Abstract
Little is known about the circumstances under which older adults initiate chronic dialysis and subsequent outcomes. Using national registry data, we conducted a retrospective analysis of 416,657 Medicare beneficiaries aged ≥67 years who initiated chronic dialysis between January 1995 and December 2008. Our goal was to define the relationship between health care intensity around the time of dialysis initiation and subsequent survival and patterns of hospitalization, use of intensive procedures (mechanical ventilation, feeding tube placement, and cardiopulmonary resuscitation), and discontinuation of dialysis before death. We found that most patients (64.5%) initiated dialysis in the hospital, including 36.6% who were hospitalized for ≥2 weeks and 7.4% who underwent one or more intensive procedures. Compared with patients who initiated dialysis in the outpatient setting, those who received the highest intensity of care at dialysis initiation (those hospitalized ≥2 weeks and receiving at least one intensive procedure) had a shorter median survival (0.7 versus 2.1 years; P<0.001), spent a greater percentage of remaining follow-up time in the hospital (median, 22.9% versus 3.1%; P<0.001), were more likely to undergo subsequent intensive procedures (44.9% versus 26.0%; adjusted hazard ratio, 2.33; 95% confidence interval [CI], 2.27 to 2.39), and were less likely to have discontinued dialysis before death (19.1% versus 26.2%; adjusted odds ratio, 0.68; 95% CI, 0.65 to 0.72). In conclusion, most older adults initiate chronic dialysis in the hospital. Those who have a prolonged hospital stay and receive other forms of life support around the time of dialysis initiation have limited survival and more intensive patterns of subsequent healthcare utilization.
Over the last decade, a growing number of older adults are initiating chronic dialysis.1 Survival among these older patients is extremely limited,2,3 and many experience functional decline,4,5 frequent hospitalization,6 and a high symptom burden7 after initiation of chronic dialysis. In this setting, patients must often make trade-offs between interventions intended to lengthen life and those directed at other treatment goals, such as maximizing quality of life and maintaining independence.
Rates of hospitalization and use of intensive procedures, such as mechanical ventilation, feeding tube placement, and cardiopulmonary resuscitation (CPR), at the end of life are exceptionally high in older dialysis patients compared with other older Medicare beneficiaries with life-limiting illness.8 Discussions about prognosis, goals, and preferences are often lacking, and patients may have little appreciation of their likelihood of clinical deterioration or knowledge of more conservative alternatives, such as hospice.9 Uncertainty about disease trajectory and prognosis can hamper formulation of future plans and treatment preferences.10–12
Prior studies have evaluated the association of patient characteristics (e.g., comorbid conditions and functional status)5,13,14 and treatment practices (e.g., early nephrology referral and type of vascular access)15,16 before and at the time of dialysis initiation with subsequent outcomes. However, many of these analyses did not capture information on clinical circumstances around the time of dialysis initiation. In reality, chronic dialysis is often initiated in the context of acute illness17,18 and rapid or unexpected loss of renal function.14
We hypothesized that illness severity around the time of dialysis initiation—as reflected in measures of health care intensity, such as length of hospitalization and use of intensive procedures—might provide information useful for anticipatory guidance and supporting treatment decisions in older adults newly initiated on chronic dialysis. To evaluate this hypothesis, we used data from the U.S. Renal Data System (USRDS), a national registry of ESRD, to identify 416,657 Medicare beneficiaries aged ≥67 years and describe the intensity of care they experienced around the time of initiation of chronic dialysis and its association with survival and patterns of future healthcare utilization.
Results
Overall, 64.5% of cohort patients initiated chronic dialysis in the inpatient setting. For those who started dialysis in the hospital, the mean length of stay ± SD was 2 weeks ± 14.6 days (median, 11.0 days [interquartile range [IQR], 7.0–18.0]), and 7.4% received at least one intensive procedure, most commonly mechanical ventilation (5.9%), followed by feeding tube placement (1.9%) and cardiopulmonary resuscitation (CPR) (0.5%). The proportion of inpatient starts increased with age from 62.7% among patients aged 67–74 years to 68.2% among patients aged ≥85 years (P<0.001). The majority of cohort members (96.2%) who initiated chronic dialysis in the hospital survived to discharge.
