Abstract
Leguminous biological nitrogen fixation (BNF) is very sensitive to environmental fluctuations. It is still contentious how BNF is regulated under stress conditions. The local or systemic control of BNF and the role played by reactive oxygen species (ROS) in such regulation have still not been elucidated completely. Cadmium, which belongs to the so-called heavy metals, is one of the most toxic substances released into the environment. The mechanisms involved in Cd toxicity are still not completely understood but the overproduction of ROS is one of its characteristic symptoms. In this work, we used a split-root system approach to study nodule BNF and the antioxidant machinery’s response to the application of a mild Cd treatment on one side of a nodulated Medicago truncatula root system. Cd induced the majority of nodule antioxidants without generating any oxidative damage. Cd treatment also provoked BNF inhibition exclusively in nodules directly exposed to Cd, without provoking any effect on plant shoot biomass or chlorophyll content. The overall data suggest that the decline in BNF was not due to a generalized breakdown of the plant but to control exerted through leghaemoglobin/oxygen availability, affecting nitrogenase function.
Key words: Antioxidant, cadmium, Medicago truncatula–Sinorhizobium meliloti symbiosis, nitrogen fixation, reactive oxygen species, split-root system.
Introduction
Legumes have the capacity to use atmospheric nitrogen (N2) as a nitrogen source so they can fulfil their nitrogen demand. This ability comes from the symbiotic relationship between legumes and soil bacteria that leads to the formation of root nodules, where biological nitrogen fixation (BNF) takes place (Oldroyd et al., 2011). However, the yield of legume crops is often limited by the extreme sensitivity of BNF to environmental fluctuations (Zahran, 1999). Several factors, including oxygen availability and plant carbon and nitrogenous status, are linked to BNF inhibition under abiotic stresses, but the mechanism responsible for the inhibition remains largely unknown. Carbon status might be regulated by either the shoot through photosynthesis inhibition or a direct carbon shortage in the nodules due to inhibition of sucrose synthase (González et al., 1995). Oxygen availability can be regulated by different factors including the O2-binding protein leghaemoglobin (Lb) or the variable oxygen diffusion barrier (ODB) in the nodule endodermis, which maintains the microaerobic environment within the infected zone of the nodules (Minchin, 1997; Minchin et al., 2008). One open question is whether the origin of BNF inhibition comes from direct inhibition within the nodules or as systemic feedback signalling from the shoot (Serraj et al., 2001; Marino et al., 2007; Gil-Quintana et al., 2013a , 2013b ).
Cadmium, which belongs to the so-called heavy metals, is one of the most toxic substances released into the environment. It mainly enters the environment as a consequence of human activity from industrial processes and by the use of fertilizers and pesticides (Verbruggen et al,. 2009; Clemens et al., 2013). Exposure to Cd, even at low concentrations, can lead to irreversible cell damage in virtually every living organism (Verbruggen et al,. 2009; Clemens et al., 2013). Some effects of Cd accumulation in plants include leaf chlorosis, root necrosis, and a decrease in plant growth (Gallego et al., 2012). The mechanisms involved in Cd toxicity are still not completely understood but the overproduction of reactive oxygen species (ROS) is one of its associated characteristic symptoms (Cuypers et al., 2010).
ROS, such as hydrogen peroxide, singlet oxygen, and superoxide anion, are constantly produced in association with aerobic life (Apel and Hirt, 2004). In nodules, high respiration rates—together with high concentrations of Lb and the abundance of catalytic Fe—enhance, among other things, nodule capacity to generate ROS (Marino et al., 2009). Under stress conditions ROS play a dual role: they may act as both signal transduction molecules and toxic agents oxidizing cellular macromolecules (Apel and Hirt, 2004; Suzuki et al., 2012). Thus, maintaining a redox balance must be strictly controlled to avoid ROS deleterious effects. To do so, plants possess a complex array of detoxification mechanisms including enzymes such as catalases or superoxide dismutases (SODs) and metabolites like glutathione and ascorbate (Apel and Hirt, 2004).
Legume nodules are an excellent means to illustrate the dual signalling and toxicity roles of ROS. For instance, disruption of nodule functionality due to natural ageing or to exposure to severe changes in the environment, like drought, salinity, or darkness, has been suggested to be provoked by the oxidative damage associated with ROS overproduction during these processes (Becana et al., 2010). However, in nodules, ROS also have an important role in signalling processes. For example, the application of a low concentration of paraquat, a ROS generator, provoked BNF inhibition associated with ROS as signalling molecules rather than as deleterious molecules (Marino et al., 2006). Moreover, in Medicago truncatula an RNA interference approach directed against MtRbohA, a nodule-enhanced NADPH oxidase gene, provoked a decrease in nodule nitrogen-fixation activity and the modulation of genes encoding the microsymbiont nitrogenase (Nase), suggesting a positive role of ROS in proper nodule functioning (Marino et al., 2011). Similarly, an NADPH oxidase of common bean has been shown to be crucial for successful rhizobial colonization (Montiel et al., 2012).
The mechanism underlying ROS generation upon Cd exposure remains to be elucidated. Cd itself is not redox active since it is not able to trigger the Fenton reaction (Salin, 1988), but it has been suggested that Cd induction of ROS production can be related to suppression of antioxidant defences (Sandalio et al., 2001), to disruption of the electron transport chain, and somehow to the activation of plasma membrane NADPH oxidases (Romero-Puertas et al., 2004; Cuypers et al., 2010). It has been widely reported that Cd impairs nodule functioning associated with photosynthesis and chlorophyll depletion and to nodule oxidative damage in soybean (Balestrasse et al., 2006), alfalfa (Shvaleva et al., 2010), white lupin (Carpena et al., 2003), and mung bean (Muneer et al., 2012), among other legumes. Moreover, it has been suggested that resistance or minor sensibility to Cd depends on the plant’s ability to increase its antioxidant defences (Noctor and Foyer, 1998; Paradiso et al., 2008).
However, little is known about the cause of the impairment of nodule function under mild stress and the potential relationship of the induction of ROS production with this BNF inhibition. Therefore, the aim of this work was to better understand nitrogen-fixation regulation under abiotic stress. We used the model legume M. truncatula grown in a split-root-system, in which only one side of a nodulated root was exposed to Cd. This system allowed us to uncouple the direct effect of Cd on nodules from the consequences derived from a systemic effect of Cd on the aerial part of the plant. Moreover, this approach was useful to investigate how nodule metabolism and antioxidant machinery are modulated under these conditions and their relationship with BNF inhibition. This work represents, to our knowledge, the first time that the effects of Cd on M. truncatula nodule physiology have been studied.
