Abstract
Isoprene emissions importantly protect plants from heat stress, but the emissions become inhibited by instantaneous increase of [CO2], and it is currently unclear how isoprene-emitting plants cope with future more frequent and severe heat episodes under high [CO2]. Hybrid aspen (Populus tremula x Populus tremuloides) saplings grown under ambient [CO2] of 380 μmol mol−1 and elevated [CO2] of 780 μmol mol−1 were used to test the hypothesis that acclimation to elevated [CO2] reduces the inhibitory effect of high [CO2] on emissions. Elevated-[CO2]-grown plants had greater isoprene emission capacity and a stronger increase of isoprene emissions with increasing temperature. High temperatures abolished the instantaneous [CO2] sensitivity of isoprene emission, possibly due to removing the substrate limitation resulting from curbed cycling of inorganic phosphate. As a result, isoprene emissions were highest in elevated-[CO2]-grown plants under high measurement [CO2]. Overall, elevated growth [CO2] improved heat resistance of photosynthesis, in particular, when assessed under high ambient [CO2] and the improved heat resistance was associated with greater cellular sugar and isoprene concentrations. Thus, contrary to expectations, these results suggest that isoprene emissions might increase in the future.
Key words: BVOCs, foliage traits, future emissions, heat stress, isoprene CO2 response, temperature response.
Introduction
Isoprene is the most abundant reactive volatile hydrocarbon emitted from a wide range of plant species (Fineschi et al., 2013; Monson et al., 2013; Sharkey et al., 2013). As a highly reactive volatile, isoprene significantly influences air quality by participating in ozone-forming reactions, and can also influence climate by participating in secondary organic aerosol formation (Claeys et al., 2004).
Isoprene as a small liphophilic molecule further plays important biological roles in protecting plants from abiotic stresses, in particular conferring greater heat resistance (Possell and Loreto, 2013; Sharkey et al., 2008). Isoprene can directly stabilize biomembranes avoiding excessive fluidity at high temperatures (Sharkey et al., 2001; Singsaas et al., 1997; Siwko et al., 2007), but isoprene can also quench reactive oxygen species formed under heat stress (Affek and Yakir, 2002; Loreto et al., 2001; Vickers et al., 2009a , 2009b ). There are multiple defences against sustained heat stress, including synthesis of polyterpenoids such as zeaxanthin (e.g. Tardy and Havaux, 1997), accumulation of osmotica (e.g. Hüve et al., 2006), and synthesis of heat-shock proteins (e.g. Riezman, 2004). However, rapid synthesis of volatile isoprene is especially advantageous in environments with intermittent heat periods such as those occurring during sunflecks when elicitation of other protective mechanisms is too slow (Behnke et al., 2007, 2013; Niinemets and Monson, 2013; Singsaas et al., 1999; Singsaas and Sharkey, 1998), but leaves may rapidly heat up to temperatures 45–50 °C (Singsaas et al., 1999; Singsaas and Sharkey, 1998; Valladares and Niinemets, 2007).
In plants, isoprene is formed in plastids from its immediate precursor dimethylallyl diphosphate (DMADP) by isoprene synthase (for recent reviews see Li and Sharkey, 2013b ; Rosenkranz and Schnitzler, 2013; Sharkey et al., 2013). Isoprene emissions increase hyperbolically with increasing light intensity and depend on temperature and ambient CO2 concentration according to asymmetric response curves with optima at leaf temperature of ~40–45 °C and at intercellular [CO2] of ~100–150 μmol mol−1 (Li and Sharkey, 2013b ; Loreto and Sharkey, 1990; Monson et al., 2012; Sun et al., 2012). Isoprene emission responses to short-term modifications in environmental drivers have been simulated assuming independent controls by different environmental drivers (for recent reviews see Grote et al., 2013; Monson et al., 2012). Based on the instantaneous CO2 response curves of isoprene emission, it has been suggested that isoprene emissions will decline in the future due to increases in atmospheric [CO2] (e.g. Arneth et al., 2007; Heald et al., 2009; Wilkinson et al., 2009). Such a reduction of isoprene emissions in future atmospheres would imply reduced capacity of plants to cope with recurrent heat episodes by isoprene emission. However, short-term fluctuations in all environmental drivers can modify the size of DMADP pool, and thus the environmental controls on isoprene emission are interactive rather than additive (Li and Sharkey, 2013a , 2013b ; Rasulov et al., 2009b , 2010; Sun et al., 2012), suggesting that direct extrapolation based on additive dependencies is not warranted.So far, information on the CO2 sensitivity of isoprene emissions under heat stress is limited. Way et al. (2011) observed that grey poplar (Populus x canescens) plants grown and measured at sub-ambient [CO2] of 190 μmol mol−1 had higher isoprene emission rate than plants grown and measured at elevated [CO2] of 590 μmol mol−1 at 30–42 °C. On the other hand, Rasulov et al. (2010) reported that inhibition of isoprene emission at the measurement [CO2] of 800 μmol mol−1 relative to 390 μmol mol−1 was lost at temperatures higher than 35 °C. Given the strongly non-linear response of isoprene emissions to [CO2], these discrepancies might reflect different [CO2] contrasts in these studies, and suggest that the sub-ambient compared with elevated [CO2] contrast is not appropriate to extrapolate isoprene emission responses to elevated temperatures from current ambient [CO2] to future conditions.
Furthermore, isoprene emissions can acclimate to growth [CO2] concentration, resulting in altered [CO2] sensitivity of isoprene emission as well as in changes in the emission capacity (Calfapietra et al., 2007, 2008, 2013; Sun et al., 2012; Wilkinson et al., 2009). In fact, there is little evidence of downregulation in isoprene emission capacity under elevated [CO2], and the emission capacity may even increase in elevated-[CO2]-acclimated plants (Li et al., 2009; Sharkey et al., 1991; Sun et al., 2012). Such an elevation of emission capacity may partly compensate for reduction of emissions due to limited DMADP pool size under high ambient [CO2], especially under high light (Sun et al., 2012). However, the overall effect of [CO2] acclimation on isoprene emissions under high temperatures will depend on temperature-dependent changes in [CO2] sensitivity of emissions.
As a further complication, there is evidence of enhanced thermal sensitivity of photosynthetic apparatus in both isoprene- and non-isoprene-emitting species acclimated to elevated [CO2] (Darbah et al., 2010; Taub et al., 2000; Way et al., 2011), possibly as the result of enhanced sugar concentrations that stabilize biomembranes (Hüve et al., 2006). There is currently no information on possible modifications of temperature dependencies of isoprene emission by growth [CO2] independent of the effects of instantaneous [CO2]. This is an important gap needing urgent filling to understand the heat effects on isoprene emissions in plants grown in different [CO2] environments as well as to improve modelling of isoprene emissions to future conditions.
In this study, we asked how the acclimation to elevated [CO2] alters the heat resistance of photosynthesis and the temperature response of isoprene emission in strong isoprene-emitter hybrid aspen (Populus tremula x Populus tremuloides). We hypothesized that plants grown under elevated CO2 have greater heat tolerance of photosynthetic apparatus and sustain greater isoprene emission rates, especially under supra-optimal temperatures. The results of this study will provide novel insight into the effects of CO2 acclimation on isoprene emissions, and into the role of isoprene in thermotolerance in future climates. Although global change is expected to alter moderately average temperatures (Meehl et al., 2007), temperatures strongly fluctuate during the day due to changes in radiation input, occasionally exceeding the threshold for leaf damage during heatflecks (Behnke et al., 2007; Singsaas et al., 1999; Singsaas and Sharkey, 2000; Way et al., 2011). It is these rapid and potentially damaging high-temperature excursions that are expected to become more frequent in climates with overall warmer temperatures. Thus, rapid protection of plant photosynthetic apparatus by adaptive features such as isoprene emission can potentially play a major role in vegetation responses to future climates.
Material and methods
Plant material and growth system
For these experiments, 2-year-old saplings of hybrid aspen (P. tremuloides Michx. x P. tremula L.) clone H200 were used (Rasulov et al., 2009a , 2011; Vahala et al., 2003 for details of the genotype). Before the start of the experimental treatments, the saplings were kept in cold room at −2 °C in the dormant state. Dormant plants were planted in 3 L plastic pots filled with sand and peat mixture (1:1), and dormancy was broken by transferring them to a growth room at 20 °C for 4 d. Plants with enlarged buds were installed in the whole-plant open gas-exchange/growth system for different [CO2] treatments. During plant growth, supply of nutrients and water was maintained at close to optimal levels (Sun et al., 2012, 2013 for details of plant growth).
The four-chamber whole-plant open gas-exchange/growth system’s design and operation have been described in our earlier studies (Sun et al., 2012, 2013). Briefly, each individual glass chamber had a volume of 12.5 L (diameter 0.2 m, height 0.4 m) to accommodate the entire foliage of a sapling, and the flow rate through the chamber was 7.5 L min−1, resulting in a relatively low chamber half-time of 70 s (see Niinemets, 2012 for a comparison of whole-plant gas-exchange systems). Chambers 1 and 3 were kept at the ambient [CO2] (mean±SD) of 380±10 μmol mol−1, and chambers 2 and 4 were treated with the elevated [CO2] of 780±10 μmol mol−1. Chamber air temperature was maintained at 28–30/23 °C (day/night) and relative humidity was 60%. Photoperiod length was 12h and the light intensity at the top of the plants was 500 μmol m−2 s−1 at start of the experiment, increasing to 800 μmol m−2 s−1 by the end of the experiments when the plants had filled the growth chamber (Sun et al., 2012).
After 30–40 d growth under given conditions when plants had formed a branched canopy filling the growth chamber (Sun et al., 2013 for details) plants were randomly moved out and temperature responses of foliage gas exchange were measured in individual attached fully mature (10–12 d old) leaves. The experiment was replicated four times, altogether with 16 plants in two treatment CO2 concentrations.
Measurements of temperature responses of net assimilation and isoprene emission
A Walz GFS-3000 portable gas-exchange/chlorophyll fluorescence system equipped with a LED array/PAM fluorometer 3055-FL (Walz GmbH, Effeltrich, Germany) and linked with a Fast Isoprene Sensor (Hills-Scientific, Boulder, CO, USA) was used for combined measurements of photosynthetic characteristics and isoprene emission rates as described in detail in Sun et al. (2012). The isoprene analyser was calibrated frequently with a standard gas containing 4.47 μmol mol−1 isoprene in N2 (Hills-Scientific).
The measurements were started by clamping the leaf in the cuvette and establishing the standard conditions of leaf temperature 30 °C, light intensity 500μmol m−2 s−1, and relative humidity 60%, corresponding to the environmental conditions during plant growth. Temperature responses of net assimilation and isoprene emission were measured after steady-state conditions had been established in the standard conditions at both the growth light intensity of 500μmol m−2 s−1 and the strong light intensity of 2000μmol m−2 s−1, and at both CO2 concentrations of 380 and 780μmol mol−1 using separate leaves for each combination of light and [CO2]. We denote the growth [CO2] treatments (380 and 780 μmol mol−1) as ambient and elevated, and measurement CO2 concentrations (380 and 780 μmol mol−1) as 380 and 780, yielding four combinations of growth and measurement CO2 concentrations: ambient (380), ambient (780), elevated (380), and elevated (780).
