Abstract
Vascular growth factors play an important role in maintaining the structure and integrity of the glomerular filtration barrier. In healthy adult glomeruli, the proendothelial survival factors vascular endothelial growth factor-A (VEGF-A) and angiopoietin-1 are constitutively expressed in glomerular podocyte epithelia. We demonstrate that this milieu of vascular growth factors is altered in streptozotocin-induced type 1 diabetic mice, with decreased angiopoietin-1 levels, VEGF-A upregulation, decreased soluble VEGF receptor-1 (VEGFR1), and increased VEGFR2 phosphorylation. This was accompanied by marked albuminuria, nephromegaly, hyperfiltration, glomerular ultrastructural alterations, and aberrant angiogenesis. We subsequently hypothesized that restoration of angiopoietin-1 expression within glomeruli might ameliorate manifestations of early diabetic glomerulopathy. Podocyte-specific inducible repletion of angiopoietin-1 in diabetic mice caused a 70% reduction of albuminuria and prevented diabetes-induced glomerular endothelial cell proliferation; hyperfiltration and renal morphology were unchanged. Furthermore, angiopoietin-1 repletion in diabetic mice increased Tie-2 phosphorylation, elevated soluble VEGFR1, and was paralleled by a decrease in VEGFR2 phosphorylation and increased endothelial nitric oxide synthase Ser1177 phosphorylation. Diabetes-induced nephrin phosphorylation was also reduced in mice with angiopoietin-1 repletion. In conclusion, targeted angiopoietin-1 therapy shows promise as a renoprotective tool in the early stages of diabetic kidney disease.
Diabetic nephropathy (DN) is the leading cause of ESRD characterized by structural changes in the kidney glomerular filtration barrier, including podocyte foot process fusion, basement membrane thickening, mesangial expansion, and abnormal angiogenesis leading to albuminuria.1,2
Healthy adult podocytes express vascular endothelial growth factor-A (VEGF-A) and angiopoietin-1 (Angpt1).3,4 VEGF-A binds to its receptors VEGFR1 and VEGFR2; the former also exists as a soluble form (sVEGFR1), which is an inhibitor of VEGF-A signaling.5 Angpt1 phosphorylates the TEK tyrosine kinase (Tie-2) receptor.5 VEGFR2 and Tie-2 are mainly expressed by glomerular endothelia, and their concurrent activation is predicted to lead to endothelial survival and vessel stabilization.3,5,6 In otherwise healthy animals, podocyte VEGF-A depletion leads to proteinuria, whereas downregulation of podocyte Angpt1 does not appear to compromise glomerular biology.7,8 Several reports have suggested that DN kidneys have altered expression of VEGF-A, Angpt1, and also Angpt2, the natural antagonist of Angpt1.8–10 In diabetic mice, deletion of podocyte Angpt1,8 or further overexpression of VEGF-A in podocytes, worsens glomerular morphology and enhances proteinuria.11 Viral delivery of COMP-Angpt112 or either glomerular transgenic or systemic overexpression of sVEGFR1 ameliorates albuminuria in DN.13,14
Here we demonstrate an Angpt1 deficiency and enhanced VEGF-A signaling in diabetic mouse kidneys. This growth factor milieu is predicted to destabilize endothelia and enhance angiogenesis,5 an early feature of diabetic glomerulopathy.2 Accordingly, we hypothesized that site-directed Angpt1 therapy would ameliorate early diabetic glomerulopathy; to test this we used an inducible podocyte-specific transgenic Angpt1 strategy.
Results
Angpt1 Induction in Nondiabetic Mice
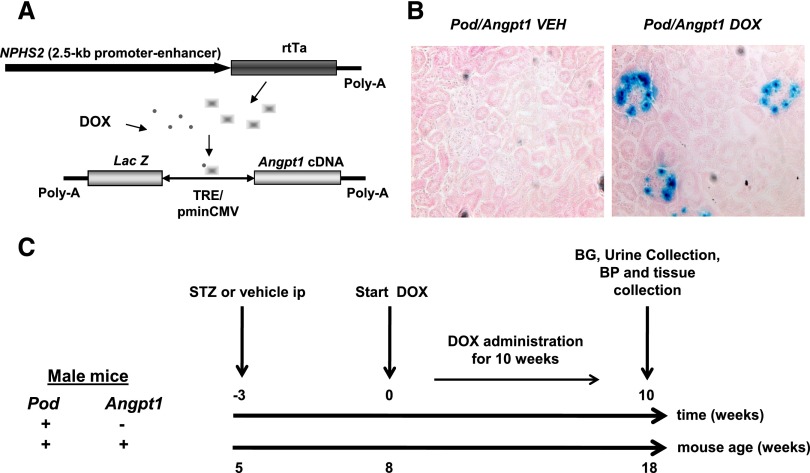
As a prelude to the DN study, our initial experiments examined whether inducible podocyte-specific Angpt1 upregulation had any effect on nondiabetic mice. We bred FVB/N mice homozygous for podocin-rtTA with BL6/CBA mice heterozygous for pTRE bidirectional LacZ/Angpt1 construct (Figure 1A) to generate two genotypes called Pod/Angpt1 and Pod/+ with a similar complements of background alleles. From 8 weeks of age, mice of both sexes were administered doxycycline or vehicle for 10 weeks, at which point they were analyzed. LacZ expression was evident only in the podocytes of Pod/Angpt1 mice administered doxycycline (Figure 1B); these animals had a two- to three-fold increase in renal cortical Angpt1 protein but no changes in Angpt2, Tie-2, phosphorylated Tie-2 (Tie-2-P)/total Tie-2 ratio, or VEGF-A (Supplemental Figure 1, A–E, and Supplemental Figure 2). Doxycycline -treated Pod/Angpt1 mouse kidneys were morphologically similar to controls; they had smooth outlines and were neither swollen nor contracted (data not shown); kidney/body weight was not different between groups (Supplemental Table 1). Transgenic Angpt1 upregulation did not alter 24-hour albumin excretion (Supplemental Figure 1F), creatinine clearance, systolic and diastolic blood pressure, or glomerular ultrastructure (Supplemental Table 1). Thus, podocyte Angpt1 upregulation in otherwise healthy animals did not alter glomerular structure and function. We therefore reasoned that this transgenic strategy could be used as a means of manipulating Angpt1 levels in diabetic glomeruli.