Materials and methods
Plant growth and Cd treatment
M. truncatula cv Jemalong J6 was used throughout the experiments. Surface-sterilized seeds were placed on 0.4% agar plates in the dark for 24h at 4 °C and then for 3 days at 14 °C. The root tip of germinated seeds was removed and plants were transferred into modified Farhaeus agar plates to induce the production of new roots. Ten days later plants were transferred into a double pot of 2×250ml with a 1:2 sand/vermiculite mixture in a controlled environment chamber (25/22 °C day/night; 75% hygrometry; 200 µE·m−2·s−1 light intensity and 16/8h light/dark photoperiod), and watered with a nitrogen-free nutrient solution (Rigaud and Puppo, 1975). Each side of the root system was inoculated with a Sinorhizobium meliloti 2011 strain. Five weeks after the transfer, plants were divided randomly in two groups: one control (C) and one treatment (T). For T plants, one pot was daily irrigated at field capacity with nutrient solution supplemented with 1mM CdCl2 while in the other pot the nutrient solution was supplemented with 1mM CaCl2 (Fig. 1A, B). For C plants both pots were daily irrigated at field capacity with nutrient solution supplemented with 1mM CaCl2. Seventy-two hours later plant material was harvested, immediately frozen in liquid nitrogen, and stored at −80 °C.
Fig. 1.
M. truncatula plants grown in split-root-system (A) leading to a root system divided into two equal parts (B). Evans blue staining of M. truncatula secondary root apex to detect cell death after Cd treatment (C). This figure is available in colour at JXB online.
Detection of cell death
To determine cell viability under Cd treatment, roots were infiltrated with a 0.25% (w/v) aqueous solution of Evans blue (Rodriguez-Serrano et al., 2006)
Cd content determination
To analyse Cd content, shoots and nodulated roots were harvested. Roots were thoroughly rinsed with deionized water to eliminate the Cd that could be superficially adsorbed. Plant material was oven-dried at 70 °C for 48h, and the dry weight was recorded. Subsamples (0.4g) of dried plant tissue were digested with a mixture of HNO3/HClO4 (Zhao et al., 1994). Cd content in the digest was determined using flame atomic absorption spectrometry (Varian, Palo Alto, CA, USA).
Chlorophyll determination
The content of chlorophyll a and b was determined spectrophotometrically as described by Arnon (1949) in 80% aqueous acetone extracts by measuring absorbance at 645 and 663nm.
Nitrogen fixation
Nitrogen fixation was determined through acetylene-reduction assay. Freshly harvested nodulated roots from detopped plants were placed in rubber-capped 30ml glass bottles filled with an acetylene/air mixture (C2H2/air, 1:10 v/v). After 1h of incubation at 25 °C the amount of ethylene in the gas phase was determined by gas chromatography using a 6890N Network GC system (Agilent Technologies, La Jolla, CA, USA).
Lipid peroxidation
Lipid peroxidation in nodules was assayed by measuring the concentration of malondialdehyde (MDA), the major 2-thiobarbituric acid-reacting substance (TBARS). Modifications as proposed by Hodges et al. (1999) were carried out to correct interference generated by non-specific turbidity, thiobarbituric acid–sugar complexes, and other non-TBARS compounds absorbing at 532nm.
Total RNA isolation, reverse transcription, and gene-expression analysis
Nodules were homogenized in liquid nitrogen with a mortar and pestle and total RNA extraction and reverse transcription were assayed as described in Andrio et al. (2013). Quantitative real-time PCR was carried out using the qPCR Mastermix Plus for SYBR Green I reagent (Eurogentec, Angers, France). Reactions were run on the Chromo4 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) and quantification was performed with Opticon Monitor analysis software version 3.1 (Bio-Rad). Every reaction was set up in three technical replicates. The PCR program used was as follows: polymerase activation (95 °C for 5min), amplification and quantification cycles repeated 40 times (94 °C for 15 s, 60 °C for 1min), and melting curve (40–95 °C with one fluorescence read every 0.5 °C). The mRNA levels were normalized against two endogenous controls: 40S Ribosomal Protein S8 (TC137982) and Mtc27 (TC132510) (Van de Velde et al., 2006) were used for M. truncatula and Smc00324 and ribosomal rna16S housekeeping genes were used to normalize the bacterial mRNA levels (Becker et al., 2004). The following formula was used to calculate the relative expression ratio: 2−ΔCT, with ΔCT = CT gene of interest−CT houskeeping gene. For each experiment, stability of the reference genes across samples was tested using geNorm software (Vandesompele et al., 2002). The absence of contamination with genomic DNA was tested by PCR in all RNA samples, prior to reverse transcription, using the primers of the housekeeping genes. The gene-specific primers used are listed in Table S1.
Protein extraction and enzyme activities
Nodules were homogenized with a mortar and pestle in 50mM Tris/HCl, 0.1mM EDTA, 0.2% Triton X-100, 1mM PMSF, 5 µM E64 (3 µl/ml), and 2mM dithiothreitol (pH 7.8) at 0–2 °C (5ml g−1 fresh weight). The homogenate was centrifugated for 30min at 20 000g at 4 °C. An aliquot of the supernatant was retained for plant-fraction protein determination (Bradford, 1976) and the rest was used to measure the following enzyme activities: NADP+-dependent isocitrate dehydrogenase (ICDH; EC 1.1.1.42), ascorbate peroxidase (APX; EC 1.11.1.11), catalase (CAT; EC 1.11.1.6), glutathione reductase (GR; EC 1.6.4.2), and unspecific peroxidases (guaiacol peroxidases, GPX; EC 1.11.1.7). ICDH was determined as described by Marino et al. (2006), and APX, CAT, GR, and GPX were assayed as described by Zabalza et al. (2007).
Western blot
Sodium dodecyl sulphate/polyacrylamide gel electrophoresis (SDS/PAGE) was performed according to Laemmli (1970) with a 1 mm-thick 12% acrylamide (w/v) resolving gel and a 4.6% (w/v) stacking gel in a vertical electrophoresis cell (Mini- Protean III; Bio-Rad) at 150V for 60min. Gels were electroblotted onto polyvinylidene fluoride membrane for 75min at 100V in a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad). Blots were blocked in 5% (w/v) skim milk in 20mM Tris-buffered saline at 4 °C overnight. We used α-sucrose synthase (SS) (1:5000), α-Lb (1.2000), and α-nitrogenase (1:2000) as primary antibodies. The secondary antibody was goat anti-rabbit horseradish peroxidase conjugate (1:50 000, Sigma-Aldrich). Immunoreactive bands were visualized with a highly sensitive chemiluminescent substrate for peroxidase detection (GE Healthcare Europe GmbH, Freiburg, Germany).
Statistical analyses
All the data presented are given as means with standard errors. The significance of the results was assessed using multiple-factor analysis of variance (P value <0.05).