During response-curve measurements, leaf temperature was changed from the stabilization temperature of 30 °C to higher temperatures in steps of 5 °C up to 50 °C. The leaf was maintained at every temperature for 8min that was sufficient for establishment of steady-state conditions for measurements under 35–45 °C. This time period is comparable to past studies investigating the effect of heat- flecks on isoprene emission and photosynthesis (Behnke et al., 2007, 2013; Way et al., 2011). Time-dependent reductions in net assimilation rate were observed at 50 °C and sometimes at 45 °C as reported in other studies, likely reflecting time-dependent accumulation of reactive oxygen species and damage to photosynthetic apparatus (Hüve et al., 2006, 2011). Analogous time-dependent decreases can be sometimes observed for isoprene emissions (Loreto et al., 2006; Rasulov et al., 2010; Singsaas and Sharkey, 2000), mostly resulting from time-dependent reductions in the pool size of DMADP, the substrate for isoprene formation (Rasulov et al., 2010). In the case of time-dependent changes, there will be no true steady state and therefore, standardizing the time of sampling is highly recommended (Niinemets et al., 2010a , 2010d) as it allows for comparison of all leaves at a common heat dose.
Net assimilation, transpiration, and isoprene emission rates and steady-state fluorescence yield, F, were recorded during the last 30 s measurement period at the given temperature. Thereafter, a saturating pulse of white light was given to measure the maximum light-adapted quantum yield of photosystem II (PSII), F m′. The effective quantum yield of PSII (ΦPSII) was determined as (F m′−F)/F m′.
Isoprene concentration in leaf intercellular air space (C iso,i) was calculated as:
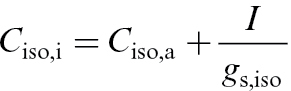 |
(1) |
where C iso,a is the isoprene concentration in the leaf chamber, and g s,iso is the stomatal conductance for isoprene. g s,iso was estimated as the product of stomatal conductance to water vapour and the ratio of the binary diffusion coefficients for isoprene (D iso) and water vapour (D H2O) (Niinemets and Reichstein, 2003). The temperature relationships of D iso and D H2O were developed based on the Chapman and Enskog theory of gas diffusion by intermolecular collision as in Niinemets and Reichstein (2003). The ratio D iso/D H2O was essentially independent of temperature, and an average value of 0.339 was used. The corresponding equilibrium concentration of isoprene in leaf liquid phase (nmol m−3) is given as C iso,w=C iso,i P/H pc, where P is the air pressure (Pa) and H pc is the Henry’s law constant for isoprene. A value of H pc of 9950 Pa m3 mol−1 at 30 °C was estimated from available data at 25 °C assuming an enthalpy of volatilization of 37 kJ mol−1 (Copolovici and Niinemets, 2005; Niinemets and Reichstein, 2003).
Overall, photosynthetic capacities (light-saturated net assimilation rate at an ambient CO2 concentration of 380 μmol mol−1 and leaf temperature of 30 °C) between about 7 to 20 μmol m−2 s−1, and isoprene emission capacities between about 10 to 40 nmol m−2 s−1 observed across the leaves measured in plants grown under ambient and elevated CO2 concentrations are similar to the values of these traits in Populus spp. observed in other studies (Calfapietra et al., 2007; Loreto et al., 2007; Niinemets et al., 2010c ; Pegoraro et al., 2004; Rasulov et al., 2010, 2011; Rosenstiel et al., 2003; Wiberley et al., 2008, 2009; Wilkinson et al., 2009). Lower values of isoprene emission from poplar species have been reported in some other studies, including emissions from young leaves (Centritto et al., 2004), from shaded leaves (Loreto et al., 2007), and from tissue-cultured plants with extremely thin leaves (Schnitzler et al., 2004). As isoprene synthase content is low in young, shaded, and morphologically weakly developed leaves (Calfapietra et al., 2007; Mayrhofer et al., 2005; Niinemets et al., 2010c ), lower estimates of isoprene emission rate in these other studies with poplars likely reflect low isoprene synthase activity in these studies. Thus, we conclude that our estimates of isoprene emission capacity are representative of poplar species.
Estimation of relative changes in net assimilation and isoprene emission rates
To compare the temperature treatment effects independent of differences in the capacities for net assimilation and isoprene emission, we calculated the normalized changes in these traits. Relative change in net assimilation rate, R A, was calculated as:
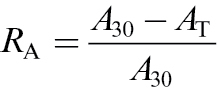 |
(2) |
where A 30 is the net assimilation rate at 30 °C and A T that at given temperature T. An increase in R A reflects a reduction in A T compared with A 30. Relative change in the effective quantum yield of PSII was calculated analogously. Relative change in isoprene emission rate due to changes in temperature, R I, was calculated as:
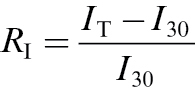 |
(3) |
where I 30 is the isoprene emission rate at 30 °C and I T the emission rate at given temperature T. An increase in R I corresponds to an increase in I T relative to I 30. As net assimilation rate generally decreased and isoprene emission rate increased at temperatures above 30 °C, relative changes in net assimilation and isoprene emission were defined differently to have positive values for both R A and R I across the whole temperature range.
Electrolyte leakage in response to heat stress
Leaf relative electrolyte leakage, a measure of membrane integrity, was assessed by changes in electrical conductivity of distilled water after soaking the treated leaves (Bajji et al., 2002; Kocheva et al., 2005; Scotti Campos et al., 2003). Detached leaves enclosed in plastic bags were immersed in water at a given temperature (25, 50, and 52 °C) for 5min. Then, three freshly cut discs (7mm in diameter each) from the treated leaf were immediately soaked in 5ml of distilled water at 25 °C. Conductivity of the water was measured in 24h after disc soaking using a conductometer HandyLab LF1 (Schott GmbH, Mainz, Germany). Thereafter, the same flasks with leaf discs were heated in a boiling-water bath for 10min and left to cool for 1h. The solution conductivity was measured again at 25 °C, and the relative electrical conductivity of the sample was expressed as a percentage of the maximum conductivity observed after boiling.
Foliage morphological and anatomical measurements
After gas-exchange measurements, leaf samples were taken for structural and chemical analyses. Leaf fresh mass and leaf area were determined immediately and dry mass after drying the leaves in a ventilated oven at 70 °C for 48h. Key foliage structural, anatomical, and chemical traits, including leaf dry mass per unit area (M A), nitrogen and carbon contents, leaf thickness, exposed mesophyll and chloroplast surface area, and number of chloroplasts for leaves developed under the CO2 treatments (10–16 replicates per treatment) have been reported by Sun et al. (2012, 2013). Elevated-[CO2]-grown plants had about 15% thicker leaves with 35% greater M A and 20% greater chloroplast exposed surface area per leaf area (Sun et al., 2012). In addition, the cross-sectional area of chloroplasts covered by starch granules per chloroplast area (a chl,s/a chl) was about 50% greater under elevated [CO2] (Sun et al., 2012). Here we use these data to estimate the distribution of leaf water among different leaf fractions to gain insight into possible differences in leaf sugar distribution and isoprene partitioning among leaf gas and liquid phases. Although water solubility of isoprene is relatively small with the dimensionless Henry’s law constant [H cc= H pc/(RT k), where R is the gas constant and T k the absolute temperature] at 30 °C being 3.95mol m−3 air (mol m−3 water), still a significant fraction of whole-leaf isoprene pool can be in the liquid phase depending on the relative size of leaf gas and liquid phases.
First, the volume fraction of mesophyll without intercellular air space (f t,mes) was calculated as:
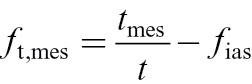 |
(4) |
where t mes is the mesophyll thickness, t the leaf thickness, and f ias is the fraction of intercellular air space. The volume fraction of chloroplasts (f t,chl) was calculated as the product of f t,mes and the ratio of cross-sectional areas of chloroplasts to mesophyll cells (a chl/a mes). The fraction of water in leaf mesophyll, F W,mes, was approximated by:
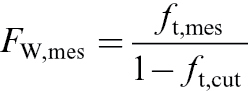 |
(5) |
where f t,cut is the volume fraction of cuticle with outer thickened cell walls (Niinemets, 1999). Equation 5 assumes that leaf water is uniformly distributed among epidermis and mesophyll cells. The correction, f t,cut, was minor for hybrid aspen, but was included for internal consistency. Finally, the volume fraction of water in chloroplasts, F W,chl, was calculated as:
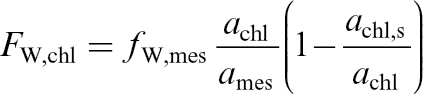 |
(6) |
The second term in this equation, 1−a chl,s/a chl, accounts for the reduction of chloroplastic water volume due to presence of starch granules.
Leaf sugar analysis
Content of soluble sugars was measured with the phenol sulphuric acid method of Dubois et al. (1956) as modified by Chow and Landhäusser (2004). The method is based on formation of orange-red colour as the result of condensation of furan derivatives produced under acidic conditions with phenol (Dubois et al., 1956). The soluble sugars were extracted in distilled water at 100 °C for 30min, the extract was treated with the phenol/sulphuric acid reagent as in Chow and Landhäusser (2004) and the absorbance was measured at 485nm with a Shimadzu UV2550PC spectrophotometer (Shimadzu, Kyoto, Japan). The standard curve was developed for sucrose and finally the sugar content was expressed in C6 sugar units. Leaf sugar concentration was calculated both per unit leaf dry mass and per unit leaf water.
Data analyses
[CO2] treatment effects on leaf traits were compared by one-way ANOVA followed by Tukey’s test. Within treatments, paired- sample t-tests were used to compare the physiological traits measured repeatedly under different measurement light and [CO2] conditions. Correlative relationships of R A (Eq. 2) versus R I (Eq. 3) were analysed by linear regressions and, whenever pertinent, by second-order polynomial regressions. Covariation analyses (ANCOVA) were employed to compare these relationships among the [CO2] treatments and at different measurement [CO2] and light intensities. In these analyses, the significance of the interaction term (treatment with covariate) was tested first (separate-slope model) and whenever the interaction term was non-significant the model was refitted without the interaction term (common-slope model). SPSS 17.0 (IBM SPSS Statistics) was used for all analyses and all statistical relationships were considered significant at P<0.05.
