, wide range C-reactive protein.
All person-visits statistic is participant-based (n=478).
Based on mean of values from two visits.
Adjusted for age, sex, and height.
Protein to creatinine ratio>2.
wrCRP measured at V1b, V3 and V5.
At V2 and V4 (3 years into the study), two thirds of the subjects were treated with antihypertensive medications. Most subjects were taking either an angiotensin-converting enzyme inhibitor (ACEI) or an angiotensin receptor blocker (ARB); 14% of treated children were taking a calcium channel blocker alone or in combination, and only 7% of children were taking two or more antihypertensive medications. At V6 (5 years into the study), a slightly higher percentage of children was receiving antihypertensive medications (61% ACEI or ARB, 18% calcium channel blocker, and 11% combination therapy).
Median systolic BP (SBP) z score was 0.4 at V2, 0.0 at V4, and 0.1 at V6; median diastolic BP (DBP) z score was 0.5 at V2, 0.2 at V4, and 0.4 at V6. Median LVMI was 33.6 g/m2.7 at V2, 31.5 g/m2.7 at V4, and 28.6 g/m2.7 at V6. The percentage of children with LVH was 16% at V2, 13% at V4, and 11% at V6 (Table 1). The prevalence of LVH in children within 1 year of reaching ESRD (dialysis or transplant) or death was 37% at V2 (n=30), 25% at V4 (n=28), and 29% at V6 (n=7), which is substantially larger than the overall prevalence of LVH at each visit. This difference in the prevalence of LVH directed our approach in analysis by adjusting for informative dropout to provide unbiased estimates of these rates.
Effects of BP and Time on LVMI
Results from the longitudinal mixed model components of the joint models predicting LVMI using SBP and DBP are shown in Tables 2 and 3, respectively. When adjusted for SBP and confounders, LVMI had an average decrease of 36.1% (P<0.001) from V2 to V4 and 40.2% (P=0.01) from V2 to V6. For every 1 SD increase in SBP z score, LVMI increased by 3.4% (P=0.01) at V2, and this effect increased to 7.3% (P<0.001) at V4 and 7.9% (P=0.004) at V6.
Table 2.
Results from multivariate model predicting LVMI with SBP
| Covariatea | Estimated Change (95% Confidence Interval) | P Value |
|---|---|---|
| SBP z score × V2b,c | 3.4% (0.7% to 6.2%) | 0.01 |
| SBP z score × V4 | 7.3% (3.8% to 10.8%) | <0.001 |
| SBP z score × V6 | 7.9% (2.5% to 13.7%) | 0.004 |
| V4 versus V2 | −36.1% (−49.5% to −19.3%) | <0.001 |
| V6 versus V2 | −40.2% (−59.6% to −11.3%) | 0.01 |
| Average height (cm) × V2d,e | −0.5% (−0.7% to −0.3%) | <0.001 |
| Average height (cm) × V4 | −0.2% (−0.5% to 0.1%) | 0.22 |
| Average height (cm) × V6 | −0.2% (−0.5% to 0.2%) | 0.39 |
| GFR | 0.0% (−0.1 to 0.1%) | 0.97 |
| Female sex | −3.2% (−7.6 to 1.4%) | 0.17 |
| African-American race | 4.1% (−1.5% to 10.1%) | 0.15 |
| Anemia | 6.2% (1.8% to 10.9%) | 0.006 |
| ACEI or ARB | −0.5% (−4.7% to 3.9%) | 0.82 |
| Other antihypertensive medication | 9.8% (2.3% to 17.8%) | 0.009 |
Visit is a categorical variable. Additional covariates in the model (nonsignificant) are age, CKD diagnosis, duration of CKD, and duration of CKD–visit interaction.
Overall test for SBP–visit interaction (P=0.10).
Slope of V2 SBP z score versus average of V4 and V6 SBP z score slopes (P=0.04).
Overall test for height–visit interaction (P<0.001).
Slope of V2 height versus average of V4 and V6 heights (P<0.001).
Table 3.