Results
Cd exposure provokes local nitrogen-fixation inhibition without affecting aerial parts of the plant
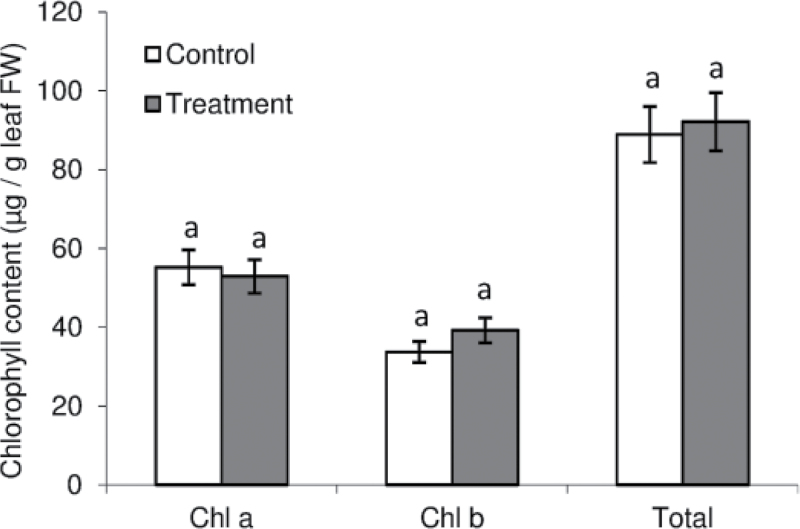
Split-root system (SRS) approaches are very useful to study local or systemic effects of a stress applied on the root system, in this case Cd. When using a SRS approach to study root responses to an environmental change it is suitable that the applied stress provokes a minimum effect on the aerial part for better discrimination between local or systemic effects. To use the lowest Cd dose that in our conditions could lead to nitrogen-fixation impairment, we first screened the effect of different Cd treatments on the Nase activity of M. truncatula nodules. We applied six different concentrations of CdCl2 (0, 0.1, 0.2, 0.5, 1, and 2mM) for 3 days, after which we selected 1mM, the lowest concentration that in our conditions provoked nitrogen-fixation inhibition (data not shown). Plants in the SRS (Fig. 1A, B) were divided into two groups: one control (C) with both sides of the root system irrigated with nutrient solution and one Cd-treated (T) with one half of the root system treated with Cd (split-treatment, ST) and the other half irrigated with nutrient solution (split-control, SC). Leaf chlorosis, root necrosis, and a decrease in plant growth are some of the most characteristic symptoms of Cd toxicity (Gallego et al., 2012). The aim of our work was to apply a slight Cd stress to avoid these symptoms. Thus, we used these three parameters as indicators of the severity of our treatment. Cd application did not affect shoot biomass (Table 1), nor chlorophyll a and b content (Fig. 2), indicating that 3 days of Cd exposure did not provoke damage to the aerial part. Root and nodule biomass were similar in ST, SC, and C roots (Table 1) and no root necrosis could be observed as determined by Evans blue staining (Fig. 1C).
Table 1.
Biomass (fresh weight) of control and Cd-treated M. truncatula nodules grown in SRS. Root and nodule fresh weight values of control plants correspond to the mean of half roots
| Shoot (g) | Root (g) | Nodules (mg) | |
|---|---|---|---|
| C | 0.74±0.05 | 0.56±0.04 | 25.67±3.02 |
| SC | 0.66±0.05 | 25.94±4.11 | |
| ST | 0.58±0.03 | 24.41±2.57 | |
| T | 0.75±0.04 |
Each value represents mean±SE for n=8.
Fig. 2.
Chlorophyll content in control and Cd-treated M. truncatula plants grown in a SRS. Each bar shows mean±SE for n=6. FW, fresh weight.
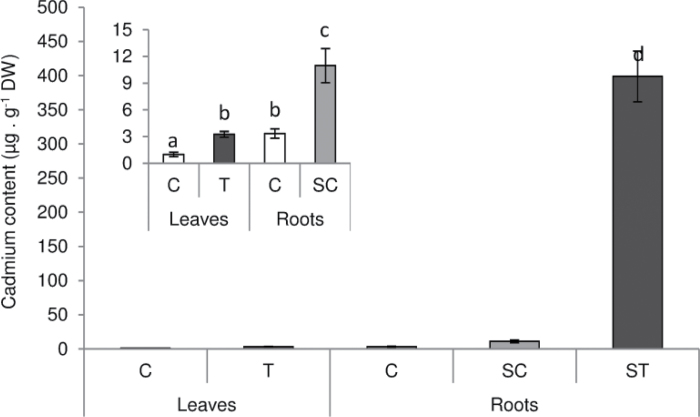
Cd content in ST roots was close to 400 µg g−1 dry weight, about 100-fold higher than in C roots (Fig. 3). Cd can enter the roots through symplastic and apoplastic pathways and is translocated via the vascular system to the rest of the plant (Mendoza-Cózatl et al., 2011). In this work, at the time of harvest, only approximately 1% of the Cd taken up by ST roots was translocated to shoots or SC roots (Fig. 3).
Fig. 3.
Cd content in leaves and roots of control and Cd-treated M. truncatula plants grown in a SRS. Each bar represents mean±SE for n=8. Different letters represent significant differences (P≤0.05). The inset shows the detail for C, T, and SC plants. DW, dry weight.
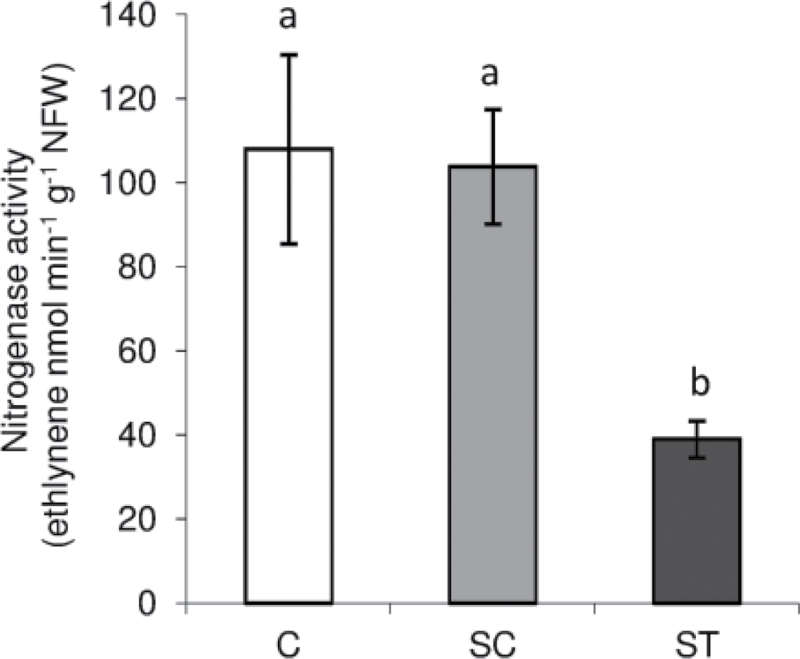
Nase activity in ST nodules was dramatically reduced while the activity in SC nodules was similar to control plants (Fig. 4). This result shows that nitrogen fixation activity under Cd stress is locally controlled. It has been previously shown that acetylene may inhibit Nase activity and that it can affect the functioning of the ODB (Minchin et al., 1983, 1986). In this work BNF was determined by the traditional acetylene-reduction assay. The limitations associated with this technique must be taken into account to interpret the approximately 60% BNF decline in ST nodules compared to SC nodules not as a quantitative measurement but as a rather qualitative one, showing that BNF was inhibited by Cd treatment only in ST nodules.
Fig. 4.
Nase activity of control and Cd-treated M. truncatula nodules grown in a SRS. Each bar represents mean±SE for n=8. Different letters represent significant differences (P≤0.05). NFW, nodule fresh weight.