Results
Effects of growth [CO2] on leaf structure and chemistry
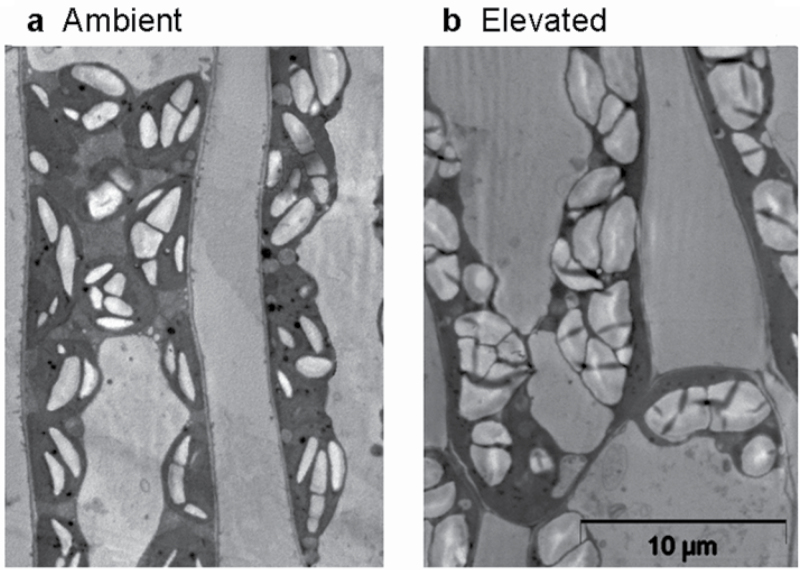
As we have demonstrated previously in hybrid aspen (P. tremula x P. tremuloides), elevated [CO2] resulted in thicker leaves with greater leaf dry mass per unit area and more chloroplasts per unit leaf surface area, overall indicating more advanced mesophyll development (Sun et al., 2012, 2013). Here we analyse additional traits with importance in leaf heat resistance (within-leaf distribution of sugars and isoprene). Elevated growth [CO2] resulted in greater leaf fresh mass per unit leaf area (M F) and mass of water per leaf area (M WA), although there was no significant treatment effect on mass of water per leaf volume (M WV) (Table 1). The fractions of mesophyll cells and intercellular air space of total leaf volume did not differ among the treatments, but the volume percentage of chloroplasts was higher in elevated-[CO2]-grown leaves (Table 1). Although starch granules comprised a greater proportion of chloroplast volume in leaves under elevated [CO2] treatment (Fig. 1), the overall fraction of leaf water in chloroplasts (Eq. 6) was higher under elevated [CO2] (Table 1). Leaf soluble sugar contents per dry mass (S D) and per leaf water (S W) were greater in leaves under elevated [CO2] (Table 1).
Table 1.
Foliage anatomical, morphological, and chemical traits of hybrid aspen ( P. tremula x P. tremuloides) trees grown under ambient (380 μmol mol−1) and elevated (780 μmol mol−1) atmospheric CO2 concentrations
| Trait | Treatment | P | |
|---|---|---|---|
| Ambient | Elevated | ||
| Leaf fresh mass per unit leaf area (g m−2) (M F) | 153.5±3.7 | 180.9±4.7 | <0.0001 |
| Mass of water per leaf area (g m−2) (M WA) | 125±5 | 142.4±3.2 | 0.001 |
| Mass of water per leaf volume (g cm−3) (M WV) | 0.713±0.040 | 0.728±0.017 | 0.76 |
| Percentage of intercellular air space (%) (f ias) | 26.2±1.1 | 24.3±1.1 | 0.21 |
| Percentage of mesophyll cells of total leaf volume (without air spaces) (%) (f t,mes, Eq. 4) | 59.2±2.1 | 61.4±3.4 | 0.25 |
| Percentage of chloroplasts of total leaf volume (without air spaces) (%) (f Chl) | 11.4±1.3 | 29±5 | 0.02 |
| Percentage of leaf water in mesophyll (%) (F W,mes, Eq. 5) | 46.1±3.0 | 51.0±2.4 | 0.25 |
| Percentage of leaf water in chloroplasts (%) (F W,Chl, Eq. 6) | 7.72±0.45 | 13.0±1.1 | 0.004 |
| Sugar content in leaf water (g g−1) (S W) | 0.036±0.007 | 0.053±0.008 | 0.002 |
| Sugar content per dry mass (g g−1) (S D) | 0.1293±0.0035 | 0.1567±0.0033 | <0.0001 |
Data are means±SE of four independent samples (trees). Means were compared using ANOVA. Leaf dry mass per unit area was 28.8±0.6g m−2 for plants grown under ambient and 38.6±0.8g m−2 for plants grown under elevated [CO2] (P<0.001) (Sun et al., 2012).
Fig. 1.
Transmission electron microscopy (TEM) images of leaf palisade mesophyll cells in hybrid aspen (P. tremula x P. tremuloides) leaves developed under the ambient CO2 concentration of 380 μmol mol−1 (a) and elevated CO2 concentration of 780 μmol mol−1 (b). The cells were viewed at 2100× magnification with a Philips Tecnai 10 TEM microscope (FEI, Eindhoven, Netherlands) using an accelerating voltage of 80kV.
Dependencies of net assimilation and isoprene emission rates and intercellular isoprene concentration on temperature: general patterns
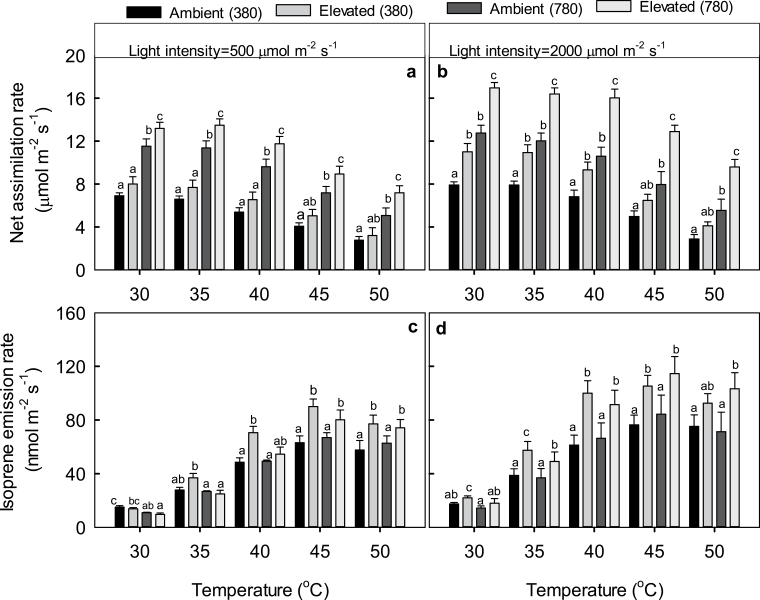
Net assimilation rate (A) of hybrid aspen leaves was the highest at leaf temperatures between 30 and 35 °C (Fig. 2a, b), whereas isoprene emission rate (I) increased up to temperatures of 45–50 °C (Fig. 2c, d). Temperature responses were broadly similar under moderately high light intensity of 500 μmol m−2 s−1 and strong light intensity of 2000 μmol m−2 s−1 (compare Fig. 2a and b, and Fig. 2c and d).
Fig. 2.
Temperature response of net assimilation rate (a, b), and isoprene emission rate (c, d) in hybrid aspen leaves under different growth and measurement CO2 concentrations and at different light intensities. Data in (a) and (c) correspond to measurements under a moderate light intensity of 500 μmol m−2 s−1 and (b) and (d) to measurements under a strong light intensity of 2000 μmol m−2 s−1. Ambient (380) and elevated (380) denote plants grown under the ambient [CO2] of 380 μmol mol−1 and elevated [CO2] of 780 μmol mol−1, and both measured at [CO2] of 380 μmol mol−1. Ambient (780) and elevated (780) plants were grown under the ambient [CO2] of 380 μmol mol−1 and elevated [CO2] of 780 μmol mol−1, and both measured at [CO2] of 780 μmol mol−1. Data are means (+SE) of 8–10 replicate leaves. At each individual temperature different letters at the top of each bar indicate statistically significant differences at a given temperature (P<0.05).
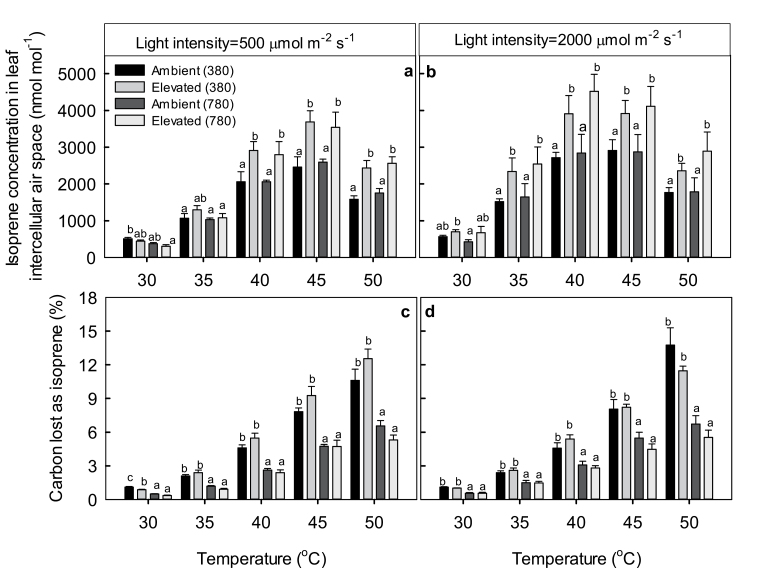
The temperature dependence of the concentration of isoprene in leaf intercellular air space (C iso,i) mirrored the temperature response of isoprene emission (Fig. 3a, b), whereas the fraction of carbon lost due to isoprene emission was the highest at 50 °C, reaching up to 15% of net assimilation rate, i.e. almost an order of magnitude increase compared to the carbon lost at 30 °C (Fig. 3c, d).
Fig. 3.
Temperature response of isoprene concentration in leaf intercellular air space (C iso,i, Eq. 1) (a, b) and the percentage of carbon lost as isoprene (c, d) in hybrid aspen leaves grown under different growth CO2 environments and measured under different growth and light conditions. Data are means (+SE) of 8–10 replicate leaves. Data presentation and statistics as in Figure 2.
Effects of growth [CO2] and measurement [CO2] and light intensity on net assimilation and isoprene emission rates under different temperatures
Measurement [CO2] (instantaneous change in [CO2]) generally increased the net assimilation rate (A) (Fig. 2a, b), although the increase was weaker for plants grown under ambient [CO2] than in plants grown under elevated [CO2], especially under moderate light intensity of 500 μmol m−2 s−1 (see Fig. 2a, b). At the moderate light intensity of 500 μmol m−2 s−1 and measurement [CO2] of 380 μmol mol−1, A was similar among plants grown at ambient and elevated [CO2] (Fig. 2a), but when measured at [CO2] of 780 μmol mol−1, A of elevated-[CO2]-grown plants was higher than in ambient-[CO2]-grown plants at a given temperature (Fig. 2a). Furthermore, under the strong light intensity of 2000 μmol m−2 s−1, A in elevated-[CO2]-grown plants was significantly higher than that in ambient-[CO2]-grown plants at both measurement CO2 concentrations of 380 and 780 μmol mol−1 (Fig. 2b).