Results from multivariate model predicting LVMI with DBP
| Covariatea | Estimated Change (95% Confidence Interval) | P Value |
|---|---|---|
| DBP z score × V2b,c | −0.1% (−3.1% to 3.1%) | 0.97 |
| DBP z score × V4 | 4.9% (1.0% to 8.9%) | 0.01 |
| DBP z score × V6 | −1.1% (−6.7% to 4.8%) | 0.70 |
| V4 versus V2 | −36.1% (−49.3% to −19.5%) | <0.001 |
| V6 versus V2 | −33.1% (−55.2% to −0.1%) | 0.05 |
| Average height (cm) × V2d,e | −0.5% (−0.8% to −0.3%) | <0.001 |
| Average height (cm) × V4 | −0.2% (−0.5% to 0.0%) | 0.10 |
| Average height (cm) × V6 | −0.3% (−0.6% to 0.1%) | 0.14 |
| GFR | 0.0% (−0.1% to 0.1%) | 0.54 |
| Female sex | −2.9% (−7.5% to 1.8%) | 0.22 |
| African-American race | 6.4% (0.6% to 12.7%) | 0.03 |
| Anemia | 6.7% (2.2% to 11.4%) | 0.003 |
| ACEI or ARB | −0.9% (−5.3% to 3.6%) | 0.68 |
| Other antihypertensive medication | 10.7% (3.1% to 18.9%) | 0.005 |
Visit is a categorical variable. Additional covariates in the model (nonsignificant) are age, CKD diagnosis, duration of CKD, and duration of CKD–visit interaction.
Overall test for DBP–visit interaction (P=0.07).
Slope of V2 DBP z score versus average of V4 and V6 DBP z score slopes (P=0.41).
Overall test for height–visit interaction (P=0.001).
Slope of V2 height versus average of V4 and V6 heights (P=0.002).
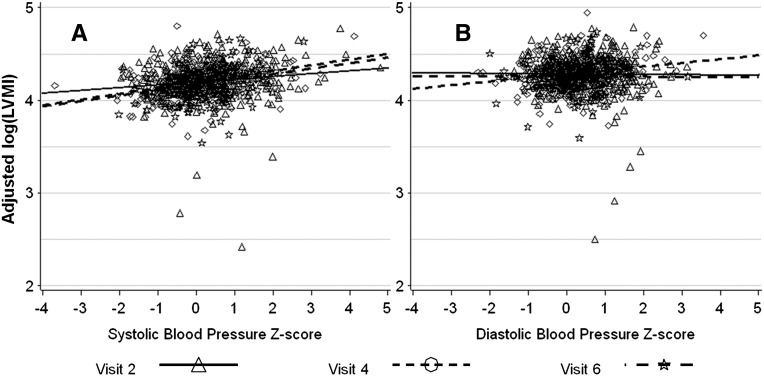
When adjusted for DBP and confounders, LVMI had an average decrease of 36.1% (P<0.001) from V2 to V4 and 33.1% (P=0.05) from V2 to V6. For every 1 SD increase in DBP z score, LVMI was unchanged at V2, increased by 4.9% (P=0.01) at V4, and decreased by 1.1% (P=0.70) at V6. This result is illustrated in Figure 1, which shows the relationship between SBP and DBP z scores and LVMI. Figure 1 also shows that, after adjusting for the covariates in the multivariate models, there was a slight positive relationship between SBP z score and log(LVMI) at all visits and no relationship between DBP z score and log(LVMI) at V2 and V6, with a slightly increased slope at V4. Based on the multivariate models, there was no slope–visit interaction effect and therefore, no change in slope over time. The estimated SBP effect in the reduced model without interaction was 5.2%, and the DBP effect was 1.5%.
Figure 1.
Partial residual plot of Log(LVMI) versus (A) SBP and (B) DBP z scores. BP was adjusted for age, sex, and height.
Effects of BP and Time on LVH
Tables 4 and 5 show the results of multivariate logistic models predicting LVH with SBP and DBP, respectively. SBP z score at V2 and V4 was a significant predictor, with 1.82 and 2.68 increased odds of having LVH per unit z-score increase in SBP at V2 and V4, respectively. DBP z score, although not a significant predictor, showed increased odds at V2 and V4 as well. Female sex increased the odds of having LVH by more than four and anemia by more than three. The use of antihypertensive medications other than ACEIs or ARBs was associated with an increase in the odds of having LVH in both models, although it was a significant predictor only in the DBP model. Kidney function (measured GFR) was not a significant predictor of LVH.
Table 4.