Figure 1.
Transgene constructs and experimental plan. Diagram of the constructs (A) used for the generation of transgenic mice. The construct above corresponds to a 2.5-kb fragment of the NPHS2 (podocin: Pod) promoter-enhancer region driving the expression of rtTA. In the presence of doxycycline (DOX), but not vehicle (VEH), rtTA binds to tetracycline-response operon promoter element (TRE) and initiates transcription from the cytomegalovirus (CMV) promoter of the LacZ/Angpt1 cDNAs. Nuclear X-gal staining of glomerular podocytes (B) was seen only in Pod/Angpt1 mice administered with DOX (X-Gal and eosin staining, magnification ×40). (C) The experimental plan followed in the diabetes study. BG, blood glucose; STZ, streptozotocin.
Angpt1 Deficiency Is Attenuated by Targeted Glomerular Angpt1 Repletion in Diabetic Mice
Five-week-old male Pod/Angpt1 and Pod/+ mice were made diabetic by daily injection of streptozotocin; in addition a group of Pod/+ mice were administered citrate buffer as nondiabetic controls. Three weeks later (8 weeks old mice), we isolated glomeruli and found that glomerular Angpt1 mRNA was decreased in diabetic mice, with no significant changes in Angpt2 mRNA between nondiabetic and diabetic mice (Figure 2A). To obtain insight in Angpt regulation in human podocytes, we performed in vitro studies with cells incubated in high or normal glucose conditions. Using quantitative RT-PCR, ANGPT1 mRNA was significantly downregulated in high glucose–treated cells compared with normal glucose–treated cells (Figure 2A). ANGPT2 transcripts were not detected in cultured podocytes (not shown).
Figure 2.
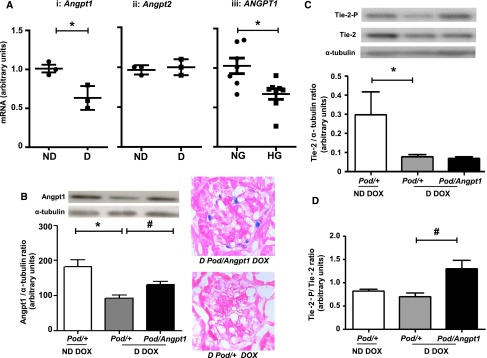
Diabetes-mediated Angpt imbalance is ameliorated in mice with Angpt1 repletion. In 8-week-old mice (before treatment with vehicle or doxycycline [DOX]) diabetes led to a decrease in Angpt1 mRNA in isolated glomeruli (D, diabetic; ND, nondiabetic; n=3/group; *P=0.02) (Ai); there was no change in Angpt2 mRNA (Aii). In human podocytes ANGPT1 mRNA was downregulated in high glucose (HG) compared with normal glucose (NG) conditions (Aiii) (n=7–8/group; *P=0.009). In mice with 10 weeks of DOX administration, renal cortical Angpt1 protein was decreased in diabetic mice compared with nondiabetic mice (n=8–11/group; *P=0.002); Angpt1 repletion in diabetic mice resulted in a 20%–30% increase in Angpt1 levels in diabetic mice (n=7–11/group; #P=0.02) (B). X-gal staining of D Pod/Angpt1 mice demonstrated positive expression in glomerular podocytes, indicating the transgene system was working in diabetic mice (B) (X-Gal and eosin staining, magnification x60). Kidney cortical Tie-2 was downregulated in diabetic animals compared with nondiabetic animals (n=7–8/group; *P=0.02) (C); in Pod/Angpt1 DOX diabetic mice, we observed a significant upregulation of Tie-2-P/Tie-2 ratio compared with diabetic control mice (n=7–8/group; #P=0.003) (D). *indicates comparisons between nondiabetic and diabetic state or normal and high glucose conditions; # indicates the comparisons between diabetic states without and with Angpt1 repletion.
We subsequently examined the effect of restoring Angpt1 in 8-week-old male mice that had been exposed to diabetes for 3 weeks. Nondiabetic Pod/+, diabetic Pod/Angpt1, and diabetic Pod/+ groups were fed doxycycline for 10 weeks and studied in detail when they were 18 weeks old (i.e., 13 weeks after induction of diabetes) (Figure 1C). We focused on two main comparisons: nondiabetic Pod/+ doxycycline versus diabetic Pod/+ doxycycline to assess the effect of diabetes, and diabetic Pod/+ doxycycline versus diabetic Pod/Angpt1 doxycycline to assess the effects of Angpt1 restoration in a diabetic milieu.
After 13 weeks of diabetes, the renal cortical Angpt1 protein expression was still significantly reduced (Figure 2B) in diabetic Pod/+ doxycycline compared with nondiabetic Pod/+ doxycycline mice. Diabetes also led to a significant downregulation of renal cortical Tie-2 protein expression (Figure 2C) but not phosphorylated Tie-2/total Tie-2 (Figure 2D). Podocyte-specific Angpt1 repletion in diabetic mice attenuated the Angpt1 deficiency found in diabetic Pod/+ doxycycline animals (Figure 2B). Diabetic Pod/Angpt1 had similar Tie-2 protein levels compared with diabetic Pod/+ doxycycline mice (Figure 2C), but Tie-2-P/total Tie-2 ratio was significantly increased (Figure 2D).
Effect of Targeted Angpt1 Repletion on Renal Clinical Parameters in Diabetic Mice
Thirteen weeks of diabetes increased circulating glucose levels and raised kidney/body weight in diabetic Pod/+ doxycycline compared with nondiabetic Pod/+ doxycycline mice; Angpt1 repletion did not alter either of these parameters (Table 1). Albuminuria was increased in diabetic Pod/+ doxycycline compared with nondiabetic Pod/+ doxycycline mice, and this was significantly attenuated by 70% in diabetic Pod/Angpt1 doxycycline mice (albuminuria expressed as geometric mean [95% confidence interval (CI)], µg/24 hours was as follows: nondiabetic Pod/+ doxycycline mice, 69 [46.7 to 102]; diabetic Pod/+ doxycycline mice, 2101 [951 to 4643]; diabetic Pod/Angpt1 doxycycline mice, 508 [326 to 794]) (Figure 3A). Diabetes also led to an increase in creatinine clearance and systolic BP, but Angpt1 repletion had no effect on these parameters (Table 1).