Higher measurement [CO2] inhibited isoprene emission rate in elevated-[CO2]-grown plants at temperatures of 30–35 °C under moderately high light (Fig. 2c) and at 30 °C under strong light (Fig. 2d), but the [CO2] inhibition was lost at higher temperatures (Fig. 2c, d). At temperatures higher than 35 °C under moderately high light and higher than 30 °C under strong light, the isoprene emission rate in elevated-[CO2]-grown plants exceeded that in ambient-[CO2]-grown plants (Fig. 2c, d).
The variations in C iso,i among the growth [CO2] treatments and measurement [CO2] and light intensities reflected the differences in isoprene emission rate (see Fig. 2c, d and Fig. 3a, b). Thus, C iso,i was greater at stronger light, did not depend on measurement [CO2], and was greater in elevated-[CO2]-grown plants above 35 °C under the moderate light intensity of 500 μmol m−2 s−1 and above 30 °C under the strong light intensity of 2000 μmol m−2 s−1 (Fig. 3a, b). Given the size of the leaf gas- and liquid-phase pools (Table 1), the predicted amount (concentration multiplied by the volume of a given leaf phase) of isoprene in leaf gas- and liquid-phase pools at 30 °C was roughly similar (0.81-fold lower in the leaf liquid phase for ambient-[CO2]-grown plants and 0.89-fold lower for elevated-[CO2]-grown leaves). However, given the greater fraction of leaf water in chloroplasts in elevated-[CO2]-grown plants (Table 1), the total amount of isoprene associated with chloroplasts was also greater in elevated-[CO2]-grown plants.
The fraction of carbon lost as isoprene was always larger at the measurement [CO2] of 380 μmol mol−1 than at 780 μmol mol−1 (Fig. 3c, d). Growth [CO2] effects on relative carbon loss were minor, with the only significant difference being the greater carbon loss at 30 °C under moderate light intensity and at measurement [CO2] of 380 μmol mol−1 in ambient-[CO2]-grown plants (Fig. 3c).
Relationship of assimilation and isoprene emission rate with temperature
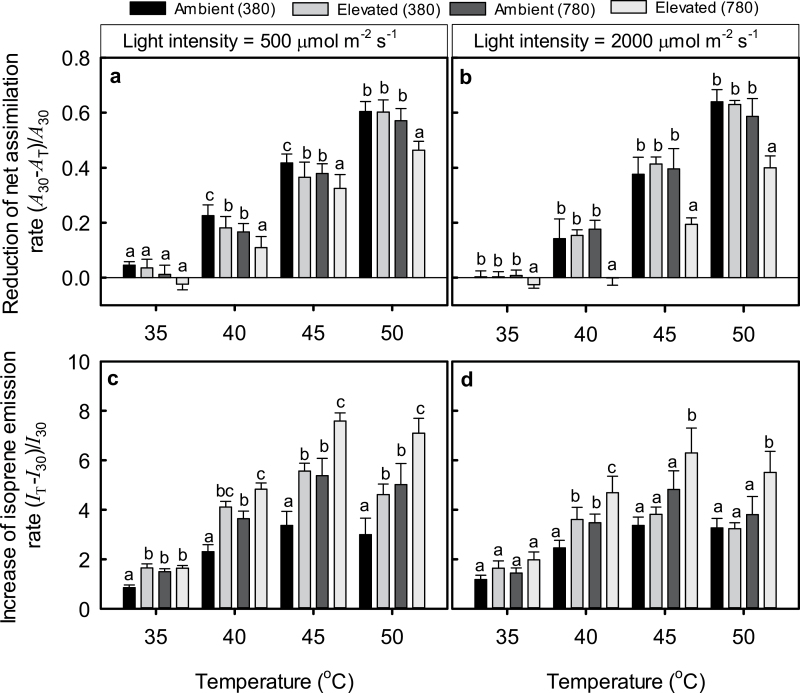
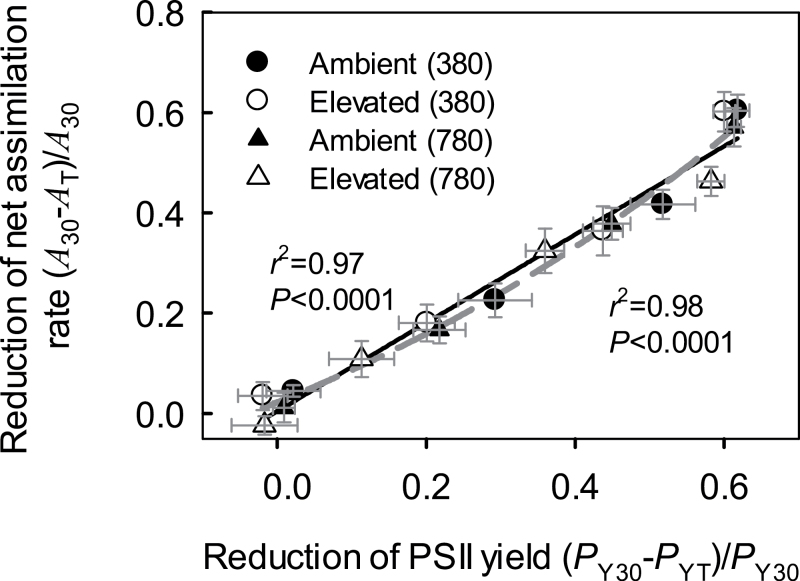
The relative reduction in net assimilation rate (Eq. 2, R A) increased almost linearly over the temperature range of 35–50 °C (Fig. 4a, b; r 2>0.9 for linear regressions). In contrast, the relative increase of isoprene emission rate (Eq. 3, R I) tended to be curvilinearly related to temperature, reaching a maximum at ~45 °C (Fig. 4c, d). R A at given temperature was lower in elevated-[CO2]-grown leaves measured at 780 μmol mol−1 (Fig. 4a, b), except at 35 °C under the light intensity of 500 μmol m−2 s−1 (Fig. 4a). At this light intensity, R A of ambient-[CO2] grown plants measured at 380 μmol mol−1 was greater than that for the rest of the treatments at 40 and 45 °C (Fig. 4a). Reductions in the effective quantum yield of PSII paralleled changes in R A, being smaller in elevated-[CO2]-grown plants measured at [CO2] of 780 μmol mol−1 than in the other treatments (P<0.001). The reductions in the effective PSII quantum yield and in R A were strongly correlated across the different measurement conditions and treatments (Fig. 5).
Fig. 4.
Relative reduction of net assimilation rate (Eq. 2) (a, b) and relative increase of isoprene emission rate (Eq. 3) (c, d) with increasing temperature in hybrid aspen leaves grown under different [CO2] of 380 μmol mol−1 (ambient) and 780 μmol mol−1 (elevated) and measured under different [CO2] and light conditions. Measurement CO2 concentrations, 380 or 780 μmol mol−1, are shown in parentheses for each treatment. Data are means (+SE) of 8–10 replicate leaves.
Fig. 5.
Relationship of the decrease of net assimilation rate (Eq. 2) with the reduction in effective quantum yield of PSII over the temperature range of 30–50 °C (n=8–10 for individual data points). Temperature responses of the change in net assimilation rate are illustrated in Figure 4a, c. The measurements conducted at light intensities of 500 and 2000 μmol m−2 s−1 were pooled. The decrease of PSII quantum yield was calculated as (Y 30−Y T)/Y 30, where Y 30 is the yield at 30 °C and Y T the PSII yield at any other measurement temperature between 30 and 50 °C. Error bars denote ±SE. Data were fitted by linear regression.
The values of R I in ambient-[CO2] grown plants measured at [CO2] of 380 μmol mol−1 and light intensity of 500 μmol m−2 s−1 were less than for the rest of the treatments (Fig. 4c), while at higher light level this was the case at 40 °C (Fig. 4d). On the other hand, R I in elevated-[CO2]-grown plants measured at 780 μmol mol−1 was consistently higher than for the rest of the treatments (Fig. 4c, d), except for 35 °C at the higher light intensity (Fig. 4d).
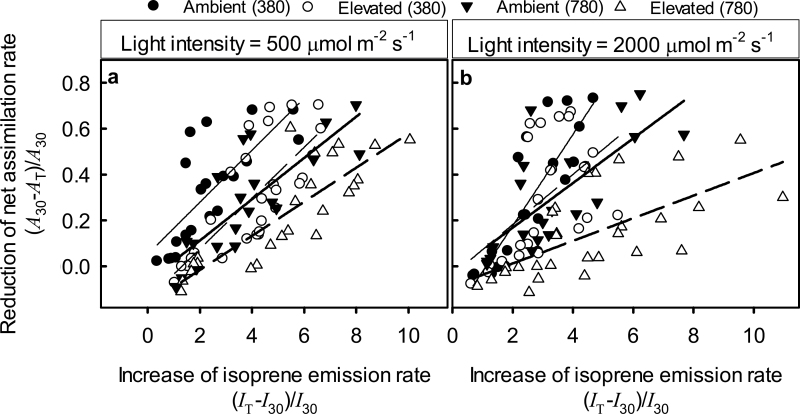
The reduction in net assimilation rate was positively correlated with the increase in isoprene emission rate (Fig. 6a, b). For measurements under lower light intensity of 500 μmol m−2 s−1, the interaction of R I with treatment was not significant (P>0.8). According to the common-slope ANCOVA, ambient-[CO2]-grown leaves measured at 380 μmol mol−1 had greater R A at given R I than that in the other treatments, while elevated-CO2-grown leaves measured at 780 μmol mol−1 had lower R A at given R I than that in the other treatments (Fig. 6a; P<0.001 for both comparisons). At higher light of 2000 μmol m−2 s−1 the interaction term was significant, indicating that the slope for elevated-[CO2]-grown plants measured at 780 μmol mol−1 was less than that for the other growth and measurement [CO2] combinations (Fig. 6b; P<0.001).
Fig. 6.
Correlations of the decrease of net assimilation rate (R A, Eq. 2) with the increase of isoprene emission rate (R I) during heat stress in hybrid aspen leaves under different growth (ambient of 380 μmol mol−1 and elevated of 780 μmol mol−1) and measurement [CO2] conditions (380 and 780 μmol mol−1). The measurements were conducted under a moderate light intensity of 500 μmol m−2 s−1 (a) and under a strong light intensity of 2000 μmol m−2 s−1 (b). Linear regression lines are shown to highlight the trends: ambient (380), thin solid line [r 2=0.56 for (a) and r 2=0.69 for (b), P<0.001 for both]; elevated (380), thin dashed line [r 2=0.58 for (a) and r 2=0.28 for (b), P<0.0001 for (a) and P<0.01 for (b)]; ambient (780), thick solid line [r 2=0.64 for (a) and r 2=0.54 for (b), P<0.001 for both]; and elevated (780), thick dashed line [r 2=0.71 for (a) and r 2=0.43 for (b), P<0.001 for both].
