Abstract
The cells in a human body have identical DNA sequences, yet the body has >200 cell types with different phenotypes. The basis for this nongenetic cellular memory, which records developmental and environmental cues, is epigenetics. The epigenome includes covalent modifications of the DNA and its associated proteins and defines DNA accessibility to the transcriptional machinery. Notably, the epigenome has emerged as an important mediator of the long-term programming effect of environmental exposure, and multiple lines of evidence point to the epigenome as an important missing link in our understanding of CKD development. For example, recent studies identified epigenetic differences in the enhancer regions of fibrosis-related genes in diseased human kidney samples. Furthermore, chromatin profiling and epigenome analysis are powerful tools for annotating gene regulatory regions that can be harnessed to interpret disease-causing polymorphisms for complex traits such as CKD. This review highlights the results of studies investigating the renal epigenome and discusses the significance of these findings and future directions in the context of novel diagnostic and treatment strategies for CKD.
What Is Epigenetics?
The original definition of epigenetics comes from Waddington. He defined epigenetics as the idea that phenotype arises from genotype through programmed change. This classic definition is mostly used in developmental biology. The modern definition of epigenetics is the heritable information during cell division other than the DNA sequence itself. An epigenetic system therefore should be heritable, self-perpetuating, but also reversible. Cytosine methylation and other modification of cytosines in the DNA clearly fulfill these criteria. In addition, histone modifications and noncoding RNAs are often discussed as part of the epigenome. The epigenome can act as a sensor of environmental stress and through phenotypic changes they may potentially also promote evolution.
Why Do We Need to Know about the Cell Type–Specific Epigenome?
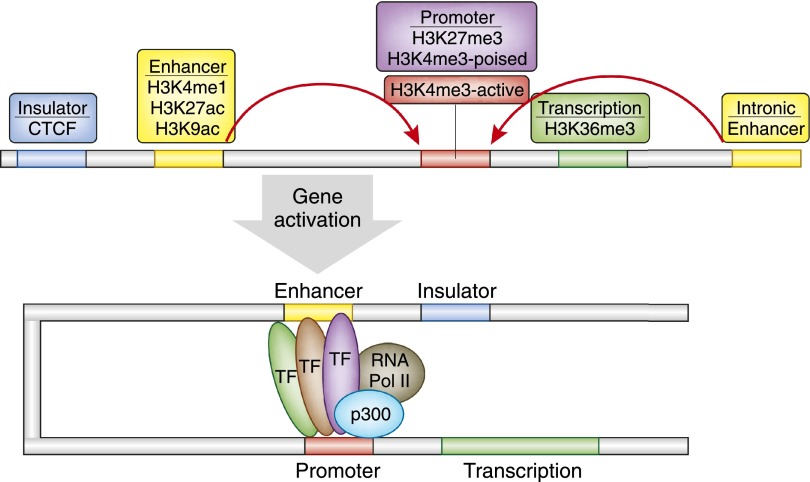
We learned that different cells, even when they are derived from the same person, respond very differently to environmental cues. For example, following TGF-β treatment, certain cells proliferate, others die, and some transform and produce cytokines or matrix. On the mechanistic level, a critical observation is that transcription factor availability and the promoter sequence information alone do not determine the regulatory outcome on a target gene (i.e., binding to targets and transcription). The structural basis for this is that the DNA is present in the nucleus in a highly organized form. Small basic proteins called histones (H1–4 isoforms) wrap the DNA and guide transcription factor binding (Figure 1). Histone tails can undergo >60 different types of modification, including methylation, acetylation, phosphorylation, sumoylation, and ubiquitination. Modifications of the fourth, ninth, 27th, and 36th lysine (K) residues of H3 histone seem to be especially important. These residues are abbreviated as H3K4, H3K9, and H3K27, and H3K36.1,2 A typical transcription unit in a multicellular eukaryote contains both clusters of proximal promoter elements and five types of cis-acting regulatory sequences (promoters, enhancers, silencers, insulators, and locus control regions) (Figure 2). For target gene transcription to take place, both long- and short-range regulatory regions are needed. Simultaneous binding of transcription factors to the long- and short-range regulatory regions and to each other results in genomic DNA loops that join distant regulatory DNA sequences together.3–5 The long- and short-range regulatory elements in the noncoding region of the genome define the regulatory syntax for the cell type–specific gene regulation. This can explain why the same transcription factor has different targets in different cell types despite the same promoter sequence.