Results from the multivariate logistic model predicting LVH with SBP
| Covariatea | Odds Ratio | Lower Confidence Limit | Upper Confidence Limit | P Value |
|---|---|---|---|---|
| SBP z score × V2 | 1.82 | 1.14 | 2.91 | 0.01 |
| SBP z score × V4 | 2.68 | 1.53 | 4.70 | <0.001 |
| SBP z score × V6 | 1.68 | 0.64 | 4.40 | 0.29 |
| Female sex | 4.26 | 1.87 | 9.71 | <0.001 |
| African-American race | 1.82 | 0.75 | 4.41 | 0.18 |
| Anemia | 2.96 | 1.40 | 6.25 | 0.005 |
| ACEI or ARB | 0.66 | 0.31 | 1.42 | 0.29 |
| Other antihypertensive medication | 2.65 | 0.88 | 8.04 | 0.08 |
Visit is a categorical variable. Additional covariates in the model (nonsignificant) are GFR, height, age, CKD diagnosis, duration of CKD, and duration of CKD–visit interaction.
Table 5.
Results from the multivariate logistic model predicting LVH with DBP
| Covariatea | Odds Ratio | Lower Confidence Limit | Upper Confidence Limit | P Value |
|---|---|---|---|---|
| DBP z score × V2 | 1.56 | 0.90 | 2.69 | 0.11 |
| DBP z score × V4 | 1.66 | 0.94 | 2.96 | 0.08 |
| DBP z score × V6 | 1.03 | 0.37 | 2.91 | 0.95 |
| Female sex | 4.54 | 1.97 | 10.50 | <0.001 |
| African-American race | 2.17 | 0.89 | 5.33 | 0.09 |
| Anemia | 3.03 | 1.44 | 6.40 | 0.004 |
| ACEI or ARB | 0.71 | 0.33 | 1.55 | 0.39 |
| Other antihypertensive medication | 3.11 | 1.01 | 9.54 | 0.05 |
Visit is a categorical variable. Additional covariates in the model (nonsignificant) are GFR, height, age, CKD diagnosis, duration of CKD, and duration of CKD–visit interaction.
We calculated estimated rates of LVH at each visit from the joint model. For the systolic model, LVH rates were 15.3% (SE=0.60) at V2, 13.6% (SE=0.74) at V4, and 12.6% (SE=0.89) at V6; for the diastolic model, rates were 15.1% (SE=0.56) at V2, 13.6% (SE=0.59) at V4, and 12.6% (SE=0.79) at V6. We also performed a sensitivity analysis of the results of the joint model for the association of BP with both LVMI and LVH (Supplemental Table 1). In addition to the two general estimating equations and (unadjusted) linear mixed models, a joint model with a Weibull distribution for time to dropout as well as the better fitting lognormal model were included. The results of this analysis indicated the stability of the mixed and joint models.
Discussion
Main Findings
This study is the largest longitudinal study analyzing the effect of BP on cardiac structure in children with CKD stages 2–4. It confirms our previous cross-sectional findings that LVH is strongly associated with hypertension in children with CKD.2 Furthermore, we show that there was a positive association between BP and LVMI in this population over a 4-year follow-up period. We found a similar association between BP and LVH corresponding to regression of LVH over time, with a decline in the estimated prevalence of LVH after the 4-year period of observation, based on the joint model.