Cd up-regulates nodule antioxidant machinery
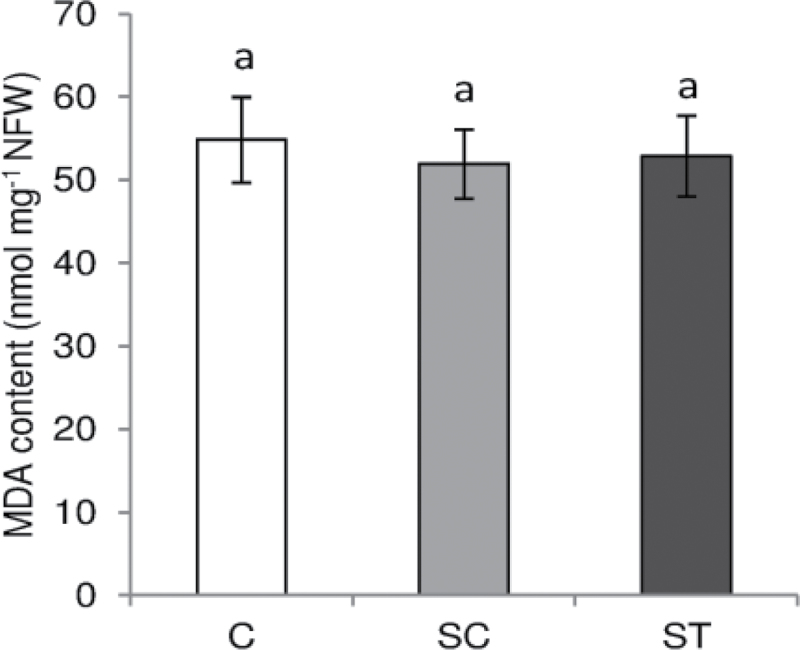
Cd is widely known to generate lipid oxidation (Cuypers et al., 2010). Thus, we measured lipid oxidation through the determination of MDA content as a marker of oxidative damage in nodules. No significant modification in MDA content was observed in ST nodules compared to SC and C nodules (Fig. 5). According to this result, Cd did not provoke a generalized oxidative damage in nodules at the time of harvest.
Fig. 5.
MDA content of control and Cd-treated M. truncatula nodules grown in a SRS. Each bar represents mean±SE for n=6. Different letters represent significant differences (P≤0.05). NFW, nodule fresh weight.
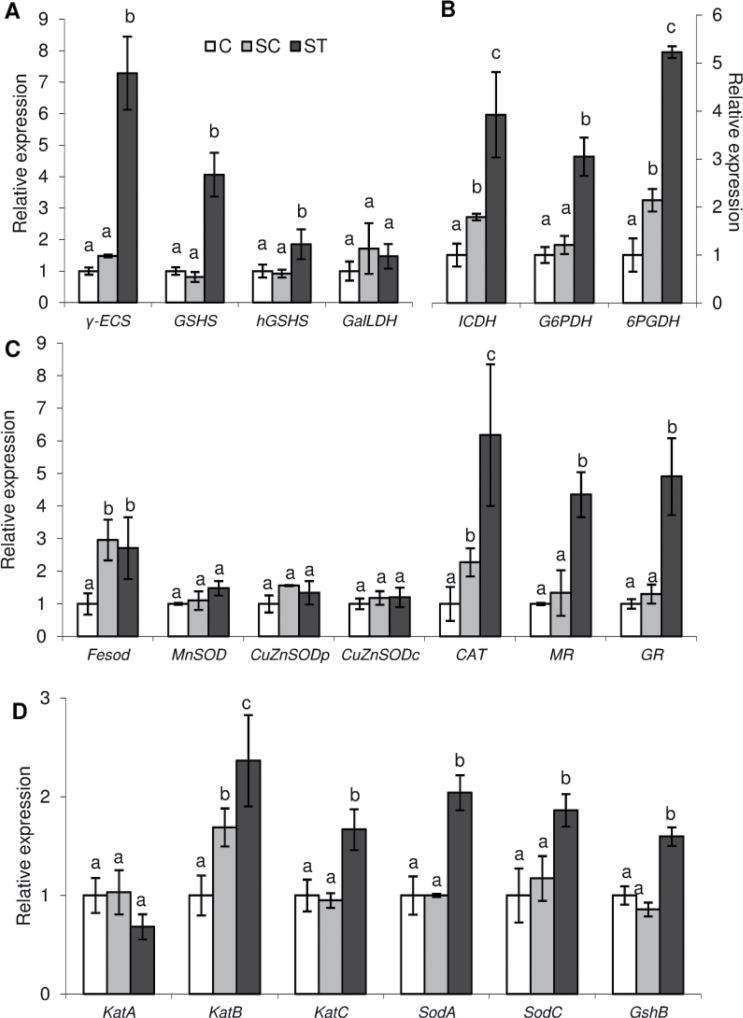
To investigate how nodular antioxidant defence responded to Cd and whether its control was local or systemic we determined by quantitative PCR the expression of genes encoding some of the nodule main antioxidant machinery components, from both the plant and the bacterial partners (Fig. 6). For the plant side, these include SODs, a family of metalloenzymes harbouring Fe, Mn, or CuZn in the active site that detoxify O2 − to H2O2, and CAT, an enzyme that directly detoxifies H2O2. We also investigated components of non-enzymatic antioxidant biosynthesis such as γ-glutamyl-cysteine synthetase (γ-ECS), glutathione synthetase and homoglutathione synthetase for thiol synthesis, and galactono-1,4-lactone dehydrogenase, the enzyme catalysing the last step in the main pathway of ascorbate biosynthesis. We also analysed the expression of genes encoding monodehydroascorbate reductase (MR) and GR, enzymes of the ascorbate-glutathione cycle. Moreover, NADPH recycling has been described as a factor limiting plant antioxidant capacity (Foyer and Noctor, 2009). Thus, we determined the expression of genes encoding the main NADPH-generating enzymes which include ICDH and the pentose-phosphate pathway NADP+-generating glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase. From the microsymbiont we analysed the gene expression of bacterial antioxidants including SODs (SodA, SodC), catalases (KatA, KatB, KatC), and glutathione synthetase (Gshb).
Fig. 6.
Expression quantification by quantitative PCR of genes encoding (A) plant components of non-enzymatic antioxidant machinery, (B) plant NADPH-generating enzymes, (C) plant antioxidant enzymes, and (D) bacterium antioxidant components. Each bar represents mean±SE for n=6. Different letters represent significant differences (P≤0.05). GSHS, glutathione synthetase; hGSHS, homoglutathione synthetase; GalLDH, galactono-1,4-lactone dehydrogenase; G6PDH, glucose-6-phosphate dehydrogenase; 6PGDH, 6-phosphogluconate dehydrogenase.