Figure 1.
Epigenetic modifications. DNA in the genome is wrapped around nucleosomes, made of histones. Histone tails can undergo multiple different type of modifications: methylation, acetylation, ubiquitination, sumoylation, and phosphorylation. Transcription factors can bind only to open chromatin areas; these areas can be identified by DNaseI hypersensitivity. Cytosines in the DNA can be modified by methylation.
Figure 2.
A model of the eukaryotic transcriptional unit. For active gene transcription to take place, transcription factors bind to active promoter (red) and enhancer (orange, yellow) regions. Promoter-enhancer units are separated by insulator sites (depicted in blue). Insulators sites are enriched for CTCF binding, and active promoters can be identified by H3K4me2/3 enrichment. Poised promoter regions are enriched in both H3K4me3 and H3K27me3 histone modification. Enhancers can be upstream or downstream of promoters, and they are enriched for H3K4me1, H3K27ac, and H3K9ac.
In this context, identifying enhancers and other gene regulatory regions is critically important. Enhancer regions are critical for defining cell type specificity. The cell type–specific master transcription factor occupies the majority of the enhancers.6 Traditionally, it has been extremely difficult to characterize enhancer regions because they are highly cell type–specific and do not have a characteristic location (they can be upstream or downstream, in plus or minus orientation).
Recently, we learned that cell type–specific gene regulatory regions (including enhancers) can be identified by the presence of specific histone tail modifications.7,8 The most commonly used method for genome-wide characterization of these regions is called ChIP-Seq (chromatin immunoprecipitation followed by next-generation sequencing). After the DNA and the DNA-associated proteins are cross-linked, DNA regions where the specific histone tail modification is present are pulled down by immunoprecipitation; the pulled (DNA) fragments are then analyzed using next-generation sequencing and the obtained sequences are computationally aligned. The genomic area where the DNA is bound to a specific histone tail is enriched by ChIP, giving rise to characteristic “peaks or mountains” (Figure 3). Specifically, H4K4me1 and H3K27ac histone tail modifications are enriched around enhancer regions. Active promoters are decorated by H4K4me3-modified histones, deposited by the large COMPASS protein complex. Promoter-enhancer units are separated by insulator regions that bind CTCF (CCCTC-binding factor) proteins. Repressed genes and regions are associated with H3K27me3-modified histones. The presence of both H4K4me3 (active) and H3K27me3 (repressed) indicates promoters that are poised for transcription. Poised promoters are characteristics of stem and progenitor cells (Figure 3). When ChIP-Seq is performed for multiple different histone tail modifications, a cell type–specific gene regulatory map can be generated (Figure 3). Active enhancers and promoter regions can also be identified by deoxyribonuclease I (DNaseI) hypersensitivity. The method is based on the fact that the DNA has to be free of nucleosomes on active enhancers and promoters so transcription factors can bind. These nucleosome-free regions are more sensitive for DNaseI digestions compared with regions that are wrapped to nucleosomes.9
Figure 3.
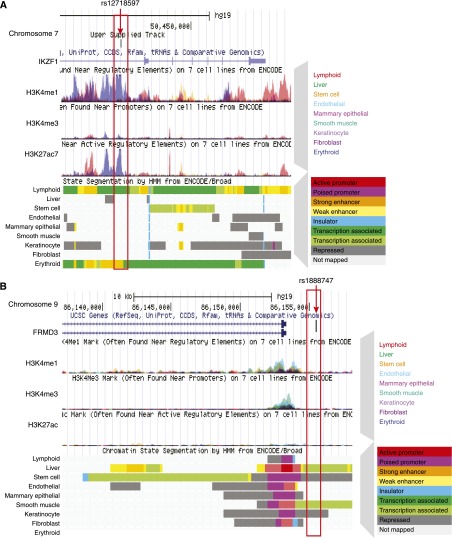
Epigenetic annotation using the ENCODE database. Epigenetic annotation of the (A) IKZF1 and (B) FRMD3 locus by the ENCODE database. (A) After the chromosomal location, the first lines show the overlapping enrichment of H3K4me1 methylation in the seven different ENCODE cell lines, followed by the H3K4me3 and H3K27ac patterns (the different colors show the overlapping enrichment in the different cell lines) and then the hidden Markov modeling–based chromatin annotation of the same locus of the nine different ENCODE cell line. The color coding is shown next to the image. Note the active promoter of these genes (shown in red), which corresponds to the enrichment for the H3K4me3 histone tail modification. Enhancers are shown in yellow (orange); they correspond to the H3K4me1 histone tail modification. The erythrocyte volume–associated polymorphism (rs12718597) is associated with an enhancer region in the erythroid cell line only. Note the blueish H3K4me1 peak at this locus and the yellow region (enhancer) chromatin annotation at the bottom. (B) The same modifications are shown for the FRMD3 locus. The genetic polymorphism associated with diabetic kidney disease rs1888747 shown in the picture is in the noncoding region of the genome. It is also outside of the promoter region of FRMD3. These data were downloaded and are available at the UCSC genome browser website (http://genome.ucsc.edu).