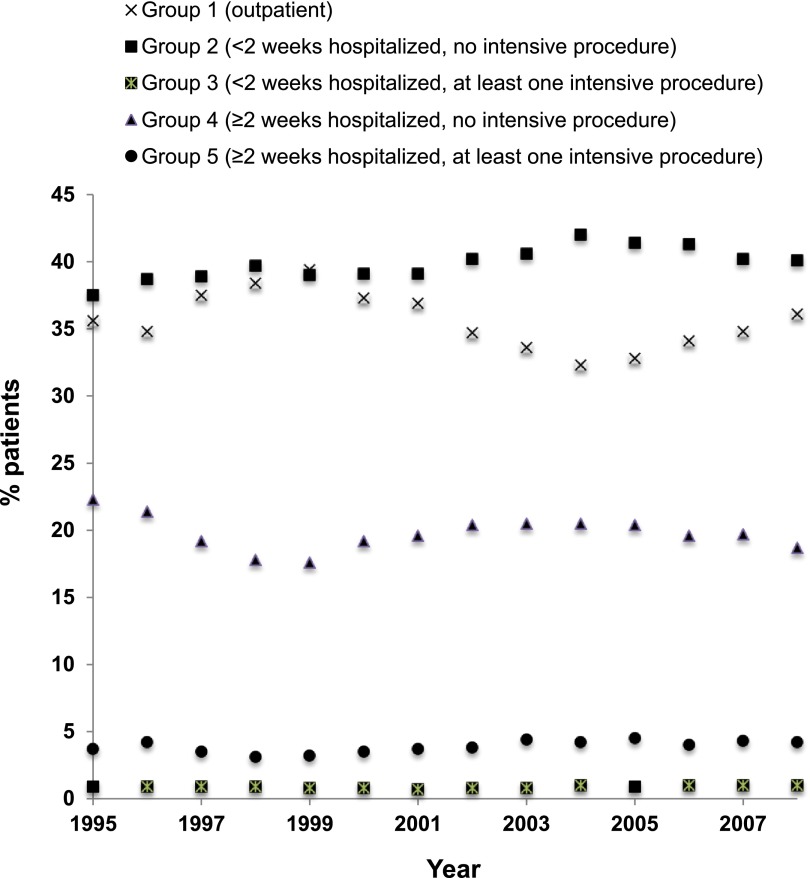
Patients were classified into five groups according to level of intensity of care received around the time of dialysis initiation with group 1 (outpatient initiation) having the lowest level of intensity and group 5 (inpatient stay ≥2 weeks, and receipt of at least one intensive procedure) having the highest (Table 1). Between 1995 and 2008, the proportion of patients initiating dialysis as an outpatient decreased (P<0.001) and the proportion who initiated dialysis during low-intensity hospital admissions (<2 weeks and no intensive procedures) increased (P<0.001) (Figure 1). However, there did not appear to be a statistically significant linear trend toward higher-intensity admissions over time.
Table 1.
Intensity of care received at dialysis initiation
| Group | Intensity of Care | Place of Initiation | Length of Index Hospitalization (wk) | Intensive Procedure Performed? | Patients, n (%) |
|---|---|---|---|---|---|
| 1 | Lowest | Outpatient | 0 | No | 147,823 (35.5) |
| 2 | Inpatient | < 2 | No | 166,549 (40.0) | |
| 3 | Inpatient | < 2 | Yes | 3763 (0.9) | |
| 4 | Inpatient | ≥ 2 | No | 82,238 (19.7) | |
| 5 | Highest | Inpatient | ≥ 2 | Yes | 16,284 (3.9) |
Figure 1.
Trends in the level of intensity of care received around the time of dialysis initiation. Most older adults initiated chronic dialysis during low-intensity hospital admissions (group 2) and this trend has been increasing over time (P<0.001).
Patients who received a higher level of intensity of care at dialysis initiation were more likely to be older, male, and black and to have nonresolving tubular necrosis listed as the cause of ESRD, a higher mean estimated GFR (eGFR) at initiation, lower rates of predialysis nephrology care, and a higher prevalence of comorbid conditions (Table 2).
Table 2.