Membrane leakiness in relation to growth [CO2] and temperature-dependent reduction in net assimilation rate
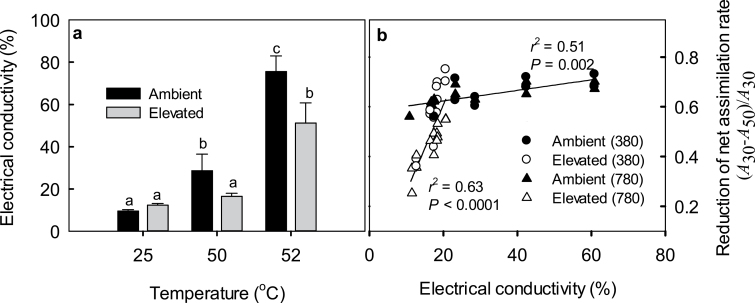
Relative electrical conductivity, the measure of membrane leakiness, was not significantly increased in elevated-[CO2]-grown plants after exposure of leaf discs to 50 °C (Fig. 7a). In contrast, in ambient-[CO2]-grown plants, exposure to 50 °C resulted in a significant increase in membrane leakiness (Fig. 7a). Exposure to severe heat stress of 52 °C resulted in enhanced membrane leakiness for both [CO2] treatments, but the leakiness was greater for ambient-[CO2]-grown plants (Fig. 7a).
Fig. 7.
Mean (+SE) relative leaf electrolyte leakage in response to heat stress (a) and correlations of the decrease of net assimilation rate (see Fig. 4a, c) with leaf electrical conductivity at 50 °C (b) in hybrid aspen leaves grown under ambient [CO2] of 380 μmol mol−1 and elevated [CO2] of 780 μmol mol−1. In (a) the data are means of 8–10 replicate leaves and means with different letters are significantly different at P<0.05 according to one-way ANOVA. In (b) the data correspond to individual measurements, data labels are as in Fig. 5, and the measurements conducted at light intensities of 500 and 2000 μmol m−2 s−1 were pooled. Data in (b) were fitted by linear regressions.
Relative electrical conductivity at 50 °C was correlated with the reduction in net assimilation rate (Fig. 7b) and PSII quantum yield (data not shown). However, the slope of this relationship was shallower in ambient-[CO2]-grown plants (P<0.001 for the interaction term of electrical conductivity × growth [CO2]; Fig. 7b), indicating that in ambient-[CO2]-grown plants a given reduction in net assimilation rate observed immediately at the end of the exposure period was associated with greater electrolyte leakage over the following 24h soaking of leaf discs.
Discussion
Elevated-[CO2]-driven modifications in leaf chemistry, structure, and photosynthesis
Elevated growth [CO2] resulted in greater leaf fresh mass per unit leaf area (M F), mass of water per leaf area (M WA), and fraction of water in chloroplasts (F W,Chl) (Table 1). However, the mass of leaf water per leaf volume was not significantly different among the treatments, indicating that greater mass of water per leaf area resulted from thicker leaf mesophyll in elevated-[CO2]-grown plants (Sun et al., 2012), as has been consistently observed (e.g. Miyazawa et al., 2011; Sims et al., 1998a , 1998b ), and suggested to reflect morphological ‘upregulation’ (Luo et al., 1997).
Elevated [CO2] also resulted in greater starch grain number and size inside the chloroplasts (Fig. 1) and enhanced leaf sugar content per dry mass and concentration in leaf water (Table 1), as has been demonstrated in numerous studies (see Makino and Mae, 1999 for reviews; Saxe et al., 1998). Furthermore, greater fraction of leaf water in chloroplasts in elevated-[CO2]-grown plants (Table 1) further suggests that a greater fraction of leaf sugar and liquid-phase isoprene is associated with chloroplasts in elevated-[CO2]-grown than in ambient-[CO2]-grown plants.
Elevated [CO2] is often associated with ‘downregulation of photosynthesis’, defined as reduced photosynthesis observed at the same given ambient [CO2] (Curtis and Wang, 1998; Johnson, 2006; Luo et al., 1997; Nowak et al., 2004). This downregulation is mainly associated with reduced nitrogen content and may also reflect feedback-inhibition of photosynthesis due to enhanced sugar concentrations (Curtis and Wang, 1998; Jeannette et al., 2000; Johnson, 2006; Luo et al., 1997; Myers et al., 1999; Nowak et al., 2004). However, in our study at optimum nutrient supply we actually observed enhanced photosynthetic capacity in elevated-[CO2]-grown plants (Sun et al., 2012; Fig. 2b), indicating no downregulation nor stronger feedback inhibition despite higher sugar concentrations.
Temperature responses of net assimilation and isoprene emission under different environmental conditions
Isoprene is formed in chloroplasts by isoprene synthase from its immediate precursor dimethylallyl diphosphate (DMADP) (see Li and Sharkey, 2013b for a recent review). The major source of chloroplastic DMADP is the plastidic 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway that starts with condensation of pyruvate and glyceraldehyde 3-phosphate (GAP) (Lichtenthaler, 1999; Schwender et al., 1997). Although there may be some contribution of the cytosolic mevalonic acid pathway because isopentenyl diphosphate, the isomer of DMADP, may be transferred between the cytosol and plastid, mevalonic acid pathway contribution is generally minor (Bick and Lange, 2003; Laule et al., 2003).
As both the isoprene synthase and the main pathway for DMADP formation are in chloroplasts, and chloroplastic DMADP formation under non-stressed conditions mainly relies on recently fixed carbon—in particular, on primary photosynthetic metabolite GAP—isoprene emission is strongly associated with photosynthetic carbon metabolism (for recent reviews see Li and Sharkey, 2013b ; Monson, 2013). Pyruvate for DMADP synthesis is assumed to be of cytosolic origin and transported to the chloroplasts in the form of phosphoenolpyruvate (PEP) by a PEP transporter in exchange for inorganic phosphate (Pi) (Li and Sharkey, 2013b ; Monson, 2013), but there is also evidence that pyruvate can be formed in chloroplasts from 2-phosphoglycerate (see Rasulov et al., 2011 for a discussion). At any rate, 13C-labelling experiments demonstrate that in unstressed plants 85–90% of the carbon in isoprene is derived from recently assimilated photosynthates (Delwiche and Sharkey, 1993; Funk et al., 2004; Karl et al., 2002). However, there are important discrepancies among isoprene emission and photosynthesis as demonstrated by our study and past observations, indicating significant differences in the regulation of isoprene emission and net assimilation rates: (i) the optimum temperature for isoprene emission is greater than that for net assimilation (Fig. 2; see also e.g. Harley et al., 1996; Niinemets et al., 1999) and, as the result, the fraction of carbon lost due to isoprene emission increases at higher temperatures (Fig. 3c, d); (ii) isoprene emission more strongly responds to light than net assimilation rate and is saturated at greater light intensity (Fig. 2 and 3; see also e.g. Harley et al., 1996; Monson et al., 2012; Niinemets et al., 1999, 2010d ); and (iii) isoprene emission rate is inhibited by above-ambient [CO2] concentrations, while net assimilation rate increases (Fig. 2; see Li and Sharkey, 2013b ; Monson, 2013 for reviews). In fact, isoprene emission is even suppressed at current ambient [CO2] relative to sub-ambient [CO2] (e.g. Guidolotti et al., 2011; Niinemets et al., 2010b ; Wilkinson et al., 2009). On the other hand, acclimation of capacities for photosynthesis and isoprene emission to growth [CO2] seems to occur in similar manner. When there is a downregulation in photosynthetic capacity in elevated-[CO2]-grown leaves, isoprene emission capacity is often reduced as well; in contrast, when photosynthetic capacity is enhanced upon acclimation to elevated [CO2], isoprene emission capacity seems to increase as well (see Sun et al., 2012 for a literature review of case studies). In Sun et al. (2012) this enhanced emission capacity became evident by increased isoprene emission rate at 30 °C under high light intensity of 2000 μmol m−2 s−1 and our study further demonstrates that this enhancement is maintained over the entire temperature response (Fig. 2d).
Apart from these general observations, our study demonstrates several important novel aspects of environmental responses of isoprene emission under high temperature and in plants developed in different atmospheric [CO2]: (i) the CO2 sensitivity of isoprene emission was lost at temperatures higher than 35–40 °C (Fig. 2c, d); (ii) as a result of the loss of [CO2] sensitivity of emissions, isoprene emission rates in elevated-[CO2]-grown plants exceeded the emissions in ambient-[CO2]-grown plants at higher temperatures of 40–50 °C; and (iii) high-temperature emission enhancement was maintained not only at high but also at moderate light intensity (Fig. 2c, d, Fig. 4c, d). In the following, we analyse the possible factors responsible for the loss of CO2 sensitivity at higher temperatures.
Loss of CO2 sensitivity of isoprene emission at elevated temperatures
Given that the reduction of isoprene emission at higher measurement [CO2] is associated with reduction in chloroplastic DMADP pool size (Li and Sharkey, 2013b ; Rasulov et al., 2009b ; Wilkinson et al., 2009), loss of CO2 sensitivity at higher temperatures suggests that DMADP should have become more readily available under high [CO2] and temperature. Three hypotheses have been offered to explain why DMADP pool size is reduced under high [CO2]. According to the first hypothesis, transport of PEP to chloroplasts by PEP/Pi antiporter becomes limited at higher [CO2] due to a reduction of cytosolic PEP level by faster reaction of PEP carboxylase; this reduces pyruvate availability for DMADP synthesis and ultimately chloroplastic DMADP pool size (Rosenstiel et al., 2003; Wilkinson et al., 2009). However, it is difficult to explain the lack of [CO2] sensitivity at higher temperature by this mechanism as PEP carboxylase activity is expected to increase at higher temperature, thereby suppressing cytosolic PEP concentration even more.
Alternatively, Li and Sharkey (2013b ) suggested that reduction of chloroplastic Pi due to feedback limitation of photosynthesis under high [CO2], i.e. inability of starch and sucrose synthesis reactions to keep up with synthesis of triose phosphates, especially at lower temperature, reduces PEP/Pi transport activity and thereby leads to reduced chloroplastic PEP levels. The third hypothesis was based on the observations that feedback inhibition of photosynthesis is also associated with a reduction of ATP synthesis rate (Sharkey, 1985; Socias et al., 1993). Thus, decreases in ATP availability have been suggested to be responsible for the reduced rate of DMADP formation (Rasulov et al., 2009b ). As cytosolic sucrose synthesis very strongly responds to temperature (Sage and Sharkey, 1987), increased sucrose synthesis reduces triose phosphate concentrations and increases equilibrium Pi concentrations, thereby enhancing both PEP transporter activity (Li and Sharkey, 2013b ), but also ATP synthesis rate.