Methylation of cytosines is another key determinant of transcription. The majority of the genome has low cytosine/guanine content, and these cytosines are usually methylated. Cytosine/guanine-rich regions are organized into CpG islands (CGIs) in the genome. These are short, stretched (about 300–3000 bp) regions characterized by high cytosine/guanine content (>60%). These CGIs are enriched on gene promoter regions, and cytosines in CGIs are usually unmethylated. Promoter methylation level plays a key role in the regulation of gene transcriptions. Methylations of the promoter regions of tumor suppressors play important role in tumor suppressor silencing and ultimately carcinogenesis. Two basic models have evolved: In the first, DNA methylation can directly repress transcription by blocking transcriptional activators from binding to cognate DNA sequences; in the second, methyl-binding proteins recognize methylated DNA and recruit corepressors to silence gene expression directly. In addition, binding of the CTCF protein to cytosines is very sensitive to CpG methylation. As CTCF plays role in chromatin looping, it indicates an interesting and important interplay between the different epigenetic modifications. Genome-wide studies identified that methylation of regions located next to but outside of the CGIs (so-called CpG shore regions) plays a key role in diverse biologic processes, including cell type specification.10 Gene body regions can also be methylated, but their functional significance is debated. These regions may play a role in alternative splicing, and by suppressing cryptic transcription sites they may increase the efficiency of the RNA polymerase.
There are multiple different methods for analyzing cytosine methylation levels. These methods are based on three different principles. Bisulfite converts unmethylated cytosines in the DNA, while methylated cytosines remain. Bisulfite-converted DNA then can be analyzed multiple ways, for example, by sequencing (by whole genome-wide bisulfite sequencing), by arrays (used in the Infinium arrays sold by Illumina), and by mass spectrometry (e.g., by the Sequenom MassArray Epityper). Methylated cytosines can also be identified by methylcytosine-binding proteins, and these regions can be pulled down (e.g., by a method called methylcytosine binding and immunoprecipitation). In addition, methylation levels can be analyzed by methylation-sensitive and -insensitive enzymes that cut the same sequences around methylated and unmethylated cytosines differentially. This principle can then be coupled to microarrays or next-generation sequencing using such methods as HpaII tiny fragment enrichment by ligation-mediated PCR, methylation restriction enzyme–mediated sequencing, or reduced representation bisulfite sequencing.11
Cytosine methylation levels are believed to be the highest in fully differentiated cells. Cytosine methylation is erased after fertilization, except for the imprinted regions.12 During cell type–specific differentiation, the cytosine methylation level increases mainly by DNA methyl transferases (DNMTs). DNMT3A and 3B participate in de novo DNA methylation of unmethylated cytosines. DNMT1 is responsible for maintaining the already established DNA methylation levels during cell division.
Higher-order chromatin structure and looping bring together proximal promoter and distal enhancer elements, thereby playing an important role in regulating transcription. New methods, including chromatin conformation capture and sequencing (3C, 4C, 5C, and Hi-C), are emerging to detect and analyze these interactions.13 Furthermore, different long and short noncoding RNAs are important for fine-tuning transcription. New discoveries identified a novel class of RNA, the enhancer RNA. Enhancer RNAs play a role in establishing long-range relationships between enhancer and promoter regions to regulate transcription.14,15 In summary, the epigenome is the key determinant of cell types and transcriptional outcome.