Prevalence of LVH
Previous single- and multicenter studies have reported a prevalence of LVH in children with mild to moderate CKD to vary widely from 17% to 49%, depending on the definition of LVH and the stage of CKD.1–5,16 Previous cross-sectional analysis in the CKiD cohort (n=366) reported a 17% prevalence of LVH at baseline.2 Our present longitudinal study, in a larger population (n=478), showed similar prevalence of LVH at baseline (V2) of 16%. Using the same normative data, a recent smaller, single-center cross-sectional study in 49 nonhypertensive CKD children found a 49% prevalence of LVH (24 of 49 subjects).3 Although the mean GFR in that study was lower (26.1 ml/min per 1.73 m2) than in our study (43.8 ml/min per 1.73 m2), reflecting more advanced kidney disease, a significant relationship of GFR with LVH was not found, suggesting that the difference is not explained by declining kidney function. Similarly, our previous cross-sectional study2 as well as current analysis showed no association of kidney function (measured GFR) with LVH. In contrast, reduced kidney function assessed by cystatin C was associated with a higher prevalence of LVH in the recent report from the Chronic Renal Insufficiency Cohort Study in adults.17
Effects of BP and Time on LVMI
The link between BP and LVMI in CKD children has been debated in the literature.1–4,15 One of the important findings of our current longitudinal study is the confirmation of a strong association between casual BP and LVMI in the CKiD population. The multivariate model predicting LVMI with SBP also showed that, after adjusting for SBP and confounders (including age), LVMI had an average decrease of 40% over 4 years. When adjusted for DBP and confounders, LVMI also had a decrease of 33%. The effect of DBP in this context was significant only at V4. The adjustment for age was necessary, because LVMI naturally decreases as children get older when LVM is indexed to height (meter2.7).18 For example, LVMI decreases from 70 g/m2.7 in 1-year-old children to 40 g/m2.7 in female and 45 g/m2.7 in male adolescents. A recent smaller, single-center study3 found that SBP was the only BP measure that maintained a consistent association with LVMI after adjustment for confounders.
Effects of BP and Time on LVH
Among the aims of the CKiD study is the identification of risk factors in the development or progression of cardiovascular disease in CKD. In this regard, we have identified four important independent predictors of LVH in the CKiD population, including SBP, anemia, female sex, and use of antihypertensive medications other than ACEI or ARB. To avoid misclassification of diagnosis of LVH, our analysis also included adjustment for height. This adjustment was required, because the study participants had significantly reduced height (median height percentiles 25–29) relative to age. We found no significant independent relationship between height and LVH in the multivariable analysis.
Previous studies in both children and adults have found a significant relationship between low hemoglobin and increased LVMI.1,16,19,20 Low hemoglobin was a significant predictor in our study as well; anemia increased the risk of LVH by more than three times, illustrating the need for appropriate treatment of anemia in children with CKD.
The likelihood of developing LVH was four times higher in females than males, suggesting the possibility that hormonal factors might be important. Several studies have shown sex differences in cardiac structure.21–23 For example, in animal models of pressure overload, females did develop more hypertrophy than males.21 Furthermore, an association between estrogen gene polymorphism and LVM has been reported.23 However, this finding may be related to unknown factors affecting the normative values used for LVMI and represent a limitation to our findings.
The use of antihypertensive medication other than ACEI or ARB was a significant predictor of LVH in the multivariate analysis (only for DBP), whereas both ACEI and ARB medications, although not significant, seemed to be protective for the development of LVH. Both ACEI and ARB are known to decrease myocardial fibrosis in hypertensive heart disease. This effect could occur independently of any regression of LVH.24–26 In a baseline cross-sectional analysis,15 the use of antihypertensive medication other than ACEI or ARB was also associated with uncontrolled BP. It is possible that the association with the lower prevalence of LVH is related to a better control of hypertension with ACEI and ARB in our study. However, we cannot rule out selection by indication as a possible explanation for the medication effect.
Some of the initial subjects dropped out of the study because of progression to ESRD. These children with more advanced CKD had almost two times higher (37% at V2 and 29% at V6) prevalence of LVH before exiting the study than the overall cohort. This finding may explain, at least in part, why the prevalence of LVH decreased over time in children who remained in the study. To account for the additional information not provided by subjects who experienced ESRD or died, we used a shared parameter joint (linear or logistic) model for the longitudinal outcome of interest (in our case, LVMI or LVH) and the time to ESRD or death as described below. This model also allowed us to estimate the rates of LVH at each visit adjusted for informative dropout as presented above.
Strengths and Limitations
This study has important strengths, including a large sample size, standardized demographic, clinical, laboratory, and ECHO data, and precise measurement of GFR with iohexol infusion.27 In addition, we report on longitudinal data of most children on this cohort, which confirmed many of the preliminary findings on the cross-sectional analysis. Conversely, the current analysis was limited to casual BP data without 24-hour ambulatory BP monitoring. Hence, we plan to perform the analysis in the near future after sufficient data on ambulatory BP monitoring become available. At the same time, the association of office BP with cardiac structure is of important clinical significance, because casual BP is still much preferred, and also, it is the dominant method of BP evaluation in clinical practice. Additional studies are also warranted to examine the effect of other factors (e.g., anemia) on LVH changes over time. However, the main focus of our work was the BP effect on LVH over time. Therefore, methodological and analytical considerations directly reflect this main objective.