However, Way et al. (2011) did not observe higher-temperature reduction of inhibition of isoprene emission by [CO2] of 590 μmol mol−1 compared with sub-ambient level of 190 μmol mol−1. The discrepancy among our results and the study of Way et al. (2011) likely reflects the strong non-linearity in the [CO2] responses of DMADP pool size and isoprene emission. Due to this non-linearity, in relative terms, temperature enhancement of sucrose synthesis rate cannot release as much Pi for sub-ambient versus supra-ambient (Way et al., 2011) than for ambient versus supra-ambient measurement [CO2] contrast.
On the other hand, emissions at a higher temperature consume much larger amounts of carbon, both in absolute terms and relative to net assimilation rate, than emissions at lower temperatures (Fig. 3c, d). This increase, of almost an order of magnitude, is large enough that it could significantly reduce triose phosphate concentrations and thereby partly restore chloroplastic Pi level. However, the situation may be further complicated by the onset of the use of alternative carbon sources. It has been reported that the percentage of carbon derived from recently assimilated photosynthates is reduced under heat stress (Funk et al., 2004). Analogously, drought-stress experiments indicate that even complete inhibition of photosynthesis by severe stress moderately inhibits isoprene emission as isoprene supply can be maintained on the basis of alternative ‘older’ carbon sources not readily labelled by 13C (Brilli et al., 2007; Karl et al., 2002; Li and Sharkey, 2013b ; Schnitzler et al., 2004; Wolfertz et al., 2003). As already suggested in the other studies under drought stress, we hypothesize that such an ‘older’ carbon source for enhanced isoprene emissions under heat stress is most likely chloroplastic starch. Use of old or temporary stored photosynthates through the pentose phosphate pathway or glycolysis could supply the substrates of GAP and pyruvate for DMADP formation. The pentose phosphate pathway is an alternative route for the use of stored photosynthates, generating NADPH and sugar phosphates, particularly in stressed plants (Eicks et al., 2002; Fettke et al., 2011). In fact, heat stress triggers starch hydrolysis (e.g. Hüve et al., 2012), and thus could compensate for possible heavier competition for triose phosphates by enhanced rate of sucrose synthesis. In this study, elevated-[CO2]-grown plants had higher soluble sugar and starch contents (Table 1, Fig. 1), and thus more readily available alternative carbon sources can partly explain their higher isoprene emission rates at high temperatures.
Heat-stress resistance under different growth [CO2] and the role of isoprene
High temperature results in excessive membrane fluidity and membrane leakiness, loss of compartmentalization, and reduction of physiological functions such as photosynthetic decline (Balogh et al., 2011; Hald et al., 2008) as was also observed in our study at 50 °C in ambient-[CO2]-grown and at 52 °C in elevated-[CO2]-grown plants (Fig. 7a). Greater heat resistance in elevated-[CO2]-grown plants is in agreement with several past studies (Darbah et al., 2010; Taub et al., 2000; Way et al., 2011). This enhancement is common in both non-isoprene- and isoprene-emitting species, but isoprene-emitting species seem to have higher heat-stress resistance (Darbah et al., 2010; Way et al., 2011). Electrolyte leakage, an integrated estimate over 24h following heat stress, was correlated with the reduction in net assimilation rate, but the slope differed among elevated- and ambient-[CO2]-grown plants. We suggest that this is indicative of greater damage at the given short-term reduction in net assimilation rate. In fact, once the stress threshold has been reached, there is a time-dependent reduction in net assimilation rate even after return to lower temperature (Hüve et al., 2011).
The enhancement of thermal tolerance under elevated growth [CO2] has been associated with greater sugar concentrations that stabilize membranes under stress (Livingston et al., 2009; Nagao et al., 2005). In our study, elevated growth [CO2] resulted in greater leaf sugar content in leaf water (Table 1). However, the overall effect of sugars on heat tolerance may depend on the subcellular distribution of sugars. Given the distribution of water within the leaf, greater proportion of sugars was associated with chloroplasts under elevated [CO2], and thus, sugar concentrations were likely elevated both in the cytosol and in the chloroplasts in elevated-[CO2]-grown plants. Thus, higher sugar concentrations seemed to contribute to the higher thermal tolerance by maintaining lower membrane leakage of hybrid aspen plants grown under elevated [CO2].
In addition to sugars, isoprene, a liphophilic and highly reactive molecule, participates in protecting plants under heat stress. Isoprene has been hypothesized to (i) stabilize and protect membranes against high temperature (Sharkey et al., 2001; Singsaas et al., 1997; Velikova et al., 2011), (ii) serve as antioxidant, eliminating reactive oxygen species produced by heat stress (Possell and Loreto, 2013), and (iii) consume excess energy, especially under high light (Sanadze, 2004, 2010). Given the enhanced isoprene emissions (Fig. 2c, d) and greater intercellular isoprene concentrations in elevated-[CO2]-grown plants (Fig. 3a, b) that were also associated with a greater liquid-phase pool of isoprene (see Table 1 for the distribution of leaf water), higher heat-stress resistance of these plants can at least partly be attributed to enhanced isoprene production. Clearly, the stronger the temperature-dependent reduction in net assimilation rate the greater the enhancement of isoprene emission rate (Fig. 6). This uncoupling of isoprene emissions from photosynthesis at temperatures high enough to lead to severe reductions in net assimilation rates is consistent with the involvement of isoprene in heat protection.
Nevertheless, there was a certain mismatch between the reductions in net assimilation rate and increases in isoprene emission rate at 45–50 °C (Fig. 4c, d), suggesting that isoprene emissions themselves became limited by excess temperatures. Such a limitation was less evident for elevated-[CO2]-grown plants at higher measurement [CO2] of 780 μmol mol−1 (Fig. 4c, d). However, different levels of protection could be achieved by given enhancement of isoprene emission rate (Fig. 6), questioning the direct involvement of isoprene in heat protection. Nevertheless, photosynthesis clearly was more stable in elevated-[CO2]-grown plants measured at their corresponding growth [CO2], and this was accompanied by higher intercellular isoprene concentration (Fig. 3a, b). Thus we suggest that both isoprene and sugars are involved in heat-stress resistance and that differences in the stability of photosynthesis under heat stress at given isoprene emission rate possibly reflect differences in the basal level of heat tolerance provided by sugars.
Conclusions
The results of this study demonstrate that heat resistance of hybrid aspen was strongly enhanced by elevated growth [CO2] and this was associated both with more stable net assimilation rates and particularly strong enhancement of isoprene emissions under heat stress. The evidence of loss of CO2 inhibition of isoprene emission at higher temperatures as well as maintenance of enhanced isoprene emission capacity in elevated-[CO2]-grown plants, both under moderately elevated temperatures that may be experienced during heat waves and under temperatures resulting in severe heat stress that can occur upon exposure to heatflecks during the day (Fig. 2c, d; Li et al., 2011; Li and Sharkey, 2013b ; Rasulov et al., 2010), potentially has major implications for predicting isoprene emissions in future climates. Contrary to past suggestions, our results suggest that isoprene might protect leaf photosynthetic function against heat stress more effectively under future elevated [CO2] conditions.
Acknowledgements
We acknowledge the financial support from the Estonian Ministry of Science and Education (institutional grant IUT-8-3), the Estonian Science Foundation (grant 9253), the European Commission through the European Regional Fund (the Centre of Excellence in Environmental Adaptation), the European Social Fund (Doctoral Studies and Internationalization Programme DoRa), and the European Research Council (advanced grant 322603, SIP-VOL+).
Glossary
Abbreviations:
- DMADP
dimethylallyl diphosphate
- GAP
glyceraldehyde 3-phosphate
- PEP
phosphoenolpyruvate
- Pi
inorganic phosphate
- PSII
photosystem II.