Epigenetics, Differentiation and Cell Type Specification
Cell type–specific gene regulatory elements (and the “histone code”) are established during development and differentiation. Later, these regions are maintained during somatic cell division to preserve cell identity. During development and differentiation, cell type–specific master or pioneering transcription factors have the capacity to bind to DNA that is wrapped to nucleosomes. These pioneering transcription factors seem to be different from other transcription factors. After the initial binding they recruit other chromatin-modifying enzymes to establish the cell type–specific gene regulatory landscape, including active enhancers and promoters, whereas other regions become repressed. The DNA template probably plays a critical role in setting up the cell type–specific epigenetic landscape (cytosine methylation and histone modifications).16 Once cell type–specific gene regulatory regions are established, they are strictly maintained during cell division. Therefore, it is believed that the epigenome can provide the best characterization of the cell type, as transcript levels fluctuate rapidly after external stimuli.
Very little is known about epigenetic changes during kidney development. A recent paper from the McMahon group analyzed Six2 and β-catenin–binding sites in developing mouse kidneys. Six2 is one of the critical renal-specific pioneering transcription factors.17 Pioneering transcription factors usually work together with other chromatin modifiers, including histone deacetylases (HDACs). Work from El-Dahr and colleagues indicates that genetic deletion of HDACs during kidney development has a deleterious effect on kidney maturation in mouse models supporting the role of HDACs during kidney development.18 Future studies aiming to understand epigenetic changes during kidney differentiation will be important to understand cell type–specific differences during development and disease.
Epigenetics and the Missing Heredity
Monogenic diseases are typically caused by nonsynonymous coding mutations. These mutations target conserved amino acids, resulting in a loss of functional protein expression and disease development. Identifying causal genes for complex traits, such as diabetic and hypertensive chronic kidney disease, has been challenging. Large genome-wide association studies are being performed to uncover genetic polymorphisms associated with disease development. Recent studies indicate that >90% of the identified genetic variants associated with complex traits and disease development is localized to the noncoding region of the genome. It is unclear how these noncoding polymorphisms lead to specific diseases only in target organs. Recent reports indicate that the cellular epigenome and chromatin annotation maps might help us understand the disease-causing role of genetic polymorphisms. A strong association between disease-causing genetic polymorphism and cell type–specific gene regulatory regions has been described. Reports indicate that, for example, single-nucleotide polymorphisms (SNPs) associated with blood cell phenotypes are localized to noncoding, but blood cell–specific gene regulatory regions4,19 (Figure 3A). Interestingly, most often these polymorphisms are localized not to promoter regions but to enhancers. Figure 3 shows a polymorphism associated with diabetic kidney disease development localizing to the 5′ untranslated region of the FRMD3 gene.20 Histone tail modification tracks from the nine different cell lines show that the polymorphism is clearly outside of the coding or even the promoter region of FRMD3. Kidney-specific gene regulatory maps would help determine whether this SNP is on a kidney specific gene regulatory region, explaining kidney disease development.
Understanding cell type–specific gene regulatory regions is a critically important task. Currently, significant National Institutes of Health efforts are directed to the ENCyclopedia Of DNA Elements (ENCODE) Project, aimed at characterizing cell type–specific gene regulatory regions.21–24 This project provided a new user encyclopedia for the human genome, defining functional elements: gene regulatory regions, new transcript variants, and new transcriptional units,25 indicating that we can assign function to close to 80% of the noncoding region of the genome. The ENCODE project uses cultured human cell lines of different origins, including endothelial, fibroblast, myocyte, stem cell, erythroid, epithelial, and lymphoid origins. Unfortunately, kidney cells are not included in the nine cell types prioritized in the ENCODE project. This critical information could help us unravel the mechanism how noncoding SNPs influence complex trait development, such as CKD and diabetic kidney disease.3,4 Furthermore, by understanding gene regulatory regions, we would be able to predict the function of cis-regulatory sequences, define transcriptional regulatory networks, and understand the dynamic properties of kidney cells (i.e., predict the transcriptional effect of environmental stimuli).