Characteristics of patients who initiated dialysis (n=416,657) according to level of intensity of care received around the time of initiation
| Characteristic | Intensity of Care (95% CI) (%)a | Trend P Value | ||||
|---|---|---|---|---|---|---|
| Group 1 (Lowest) (n=147,823) | Group 2 (n=166,549) | Group 3 (n=3763) | Group 4 (n=82,238) | Group 5 (Highest) (n=16,284) | ||
| Men | 53.4 (53.2 to 53.7) | 48.6 (48.4 to 48.9) | 47.9 (46.4 to 49.5) | 52.2 (51.8 to 52.5) | 49.5 (48.8 to 50.3) | <0.001 |
| Race | ||||||
| White | 74.2 (74.0 to 74.5) | 75.6 (75.4 to 75.8) | 73.7 (72.2 to 75.1) | 76.2 (75.9 to 76.4) | 73.9 (73.2 to 74.6) | Reference |
| Black | 19.5 (19.3 to 19.7) | 20.6 (20.4 to 20.8) | 22.6 (21.3 to 24.0) | 20.5 (20.3 to 20.8) | 22.6 (22.0 to 23.2) | <0.001 |
| Other | 5.6 (5.5 to 5.8) | 3.3 (3.2 to 3.3) | 3.2 (2.7 to 3.8) | 2.7 (2.6 to 2.8) | 2.9 (2.7 to 3.2) | <0.001 |
| Age | ||||||
| 67–74 yr | 45.2 (45.0 to 45.5) | 42.8 (42.5 to 43.0) | 47.5 (45.9 to 49.1) | 39.4 (39.1 to 39.8) | 42.6 (41.9 to 43.4) | Reference |
| 75–79 yr | 26.7 (26.4 to 26.9) | 26.7 (26.5 to 26.9) | 26.2 (24.8 to 27.6) | 27.5 (27.2 to 27.8) | 27.9 (27.2 to 28.5) | <0.001 |
| 80–84 yr | 18.3 (18.2 to 18.6) | 19.4 (19.2 to 19.6) | 16.4 (15.3 to 17.6) | 20.6 (20.3 to 20.9) | 19.2 (18.6 to 19.8) | <0.001 |
| ≥85 yr | 9.7 (9.6 to 9.9) | 11.1 (11.0 to 11.3) | 9.9 (9.0 to 10.9) | 12.4 (12.2 to 12.7) | 10.4 (9.9 to 10.8) | <0.001 |
| Cause of ESRD | ||||||
| Hypertension | 32.1 (31.9 to 32.4) | 32.4 (32.1 to 32.6) | 34.1 (32.6 to 32.6) | 29.1 (28.8 to 29.4) | 28.8 (28.1 to 29.5) | Reference |
| Nonresolving tubular necrosis | 1.5 (1.4 to 1.5) | 1.2 (1.2 to 1.3) | 3.4 (2.8 to 4.0) | 4.5 (4.4 to 4.6) | 12.8 (12.3 to 13.3) | <0.001 |
| Diabetes mellitus | 40.5 (40.3 to 40.8) | 41.2 (40.9 to 41.4) | 40.0 (38.4 to 41.5) | 39.2 (38.8 to 39.5) | 34.6 (33.9 to 35.4) | <0.001 |
| GN | 7.3 (7.2 to 7.5) | 6.5 (6.3 to 6.6) | 4.9 (4.3 to 5.7) | 6.2 (6.0 to 6.3) | 4.7 (4.4 to 5.0) | <0.001 |
| Other | 13.3 (13.1 to 13.5) | 13.9 (13.7 to 14.0) | 12.2 (11.2 to 13.3) | 15.2 (15.0 to 15.5) | 12.5(12.0 to 13.0) | <0.001 |
| Unknown | 5.3 (5.2 to 5.4) | 5.0 (4.9 to 5.1) | 5.5 (4.8 to 6.3) | 5.9 (5.7 to 6.0) | 6.6 (6.2 to 7.0) | <0.001 |
| Median eGFR (IQR) (ml/min per 1.73 m2) | 9.4 (7.1–12.6) | 8.9 (6.6–12.2) | 9.2 (6.8–12.6) | 9.8 (7.1–13.6) | 10.6 (7.7–14.7) | <0.001 |
| Comorbid conditions | ||||||
| Coronary arterial disease | 59.2 (58.9 to 59.4) | 69.5 (69.3 to 69.7) | 75.9 (74.5 to 77.2) | 76.0 (75.7 to 76.3) | 77.9 (77.2 to 78.5) | <0.001 |
| Peripheral arterial disease | 27.7 (27.5 to 28.0) | 33.9 (33.7 to 34.1) | 35.4 (33.9 to 37.0) | 38.1 (37.8 to 38.4) | 36.9 (36.1 to 37.6) | <0.001 |
| Congestive heart failure | 62.6 (62.3 to 62.8) | 77.4 (77.2 to 77.6) | 87.6 (86.5 to 88.6) | 84.5 (84.3 to 84.8) | 86.6 (86.0 to 87.0) | <0.001 |
| Hypertension | 90.0 (89.9 to 90.2) | 98.0 (97.9 to 98.1) | 97.8 (97.3 to 98.2) | 96.7 (96.6 to 96.8) | 95.6 (95.3 to 95.9) | <0.001 |
| COPD and/or emphysema | 33.7 (33.5 to 33.9) | 41.9 (41.7 to 42.2) | 52.2 (50.6 to 53.8) | 49.0 (48.7 to 49.3) | 55.1 (54.3 to 55.8) | <0.001 |