Table 1.
Clinical and biochemical characteristics of nondiabetic and diabetic mice with podocyte-specific Angpt1 repletion and relative controls
| Variable | Nondiabetic Pod/+ DOX | Diabetic Pod/+ DOX | Diabetic Pod/Angpt1 DOX |
|---|---|---|---|
| Fed glycemia (mM) | 5.5±0.3 | 33.1±0.8a | 30.7±1.9 |
| Body weight (g) | 28.8±0.6 | 26.1±0.7a | 24.0±0.7 |
| Kidney weight/body weight (mg/g) | 6.3±0.1 | 8.5±0.2a | 8.5±0.4 |
| Creatinine clearance (µl/min per g body weight) | 11.5±1.9 | 45.7±9.2a | 37.2±10 |
| Systolic BP (mmHg) | 119±3 | 127±3 | 123±2 |
Values are expressed as the mean ± SEM. Diabetic animals had a higher fed glycemia, lower body weight, increased kidney-to-body weight ratio, and increased creatinine clearance compared with nondiabetic animals. No differences were observed between diabetic animals with and those without Angpt1repletion (n=6–12/group). DOX, doxycycline.
P<0.05.
Figure 3.
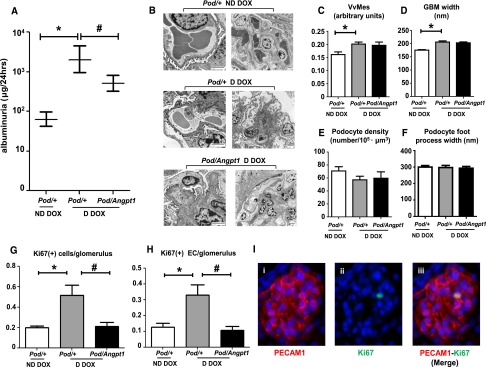
Angpt1 repletion ameliorates diabetes-mediated albuminuria and glomerular endothelial cells proliferation. Albuminuria (geometric mean, 95% confidence intervals) was increased in diabetic mice compared with nondiabetic mice (n=10–12/group; *P=0.001) (A); significant amelioration of albuminuria was observed in diabetic mice with restored Angpt1 levels (n=10–12/group; #P=0.003). Representative image on transmission electron microscopy (scale bar: 2 μm) (B) and quantitative electron microscopy analysis of glomerular parameters: mesangial volume fraction (VvMes) (C), glomerular basement (GBM) width (D), podocyte density (E), and foot process width (F) in nondiabetic Pod/+, diabetic Pod/+ and Pod/Angpt1 mice after 10 weeks of doxycycline (DOX) administration. VvMes and GBM width were increased in diabetic compared with nondiabetic mice (n=9–12/group; *P<0.01); Angpt1 repletion did not affect these parameters. Angpt1 repletion prevented the diabetes-induced increase in total glomerular Ki67-positive cells and proliferating endothelia (EC) (G and H, n=4/group; *P<0.01, #P<0.01). Representative image showing a Ki67-positive glomerular endothelial cell (I) (magnification ×60). D, diabetic; ND, nondiabetic. *indicates comparisons between nondiabetic and diabetic states; # indicates the comparisons between diabetic states without and with Angpt1 repletion.
Effect of Targeted Angpt1 Repletion on Glomerular Structure in Diabetic Mice
To determine whether changes in albumin excretion were associated with alterations in glomerular structure, detailed electron microscopy analyses were performed. Diabetic Pod/+ doxycycline mice showed mesangial expansion and increased glomerular basement membrane thickness compared with nondiabetic Pod/+ doxycycline mice; restoring Angpt1 levels in diabetes did not affect these parameters (Figure 3, C and D). No significant differences were observed in podocyte density and foot processes width (Figure 3, E and F) between any of the groups of mice studied. There was also no apparent alterations (e.g., cell swelling, cysts) observed in glomerular endothelial cell morphology after induction of diabetes with or without Angpt1 repletion. Notably, the total numbers of proliferating glomerular cells, as well as the numbers of proliferating glomerular endothelia, were elevated in diabetic Pod/+ doxycycline mice compared with nondiabetic Pod/+ doxycycline mice; restoring the Angpt1 levels significantly ameliorated these effects (Figure 3, G–I). Assessment of glycocalyx using wheat germ agglutinin immunohistochemistry, which labels N-acetyl glucosamine and N-acetyl neuraminic acid oligosaccharide moieties15 (Supplemental Figure 3) did not demonstrate any difference in the three groups of animal studied (percentage area with glomerular staining: nondiabetic Pod/+ doxycycline mice, 13.1%±1.6%; diabetic Pod/+ doxycycline mice, 14.6%±0.8%; diabetic Pod/Angpt1 doxycycline mice, 11.3%±1.6%).