Epigenome as the Environmental Footprint
While the epigenome is stable and is maintained during somatic cell divisions, it can also be reversible and act as an environmental sensor. Therefore, it is believed that we will likely be able to use the cellular epigenome as an environmental footprint. Although we do not fully understand the molecular mechanism of long-term environmental programming, we know that cells constantly adjust their metabolic state to nutrient availability.26,27 In yeast, for example, nutrient availability is the key determinant of fate. Most chromatin-modifying enzymes require substrates or cofactors that are intermediates of cell metabolism; for example, acetyl and methyl groups are needed for histone tail acetylation and methylation. In vitro evidence indicates that fluctuations of metabolite levels modulate activities of chromatin-modifying enzymes and therefore can influence chromatin dynamics. Although it has not been systematically evaluated, metabolite fluctuation in diabetes could potentially induce a shift in the cellular epigenome. Cells are likely to be even more sensitive to this “epigenetic shift” during development and differentiation, when gene regulatory regions are established. These developmentally wired networks might play important roles in phenotype development by modulating how cells later respond to stimuli. Animal model experiments confirmed that altered nutrient availability and metabolite fluctuation during development induce alterations in the epigenome.28 For example, intrauterine growth retardation (induced by limiting the blood flow to the uterus) in rats causes altered insulin secretion and metabolic syndrome in adult animals. Nutrient restriction altered cytosine methylation profiles and the expression of a key pancreatic β cell–specific transcription factor, Pdx1. Reduced Pdx1 level likely contributed to the impaired insulin secretion seen later in life in these animals.29 Here the cytosine methylation profile serves as a footprint of the adverse intrauterine environment. Similarly altered cytosine methylation patterns have been described after different environmental toxin exposures.30,31 More important in rodent models, toxin-induced changes were stable over multiple generations. Future studies shall determine whether we can use the epigenome to detect environmental exposure in humans similarly to rodent models.
Opportunities for Epigenetic Research in Nephrology: Epigenetics, Environment, and Diabetic Kidney Disease
Multiple different approaches have been taken to decipher the mechanism of diabetic kidney disease. Although the genetics of this disease remains obscure, it has long been proposed that fetal programming plays a role in CKD development.32 Both human and animal studies support the hypothesis that low birthweight—a marker of adverse intrauterine circumstances—is associated with a congenital deficit in nephron number.1 Reduced nephron number causes a compensatory glomerular hypertrophy, leading to the development of salt-sensitive hypertension and glomerulosclerosis, which then starts a vicious cycle of further nephron loss and ultimately CKD and ESRD.33 Recent studies also emphasize the critical role of metabolic programming in diabetic kidney disease. A landmark study demonstrated that good glycemic control profoundly reduces the development of renal and cardiovascular complications.34 Surprisingly, patients with poor metabolic control continue to develop diabetic kidney disease at a significantly higher rate, even after they had similar level of glycemic control for 25 years, indicating that early metabolic control has a long-term effect on clinical outcomes, a concept called “metabolic memory.”35 Despite the strong clinical observational evidence, the mechanism of fetal and adult programming in development of diabetic kidney disease remains elusive. Epigenetic changes are proposed as a molecular mechanism of metabolic memory and fetal programming.36 Cell culture–based studies support that the epigenome could be the important missing link.37 Endothelial cells incubated in high glucose medium change their histone tail modification patterns, and these epigenetic changes persist even after the cells placed back into normal glucose medium.38 Diabetic animal models and cells obtained from diabetic animals also show significant differences in their epigenome.39 Defining a connection between epigenetic dysregulation and development of diabetic kidney disease could profoundly change our understanding of the disease and drive the development of new diagnostics and therapeutics for a condition that is responsible for more than 50% of all ESRD cases in the United States.
A critical question remains whether epigenetic changes can be observed in patients with diabetes and diabetic complications. Our laboratory recently performed genome-wide cytosine methylation analysis of microdissected control and diseased kidney epithelial cells (including some from diabetic kidney disease). We found that the epigenome of control and CKD kidneys are significantly different. We identified more than 4000 differentially methylated regions in CKD samples. Gene ontology analysis showed that the differentially methylated loci were related to development and fibrosis. Interestingly, we did not observe significant differences in promoter methylation levels, but differentially methylated regions were enriched on enhancer regions.40 This observation will help to refocus our attention to enhancers from promoter regions. In a different study, cytosine methylation patterns of mixed peripheral blood monocytes obtained from patients with CKD were also different from those in controls.41 Because the epigenome is cell type specific, it is not clear whether these studies identified changes that are specific to the disease state or just detected minor differences in cell type heterogeneity. In summary, clinical observational evidence suggest the long-term programming or memory role of the environment in development of CKD or diabetic kidney disease, and initial human studies indicate epigenetic differences in CKD samples, raising the possibility that epigenetics plays an important role in CKD development.
Clinical Implications: Therapeutics and Diagnostics
There are two key areas where epigenetics could enter nephrology clinics relatively soon: therapeutics and diagnostics. Epigenetic-based therapies that inhibit cytosine methylation (DNMT inhibitors) or HDACs have already entered clinical practice in the cancer field.