On the plant side, the three genes of (homo)glutathione synthesis were locally up-regulated by Cd (Fig. 6A). Among these three genes, γ-ECS presented the highest induction, about seven times in ST nodules compared to C and SC nodules (Fig. 6A). Similarly, the three genes encoding NADPH-generating enzymes were induced in ST nodules (Fig. 6B). Interestingly, ICDH and 6-phosphogluconate dehydrogenase were slightly induced in SC compared to C nodules (Fig. 6B). Among the genes encoding antioxidant enzymes, FeSOD, CAT, MR, and GR were induced in ST nodules compared to C nodules (Fig. 6C). Among them, MR and GR were exclusively induced in ST nodules whereas CAT was partially induced in SC nodules compared to C nodules (Fig. 6C). FeSOD was the unique gene that experienced an induction in SC nodules similar to that of ST nodules upon Cd exposure (Fig. 6C). At the bacterial level KatC, SodA, SodC, and Gshb were locally induced and KatB was induced in both SC and ST nodules (Fig. 6D).
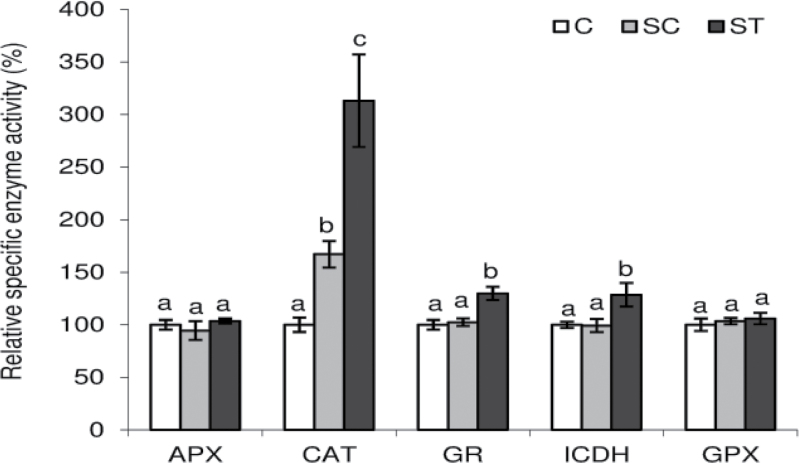
We also determined the nodule plant fraction enzyme activities of APX, CAT, GR, ICDH, and unspecific peroxidases (GPX). GR and ICDH activities were locally induced (Fig. 7). In agreement with its gene expression, CAT activity was enhanced both in SC and ST nodules but the induction was 2-fold higher in the nodules directly exposed to Cd (ST). GPX and APX activities did not respond to Cd (Fig. 7).
Fig. 7.
Enzyme activity of M. truncatula nodule APX, CAT, GR, ICDH, and GPX. Each bar represents mean±SE for n=6. Different letters represent significant differences (P≤0.5).
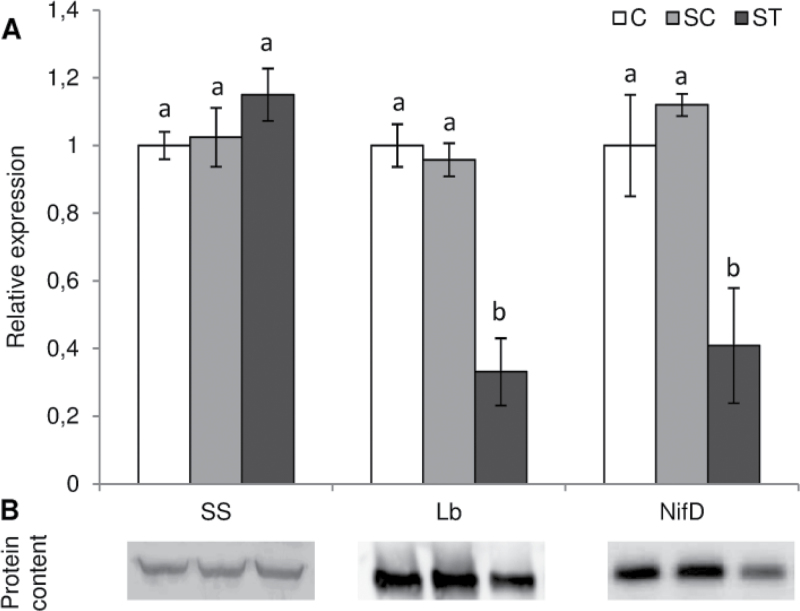
Cd effect on nitrogen fixation is related to Lb and Nase down-regulation
To gain further knowledge on the potential responsible agents of nitrogen fixation activity decline we determined by quantitative PCR the expression of genes encoding some of the main actors of nodule response to abiotic stresses: SS, Lb, and the Nase component NifD. The SS expression level was similar in every treatment. However both Lb and NifD expression declined locally in ST nodules (Fig. 8A). We also determined by Western blot the protein amounts of SS, Lb, and NifD with specific antibodies (Fig. 8B). In accordance with gene-expression levels, SS protein content did not vary while Lb and NifD declined locally in the nodules directly exposed to Cd (Fig. 8B).
Fig. 8.
Gene expression (A) and protein content (B) of SS, Lb, and a Nase component (NifD) of control and Cd-treated M. truncatula nodules grown in a SRS. Each bar represents mean±SE for n=6. Different letters represent significant differences (P≤0.05).
Discussion
The decline of nitrogen fixation under abiotic stress has been related to a shortage of carbon due to a reduction of plant photosynthetic capacity or to a direct shortage in the nodule, related to down-regulation of SS, the enzyme that catalyses the first step of sucrose hydrolysis before entering the glycolytic pathway (González et al., 1995). There is also evidence, mainly obtained in legumes of tropical origin, that BNF inhibition is due to feedback control related to plant ‘nitrogenous status’ (King and Purcell, 2005). Finally, it has been shown that oxygen is a key factor in BNF regulation, related both to the need for oxygen in obtaining energy and to ROS damage of nodule components such as Lb or Nase (Becana et al., 2010). Moreover, Lb oxidation increases nodule free oxygen that can directly inhibit Nase gene expression and activity (Becana et al., 2010). Nodule permeability to oxygen through the operation of ODB can also participate in BNF regulation upon changes in the environment (Minchin, 1997).
In nodules of several legumes, many environmental constraints, such as dark stress (Gogorcena et al., 1997), drought (Gogorcena et al., 1995), or excess nitrogen (Escuredo et al., 1996), are known to induce ROS overproduction, causing BNF inhibition. These stresses affect many plant metabolic processes including a decrease of nodule antioxidant capacity as a consequence of oxidative damage that may even lead to cell death. Thus, these stresses provoke a rather generalized harmful effect in the plant which is often associated with a deleterious effect on plant shoot performance. To study oxidative stress-avoiding effects on shoot and oxidative damage, Marino et al. (2006) applied a low concentration of paraquat to nodulated pea plants, which provoked an effect on BNF before any other effect on plant performance was evident. The authors suggested that the BNF decline was related to SS down-regulation and to a limitation in carbon supply to bacteroids (Marino et al., 2006).