Effect of Targeted Glomerular Angpt1 Repletion on Molecular Parameters in Diabetic Mice
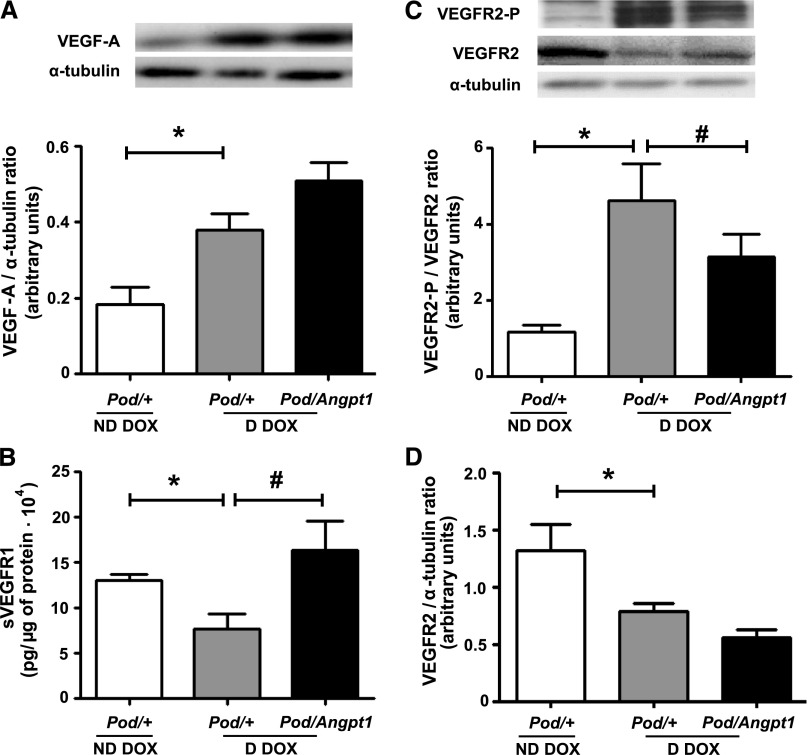
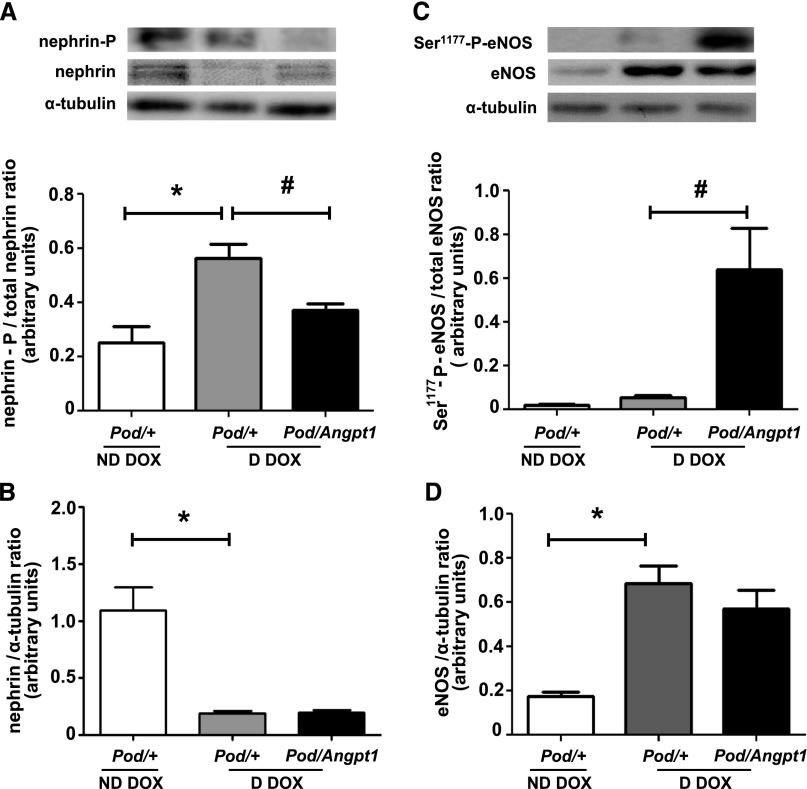
Because VEGF-A is considered to have profound effects on glomerular biology, we evaluated whether Angpt1 repletion altered VEGF-A levels and signaling in diabetic mice. Compared with nondiabetic Pod/+ doxycycline mice, diabetic Pod/+ doxycycline animals exhibited a 2.2-fold increase in renal cortical protein levels of VEGF-A (Figure 4A); although total VEGFR2 levels were reduced, diabetes led to a 4-fold increase in phosphorylated VEGFR2 (Figure 4, C and D). Transgenic Angpt1 expression in diabetic mice did not alter VEGF-A or total VEGFR2 levels (Figure 4, A and D) but significantly reduced the diabetes-induced increase in VEGFR2 phosphorylation (Figure 4C). The expression of sVEGFR1 in the kidney cortex was downregulated in diabetic Pod/+ doxycycline mice compared with nondiabetic Pod/+ doxycycline mice, and this reduction was prevented by Angpt1 repletion during diabetes (Figure 4B). The slit diaphragm protein nephrin was downregulated in diabetes and restoring Angpt1 did not modify its levels (Figure 5B); conversely, diabetes-induced increase in nephrin phosphorylation was reduced in mice with Angpt1 repletion (Figure 5A). Endothelial nitric oxide synthase (eNOS) was upregulated in diabetes and not modified by Angpt1 repletion (Figure 5D); however, restoring Angpt1 levels increased eNOS Ser1177 phosphorylation in diabetic mice (Figure 5C).
Figure 4.
Angpt1 repletion ameliorates renal cortical levels of VEGF-A/VEGFR phosphorylation in diabetic mice. At 10 weeks of doxycycline (DOX) administration, VEGF-A (A) and VEGFR2-P (C) were upregulated in diabetic compared with nondiabetic mice (*P<0.01), while VEGFR2 (D) was downregulated (*P=0.02). Angpt1 repletion in diabetic mice downregulated diabetes-induced VEGFR2 phosphorylation (#P=0.04) (C) and prevented the diabetes-induced sVEGFR1 downregulation (#P=0.005) (B) (n=8–12/group in all analyses). D, diabetic; ND, nondiabetic. *indicates comparisons between nondiabetic and diabetic states; # indicates the comparisons between diabetic states without and with Angpt1 repletion.
Figure 5.
Angpt1 repletion modulates renal cortical levels of nephrin and eNOS phosphorylation in diabetic mice. Diabetes was paralleled by a downregulation of nephrin (B) and by an increase in its phosphorylation (*P<0.01) (A); nephrin downregulation was not affected by Angpt1 repletion, which was, in contrast, associated with a reduction in diabetes-induced nephrin phosphorylation (#P=0.01) (A and B). eNOS was upregulated in diabetic mice and this was not affected by Angpt1 repletion (*P=0.01) (D). An increase in eNOS phosphorylation in Ser1177 was observed only in diabetic mice with Angpt1 repletion (#P=0.01) (C) (n=4–8/group in all analyses). D, diabetic; DOX, doxycycline; ND, nondiabetic. *indicates comparisons between nondiabetic and diabetic states; # indicates the comparisons between diabetic states without and with Angpt1 repletion.