References
- Affek HP, Yakir D. 2002. Protection by isoprene against singlet oxygen in leaves. Plant Physiology 129, 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneth A, Niinemets Ü, Pressley S, et al. 2007. Process-based estimates of terrestrial ecosystem isoprene emissions: incorporating the effects of a direct CO2-isoprene interaction. Atmospheric Chemistry and Physics 7, 31–53 [Google Scholar]
- Bajji M, Kinet J-M, Lutts S. 2002. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regulation 36, 61–70 [Google Scholar]
- Balogh G, Maulucci G, Gombos I, et al. 2011. Heat stress causes spatially-distinct membrane re-modelling in K562 leukemia cells. PloS ONE 6, e21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke K, Ehlting B, Teuber M, Bauerfeind M, Louis S, Hänsch R, Polle A, Bohlmann J, Schnitzler J-P. 2007. Transgenic, non-isoprene emitting poplars don’t like it hot. The Plant Journal 51, 485–499 [DOI] [PubMed] [Google Scholar]
- Behnke K, Ghirardo A, Janz D, et al. 2013. Isoprene function in two contrasting poplars under salt and sunflecks. Tree Physiology 33, 562–578 [DOI] [PubMed] [Google Scholar]
- Bick JA, Lange BM. 2003. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membranes. Archives of Biochemistry and Biophysics 415, 146–154 [DOI] [PubMed] [Google Scholar]
- Brilli F, Barta C, Fortunati A, Lerdau M, Loreto F, Centritto M. 2007. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. The New Phytologist 175, 244–254 [DOI] [PubMed] [Google Scholar]
- Calfapietra C, Pallozzi E, Lusini I, Velikova V. 2013. Modification of BVOC emissions by changes in atmospheric [CO2] and air pollution. In: Niinemets Ü, Monson RK, eds. Biology, controls and models of tree volatile organic compound emissions. Tree Physiology 5. Berlin: Springer, 253–284 [Google Scholar]
- Calfapietra C, Scarascia Mugnozza G, Karnosky DF, Loreto F, Sharkey TD. 2008. Isoprene emission rates under elevated CO2 and O3 in two field-grown aspen clones differing in their sensitivity to O3 . The New Phytologist 179, 55–61 [DOI] [PubMed] [Google Scholar]
- Calfapietra C, Wiberley AE, Falbel TG, Linskey AR, Scarascia, Mugnozza G, Karnosky DF, Loreto F, Sharkey TD. 2007. Isoprene synthase expression and protein levels are reduced under elevated O3 but not under elevated CO2 (FACE) in field-grown aspen trees. Plant, Cell and Environment 30, 654–661 [DOI] [PubMed] [Google Scholar]
- Centritto M, Nascetti P, Petrilli L, Raschi A, Loreto F. 2004. Profiles of isoprene emission and photosynthetic parameters in hybrid poplars exposed to free-air CO2 enrichment. Plant, Cell and Environment 27, 403–412 [Google Scholar]
- Chow PS, Landhäusser SM. 2004. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiology 24, 1129–1136 [DOI] [PubMed] [Google Scholar]
- Claeys M, Graham B, Vas G, et al. 2004. Formation of secondary organic aerosols through photooxidation of isoprene. Science 303, 1173–1176 [DOI] [PubMed] [Google Scholar]
- Copolovici LO, Niinemets Ü. 2005. Temperature dependencies of Henry’s law constants and octanol/water partition coefficients for key plant volatile monoterpenoids. Chemosphere 61, 1390–1400 [DOI] [PubMed] [Google Scholar]
- Curtis PS, Wang X. 1998. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113, 299–313 [DOI] [PubMed] [Google Scholar]
- Darbah JNT, Sharkey TD, Calfapietra C, Karnosky DF. 2010. Differential response of aspen and birch trees to heat stress under elevated carbon dioxide. Environmental Pollution 158, 1008–1014 [DOI] [PubMed] [Google Scholar]
- Delwiche CF, Sharkey TD. 1993. Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant, Cell and Environment 16, 587–591 [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28, 350–356 [Google Scholar]
- Eicks M, Maurino V, Knappe S, Flügge U-I, Fischer K. 2002. The plastidic pentose phosphate translocator represents a link between the cytosolic and the plastidic pentose phosphate pathways in plants. Plant Physiology 128, 512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettke J, Malinova I, Albrecht T, Hejazi M, Steup M. 2011. Glucose-1-phosphate transport into protoplasts and chloroplasts from leaves of Arabidopsis . Plant Physiology 155, 1723–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineschi S, Loreto F, Staudt M, Peñuelas J. 2013. Diversification of volatile isoprenoid emissions from trees: evolutionary and ecological perspectives In: Niinemets Ü, Monson RK, eds. Biology, controls and models of tree volatile organic compound emissions. Tree Physiology 5. Berlin: Springer, 1–20 [Google Scholar]
- Funk JL, Mak JE, Lerdau MT. 2004. Stress-induced changes in carbon sources for isoprene production in Populus deltoides . Plant, Cell and Environment 27, 747–755 [Google Scholar]
- Grote R, Monson RK, Niinemets Ü. 2013. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK, eds. Biology, controls and models of tree volatile organic compound emissions. Tree Physiology, 5. Berlin: Springer, 315–355 [Google Scholar]
- Guidolotti G, Calfapietra C, Loreto F. 2011. The relationship between isoprene emission, CO2 assimilation and water use efficiency across a range of poplar genotypes. Physiologia Plantarum 142, 297–304 [DOI] [PubMed] [Google Scholar]
- Hald S, Pribil M, Leister D, Gallois P, Johnson GN. 2008. Competition between linear and cyclic electron flow in plants deficient in Photosystem I. Biochimica et Biophysica Acta Bioenergetics 1777, 1173–1183 [DOI] [PubMed] [Google Scholar]
- Harley P, Guenther A, Zimmerman P. 1996. Effects of light, temperature and canopy position on net photosynthesis and isoprene emission from sweetgum (Liquidambar styraciflua) leaves. Tree Physiology 16, 25–32 [DOI] [PubMed] [Google Scholar]
- Heald CL, Wilkinson MJ, Monson RK, Alo CA, Wang G, Guenther A. 2009. Response of isoprene emission to ambient CO2 changes and implications for global budgets. Global Change Biology 15, 1127–1140 [Google Scholar]
- Hüve K, Bichele I, Ivanova H, Keerberg O, Pärnik T, Rasulov B, Tobias M, Niinemets Ü. 2012. Temperature responses of dark respiration in relation to leaf sugar concentration. Physiologia Plantarum 144, 320–334 [DOI] [PubMed] [Google Scholar]
- Hüve K, Bichele I, Rasulov B, Niinemets Ü. 2011. When it is too hot for photosynthesis: heat-induced instability of photosynthesis in relation to respiratory burst, cell permeability changes and H2O2 formation. Plant, Cell and Environment 34, 113–126 [DOI] [PubMed] [Google Scholar]
- Hüve K, Bichele I, Tobias M, Niinemets Ü. 2006. Heat sensitivity of photosynthetic electron transport varies during the day due to changes in sugars and osmotic potential. Plant, Cell and Environment 29, 212–228 [DOI] [PubMed] [Google Scholar]
- Jeannette E, Reyss A, Grégory N, Gantet P, Prioul JL. 2000. Carbohydrate metabolism in a heat-girdled maize source leaf. Plant, Cell and Environment 23, 61–69 [Google Scholar]
- Johnson DW. 2006. Progressive N limitation in forests: review and implications for long-term responses to elevated CO2 . Ecology 87, 64–75 [DOI] [PubMed] [Google Scholar]
- Karl T, Fall R, Rosenstiel TN, Prazeller P, Larsen B, Seufert G, Lindinger W. 2002. On-line analysis of the 13CO2 labeling of leaf isoprene suggests multiple subcellular origins of isoprene precursors. Planta 215, 894–905 [DOI] [PubMed] [Google Scholar]
- Kocheva KV, Georgiev GI, Kochev VK. 2005. A diffusion approach to the electrolyte leakage from plant tissues. Physiologia Plantarum 125, 1–9 [DOI] [PubMed] [Google Scholar]
- Laule O, Fürholz A, Chang HS, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange M. 2003. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana . Proceedings of the National Academy of Sciences USA 100, 6866–6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Chen Y, Shi Y, He X, Chen X. 2009. Impact of elevated CO2 and O3 concentrations on biogenic volatile organic compounds emissions from Ginkgo biloba . Bulletin of Environmental Contamination and Toxicology 82, 473–477 [DOI] [PubMed] [Google Scholar]
- Li Z, Ratliff EA, Sharkey TD. 2011. Effect of temperature on postillumination isoprene emission in oak and poplar. Plant Physiology 155, 1037–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sharkey TD. 2013a. Metabolic profiling of the methylerythritol phosphate pathway reveals the source of post-illumination isoprene burst from leaves. Plant, Cell and Environment 36, 429–437 [DOI] [PubMed] [Google Scholar]
- Li Z, Sharkey TD. 2013b. Molecular and pathway controls on biogenic volatile organic compound emissions. In: Niinemets Ü, Monson RK, eds. Biology, controls and models of tree volatile organic compound emissions. Tree Physiology 5. Berlin: Springer, 119–151 [Google Scholar]
- Lichtenthaler HK. 1999. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annual Review of Plant Physiology and Plant Molecular Biology 50, 47–65 [DOI] [PubMed] [Google Scholar]
- Livingston DI, Hincha D, Heyer A. 2009. Fructan and its relationship to abiotic stress tolerance in plants. Cellular and Molecular Life Sciences 66, 2007–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Barta C, Brilli F, Nogues I. 2006. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant, Cell and Environment 29, 1820–1828 [DOI] [PubMed] [Google Scholar]
- Loreto F, Centritto M, Barta C, Calfapietra C, Fares S, Monson RK. 2007. The relationship between isoprene emission rate and dark respiration rate in white poplar (Populus alba L.) leaves. Plant, Cell and Environment 30, 662–669 [DOI] [PubMed] [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S. 2001. Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiology 126, 993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD. 1990. A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta 182, 523–531 [DOI] [PubMed] [Google Scholar]
- Luo Y, Sims DA, Griffin KL. 1997. Nonlinearity of photosynthetic responses to growth in rising atmospheric CO2: an experimental and modelling study. Global Change Biology 3, 173–183 [Google Scholar]
- Makino A, Mae T. 1999. Photosynthesis and plant growth at elevated levels of CO2 . Plant and Cell Physiology 40, 999–1006 [Google Scholar]
- Mayrhofer S, Teuber M, Zimmer I, Louis S, Fischbach RJ, Schnitzler J-P. 2005. Diurnal and seasonal variation of isoprene biosynthesis-related genes in grey poplar leaves. Plant Physiology 139, 474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl GA, Stocker TF, Collins WD, et al. 2007. Global climate projections. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, eds. Climate change 2007: The physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press, 747–845 [Google Scholar]
- Miyazawa S-I, Warren CR, Turpin DH, Livingston NJ. 2011. Determination of the site of CO2 sensing in poplar: is the area-based N content and anatomy of new leaves determined by their immediate CO2 environment or by the CO2 environment of mature leaves? Journal of Experimental Botany 62, 2787–2796 [DOI] [PubMed] [Google Scholar]
- Monson RK. 2013. Metabolic and gene expression controls on the production of biogenic volatile organic compounds. In: Niinemets Ü, Monson RK, eds. Biology, controls and models of tree volatile organic compound emissions. Tree Physiology 5. Berlin: Springer, 153–179 [Google Scholar]
- Monson RK, Grote R, Niinemets Ü, Schnitzler J-P. 2012. Tansley review. Modeling the isoprene emission rate from leaves. The New Phytologist 195, 541–559 [DOI] [PubMed] [Google Scholar]
- Monson RK, Jones RT, Rosenstiel TN, Schnitzler J-P. 2013. Why only some plants emit isoprene. Plant, Cell and Environment 36, 503–516 [DOI] [PubMed] [Google Scholar]
- Myers DA, Thomas RB, DeLucia EH. 1999. Photosynthetic responses of loblolly pine (Pinus taeda) needles to experimental reduction in sink demand. Tree Physiology 19, 235–242 [DOI] [PubMed] [Google Scholar]