| Cirrhosis | 1.7 (1.6 to 1.8) | 2.1 (2.0 to 2.2) | 1.7 (1.4 to 2.2) | 3.4 (3.3 to 3.5) | 2.7 (2.5 to 3.0) | <0.001 |
| Dementia | 5.5 (5.4 to 5.7) | 6.6 (6.5 to 6.7) | 8.7 (7.9 to 9.7) | 8.9 (8.7 to 9.1) | 11.6 (11.1 to 12.1) | <0.001 |
| Diabetes mellitus | 60.8 (60.5 to 61.0) | 66.2 (66.0 to 66.5) | 66.6 (65.1 to 68.1) | 67.0 (66.7 to 67.4) | 65.3 (64.5 to 66.0) | <0.001 |
| Cancer | 28.4 (28.1 to 28.6) | 31.8 (31.6 to 32.0) | 29.4 (28.0 to 31.0) | 33.9 (33.6 to 34.2) | 32.2 (31.5 to 32.9) | <0.001 |
| Late nephrology careb | 42.3 (42.0 to 42.5) | 41.6 (41.3 to 41.8) | 47.7 (46.1 to 49.3) | 54.9 (54.5 to 55.3) | 60.7 (60.0 to 61.5) | <0.001 |
| Intensive procedure | ||||||
| Mechanical ventilation | NA | NA | 80.0 (78.7 to 81.2) | NA | 78.9 (78.3 to 79.5) | NA |
| Cardiopulmonary resuscitation | NA | NA | 10.9 (10.0 to 12.0) | NA | 6.1 (5.8 to 6.5) | NA |
| Feeding tube placement | NA | NA | 15.8 (14.7 to 17.0) | NA | 28.2 (27.5 to 28.9) | NA |
COPD, chronic obstructive pulmonary disease; NA, not applicable.
Group 1: outpatient; group 2: <2 weeks hospitalized, no intensive procedure; group 3: <2 weeks hospitalized, at least one intensive procedure; group 4: ≥2 weeks hospitalized, no intensive procedure; Group 5: ≥2 weeks hospitalized, at least one intensive procedure.
≤3 months predialysis nephrology care.
The median survival of patients who received mechanical ventilation, CPR and feeding tube placement around the time of dialysis initiation was 0.9 years (IQR, 0.3–2.7 years), 0.8 years (IQR, 0.2–2.3 years), and 0.4 years (IQR, 0.2–1.4 years), respectively. Patients who received higher levels of intensity of care at dialysis initiation had more limited survival. Median survival was 2.1 years (IQR, 0.8–4.1 years) among patients in group 1 (outpatient initiation), 1.7 years (IQR, 0.6–3.6 years) in group 2 (hospital stay <2 weeks and no intensive procedure), 1.3 years (IQR, 0.4–3.1 years) in group 3 (hospital stay <2 weeks and at least one intensive procedure), 1.0 year (IQR, 0.3–2.7 years) in group 4 (hospital stay ≥2 weeks and no intensive procedure), and 0.7 years (IQR, 0.3–2.3 years) in group 5 (hospital stay ≥2 weeks and at least one intensive procedure) (P<0.001). Absolute differences in median survival across groups were most pronounced among younger patients (Figure 2A). For example, among patients aged 67–74 years, median survival ranged from 1.2 to 2.6 years across groups with differing levels of intensity of care (P<0.001) compared with 0.4–1.0 years among patients aged ≥85 years (P<0.001). After controlling for differences in patient characteristics, the relative risk of death was significantly higher among patients who received the highest compared with the lowest level of intensity of care at initiation (adjusted hazard ratio, 1.44, 95% confidence interval [CI], 1.42–1.47). The relative risks of death associated with a given level of intensity were similar or greater among older compared with younger patients (Table 3).