Animal model based experiments performed by the Zeisberg group indicate that increased cytosine methylation level of the RASAL1 gene is causally linked to kidney fibrosis development in mouse models.42 Indeed, 5′-azacitidine, a drug that inhibits cytosine methylation, successfully inhibited fibrosis development in mice. This drug has been used in clinical practice and is approved for the treatment of myelodysplastic syndrome. Although further studies are needed to examine the relationship between cytosine methylation and kidney fibrosis, the initial animal model studies are encouraging.
Another kind of epigenome modifier, HDACs, emerged to play a key role in kidney repair after AKI and glomerulosclerosis development. HDAC inhibitors were beneficial for the treatment of experimental FSGS and HIV-associated renal disease.43 Although these are interesting initial observations, the concern is that both HDAC and DNMT inhibitors have pleotropic effects and they likely modify thousands of cytosines and histone tails. Further drug development to enhance specificity will likely be needed before potential human trials.
Expectations are high in the world of diagnostics as well. The epigenome is clearly viewed as the long-term footprint of environmental exposures and as an interface between genetic variation and disease development. Cytosine methylation modifications are stable and easy to analyze; therefore, they could be used to predict risk and diagnose disease progression. Epigenome characterization is now becoming routine in cancer diagnostics and treatments, and we hope that it will come to nephrology clinics as well. A critical bottleneck is the lack of kidney tissue samples from patients with CKD.
Our knowledge in the area of epigenetics is increasing exponentially. High-resolution epigenome analyses for different human cell lines has recently been made publicly available by the ENCODE project. In addition, the International Human Epigenome Consortium is aiming to analyze 1000 human reference epigenomes from different tissues and cell types. Initial goals of these studies are to establish reference samples and define “normal” variation in the same cell types. These reference epigenomes can then be tested to understand the effect of different environmental factors on the epigenome (epigenome/environment) and understand the effect of genetic variations on the cell type–specific epigenome (epigenome/genome). It will be important to generate publicly available high-quality kidney specific datasets. It is increasingly clear that epigenetics will be a key component to the understanding of common complex diseases, including diabetic and hypertensive CKD.
Disclosures
The Susztak lab receives research support from Boehringer Ingelheim.
Acknowledgments
Work in the Susztak lab is supported by National Institutes of Health grant 5R01DK087635-05
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Woroniecki R, Gaikwad AB, Susztak K: Fetal environment, epigenetics, and pediatric renal disease. Pediatr Nephrol 26: 705–711, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohtat D, Susztak K: Fine tuning gene expression: the epigenome. Semin Nephrol 30: 468–476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ram O, Goren A, Amit I, Shoresh N, Yosef N, Ernst J, Kellis M, Gymrek M, Issner R, Coyne M, Durham T, Zhang X, Donaghey J, Epstein CB, Regev A, Bernstein BE: Combinatorial patterning of chromatin regulators uncovered by genome-wide location analysis in human cells. Cell 147: 1628–1639, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, Zhang X, Wang L, Issner R, Coyne M, Ku M, Durham T, Kellis M, Bernstein BE: Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473: 43–49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein BE, Stamatoyannopoulos JA, Costello JF, Ren B, Milosavljevic A, Meissner A, Kellis M, Marra MA, Beaudet AL, Ecker JR, Farnham PJ, Hirst M, Lander ES, Mikkelsen TS, Thomson JA, The NIH Roadmap Epigenomics Mapping Consortium : The NIH Roadmap Epigenomics Mapping Consortium. Nat Biotechnol 28: 1045–1048, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ernst J, Kellis M: Interplay between chromatin state, regulator binding, and regulatory motifs in six human cell types. Genome Res 23: 1142–1154, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW, Ching KA, Antosiewicz-Bourget JE, Liu H, Zhang X, Green RD, Lobanenkov VV, Stewart R, Thomson JA, Crawford GE, Kellis M, Ren B: Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 459: 108–112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, Wang