Ex Vivo ANGPT Determinations in Humans
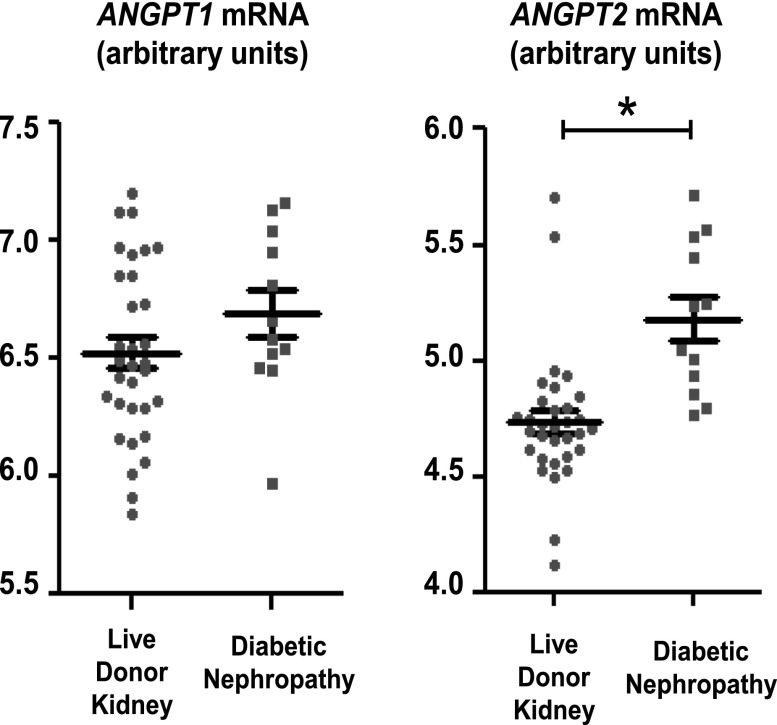
To relate to human disease the diabetes-induced Angpt imbalance observed in animals, we assessed ANGPT mRNA levels in isolated glomeruli obtained from diabetic patients and live donor kidneys (LDKs). Most patients had type 2 diabetes (see Concise Methods). The levels of ANGPT1 did not differ between patients with DN compared with LDKs, but ANGPT2 was significantly higher in DN (Figure 6). No correlation was observed with estimated GFR.
Figure 6.
ANGPT1 and ANGPT2 mRNA are dysregulated in patients with DN. ANGPT1 and ANGPT2 mRNA expression in isolated glomeruli from patients with diabetic nephropathy and live donor kidney. ANGPT2 is increased in glomeruli obtained from biopsy specimens of patients with DN (n=12) compared with biopsy specimens from live donor kidneys (n=32) (live donor kidney versus diabetic nephropathy, *P=0.00002).
Discussion
In this study, we demonstrated that the early stages of DN were associated with changes in vascular growth factors (Angpt1 deficiency/VEGF-A excess) leading to vessel destabilization, marked albuminuria, and glomerular endothelial cell proliferation. We subsequently hypothesized that therapeutically restoring local Angpt1 within glomeruli could ameliorate early diabetic glomerulopathy. Our findings demonstrated that podocyte-specific Angpt1 restoration ameliorated the diabetes-induced decrease in Angpt1 and resulted in an increase in kidney cortical Tie-2 phosphorylation; this was in contrast with nondiabetic Angpt1 overexpressing mice, suggesting that the effects of Angpt1 are context dependent. An alternative explanation for this finding is that a more permeable glomerular filtration barrier in diabetes could allow a better availability of podocyte-secreted Angpt1 for the glomerular endothelium.
Repletion of Angpt1 in diabetic mice was associated with marked attenuation of albuminuria, a key marker of early diabetic glomerulopathy.16 Prior work by Lee and colleagues12 treated db/db mice with multiple systemic injections of adenovirus for COMP-Angpt1 (a modified form of Angpt117). COMP-Angpt1 overexpression improved albuminuria, mesangial matrix expansion, glomerular basement membrane thickness and inflammation. Critically, the interpretation of the effects of COMP-Angpt1 was confounded by a significant reduction of glycemia in the diabetic animals treated with the adenovirus, which by itself would account for the amelioration of DN. In contrast, our transgenic strategy allows us to modulate glomerular Angpt1 expression in a site-specific manner without any systemic adverse effects. Genetic ablation of Angpt1 in podocytes is associated with a worsening of progression of DN secondary to further disruption of the diabetes-induced local imbalance of Angpt1 and Angpt2.8 Our study shows that restoration of Angpt1 in glomeruli improves diabetic kidney disease, which could be developed as a novel treatment strategy for DN.
How might restored levels of glomerular Angpt1 ameliorate albuminuria in diabetic mice? One potential explanation could be that restoring Angpt1 levels had a local hemodynamic effect that could drive changes in albuminuria.18 However, although early diabetes led to hyperfiltration, evidenced by an elevation in creatinine clearance, Angpt1 repletion did not significantly alter this. Furthermore, there was no significant correlation between the degree of albuminuria and creatinine clearance (data not shown). In addition, the modest diabetes-mediated increase in systolic BP was not altered by Angpt1 repletion, again suggesting a hemodynamic independent effect of Angpt1 on albuminuria.