Figure 2.
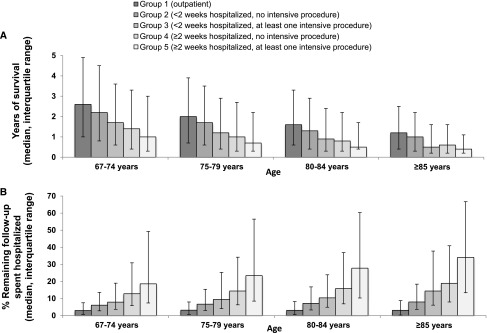
Median survival (A) and percentage of remaining follow-up spent in the hospital (B) after dialysis initiation. Error bars represent interquartile ranges. Patients who received higher levels of healthcare intensity around the time of dialysis initiation experienced more limited survival and spent more time in the hospital thereafter.
Table 3.
Adjusted associations between intensity of care around the time of dialysis initiation and death
| Intensity of Carea | Hazard Ratio per Age (95% CI) | |||
|---|---|---|---|---|
| 67–74 yr | 75–79 yr | 80–84 yr | ≥85 yr | |
| Group 1 (lowest) | Reference | Reference | Reference | Reference |
| Group 2 | 1.07 (1.05 to 1.08) | 1.07 (1.06 to 1.09) | 1.09 (1.07 to 1.11) | 1.09 (1.07 to 1.12) |
| Group 3 | 1.21 (1.15 to 1.28) | 1.26 (1.17 to 1.35) | 1.23 (1.13 to 1.34) | 1.44 (1.29 to 1.61) |
| Group 4 | 1.30 (1.28 to 1.32) | 1.29 (1.26 to 1.31) | 1.33 (1.30 to 1.36) | 1.36 (1.32 to 1.40) |
| Group 5 (highest) | 1.39 (1.35 to 1.43) | 1.42 (1.37 to 1.47) | 1.53 (1.47 to 1.60) | 1.50 (1.42 to 1.59) |
Adjusted for sex, race, cause of ESRD, eGFR, comorbid conditions, and year dialysis was initiated.
Group 1: outpatient; group 2: <2 weeks hospitalized, no intensive procedure; group 3: <2 weeks hospitalized, at least one intensive procedure; group 4: ≥2 weeks hospitalized, no intensive procedure; group 5: ≥2 weeks hospitalized, at least one intensive procedure.
The percentage of remaining follow-up time spent in the hospital increased with progressively higher levels of intensity of care at dialysis initiation. The median percentage of days spent hospitalized was 3.1% (IQR, 0.6%–7.8%) among patients in group 1, 6.7% (IQR, 3.0%–15.2%) in group 2, 9.1% (IQR, 4.1%–22.8%) in group 3, 14.4% (IQR, 6.4%–34.4%) in group 4, and 22.3% (IQR, 8.5%–55.2%) in group 5 (P<0.001). There was more heterogeneity in the percentage of remaining follow-up spent in the hospital for older patients (Figure 2B). For instance, among patients aged 65–74 years, the percentage of remaining follow-up time spent in the hospital ranged from 3.0% to 18.6% across groups with differing levels of intensity of care (P<0.001) compared with 3.1%–34.1% among patients aged ≥85 years (<em>P<0.001).
Compared with those who initiated dialysis in the outpatient setting (group 1), patients who received the most intensive care (group 5) around the time of dialysis initiation were more likely to undergo an intensive procedure during follow-up (44.9% versus 26.0%; adjusted hazard ratio, 2.33; 95% CI, 2.27 to 2.39). Among patients who died during follow-up, those in group 5 were less likely to have discontinued dialysis before death compared with patients in group 1 (19.1% versus 26.2%; adjusted odds ratio, 0.68; 95% CI, 0.65 to 0.72).
Discussion
Most members of a national cohort of older Medicare beneficiaries who initiated chronic dialysis did so in the hospital. One in three of these patients spent more than 2 weeks in the hospital, and of these, one in six received at least one other form of life support during the same admission. Patients who had received more intensive care around the time of dialysis initiation had more limited survival, spent more time in the hospital, were more likely to undergo one or more intensive procedures during follow-up, and were less likely to have discontinued dialysis before death.