There is a potential limitation imposed by the strong assumptions made by the joint longitudinal and survival model for dealing with informative dropout. Specifically, these models assume that the information in the missing data for a subject who drops out is basically available in the shared random effect. Similar criticisms have been made of selection models, which assume that the missing data can be summarized by the initial missing value of the longitudinal outcome variable. We would argue that the assumption of a shared random effect has a considerable degree of plausibility in the same way as in the linear mixed model. Mixed models having both fixed and random effects have proven their use in many years of application. In addition, the results of the sensitivity analysis, showing the stability of the mixed and joint models, validates our approach.
Conclusions
Hypertension is associated with LVH in pediatric CKD, and therefore, regression of LVH can be accomplished with better BP control. Future research studies should be focused on defining the adequate BP targets and addressing whether the improvements in BP and cardiac structure are associated with the improvement of long-term cardiovascular-related outcomes in children with CKD.
Concise Methods
Study Population
The CKiD study is a multicenter, prospective cohort study of 586 children with mild to moderate CKD being conducted at 48 pediatric nephrology centers in North America. Briefly, eligible children were between the ages of 1 and 16 years and had an estimated GFR between 30 and 90 ml/min per 1.73 m2. The CKiD study design and conduct were approved by an external advisory committee appointed by the National Institutes of Health and the Institutional Review Boards at each participating center. Each participating parent or guardian provided informed consent. Assent from child was obtained when age appropriate. The study design and objectives have been published previously.27
At all visits in this analysis, GFR was determined by directly measured plasma iohexol (GE Healthcare, Amersham Division, Princeton, NJ) disappearance curves; details of the GFR assessment method have been published previously.28 An estimated GFR value was used when a directly measured value was unavailable.29 LVMI was determined by ECHO, which was performed 1 year after study entry and then every 2 years thereafter; BP was measured at every visit. At this point, data are available from V2 to V6 for some of the children depending on enrollment date.
Casual BP Measurements
CKiD participants had casual BP measurements obtained from the right arm at study entry (baseline) and annual intervals after enrollment. Casual BP in CKiD is obtained by auscultation using an aneroid sphygmomanometer (Mabis MedicKit 5; Mabis Healthcare, Waukegan, IL); all participating sites have been provided with the same equipment by the CKiD Clinical Coordinating Centers. The Clinical Coordinating Centers also provide standardized training and certification in the auscultatory BP measurement protocol to all study personnel responsible for casual BP measurement. The specific procedure for BP determination in the CKiD study has been described elsewhere.15 The average of three BP measurements is recorded as the participants’ casual BP for that study visit. Because LVMI in CKiD is measured only at the even-numbered visits, the BP used in this analysis is the average of the BP at the corresponding even-numbered visit and the prior visit. The rationale for averaging the two BP z scores is that the average over the two visits is more representative of the 1-year time period than a single measurement, and the effect precedes the outcome (LVMI and LVH).
LVM and LVH
The specific procedure for ECHO determination of LVM and definition of LVH in the CKiD study have been described elsewhere.15 Briefly, M-Mode and Doppler ECHO are performed at individual participating centers, but reading and analyses of ECHO data are performed by the Cardiovascular Core Imaging Research Laboratory at Cincinnati Children’s Hospital Medical Center. To achieve standardization and uniformity of ECHO images across the centers, qualifying recordings were sent to each center and then certified at the Cardiovascular Core Imaging Research Laboratory. LVM was indexed (mass divided by height raised to a power of 2.7 [grams per meter2.7]) to account for body size.30 LVH was defined as LVMI≥95th percentile for normal children and adolescents.18
Statistical Analyses
For LVMI, data were analyzed using a linear mixed model, which included a random subject effect to adjust for repeated measurement of the same subjects at different visits. The analysis included an examination of residuals to check on the required assumptions of normally distributed errors with constant variance. This analysis indicated that the variance was increasing with the mean, and a logarithmic transformation was used to stabilize the variance. For this reason, the regression coefficients from the mixed model are reported as multiplicative effects (percent change).