A key finding was that diabetes led to an increase in VEGFR2 phosphorylation, which was attenuated by restoration of Angpt1 levels. The decrease in VEGFR2 phosphorylation in diabetic mice following Angpt1 repletion was accompanied by an increase in sVEGFR1. The level of VEGF-A signaling is critical in maintaining the integrity of the glomerular filtration barrier.19 In DN, components of the VEGF-A pathway, as shown in this and other studies, are upregulated,20,21 and dampening VEGF-A signaling can improve diabetic glomerulopathy and albuminuria.13,14,22 In contrast, reduced local production of glomerular VEGF-A in mice with type 1 diabetes promotes endothelial damage accelerating the progression of glomerular injury.23 Furthermore, the observed upregulation of sVEGFR in diabetic Angpt1 mice could account for the reduced glomerular permeability as sVEGFR1 podocyte-specific deletion results in podocyte cytoskeleton reorganization and proteinuria.24
This study showed that DN was associated with increased nephrin phosphorylation which was reduced in mice with Angpt1 repletion. Little is known about the effect of hyperglycemia on nephrin phosphorylation, with one study in pancreatic β cells showing elevated phosphorylation following glucose incubation.25 One might speculate that increased phosphorylation would target the nephrin protein for degradation, which would impair the glomerular filtration barrier. Indeed, we demonstrated that the total nephrin protein levels in diabetic mice was decreased compared with nondiabetic animals in accord with previous observations.26–28 Zhu and colleagues demonstrated that increased nephrin phosphorylation in podocytes leads to an alteration in the actin cytoskeleton with decreased stress fibers and increased lamellipodia.29 The reduction in nephrin phosphorylation in diabetic mice with Angpt1 repletion could account for an “improved” podocyte cell cytoskeleton structure and enhanced glomerular filtration barrier permeability to protein.30 The effect of Angpt1 on nephrin phosphorylation could be a direct effect on podocytes themselves; indeed, in rats, podocytes express Tie-23, and this study provides evidence for the presence of Tie-2 in mice podocytes (Supplemental Figure 4). Alternatively, the effect on Angpt1 on nephrin phosphorylation may be secondary to the dampening of VEGFR-2 signaling. Podocytes express VEGFR-2,13,31 which directly interacts with nephrin.30 VEGF-A–mediated nephrin phosphorylation results in reduction of VEGFR2-nephrin interactions, leading to changes in podocyte cytoskeleton reminiscent of the foot process effacement that leads to proteinuria.32
The most important effects of vascular growth factors are likely to be directly on the glomerular endothelium, which expresses both VEGFR1/2 and Tie-2.3,13,31 We demonstrated that Angpt1 repletion prevented the proliferation of glomerular endothelium seen in early diabetic glomerulopathy.2 This is likely to be due to the vascular stabilizing effect of Angpt1,33 which would prevent the leakiness of vessels, leading to reduced albuminuria.34 Part of this effect is probably due to the attenuation of VEGF-A signaling, which contributes to vascular stability by limiting blood vessel proliferation. In addition, Angpt1 increases eNOS Ser1177 phosphorylation, resulting in sustained nitric oxide levels,35 which preserves the integrity of interendothelial junctions in capillaries and inhibits angiogenesis and vascular permeability,36,37 in physiologic nondiabetic conditions.38 In our study, we demonstrated that eNOS Ser1177 phosphorylation was markedly increased in diabetic mice when Angpt1 levels were restored. Therefore, this effect could also contribute to improved vascular stability in diabetic mice with Angpt1 repletion. Interestingly, recent work in db/db mice has linked eNOS Ser1177 phosphorylation with protection from diabetic glomerulopathy.39 The endothelial glycocalyx also plays a critical role in the function of the filtration barrier.40,41 We visualized the glycocalyx using wheat germ agglutinin40,42 but did not observe any changes in the three groups of animals studied. This contrasts with the suggested role of Angpt1 in increasing glycocalyx thickness observed in isolated glomeruli of normal healthy animals.43 This may relate to the technique used in this study, and future experiments using more sensitive specific electron microscopy techniques are required.44
To date, no studies have examined the importance of glomerular angiopoietins in humans. We addressed this issue first through in vitro experiments using human podocytes and demonstrated that high glucose results in ANGPT1 mRNA downregulation confirming our in vivo murine observations. We also assessed ANGPT expression in isolated glomeruli obtained from diabetic patients and living donors. Similar to previous observations in rodents with long-term diabetes duration,8–10 analysis of human glomeruli showed a significant (but modest) imbalance in favor of ANGPT2 in patients with DN compared with control live donor kidney glomeruli; in contrast to our animal model and other studies,9 Angpt1 did not change. The most likely explanation for this is that our murine experiments examine early stages of diabetic glomerular disease, where angiogenesis is important.2
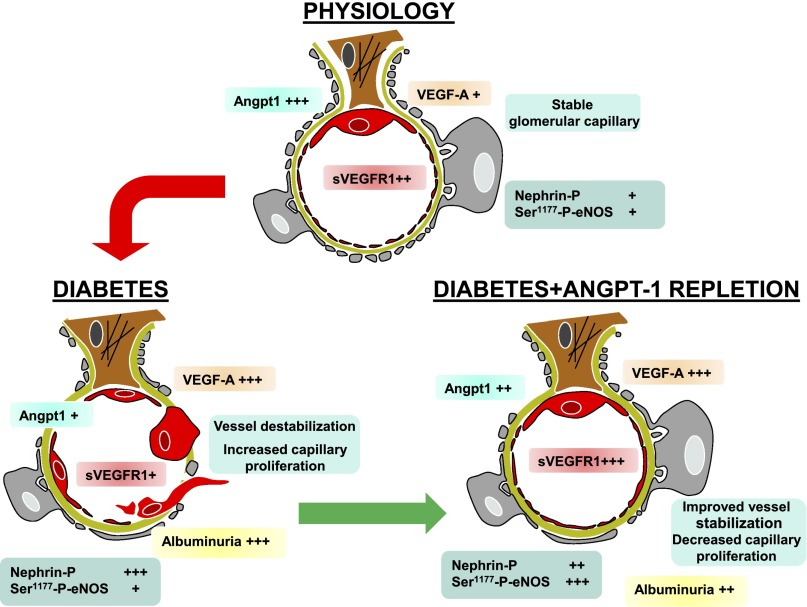
In conclusion, altering the glomerular milieu of vascular growth factors from “Angpt1 deficiency /VEGF-A excess” to “Angpt1 moderate/VEGF-A moderate” ameliorates local angiogenesis and albuminuria in experimental diabetes (Figure 7). Our finding that levels of Angpt2 transcripts were increased in glomeruli of patients with DN may also be important because (1) transgenic glomerular expression of Angpt2, an Angpt1 antagonist, in otherwise healthy mice leads to a modest increase in urinary albumin excretion,45 and (2) the balance between Angpt1 and Angpt2 may also be important in determining Tie-2 activation, as much as the absolute level of Angpt1 itself.5 Angpt1 modulation specifically targeting the glomerulus could be used as a potential therapeutical approach in the early phases of diabetic kidney disease.