Available data suggest that dialysis is often presented to patients as a “necessity” rather than as a true treatment “choice,”19 and when asked after the fact, many older patients express powerlessness about their decision to undergo chronic dialysis.9,19 While current guidelines recommend timely discussion of treatment options in the outpatient setting,20 our results demonstrate that in a sizable number of patients, the decision to initiate chronic dialysis is made in the context of severe acute illness and in concert with decisions about other intensive procedures intended to prolong life, such as mechanical ventilation, feeding tube placement, and CPR. Moreover, patients who receive more intensive care around the time of dialysis initiation are more likely to follow more intensive patterns of health care utilization in the future. Collectively, these findings argue for stronger efforts to contextualize discussions about dialysis initiation and related treatment decisions within a broader process of advance care planning to address treatment preferences in the setting of serious illness.21
Life expectancy is often important in shaping treatment preferences.22 However, in older patients with severe chronic illness and limited life expectancy, maximizing survival may be but one of several competing treatment goals. Patients may be faced with tradeoffs between prolonging life and optimizing other goals, such as preserving dignity, maximizing comfort, and maintaining quality of life.23 Prior studies have demonstrated that compared with patients who opt for conservative therapy, those who initiate chronic dialysis spend more time in a health care setting.6 Among patients newly initiated on chronic dialysis, information about how much time they can expect to spend in the hospital after dialysis initiation may be a meaningful adjunct to information on life expectancy in formulating treatment decisions. Acute illness can be particularly detrimental at older ages, resulting in persistent functional decline in a third of patients.24 Although survival was most limited at older ages, intensity of care was strongly associated with time spent in the hospital for all age groups. In patients aged ≥85 years, those who received the highest intensity of care at initiation survived a median of only 5 months after dialysis initiation and spent approximately 2 of these months in the hospital. Supplying this kind of information about anticipated outcomes to patients embarking on a course of chronic dialysis may help to provide a realistic foundation for future treatment decisions that are aligned with their goals, values, and preferences.25–27
Although we found that survival time and the proportion of hospital-free days were more limited in older adults who received more intensive care at the time of dialysis initiation, there was substantial heterogeneity in these measures among patients of similar ages who received similar levels of intensity of care. This was especially true for younger members of this cohort. For instance, patients aged 67–75 years who received the highest level of intensity of care had a median survival of 1 year, but half of patients survived for <4 months or >3 years. These findings highlight the challenges involved in applying summary prognostic information to the care of individual patients. Nevertheless, patients often appreciate the inherent uncertainty in life expectancy estimates at the individual level,28 and reasonable approximations that include information on expected range (e.g., interquartile range) may still help patients to make informed treatment choices and life plans.10 Prognostic information of this sort is not intended to replace providers’ clinical judgment about a patient’s prognosis but can be used to supplement their knowledge and increase their confidence and willingness to discuss prognosis with patients.
Although our study leverages systematically collected data on a nationally representative cohort of patients who initiated chronic dialysis, our results should be interpreted with the following limitations in mind. First, the study population was limited to older patients with Medicare parts A and B who were registered in the USRDS. The cohort does not include all patients who initiated dialysis in the hospital because those who died or recovered renal function shortly after initiation are unlikely to have been included in USRDS. This might explain why a very high percentage of cohort members who initiated dialysis in the hospital survived to discharge. If anything, our study findings thus present an overly optimistic picture of outcomes among the broader population of older adults who initiate dialysis in the hospital. Second, our findings reflect intensity of care at any point during the hospitalization in which chronic dialysis was started. Thus, for some patients this information would not be available until the time of hospital discharge or after dialysis was initiated and thus could not be used to inform decisions about dialysis initiation. Third, information on dialysis discontinuation was based on provider report, which may be subject to provider interpretation, particularly with the earlier version of the Centers for Medicare & Medicaid (CMS) Services Death Notification Form.29 Fourth, we do not have information on patients’ end-of-life care preferences, advance directives, or conversations between providers, patients, and families regarding treatment options and prognosis, which might explain some of the differences observed in use of intensive procedures around the time of dialysis initiation and thereafter. Finally, analyses of time to death and future healthcare utilization were not adjusted for possible changes in patient characteristics that may have occurred over time.
In conclusion, many older adults initiate chronic dialysis during an acute illness. Simple measures of health care intensity around the time of dialysis initiation can convey meaningful information about prognosis and future patterns of healthcare utilization. This information may be useful in setting realistic expectations for the future and guiding a range of treatment decisions and advance care planning among older adults who have initiated chronic dialysis.