In this study, we applied a dose of Cd that was low enough to avoid any change in shoot biomass (Table 1) or chlorophyll content (Fig. 2), two of the most commonly accepted markers of Cd toxicity in plant photosynthetic organs (Gallego et al., 2012). The effect of severe Cd treatments on nodule functioning has already been studied in several legumes, for instance in white lupin (Carpena et al., 2003), pigeonpea (Garg and Aggarwal, 2012), and mung bean (Muneer et al., 2012). However, in those studies Cd treatment also affected shoot biomass and/or chlorophyll content. Thus, it is not possible to distinguish whether nodule activity impairment was related to a general plant breakdown or to a direct effect on nodule components. In our study, although approximately 1% of the Cd taken up by ST roots was translocated to both shoots and SC roots, this translocation was at the time of harvest low enough to avoid toxicity in shoots (Table 1, Fig. 2) and to avoid a decline in nitrogen fixation in SC nodules (Fig. 4). Thus, this SRS approach shows that BNF inhibition provoked by Cd is due to a direct effect on nodules rather than a systemic effect through a control from the shoot. Local regulation of nitrogen fixation has already been shown under moderate water stress in pea (Marino et al., 2007) and more recently in M. truncatula (Gil-Quintana et al., 2013a ) and soybean (Gil-Quintana et al., 2013b ). The present work, with the application of Cd, which is a very mobile element (Lux et al., 2011), confirms the high and local sensitivity of the nitrogen-fixation process to environmental constraints.
To understand the mechanism of this nitrogen-fixation inhibition, we measured lipid oxidation to determine whether Cd was generating oxidative damage in nodules. MDA content was similar in control nodules (C and SC) and in nodules in direct contact with Cd (Fig. 5). Moreover, at the time of harvest root necrosis did not occur (Fig. 1C). Thus, in our conditions, the hypothesis that BNF inhibition was due to a generalized collapse of the nodule can be discarded. Furthermore, total nodule protein content was similar in C, SC, and ST nodules (data not shown). Although several studies have already studied Cd toxicity on M. truncatula shoot and root biology (Xu et al., 2010; Aloui et al., 2011), to our knowledge this work represents the first time that the effect of Cd on legume nodule functioning has been reported.
The induction of antioxidant systems appears to be a key player in the avoidance of severe effects of abiotic stress (Apel and Hirt, 2004; Suzuki et al., 2012). In legume nodules of M. truncatula plants overexpressing γ-ECS or in nodules of wild-type plants inoculated with a S. meliloti strain overexpressing γ-ECS, an increase in nitrogen-fixation capacity was reported (El Msehli et al., 2011). In common bean it seems that higher antioxidant defences would have a protective role against osmotic stress (Sassi et al., 2008). However, an increase in ascorbate content in pea nodules did not have a protective role against drought (Zabalza et al., 2008). In our work, the expression of antioxidant-enzyme-encoding genes was up-regulated (Fig. 6), including a high induction of γ-ECS (Fig. 6A). Nevertheless, antioxidant machinery induction was not sufficient to prevent BNF inhibition, showing the high sensitivity of this process to environmental fluctuations. Most of the determined antioxidants were induced only in ST nodules, but CAT (from plant or bacteria), ICDH, 6-phosphogluconate dehydrogenase, and FeSOD were also induced in SC nodules, suggesting either a systemic control or a very sensitive response to the slight increase in Cd content that was translocated from ST roots. CAT, a direct scavenger of H2O2, is one of the major antioxidants in plants (Apel and Hirt, 2004). In this work, CAT expression and activity from the plant side (Figs 6C and 7) and Katb expression from the bacterial side (Fig. 6D) were induced both in SC and ST nodules. The heterologous overexpression of a CAT gene from Brassica juncea in tobacco plants conferred to these plants an enhanced tolerance to Cd (Guan et al., 2009), limiting oxidative damage, as demonstrated by MDA monitoring. In our work the high induction of CAT might be essential to control generalized nodule oxidative damage but not enough to avoid a decline in BNF.
To gain further knowledge in the regulation of BNF in M. truncatula nodules under Cd we measured the transcription and translation of three of the most important components of nodule response to abiotic stress: SS, Lb, and NifD, one of the components of Nase enzymatic complex. SS was not affected by the stress; however, both Lb and NifD gene expression and protein content decreased. This is consistent with other studies on Cd treatment, in soybean (Balestrasse et al., 2004) or common bean (Loscos et al., 2008), where a decline in nitrogen fixation under Cd has been associated with a decrease in Lb content. However, in those studies Cd stress was very severe and oxidative damage was evident together with a down-regulation of antioxidant machinery. In our study, nodule antioxidant systems were still induced upon Cd exposure and could avoid generalized damage. However, as stated, Lb is a very sensitive protein to redox changes and its content dramatically declined in ST nodules. The function of this globin is to supply oxygen to the bacteroids, to support their energy-yielding respiration, at a tension low enough to avoid disrupting Nase, which is highly sensitive to oxygen. Thus, a Lb decline will provoke an alteration of the nodule microaerophilic environment that in turn will lead to the down-regulation of Nase expression and content (Fig. 8A, B), which ultimately will result in inhibition of nitrogen fixation (Fig. 5). ODB may also play a role in the control of nodule oxygen concentration to regulate BNF (Minchin, 1997). Lb degradation could cause a transient increase in nodule internal oxygen that could provoke ODB closure. Moreover, ROS have been also suggested to regulate ODB, although direct experimental evidence is still lacking (Minchin, 1997; Minchin et al., 2008). Thus, the interaction Lb–ROS–O2–ODB may be responsible for BNF regulation.
The heterologous overexpression of a flavodoxin from Anabaena variabilis in S. meliloti partially prevented Cd toxicity effects on Nase activity in Medicago sativa (Shvaleva et al., 2010). Flavodoxins are prokaryotic electron carrier proteins and have been suggested to play a positive role in ROS detoxification (Redondo et al., 2009). This result is in agreement with our data indicating that the effect of Cd on nitrogen-fixation capacity in M. truncatula nodules is related to a down-regulation of Nase expression and protein content, rather than SS down-regulation. In M. sativa drought-stressed plants, nitrogen fixation was suggested to be related to a direct effect on Nase (Naya et al., 2007). In that work, however, drought treatment had already provoked generalized oxidative damage (Naya et al., 2007).
Taken together, our data reveal that the application of moderate Cd treatment in SRS-grown M. truncatula plants provoked mild stress where BNF partial inhibition was controlled at the local level. We suggest that this control is exerted through Lb/oxygen availability that affects Nase function rather than being an effect provoked by a general breakdown of plant cells due to a severe oxidative damage. Interestingly, it seems that in forage legumes, which include M. sativa and M. truncatula species, mild stress provokes a control of nitrogen fixation related to Lb/oxygen availability rather than having a rapid effect on sucrose synthase, as is known in grain legumes such as pea, bean, or soybean (Arrese-Igor et al., 2012). Thus, it appears that control of nitrogen fixation is not only dependent on the type and severity of the stress but also in the legume species. Future studies using plants with altered ROS-scavenging and/or -producing mechanisms might be useful to advance our understanding of the duality of the signalling/toxic role of ROS in nitrogen-fixation regulation and in plant responses to environmental fluctuations. Besides, it is still controversial whether ROS can travel long distances in the plant, since most of them are highly reactive. Miller et al. (2009) proposed ROS as important players in systemic signals propagation in Arabidopsis.
Supplementary material
Supplementary material is available at JXB online.
Table S1. List of primers used to quantify gene-expression levels by quantitative PCR.
Acknowledgements
SS, Lb, and NifD antibodies were kindly provided by Professor Cesar Arrese-Igor.