W, Weng Z, Green RD, Crawford GE, Ren B: Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39: 311–318, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, Sheffield NC, Stergachis AB, Wang H, Vernot B, Garg K, John S, Sandstrom R, Bates D, Boatman L, Canfield TK, Diegel M, Dunn D, Ebersol AK, Frum T, Giste E, Johnson AK, Johnson EM, Kutyavin T, Lajoie B, Lee BK, Lee K, London D, Lotakis D, Neph S, Neri F, Nguyen ED, Qu H, Reynolds AP, Roach V, Safi A, Sanchez ME, Sanyal A, Shafer A, Simon JM, Song L, Vong S, Weaver M, Yan Y, Zhang Z, Zhang Z, Lenhard B, Tewari M, Dorschner MO, Hansen RS, Navas PA, Stamatoyannopoulos G, Iyer VR, Lieb JD, Sunyaev SR, Akey JM, Sabo PJ, Kaul R, Furey TS, Dekker J, Crawford GE, Stamatoyannopoulos JA: The accessible chromatin landscape of the human genome. Nature 489: 75–82, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pujadas E, Feinberg AP: Regulated noise in the epigenetic landscape of development and disease. Cell 148: 1123–1131, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris RA, Wang T, Coarfa C, Nagarajan RP, Hong C, Downey SL, Johnson BE, Fouse SD, Delaney A, Zhao Y, Olshen A, Ballinger T, Zhou X, Forsberg KJ, Gu J, Echipare L, O’Geen H, Lister R, Pelizzola M, Xi Y, Epstein CB, Bernstein BE, Hawkins RD, Ren B, Chung WY, Gu H, Bock C, Gnirke A, Zhang MQ, Haussler D, Ecker JR, Li W, Farnham PJ, Waterland RA, Meissner A, Marra MA, Hirst M, Milosavljevic A, Costello JF: Comparison of sequencing-based methods to profile DNA methylation and identification of monoallelic epigenetic modifications. Nat Biotechnol 28: 1097–1105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gehring M, Reik W, Henikoff S: DNA demethylation by DNA repair. Trends Genet 25: 82–90, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Dekker J, Marti-Renom MA, Mirny LA: Exploring the three-dimensional organization of genomes: Interpreting chromatin interaction data. Nat Rev Genet 14: 390–403, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L, Goff LA, Trapnell C, Alexander R, Lo KA, Hacisuleyman E, Sauvageau M, Tazon-Vega B, Kelley DR, Hendrickson DG, Yuan B, Kellis M, Lodish HF, Rinn JL: Long noncoding RNAs regulate adipogenesis. Proc Natl Acad Sci U S A 110: 3387–3392, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman MM, Ernst J, Wilder SP, Kundaje A, Harris RS, Libbrecht M, Giardine B, Ellenbogen PM, Bilmes JA, Birney E, Hardison RC, Dunham I, Kellis M, Noble WS: Integrative annotation of chromatin elements from ENCODE data. Nucleic Acids Res 41: 827–841, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tycko B: Allele-specific DNA methylation: Beyond imprinting. Hum Mol Genet 19[R2]: R210–R220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JS, Ma W, O’Brien LL, Chung E, Guo JJ, Cheng JG, Valerius MT, McMahon JA, Wong WH, McMahon AP: Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell 23: 637–651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Bellew C, Yao X, Stefkova J, Dipp S, Saifudeen Z, Bachvarov D, El-Dahr SS: Histone deacetylase (HDAC) activity is critical for embryonic kidney gene expression, growth, and differentiation. J Biol Chem 286: 32775–32789, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, Reynolds AP, Sandstrom R, Qu H, Brody J, Shafer A, Neri F, Lee K, Kutyavin T, Stehling-Sun S, Johnson AK, Canfield TK, Giste E, Diegel M, Bates D, Hansen RS, Neph S, Sabo PJ, Heimfeld S, Raubitschek A, Ziegler S, Cotsapas C, Sotoodehnia N, Glass I, Sunyaev SR, Kaul R, Stamatoyannopoulos JA: Systematic localization of common disease-associated variation in regulatory DNA. Science 337: 1190–1195, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman BI, Langefeld CD, Lu L, Divers J, Comeau ME, Kopp JB, Winkler CA, Nelson GW, Johnson RC, Palmer ND, Hicks PJ, Bostrom MA, Cooke JN, McDonough CW, Bowden DW: Differential effects of MYH9 and APOL1 risk variants on FRMD3 Association with Diabetic ESRD in African Americans. PLoS Genet 7: e1002150, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henikoff S: ENCODE and our very busy genome. Nat Genet 39: 817–818, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Gerstein MB, Bruce C, Rozowsky JS, Zheng D, Du J, Korbel JO, Emanuelsson O, Zhang ZD, Weissman S, Snyder M: What is a gene, post-ENCODE? History and updated definition. Genome Res 17: 669–681, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Weinstock GM: ENCODE: More genomic empowerment. Genome Res 17: 667–668, 2007 [DOI] [PubMed] [Google Scholar]