Casual BP measurements from each visit were standardized using height along with age- and sex-specific norms, with the resulting values expressed as z scores. The primary exposure in the mixed model was either SBP or DBP z score as described above. Separate models were run for each of two exposures. In addition, we were interested in whether the effect of the exposure on LVMI would increase with time. To allow for this possibility, an interaction between BP and visit was included in the model. The interaction terms allowed separate regressions on BP at each visit.
Additional covariates included in the model were sex, African-American race, CKD diagnosis (glomerular versus nonglomerular), height at each visit, age, duration of CKD in years, duration of CKD–visit interaction, GFR, ACEI or ARB use, use of hypertensive medications other than ACEI/ARB, and presence of anemia.
Subjects initiating renal replacement therapy (RRT; dialysis or a kidney transplant) during follow-up did not undergo an ECHO or regular visits in the CKiD study past these events. Because these subjects had the most advanced disease progression, we have a case of informative dropout or nonrandom missingness. In this situation, the results of the longitudinal analysis will be biased, because data on the subjects with the most advanced disease are missing. To account for informative dropout in the multivariable analysis, longitudinal LVMI and time to RRT were modeled jointly using a shared parameter model for the longitudinal and time-to-event (RRT) data.31 The two components of the joint model are linked by shared random subject effect, and this linkage provides an adjustment for nonrandom missingness. Based on previous analyses from the CKiD study, we used a parametric log-normal model for the time until event (dropout) data. Thus, estimates of effects in the longitudinal model were adjusted for informative dropout of the sickest children (those children experiencing RRT).
We analyzed data on the presence or absence of LVH at each visit using a mixed effect logistic regression model. This model also included a random subject effect, similar to the linear mixed model, and was part of a shared parameter model adjusting for informative dropout. The joint model was also used to estimate the rate of LVH at each period. This estimation was done by first using numerical integration of the conditional probability of LVH from the model (including the random effect) to estimate the marginal probability of LVH at each period for each child and then averaging these marginal estimates. For this analysis, we used the PROC NLMIXED procedure in SAS. A template of the program that we used may be found in the work by Littell et al.32 This program was modified for different joint longitudinal and survival models.
Disclosures
None.
Supplementary Material
Acknowledgments
The Chronic Kidney Disease in Children (CKiD) prospective cohort study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Child Health and Human Development, and National Heart, Lung, and Blood Institute Grants U01-DK-66143, U01-DK-66174, U01-DK-082194, and U01-DK66116.
Data were partially presented in abstract form at Kidney Week, November 10, 2011, in Philadelphia, PA.
Data were collected by the CKiD prospective cohort study, with clinical coordinating centers at Children’s Mercy Hospital and the University of Missouri at Kansas City (Bradley Warady, principal investigator) and the University of Pennsylvania (Susan Furth, principal investigator) and a data coordinating center at the Johns Hopkins Bloomberg School of Public Health (Alvaro Munoz, principal investigator).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012121197/-/DCSupplemental.
References
- 1.Matteucci MC, Wühl E, Picca S, Mastrostefano A, Rinelli G, Romano C, Rizzoni G, Mehls O, de Simone G, Schaefer F, ESCAPE Trial Group : Left ventricular geometry in children with mild to moderate chronic renal insufficiency. J Am Soc Nephrol 17: 218–226, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, Kimball T, Furth S, Warady B, CKiD Study Group : Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol 21: 137–144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha MD, Tibby SM, Rasmussen P, Rawlins D, Turner C, Dalton RN, Reid CJ, Rigden SP, Booth CJ, Simpson JM: Blood pressure control and left ventricular mass in children with chronic kidney disease. Clin J Am Soc Nephrol 6: 543–551, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson JM, Savis A, Rawlins D, Qureshi S, Sinha MD: Incidence of left ventricular hypertrophy in children with kidney disease: Impact of method of indexation of left ventricular mass. Eur J Echocardiogr 11: 271–277, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Johnstone LM, Jones CL, Grigg LE, Wilkinson JL, Walker RG, Powell HR: Left ventricular abnormalities in children, adolescents and young adults with renal disease. Kidney Int 50: 998–1006, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Malatesta-Muncher R, Wansapura J, Taylor M, Lindquist D, Hor K, Mitsnefes M: Early cardiac dysfunction in pediatric patients on maintenance dialysis and post kidney transplant. Pediatr Nephrol 27: 1157–1164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver DJ, Jr, Kimball T, Witt SA, Glascock BJ, Khoury PR, Kartal J, Mitsnefes MM: Subclinical systolic dysfunction in pediatric patients with chronic kidney disease. J Pediatr 153: 565–569, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Chinali M, de Simone G, Matteucci MC, Picca S, Mastrostefano A, Anarat A, Caliskan S, Jeck N, Neuhaus TJ, Peco-Antic A, Peruzzi L, Testa S, Mehls O, Wühl E, Schaefer F, ESCAPE Trial Group : Reduced systolic myocardial function in children with chronic renal insufficiency. J Am Soc Nephrol 18: 593–598, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Matteucci MC, Chinali M, Rinelli G, Wühl E, Zurowska A, Charbit M, Pongiglione G, Schaefer F, ESCAPE Trial Group : Change in cardiac geometry and function in CKD children during strict BP control: A randomized study. Clin J Am Soc Nephrol 8: 203–210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larstorp ACK, Okin PM, Devereux RB, Olsen MH, Ibsen H, Dahlöf B, Kjeldsen SE, Wachtell K: Regression of ECG-LVH is associated with lower risk of new-onset heart failure and mortality in patients with isolated systolic hypertension; The LIFE study. Am J Hypertens 25: 1101–1109, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, Crump J, Reisin E: Regression of left ventricular hypertrophy is a key goal of hypertension management. Curr Hypertens Rep 5: 301–308, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Pierdomenico SD, Lapenna D, Cuccurullo F: Regression of echocardiographic left ventricular hypertrophy after 2 years of therapy reduces cardiovascular risk in patients with essential hypertension. Am J Hypertens 21: 464–470, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Brown DW, Giles WH, Croft JB: Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J 140: 848–856, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Gosse P: Left ventricular hypertrophy as a predictor of cardiovascular risk. J Hypertens Suppl 23: S27–S33, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA, Chronic Kidney Disease in Children Study Group : Blood pressure in children with chronic kidney disease: A report from the Chronic Kidney Disease in Children study. Hypertension 52: 631–637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Progression of left ventricular hypertrophy in children with early chronic kidney disease: 2-year follow-up study. J Pediatr 149: 671–675, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS, Shlipak MG, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 23: 1725–1734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR: Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 22: 709–714, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Mitsnefes MM, Daniels SR, Schwartz SM, Meyer RA, Khoury P, Strife CF: Severe left ventricular hypertrophy in pediatric dialysis: Prevalence and predictors. Pediatr Nephrol 14: 898–902, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Levin A: Anemia and left ventricular hypertrophy in chronic kidney disease populations: A review of the current state of knowledge. Kidney Int Suppl 80: 35–38, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH: Hypertrophic remodeling: Gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol 32: 1118–1125, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Peter I, Shearman AM, Vasan RS, Zucker DR, Schmid CH, Demissie S, Cupples LA, Kuvin JT, Karas RH, Mendelsohn ME, Housman DE, Benjamin EJ: Association of estrogen receptor beta gene polymorphisms with left ventricular mass and wall thickness in women. Am J Hypertens 18: 1388–1395, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Leibowitz D, Dresner-Pollak R, Dvir S, Rokach A, Reznik L, Pollak A: Association of an estrogen receptor-alpha gene polymorphism with left ventricular mass. Blood Press 15: 45–50, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Brilla CG, Funck RC, Rupp H: Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 102: 1388–1393, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Devereux RB, Dahlöf B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris KE, Edelman JM, Wachtell K: Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: The Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation 110: 1456–1462, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Díez J, Querejeta R, López B, González A, Larman M, Martínez Ubago JL: Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 105: 2512–2517, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz GJ, Furth S, Cole SR, Warady B, Muñoz A: Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Muñoz A: Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 82: 445–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH: Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. J Am Coll Cardiol 20: 1251–1260, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Albert PS, Follman DA: Shared-parameter models. In: Longitudinal Data Analysis, edited by Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, Boca Raton, FL, Chapman & Hall/CRC Press, 2009, pp 433–452 [Google Scholar]
- 32.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O: SAS for Mixed Models, Section 15.6, 2nd Ed Cary, NC, SAS Institute Inc., 2006 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.