Figure 7.
Angpt1 repletion ameliorates diabetic glomerulopathy (schematic diagram).
Concise Methods
Animal Model
Five-week-old male Pod/Angpt1 and Pod/+ mice were administered streptozotocin (50 μg/g body weight) (or vehicle for controls) for 5 days and made diabetic.13 Three weeks later, animals were placed on doxycycline to induce transgenic Angpt1 expression. Glycemia was recorded with glucose oxidase method and 24-hour urine collections were carried out for albuminuria determination. Plasma and urine creatinine were measured by isotope dilution electrospray mass spectrometry and creatinine clearance determined. Systolic BP was measured by noninvasive pletismography.13
Molecular Studies
RNA was obtained from isolated glomeruli by Dynabead perfusion, and Angpt1 and Angpt2 assessed by real-time PCR.46 Western blotting was performed on renal cortical tissue for Angpt1, Angpt2, VEGF-A, eNOS, VEGFR2, VEGFR2-P (Tyr951), Tie-2 (C-terminus), Tie-2-P (Tyr1100), antiphospho eNOS Ser1177, and total and phosphorylated nephrin.
Tie-2 receptor phosphorylation was also assessed using immunoprecipitation as described.47
Immunohistochemistry
Sections from renal biopsy specimens were stained with antibodies to PECAM-1, Ki67, and FITC-conjugated wheat germ agglutinin.
Electron Microscopy
Quantitative glomerular ultrastructural analysis was performed as described.13 For immunogold studies sections were incubated with Tie-2 antibodies.
In Vitro Experiments
Conditionally immortalized human podocytes48 were exposed to media containing different glucose concentrations (25 mM for high glucose and 5 mM supplemented with mannitol for normal glucose); ANGPT1 and ANGPT2 mRNA levels were examined by real-time PCR.
Studies of Human Kidney Tissue
Glomerular ANGPT1 and ANGPT2 mRNA levels were obtained from the European Renal cDNA Bank-Kroener-Fresenius biopsy bank. Biopsy specimens were obtained from patients with DN and LDKs and analyzed by microarray technology.49
Statistical Analyses
Data are shown as mean ± SEM unless stated. Differences between two groups were analyzed by t test. When more than two groups were compared, differences were analyzed by ANOVA followed by post hoc least-square-differences test. Data for albuminuria, a non-normally distributed variable, were log transformed before analysis. Analysis was performed with SPSS20 software, and significant differences accepted at P≤0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Professor J. Kopp (Bethesda, MD) for Podocin-rtTa mice, Professor L. Holzman (University of Pennsylvania, Philadelphia, PA) for P-nephrin antisera, Professor H. Holthöfer (Dublin University, Ireland) for nephrin antisera, and Professor M. Saleem (Bristol University, United Kingdom) for human podocytes. We also thank the Biological Service Unit at King’s College London, the Electron Microscopy Unit (Newcastle University), Dr. N. Dalton and Dr. C. Turner (Guy’s and St Thomas Hospital, London, United Kingdom) for the creatinine determination and the Members of the European Renal cDNA Bank-Kroener-Fresenius biopsy bank.
This work was supported by Diabetes UK (grant 08/0003695) to L.G., D.A.L., and A.S.W.; BBSRC (grant S13745) and EFSD/Servier to L.G.; and George M. O'Brien Kidney Research Core Centre (P30-DK081943) to M.K. D.A.L. is funded by KRUK Senior Non-Clinical Fellowship and MRC New Investigator Award. A.S.W. acknowledges support from the Manchester Biomedical Research Centre, and the Manchester Academic Health Science Centre for facilitating his research.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Vasculature in Diabetic Nephropathy: All Tied Up?,” on pages 1–3.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012121218/-/DCSupplemental.
References
- 1.Gnudi L: Cellular and molecular mechanisms of diabetic glomerulopathy. Nephrol Dial Transplant 27: 2642–2649, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA: Abnormal angiogenesis in diabetic nephropathy. Diabetes 58: 1471–1478, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satchell SC, Harper SJ, Tooke JE, Kerjaschki D, Saleem MA, Mathieson PW: Human podocytes express angiopoietin 1, a potential regulator of glomerular vascular endothelial growth factor. J Am Soc Nephrol 13: 544–550, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Robert B, Zhao X, Abrahamson DR: Coexpression of neuropilin-1, Flk1, and VEGF(164) in developing and mature mouse kidney glomeruli. Am J Physiol Renal Physiol 279: F275–F282, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Potente M, Gerhardt H, Carmeliet P: Basic and therapeutic aspects of angiogenesis. Cell 146: 873–887, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Sison K, Eremina V, Baelde H, Min W, Hirashima M, Fantus IG, Quaggin SE: Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J Am Soc Nephrol 21: 1691–1701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE: Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 121: 2278–2289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizkalla B, Forbes JM, Cao Z, Boner G, Cooper ME: Temporal renal expression of angiogenic growth factors and their receptors in experimental diabetes: Role of the renin-angiotensin system. J Hypertens 23: 153–164, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto Y, Maeshima Y, Kitayama H, Kitamura S, Takazawa Y, Sugiyama H, Yamasaki Y, Makino H: Tumstatin peptide, an inhibitor of angiogenesis, prevents glomerular hypertrophy in the early stage of diabetic nephropathy. Diabetes 53: 1831–1840, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Veron D, Bertuccio CA, Marlier A, Reidy K, Garcia AM, Jimenez J, Velazquez H, Kashgarian M, Moeckel GW, Tufro A: Podocyte vascular endothelial growth factor (Vegf₁₆₄) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes. Diabetologia 54: 1227–1241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Kim W, Moon SO, Sung MJ, Kim DH, Kang KP, Jang KY, Lee SY, Park BH, Koh GY, Park SK: Renoprotective effect of COMP-angiopoietin-1 in db/db mice with type 2 diabetes. Nephrol Dial Transplant 22: 396–408, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Ku CH, White KE, Dei Cas A, Hayward A, Webster Z, Bilous R, Marshall S, Viberti G, Gnudi L: Inducible overexpression of sFlt-1 in podocytes ameliorates glomerulopathy in diabetic mice. Diabetes 57: 2824–2833, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosugi T, Nakayama T, Li Q, Chiodo VA, Zhang L, Campbell-Thompson M, Grant M, Croker BP, Nakagawa T: Soluble Flt-1 gene therapy ameliorates albuminuria but accelerates tubulointerstitial injury in diabetic mice. Am J Physiol Renal Physiol 298: F609–F616, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW: Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol 18: 2885–2893, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H: Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1: 1430–1432, 1982 [DOI] [PubMed] [Google Scholar]