Concise Methods
Study Population
Using data from the USRDS, we identified 416,657 fee-for-service Medicare beneficiaries aged ≥67 years who initiated chronic dialysis between January 1, 1995, and December 31, 2008, and for whom Medicare Parts A and B was the primary payor for health care beginning at any point during the 2-year period before dialysis initiation and continuously through dialysis initiation to end of follow-up. Patients were followed for death and hospitalization through December 31, 2008. The study was approved by the Institutional Review Board at the University of Washington.
Patient Characteristics
Demographic information, including age, sex, race, listed cause of ESRD, and eGFR at dialysis initiation were obtained from the USRDS Patients and Medical Evidence Files (derived from the CMS Medical Evidence Form 2728, which is completed by the provider after initiation). We used linked inpatient and outpatient Medicare claims to identify the following comorbid conditions present during the 2-year period before initiation based on International Classification of Diseases, Ninth Revision (ICD-9), diagnostic codes: coronary artery disease, peripheral arterial disease, congestive heart failure, chronic obstructive pulmonary disease, cirrhosis, hypertension, diabetes, dementia, and stroke. We also used inpatient and outpatient Medicare claims to determine whether patients received predialysis nephrology care, categorizing this as late care if their first visit with a nephrologist occurred during the 3 months before dialysis initiation or if they had not seen a nephrologist before initiation, and early if their first visit occurred prior to 3 months before initiation.
Predictor Variable
The primary predictor variable was level of intensity of care around the time of dialysis initiation, which was based on the following measures: site of dialysis initiation (inpatient versus outpatient), length of the index hospitalization (above versus below the mean length of stay of 2 weeks), and receipt of one or more intensive procedures (mechanical ventilation, feeding tube placement, or CPR) at any point during the index hospitalization. These measures were ascertained from the Medicare Inpatient Details File and the Medicare Physician Supplier File (which is derived from claims for procedures) supplied by USRDS. Intensive procedures were identified using ICD-9 and Healthcare Common Procedure Coding System codes.
Outcome Measures
The primary outcome measures were time to death after dialysis initiation and time spent in the hospital during follow-up. Survival was based on dates of initiation and death recorded in the USRDS Patients File. Time spent in the hospital included the hospitalization in which chronic dialysis was initiated and all subsequent hospitalizations through to the time of death or the end of follow-up.
Secondary outcome measures were future use of intensive procedures during follow-up, and among decedents, whether dialysis was discontinued prior to death. Information on dialysis discontinuation was obtained from the USRDS Death File (derived from the CMS Death Notification Form 2746, which is completed postmortem by the provider).
Statistical Analyses
The characteristics of patients who received differing levels of intensity of care at dialysis initiation were described using point estimates and 95% CIs. Tests for trend across groups were performed using regression analysis. We calculated median survival for each group using the Kaplan-Meier method, and differences were compared using the log-rank test. We measured the association between level of intensity of care at initiation and time to death using Cox proportional hazard regression models. The percentage of remaining follow-up time spent hospitalized was compared across levels of intensity of care using a Kruskal-Wallis test. Differences in survival and percentage of remaining follow-up time spent hospitalized were further evaluated among cohort members of different ages (67–74 years, 75–79 years, 80–84 years, and ≥85 years).
The association between intensity of care at dialysis initiation with future use of one or more intensive procedures was also examined using an adjusted Cox proportional hazard regression model, with censoring at the time of death or end of follow-up. Among dialysis patients who died during the follow-up period, we used a logistic regression model to examine the association between intensity of care at initiation with dialysis discontinuation prior to death.
Statistical packages used included SAS, version 9 (Cary, NC); Stata, version 11 (College Station, TX); and SPSS, version 19 (Somers, NY). Multivariate models were adjusted for all baseline characteristics.
Disclosures
None.
Acknowledgments
A.M.O. receives research funding from an interagency agreement between the Veterans Affairs Puget Sound Healthcare System and the Centers for Disease Control and Prevention and from grants from the National Institute on Aging and the Veterans Affairs Health Services Research and Development Service. She also receives royalties from UpToDate.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Older Adults with CKD and Acute Kidney Failure: Do We Know Enough for Critical Shared Decision Making?,” on pages 5–8.