- Nagao M, Minami A, Arakawa K, Fujikawa S, Takezawa D. 2005. Rapid degradation of starch in chloroplasts and concomitant accumulation of soluble sugars associated with ABA-induced freezing tolerance in the moss Physcomitrella patens . Journal of Plant Physiology 162, 169–180 [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. 1999. Research review. Components of leaf dry mass per area - thickness and density - alter leaf photosynthetic capacity in reverse directions in woody plants. The New Phytologist 144, 35–47 [Google Scholar]
- Niinemets Ü. 2012. Whole plant photosynthesis. In: Flexas J, Loreto F, Medrano H, eds. Terrestrial photosynthesis in a changing environment. A molecular, physiological and ecological approach. Cambridge: Cambridge University Press, 399–423 [Google Scholar]
- Niinemets Ü, Arneth A, Kuhn U, Monson RK, Peñuelas J, Staudt M. 2010a. The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosciences 7, 2203–2223 [Google Scholar]
- Niinemets Ü, Arneth A, Kuhn U, Monson RK, Peñuelas J, Staudt M. 2010b. The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosciences Discussions 7, 1529–1574 [Google Scholar]
- Niinemets Ü, Copolovici L, Hüve K. 2010c. High within-canopy variation in isoprene emission potentials in temperate trees: implications for predicting canopy-scale isoprene fluxes. Journal of Geophysical Research - Biogeosciences 115, G04029 [Google Scholar]
- Niinemets Ü, Monson RK. 2013. State-of-the-art of BVOC research: what do we have and what have we missed? A synthesis. In: Niinemets Ü, Monson RK, eds. Biology, controls and models of tree volatile organic compound emissions. Tree Physiology 5. Berlin: Springer, 509–528 [Google Scholar]
- Niinemets Ü, Monson RK, Arneth A, Ciccioli P, Kesselmeier J, Kuhn U, Noe SM, Peñuelas J, Staudt M. 2010d. The leaf-level emission factor of volatile isoprenoids: caveats, model algorithms, response shapes and scaling. Biogeosciences 7, 1809–1832 [Google Scholar]
- Niinemets Ü, Reichstein M. 2003. Controls on the emission of plant volatiles through stomata: sensitivity or insensitivity of the emission rates to stomatal closure explained. Journal of Geophysical Research - Atmospheres 108, 4208 [Google Scholar]
- Niinemets Ü, Tenhunen JD, Harley PC, Steinbrecher R. 1999. A model of isoprene emission based on energetic requirements for isoprene synthesis and leaf photosynthetic properties for Liquidambar and Quercus . Plant, Cell and Environment 22, 1319–1336 [Google Scholar]
- Nowak RS, Ellsworth DS, Smith SD. 2004. Functional responses of plants to elevated atmospheric CO2 - do photosynthetic and productivity data from FACE experiments support early predictions? The New Phytologist 162, 253–280 [Google Scholar]
- Pegoraro E, Rey A, Bobich EG, Barron-Gafford GA, Grieve KA, Malhi Y, Murthy R. 2004. Effect of elevated CO2 concentration and vapor pressure deficit on isoprene emission from leaves of Populus deltoides during drought. Functional Plant Biology 31, 1137–1147 [DOI] [PubMed] [Google Scholar]
- Possell M, Loreto F. 2013. The role of volatile organic compounds in plant resistance to abiotic stresses: responses and mechanisms. In: Niinemets Ü, Monson RK, eds. Biology, controls and models of tree volatile organic compound emissions. Tree Physiology 5. Berlin: Springer, 209–235 [Google Scholar]
- Rasulov B, Copolovici L, Laisk A, Niinemets Ü. 2009a. Postillumination isoprene emission: in vivo measurements of dimethylallyldiphosphate pool size and isoprene synthase kinetics in aspen leaves. Plant Physiology 149, 1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Bichele I, Laisk A, Niinemets Ü. 2010. Temperature response of isoprene emission in vivo reflects a combined effect of substrate limitations and isoprene synthase activity: a kinetic analysis. Plant Physiology 154, 1558–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Laisk A, Niinemets Ü. 2011. Induction of a longer-term component of isoprene release in darkened aspen leaves: origin and regulation under different environmental conditions. Plant Physiology 156, 816–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Hüve K, Välbe M, Laisk A, Niinemets Ü. 2009b. Evidence that light, carbon dioxide and oxygen dependencies of leaf isoprene emission are driven by energy status in hybrid aspen. Plant Physiology 151, 448–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H. 2004. Why do cells require heat shock proteins to survive heat stress? Cell Cycle 3, 61–63 [PubMed] [Google Scholar]
- Rosenkranz M, Schnitzler J-P. 2013. Genetic engineering of BVOC emissions from trees. In: Niinemets Ü, Monson RK, eds. Biology, controls and models of tree volatile organic compound emissions. Tree Physiology 5. Berlin: Springer, 95–118 [Google Scholar]
- Rosenstiel TN, Potosnak MJ, Griffin KL, Fall R, Monson RK. 2003. Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature 421, 256–259 [DOI] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD. 1987. The effect of temperature on the occurence of O2 and CO2 insensitive photosynthesis in field grown plants. Plant Physiology 84, 658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanadze GA. 2004. Biogenic isoprene (a review). Russian Journal of Plant Physiology 51, 729–741 [Google Scholar]
- Sanadze GA. 2010. Photobiosynthesis of isoprene as an example of leaf excretory function in the light of contemporary thermodynamics. Russian Journal of Plant Physiology 57, 1–6 [Google Scholar]
- Saxe H, Ellsworth DS, Heath J. 1998. Tansley review No. 98. Tree and forest functioning in an enriched CO2 atmosphere. The New Phytologist 139, 395–436 [Google Scholar]
- Schnitzler JP, Graus M, Kreuzwieser J, Heizmann U, Rennenberg H, Wisthaler A, Hansel A. 2004. Contribution of different carbon sources to isoprene biosynthesis in poplar leaves. Plant Physiology 135, 152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwender J, Zeidler J, Gröner R, Müller C, Focke M, Braun S, Lichtenthaler FW, Lichtenthaler HK. 1997. Incorporation of 1-deoxy-D-xylulose into isoprene and phytol by higher plants and algae. FEBS Letters 414, 129–134 [DOI] [PubMed] [Google Scholar]
- Scotti Campos P, Quartin V, Cochicho, Ramalho J, Nunes MA. 2003. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. Journal of Plant Physiology 160, 283–292 [DOI] [PubMed] [Google Scholar]
- Sharkey TD. 1985. Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. The Botanical Review 51, 53–105 [Google Scholar]
- Sharkey TD, Chen XY, Yeh S. 2001. Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiology 125, 2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Gray DW, Pell HK, Breneman SR, Topper L. 2013. Isoprene synthase genes form a monophyletic clade of acyclic terpene synthases in the Tps-b terpene synthase family. Evolution 67, 1026–1040 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F, Delwiche CF. 1991. High carbon dioxide and sun/shade effects on isoprene emission from oak and aspen tree leaves. Plant, Cell and Environment 14, 333–338 [Google Scholar]
- Sharkey TD, Wiberley AE, Donohue AR. 2008. Isoprene emission from plants: why and how. Annals of Botany 101, 5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims DA, Seemann JR, Luo Y. 1998a. Elevated CO2 concentration has independent effects on expansion rates and thickness of soybean leaves across light and nitrogen gradients. Journal of Experimental Botany 49, 583–591 [Google Scholar]
- Sims DA, Seemann JR, Luo Y. 1998b. The significance of differences in the mechanisms of photosynthetic acclimation to light, nitrogen and CO2 for return on investment in leaves. Functional Ecology 12, 185–194 [Google Scholar]
- Singsaas EL, Laporte MM, Shi J-Z, Monson RK, Bowling DR, Johnson K, Lerdau M, Jasentuliytana A, Sharkey TD. 1999. Kinetics of leaf temperature fluctuation affect isoprene emission from red oak (Quercus rubra) leaves. Tree Physiology 19, 917–924 [DOI] [PubMed] [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD. 1997. Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiology 115, 1413–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singsaas EL, Sharkey TD. 1998. The regulation of isoprene emission responses to rapid leaf temperature fluctuations. Plant, Cell and Environment 21, 1181–1188 [Google Scholar]
- Singsaas EL, Sharkey TD. 2000. The effects of high temperature on isoprene synthesis in oak leaves. Plant, Cell and Environment 23, 751–757 [Google Scholar]
- Siwko ME, Marrink SJ, de Vries AH, Kozubek A, Uiterkamp AJMS, Mark AE. 2007. Does isoprene protect plant membranes from thermal shock? A molecular dynamics study. Biochimica et Biophysica Acta Biomembranes 1768, 198–206 [DOI] [PubMed] [Google Scholar]
- Socias FX, Medrano H, Sharkey TD. 1993. Feedback limitation of photosynthesis of Phaseolus vulgaris L. grown in elevated CO2 . Plant, Cell and Environment 16, 81–86 [Google Scholar]
- Sun Z, Niinemets Ü, Hüve K, Noe SM, Rasulov B, Copolovici L, Vislap V. 2012. Enhanced isoprene emission capacity and altered light responsiveness in aspen grown under elevated atmospheric CO2 concentration. Global Change Biology 18, 3423–3440 [Google Scholar]
- Sun Z, Niinemets Ü, Hüve K, Rasulov B, Noe SM. 2013. Elevated atmospheric CO2 concentration leads to increased whole-plant isoprene emission in hybrid aspen (Populus tremula x Populus tremuloides). The New Phytologist 198, 788–800 [DOI] [PubMed] [Google Scholar]
- Tardy F, Havaux M. 1997. Thylakoid membrane fluidity and thermostability during the operation of the xanthophyll cycle in higher-plant chloroplasts. Biochimica et Biophysica Acta Biomembranes 1330, 179–193 [DOI] [PubMed] [Google Scholar]
- Taub DR, Seemann JR, Coleman JS. 2000. Growth in elevated CO2 protects photosynthesis against high-temperature damage. Plant, Cell and Environment 23, 649–656 [Google Scholar]
- Vahala J, Keinänen M, Schützendübel A, Polle A, Kangasjärvi J. 2003. Differential effects of elevated ozone on two hybrid aspen genotypes predisposed to chronic ozone fumigation. Role of ethylene and salicylic acid. Plant Physiology 132, 196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladares F, Niinemets Ü. 2007. The architecture of plant crowns: from design rules to light capture and performance. In: Pugnaire FI, Valladares F, eds. Handbook of functional plant ecology. Boca Raton: CRC Press, 101–149 [Google Scholar]
- Velikova V, Várkonyi Z, Szabó M, et al. 2011. Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiology 157, 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers CE, Gershenzon J, Lerdau MT, Loreto F. 2009a. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nature Chemical Biology 5, 283–291 [DOI] [PubMed] [Google Scholar]
- Vickers CE, Possell M, Cojocariu CI, Velikova VB, Laothawornkitkul J, Ryan A, Mullineaux PM, Hewitt CN. 2009b. Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant, Cell and Environment 32, 520–531 [DOI] [PubMed] [Google Scholar]
- Way DA, Schnitzler J-P, Monson RK, Jackson RB. 2011. Enhanced isoprene-related tolerance of heat- and light-stressed photosynthesis at low, but not high, CO2 concentrations. Oecologia 166, 273–282 [DOI] [PubMed] [Google Scholar]
- Wiberley AE, Donohue AR, Meier ME, Westphal MM, Sharkey TD. 2008. Regulation of isoprene emission in Populus trichocarpa leaves subjected to changing growth temperature. Plant, Cell and Environment 31, 258–267 [DOI] [PubMed] [Google Scholar]
- Wiberley AE, Donohue AR, Westphal MM, Sharkey TD. 2009. Regulation of isoprene emission from poplar leaves throughout a day. Plant Cell and Environment 32, 939–947 [DOI] [PubMed] [Google Scholar]
- Wilkinson MJ, Monson RK, Trahan N, Lee S, Brown E, Jackson RB, Polley HW, Fay PA, Fall R. 2009. Leaf isoprene emission rate as a function of atmospheric CO2 concentration. Global Change Biology 15, 1189–1200 [Google Scholar]
- Wolfertz M, Sharkey TD, Boland W, Kuehnemann F, Yeh S, Weise SE. 2003. Biochemical regulation of isoprene emission. Plant, Cell and Environment 26, 1357–1364 [Google Scholar]