- 24.ENCODE Project Consortium : A user’s guide to the encyclopedia of DNA elements (ENCODE). PLoS Biol 9: e1001046, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerstein MB, Kundaje A, Hariharan M, Landt SG, Yan KK, Cheng C, Mu XJ, Khurana E, Rozowsky J, Alexander R, Min R, Alves P, Abyzov A, Addleman N, Bhardwaj N, Boyle AP, Cayting P, Charos A, Chen DZ, Cheng Y, Clarke D, Eastman C, Euskirchen G, Frietze S, Fu Y, Gertz J, Grubert F, Harmanci A, Jain P, Kasowski M, Lacroute P, Leng J, Lian J, Monahan H, O’Geen H, Ouyang Z, Partridge EC, Patacsil D, Pauli F, Raha D, Ramirez L, Reddy TE, Reed B, Shi M, Slifer T, Wang J, Wu L, Yang X, Yip KY, Zilberman-Schapira G, Batzoglou S, Sidow A, Farnham PJ, Myers RM, Weissman SM, Snyder M: Architecture of the human regulatory network derived from ENCODE data. Nature 489: 91–100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeBerardinis RJ, Thompson CB: Cellular metabolism and disease: What do metabolic outliers teach us? Cell 148: 1132–1144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson CB: Rethinking the regulation of cellular metabolism. Cold Spring Harb Symp Quant Biol 76: 23–29, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD, Meissner A, Weng Z, Hofmann HA, Friedman N, Rando OJ: Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143: 1084–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson RF, Fazzari MJ, Niu H, Barzilai N, Simmons RA, Greally JM: Experimental intrauterine growth restriction induces alterations in DNA methylation and gene expression in pancreatic islets of rats. J Biol Chem 285: 15111–15118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerrero-Bosagna CM, Skinner MK: Environmental epigenetics and phytoestrogen/phytochemical exposures [published online ahead of print December 27, 2013]. J Steroid Biochem Mol Biol10.1016/j.jsbmb.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerrero-Bosagna C, Covert TR, Haque MM, Settles M, Nilsson EE, Anway MD, Skinner MK: Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod Toxicol 34: 694–707, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perera F, Herbstman J: Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol 31: 363–373, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hostetter TH, Rennke HG, Brenner BM: Compensatory renal hemodynamic injury: A final common pathway of residual nephron destruction. Am J Kidney Dis 1: 310–314, 1982 [DOI] [PubMed] [Google Scholar]
- 34.de Boer IH, Rue TC, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Sun W, Zinman B, Brunzell JD, White NH, Danis RP, Davis MD, Hainsworth D, Hubbard LD, Nathan DM, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group : Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: An analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 171: 412–420, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villeneuve LM, Natarajan R: The role of epigenetics in the pathology of diabetic complications. Am J Physiol Renal Physiol 299: F14–F25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko YASK, Susztak K: Epigenomics: The science of no-longer-“junk” DNA. Why study it in chronic kidney disease? Semin Nephrol 33: 354–362, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, Ren B, Natarajan R: Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem 283: 26771–26781, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M: Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 205: 2409–2417, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R: MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A 104: 3432–3437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko YAMD, Suzuki M: Park1 ASD, Izquierdo MC, Han SY, Kang HM, Si H, Hostetter TH, Pullman J, Fazzari M, Verma AK, Zheng D, Greally JM, Susztak K: Cytosine methylation changes of enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome Biol 14:R108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sapienza C, Lee J, Powell J, Erinle O, Yafai F, Reichert J, Siraj ES, Madaio M: DNA methylation profiling identifies epigenetic differences between diabetes patients with ESRD and diabetes patients without nephropathy. Epigenetics 6: 20–28, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Bechtel W, McGoohan S, Zeisberg EM, Müller GA, Kalbacher H, Salant DJ, Müller CA, Kalluri R, Zeisberg M: Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med 16: 544–550, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Beneden K, Mannaerts I, Pauwels M, Van den Branden C, van Grunsven LA: HDAC inhibitors in experimental liver and kidney fibrosis. Fibrogen Tissue Repair 6: 1, 2013 [DOI] [PMC free article] [PubMed]