- 17.Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim KT, Kim I, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY: COMP-Ang1: A designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci U S A 101: 5547–5552, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jerums G, Premaratne E, Panagiotopoulos S, MacIsaac RJ: The clinical significance of hyperfiltration in diabetes. Diabetologia 53: 2093–2104, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Johnson RJ, Croker B: Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol 18: 539–550, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Cooper ME, Vranes D, Youssef S, Stacker SA, Cox AJ, Rizkalla B, Casley DJ, Bach LA, Kelly DJ, Gilbert RE: Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes 48: 2229–2239, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Sung SH, Ziyadeh FN, Wang A, Pyagay PE, Kanwar YS, Chen S: Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J Am Soc Nephrol 17: 3093–3104, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Sivaskandarajah GA, Jeansson M, Maezawa Y, Eremina V, Baelde HJ, Quaggin SE: Vegfa protects the glomerular microvasculature in diabetes. Diabetes 61: 2958–2966, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin J, Sison K, Li C, Tian R, Wnuk M, Sung HK, Jeansson M, Zhang C, Tucholska M, Jones N, Kerjaschki D, Shibuya M, Fantus IG, Nagy A, Gerber HP, Ferrara N, Pawson T, Quaggin SE: Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 151: 384–399, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Jeon J, Leibiger I, Moede T, Walter B, Faul C, Maiguel D, Villarreal R, Guzman J, Berggren PO, Mundel P, Ricordi C, Merscher-Gomez S, Fornoni A: Dynamin-mediated Nephrin phosphorylation regulates glucose-stimulated insulin release in pancreatic beta cells. J Biol Chem 287: 28932–28942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devaraj S, Tobias P, Kasinath BS, Ramsamooj R, Afify A, Jialal I: Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arterioscler Thromb Vasc Biol 31: 1796–1804, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC: Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: Blockade of compensatory renin increase. Proc Natl Acad Sci U S A 105: 15896–15901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menne J, Meier M, Park JK, Boehne M, Kirsch T, Lindschau C, Ociepka R, Leitges M, Rinta-Valkama J, Holthofer H, Haller H: Nephrin loss in experimental diabetic nephropathy is prevented by deletion of protein kinase C alpha signaling in-vivo. Kidney Int 70: 1456–1462, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Sun N, Aoudjit L, Li H, Kawachi H, Lemay S, Takano T: Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int 73: 556–566, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Foster RR, Saleem MA, Mathieson PW, Bates DO, Harper SJ: Vascular endothelial growth factor and nephrin interact and reduce apoptosis in human podocytes. Am J Physiol Renal Physiol 288: F48–57, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Veron D, Reidy KJ, Bertuccio C, Teichman J, Villegas G, Jimenez J, Shen W, Kopp JB, Thomas DB, Tufro A: Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int 77: 989–999, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Bertuccio C, Veron D, Aggarwal PK, Holzman L, Tufro A: Vascular endothelial growth factor receptor 2 direct interaction with nephrin links VEGF-A signals to actin in kidney podocytes. J Biol Chem 286: 39933–39944, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD: Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 6: 460–463, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, Olsen BR, Alitalo K: Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol 10: 527–537, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Babaei S, Teichert-Kuliszewska K, Zhang Q, Jones N, Dumont DJ, Stewart DJ: Angiogenic actions of angiopoietin-1 require endothelium-derived nitric oxide. Am J Pathol 162: 1927–1936, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB: Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol 289: L371–L381, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa T: Uncoupling of the VEGF-endothelial nitric oxide axis in diabetic nephropathy: An explanation for the paradoxical effects of VEGF in renal disease. Am J Physiol Renal Physiol 292: F1665–F1672, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R: Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 99: 2625–2634, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng H, Wang H, Fan X, Paueksakon P, Harris RC: Improvement of endothelial nitric oxide synthase activity retards the progression of diabetic nephropathy in db/db mice. Kidney Int 82: 1176–1183, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, Bates DO, Peti-Peterdi J: Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol 23: 1339–1350, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fridén V, Oveland E, Tenstad O, Ebefors K, Nyström J, Nilsson UA, Haraldsson B: The glomerular endothelial cell coat is essential for glomerular filtration. Kidney Int 79: 1322–1330, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Kuwabara A, Satoh M, Tomita N, Sasaki T, Kashihara N: Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia 53: 2056–2065, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salmon AH, Neal CR, Sage LM, Glass CA, Harper SJ, Bates DO: Angiopoietin-1 alters microvascular permeability coefficients in vivo via modification of endothelial glycocalyx. Cardiovasc Res 83: 24–33, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hjalmarsson C, Johansson BR, Haraldsson B: Electron microscopic evaluation of the endothelial surface layer of glomerular capillaries. Microvasc Res 67: 9–17, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Davis B, Dei Cas A, Long DA, White KE, Hayward A, Ku CH, Woolf AS, Bilous R, Viberti G, Gnudi L: Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J Am Soc Nephrol 18: 2320–2329, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Long DA, Kolatsi-Joannou M, Price KL, Dessapt-Baradez C, Huang JL, Papakrivopoulou E, Hubank M, Korstanje R, Gnudi L, Woolf AS: Albuminuria is associated with too few glomeruli and too much testosterone. Kidney Int 83: 1118–1129, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan HT, Khankin EV, Karumanchi SA, Parikh SM: Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol 29: 2011–2022, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Berthier CC, Zhang H, Schin M, Henger A, Nelson RG, Yee B, Boucherot A, Neusser MA, Cohen CD, Carter-Su C, Argetsinger LS, Rastaldi MP, Brosius FC, Kretzler M: Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 58: 469–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.