Glossary
Abbreviations:
- APX
ascorbate peroxidase
- BNF
biological nitrogen fixation
- C
control
- CAT
catalase
- γ-ECS
γ-glutamyl-cysteine synthetase
- GPX
guaiacol peroxidases
- GR
glutathione reductase
- ICDH
NADP+-dependent isocitrate dehydrogenase
- Lb
leghaemoglobin
- MDA
malondialdehyde
- MR
monodehydroascorbate reductase
- Nase
nitrogenase
- ODB
oxygen diffusion barrier
- ROS
reactive oxygen species
- SC
split-control
- SOD
superoxide dismutase
- SRS
split-root system
- SS
sucrose synthase
- ST
split-treatment
- T
treatment.
References
- Aloui A, Recorbet G, Robert F, Schoefs B, Bertrand M, Henry C, Gianinazzi-Pearson V, Dumas-Gaudot E, Aschi-Smiti S. 2011. Arbuscular mycorrhizal symbiosis elicits shoot proteome changes that are modified during cadmium stress alleviation in Medicago truncatula . BMC Plant Biology 11, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrio E, Marino D, Marmeys A, de Segonzac MD, Damiani I, Genre A, Huguet S, Frendo P, Puppo A, Pauly N. 2013. Hydrogen peroxide-regulated genes in the Medicago truncatula-Sinorhizobium meliloti symbiosis. New Phytologist 198, 179–9 [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399 [DOI] [PubMed] [Google Scholar]
- Arnon DI. 1949. Copper enzymes in isolated chloroplasts - polyphenoloxidase in beta-vulgaris. Plant Physiology 24, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese-Igor C, González EM, Marino D, Ladrera R, Larrainzar E, Gil-Quintana E. 2011. Physiological responses of legume nodules to drought. Plant Stress 5 (special issue 1), 24–31 [Google Scholar]
- Balestrasse KB, Gallego SM, Tomaro ML. 2004. Cadmium-induced senescence in nodules of soybean (Glycine max L.) plants. Plant and Soil 262, 373–381 [Google Scholar]
- Balestrasse KB, Gallego SM, Tomaro ML. 2006. Oxidation of the enzymes involved in nitrogen assimilation plays an important role in the cadmium-induced toxicity in soybean plants. Plant and Soil 284, 187–194 [Google Scholar]
- Becana M, Matamoros MA, Udvardi M, Dalton DA. 2010. Recent insights into antioxidant defenses of legume root nodules. New Phytologist 188, 960–976 [DOI] [PubMed] [Google Scholar]
- Becker A, Berges H, Krol E, et al. 2004. Global changes in gene expression in Sinorhizobium meliloti 1021 under microoxic and symbiotic conditions. Molecular Plant-Microbe Interactions 17, 292–303 [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254 [DOI] [PubMed] [Google Scholar]
- Carpena RO, Vazquez S, Esteban E, Fernandez-Pascual M, de Felipe MR, Zornoza P. 2003. Cadmium-stress in white lupin: effects on nodule structure and functioning. Plant Physiology and Biochemistry 41, 911–919 [Google Scholar]
- Clemens S, Aarts MG, Thomine S, Verbruggen N. 2013. Plant science: the key to preventing slow cadmium poisoning. Trends in Plant Sciences 18, 92–99 [DOI] [PubMed] [Google Scholar]
- Cuypers A, Plusquin M, Remans T, et al. 2010. Cadmium stress: an oxidative challenge. Biometals 23, 927–940 [DOI] [PubMed] [Google Scholar]
- El Msehli S, Lambert A, Baldacci-Cresp F, Hopkins J, Boncompagni E, Smiti SA, Herouart D, Frendo P. 2011. Crucial role of (homo)glutathione in nitrogen fixation in Medicago truncatula nodules. New Phytologist 192, 496–506 [DOI] [PubMed] [Google Scholar]
- Escuredo PR, Minchin FR, Gogorcena Y, IturbeOrmaetxe I, Klucas RV, Becana M. 1996. Involvement of activated oxygen in nitrate-induced senescence of pea root modules. Plant Physiology 110, 1187–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2009. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxidants & Redox Signaling 11, 861–905 [DOI] [PubMed] [Google Scholar]
- Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Lannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP. 2012. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environmental and Experimental Botany 83, 33–46 [Google Scholar]
- Garg N, Aggarwal N. 2012. Effect of mycorrhizal inoculations on heavy metal uptake and stress alleviation of Cajanus cajan (L.) Millsp genotypes grown in cadmium and lead contaminated soils. Plant Growth Regulation 66, 9–26 [Google Scholar]
- Gil-Quintana E, Larrainzar E, Arrese-Igor C, González EM. 2013a. Is N-feedback involved in the inhibition of nitrogen fixation in drought-stressed Medicago truncatula? Journal of Experimental Botany 64, 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Quintana E, Larrainzar E, Seminario A, Día-Leal JL, Alamillo JM, Pineda M, Arrese-Igor C, Wienkoop S, González EM. 2013b. Local inhibition of nitrogen fixation and nodule metabolism in drought-stressed soybean. Journal of Experimental Botany 64, 2171–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogorcena Y, Iturbeormaetxe I, Escuredo PR, Becana M. 1995. Antioxidant defenses against activated oxygen in pea nodules subjected to water-stress. Plant Physiology 108, 753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogorcena Y, Gordon AJ, Escuredo PR, Minchin FR, Witty JF, Moran JF, Becana M. 1997. N2 fixation, carbon metabolism, and oxidative damage in nodules of dark-stressed common bean plants. Plant Physiology 113, 1193–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González EM, Gordon AJ, James C, Arrese-Igor C. 1995. The role of sucrose synthase in the response of soybean nodules to drought. Journal of Experimental Botany 46, 151–1523 [Google Scholar]
- Guan ZQ, Chai TY, Zhang YX, Xu J, Wei W. 2009. Enhancement of Cd tolerance in transgenic tobacco plants overexpressing a Cd-induced catalase cDNA. Chemosphere 76, 623–630 [DOI] [PubMed] [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK. 1999. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207, 604–611 [DOI] [PubMed] [Google Scholar]
- King CA, Purcell LC. 2005. Inhibition of N2 fixation in soybean is associated with elevated ureides and amino acids. Plant Physiology 137, 1389–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. 1970. Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- Loscos J, Matamoros MA, Becana M. 2008. Ascorbate and homoglutathione metabolism in common bean nodules under stress conditions and during natural senescence. Plant Physiology 146, 1282–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A, Martinka M, Vaculik M, White PJ. 2011. Root responses to cadmium in the rhizosphere: a review. Journal of Experimental Botany 62, 21–37 [DOI] [PubMed] [Google Scholar]
- Marino D, González EM, Arrese-Igor C. 2006. Drought effects on carbon and nitrogen metabolism of pea nodules can be mimicked by paraquat: evidence for the occurrence of two regulation pathways under oxidative stresses. Journal of Experimental Botany 57, 665–673 [DOI] [PubMed] [Google Scholar]