References
- 1.U.S. Renal Data System : USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 2.Jassal SV, Trpeski L, Zhu N, Fenton S, Hemmelgarn B: Changes in survival among elderly patients initiating dialysis from 1990 to 1999. CMAJ 177: 1033–1038, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurella M, Covinsky KE, Collins AJ, Chertow GM: Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 146: 177–183, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Jassal SV, Chiu E, Hladunewich M: Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med 361: 1612–1613, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE: Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 361: 1539–1547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson RC, Juszczak M, Davenport A, Burns A: Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 4: 1611–1619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murtagh FE, Addington-Hall J, Higginson IJ: The prevalence of symptoms in end-stage renal disease: A systematic review. Adv Chronic Kidney Dis 14: 82–99, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Wong SPY, Kreuter W, O’Hare AM: Treatment intensity at the end of life in older adults receiving long-term dialysis. Arch Intern Med 172: 661–663, discussion 663–664, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davison SN: End-of-life care preferences and needs: Perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 195–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura MK, Tan JC, O’Hare AM: Optimizing renal replacement therapy in older adults: A framework for making individualized decisions. Kidney Int 82: 261–269, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowling CB, O’Hare AM: Managing older adults with CKD: Individualized versus disease-based approaches. Am J Kidney Dis 59: 293–302, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill TM: The central role of prognosis in clinical decision making. JAMA 307: 199–200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haapio M, Helve J, Kurimo P, Forslund T, Grönhagen-Riska C, Finne P: Decline in glomerular filtration rate during pre-dialysis phase and survival on chronic renal replacement therapy. Nephrol Dial Transplant 27: 1157–1163, 2012 [DOI] [PubMed] [Google Scholar]
- 14.O’Hare AM, Batten A, Burrows NR, Pavkov ME, Taylor L, Gupta I, Todd-Stenberg J, Maynard C, Rodriguez RA, Murtagh FE, Larson EB, Williams DE: Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis 59: 513–522, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue JL, Dahl D, Ebben JP, Collins AJ: The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 42: 1013–1019, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Winkelmayer WC, Liu J, Chertow GM, Tamura MK: Predialysis nephrology care of older patients approaching end-stage renal disease. Arch Intern Med 171: 1371–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crews DC, Jaar BG, Plantinga LC, Kassem HS, Fink NE, Powe NR: Inpatient hemodialysis initiation: Reasons, risk factors and outcomes. Nephron Clin Pract 114: c19–c28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buck J, Baker R, Cannaby AM, Nicholson S, Peters J, Warwick G: Why do patients known to renal services still undergo urgent dialysis initiation? A cross-sectional survey. Nephrol Dial Transplant 22: 3240–3245, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Russ AJ, Shim JK, Kaufman SR: “Is there life on dialysis?”: Time and aging in a clinically sustained existence. Med Anthropol 24: 297–324, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moss AH: Revised dialysis clinical practice guideline promotes more informed decision-making. Clin J Am Soc Nephrol 5: 2380–2383, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Davison SN, Torgunrud C: The creation of an advance care planning process for patients with ESRD. Am J Kidney Dis 49: 27–36, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Davison SN: Facilitating advance care planning for patients with end-stage renal disease: the patient perspective. Clin J Am Soc Nephrol 1: 1023–1028, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Fried TR, Tinetti ME, Iannone L, O’Leary JR, Towle V, Van Ness PH: Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med 171: 1854–1856, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, Burant CJ, Landefeld CS: Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: Increased vulnerability with age. J Am Geriatr Soc 51: 451–458, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Kirchhoff KT, Hammes BJ, Kehl KA, Briggs LA, Brown RL: Effect of a disease-specific advance care planning intervention on end-of-life care. J Am Geriatr Soc 60: 946–950, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassel CK, Guest JA: Choosing wisely: Helping physicians and patients make smart decisions about their care. JAMA 307: 1801–1802, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Davison SN, Simpson C: Hope and advance care planning in patients with end stage renal disease: Qualitative interview study. BMJ 333: 886, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams AW, Dwyer AC, Eddy AA, Fink JC, Jaber BL, Linas SL, Michael B, O’Hare AM, Schaefer HM, Shaffer RN, Trachtman H, Weiner DE, Falk AR, American Society of Nephrology Quality, and Patient Safety Task Force : Critical and honest conversations: The evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol 7: 1664–1672, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Holley JL: A single-center review of the death notification form: Discontinuing dialysis before death is not a surrogate for withdrawal from dialysis. Am J Kidney Dis 40: 525–530, 2002 [DOI] [PubMed] [Google Scholar]