- Marino D, Frendo P, Ladrera R, Zabalza A, Puppo A, Arrese-Igor C, González EM. 2007. Nitrogen fixation control under drought stress. Localized or systemic? Plant Physiology 143, 1968–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino D, Pucciariello C, Puppo A, Frendo P. 2009. The redox state, a referee of the legume-rhizobia symbiotic game. In: Jacquot JP, ed. Advances in Botanical Research: Oxidative Stress and Redox Regulation in Plants , vol. 52 Amsterdam: Elsevier, 115–151 [Google Scholar]
- Marino D, Andrio E, Danchin EGJ, Oger E, Gucciardo S, Lambert A, Puppo A, Pauly N. 2011. A Medicago truncatula NADPH oxidase is involved in symbiotic nodule functioning. New Phytologist 189, 580–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Jobe TO, Hauser F, Schroeder JI. 2011. Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Current Opinion in Plant Biology 14, 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R. 2009. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Science Signaling 2, A26–A35 [DOI] [PubMed] [Google Scholar]
- Minchin FR. 1997. Regulation of oxygen diffusion in legume nodules. Soil Biology and Biochemistry 29, 881–888 [Google Scholar]
- Minchin FR, James EK, Becana M. 2008. Oxygen diffusion, production of reactive oxygen andnitrogen species, and antioxidants in legume nodules. In: Dilworth MJ, James EK, Sprent JI, Newton WE, eds. Leguminous Nitrogen-Fixing Symbioses. Dordrecht: Springer, 321–362 [Google Scholar]
- Minchin FR, Sheedhy JE, Witty JF. 1986. Further errors in the acetylene reduction assay: effects of plant disturbance. Journal of Experimantal Botany 37, 1581–1591 [Google Scholar]
- Minchin FR, Witty JE, Sheehy JE, Müller M. 1983. A major error in the acetylene reduction assay: decreases in nodular nitrogenase activity under assay conditions. Journal of Experimantal Botany 34, 641–649 [Google Scholar]
- Montiel J, Nava N, Cárdenas L, Sánchez-López R, Arthikala MK, Santana O, Sánchez F, Quinto C. 2012. A Phaseolus vulgaris NADPH oxidase gene is required for root infection by Rhizobia . Plant and Cell Physiology 53, 1751–1767 [DOI] [PubMed] [Google Scholar]
- Muneer S, Kim TH, Qureshi MI. 2012. Fe modulates Cd-induced oxidative stress and the expression of stress responsive proteins in the nodules of Vigna radiata . Plant Growth Regulation 68, 421–433 [Google Scholar]
- Naya L, Ladrera R, Ramos J, González EM, Arrese-Igor C, Minchin FR, Becana M. 2007. The response of carbon metabolism and antioxidant defenses of alfalfa nodules to drought stress and to the subsequent recovery of plants. Plant Physiology 144, 1104–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. 1998. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology 49, 249–279 [DOI] [PubMed] [Google Scholar]
- Oldroyd GED, Murray JD, Poole PS, Downie JA. 2011. The rules of engagement in the legume-rhizobial symbiosis. Annual Review Genetics 45, 119–144 [DOI] [PubMed] [Google Scholar]
- Paradiso A, Berardino R, de Pinto MC, di Toppi LS, Storelli MM, Tommasi F, De Gara L. 2008. Increase in ascorbate-glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant and Cell Physiology 49, 362–374 [DOI] [PubMed] [Google Scholar]
- Redondo FJ, de la Pena TC, Morcillo CN, Lucas MM, Pueyo JJ. 2009. Overexpression of flavodoxin in bacteroids induces changes in antioxidant metabolism leading to delayed senescence and starch accumulation in alfalfa root nodules. Plant Physiology 149, 1166–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud J, Puppo A. 1975. Indole-3-acetic-acid catabolism by soybean bacteroids. Journal of General Microbiology 88, 223–228 [Google Scholar]
- Rodriguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gomez M, Del Rio LA, Sandalio LM. 2006. Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell and Environment 29, 1532–1544 [DOI] [PubMed] [Google Scholar]
- Romero-Puertas MC, Rodriguez-Serrano M, Corpas FJ, Gomez M, Del Rio LA, Sandalio LM. 2004. Cadmium-induced subcellular accumulation of O2.- and H2O2 in pea leaves. Plant Cell and Environment 27, 1122–1134 [Google Scholar]
- Salin ML. 1988. Toxic oxygen species and protective systems of the chloroplast. Physiologia Plantarum 72, 681–689 [Google Scholar]
- Sandalio LM, Dalurzo HC, Gomez M, Romero-Puertas MC, del Rio LA. 2001. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. Journal of Experimental Botany 52, 2115–2126 [DOI] [PubMed] [Google Scholar]
- Sassi S, González EM, Aydi S, Arrese-Igor C, Abdelly C. 2008. Tolerance of common bean to long-term osmotic stress is related to nodule carbon flux and antioxidant defenses: evidence from two cultivars with contrasting tolerance. Plant and Soil 312, 39–48 [Google Scholar]
- Serraj R, Vadez V, Sinclair TR. 2001. Feedback regulation of symbiotic N2 fixation under drought stress. Agronomie 21, 621–626 [Google Scholar]
- Shvaleva A, de la Pena TC, Rincon A, Morcillo CN, de la Torre VSG, Lucas MM, Pueyo JJ. 2010. Flavodoxin overexpression reduces cadmium-induced damage in alfalfa root nodules. Plant and Soil 326, 109–121 [Google Scholar]
- Suzuki N, Koussevitzky S, Mittler R, Miller G. 2012. Ros and redox signalling in the response of plants to abiotic stress. Plant Cell and Environment 35, 259–270 [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 3, research0034.1–0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Velde W, Guerra JCP, De Keyser A, De Rycke R, Rombauts S, Maunoury N, Mergaert P, Kondorosi E, Holsters M, Goormachtig S. 2006. Aging in legume symbiosis. A molecular view on nodule senescence in Medicago truncatula . Plant Physiology 141, 711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C, Schat H. 2009. Mechanisms to cope with arsenic or cadmium excess in plants. Current Opinion in Plant Biology 12, 364–372 [DOI] [PubMed] [Google Scholar]
- Xu J, Wang WY, Yin HX, Liu XJ, Sun H, Mi Q. 2010. Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant and Soil 326, 321–330 [Google Scholar]
- Zabalza A, Galvez L, Marino D, Royuela M, Arrese-Igor C, González EM. 2008. The application of ascorbate or its immediate precursor, galactono-1,4-lactone, does not affect the response of nitrogen-fixing pea nodules to water stress. Journal of Plant Physiology 165, 805–812 [DOI] [PubMed] [Google Scholar]
- Zabalza A, Gaston S, Sandalio LM, del Rio LA, Royuela M. 2007. Oxidative stress is not related to the mode of action of herbicides that inhibit acetolactate synthase. Environmental and Experimental Botany 59, 150–159 [Google Scholar]
- Zahran HH. 1999. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiology and Molecular Biology Reviews 63, 968–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, McGrath SP, Crosland AR. 1994. Comparison of 3 wet digestion methods for the determination of plant sulfur by inductively-coupled plasma-atomic emission-spectroscopy (ICPAES). Communications in Soil Science and Plant Analysis 25 407–418 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.