Abstract
Purpose:
To provide an in-depth re-examination of assumed causes of tissue hypertrophy, port-wine stains, and the Sturge-Weber, Cobb, Klippel-Trénaunay, and related syndromes to support an alternative unifying pathophysiologic mechanism of venous dysplasia producing focal venous hypertension with attendant tissue responses; to provide proof of concept with new patient data; to propose a novel etiological hypothesis for the venous dysplasia in these syndromes and find supportive evidence.
Methods:
Data from 20 patients with port-wine stains and corneal pachymetry readings was collected prospectively by the author in an institutional referral-based practice. The literature was searched using MEDLINE, and articles and textbooks were obtained from the bibliographies of these publications.
Results:
Newly obtained dermatologic, corneal pachymetry, fundus ophthalmoscopic, ocular and orbital venous Doppler ultrasonography, and magnetic resonance imaging findings in patients with the Sturge-Weber syndrome or isolated port-wine stains, along with published data, reveal diffusely thickened tissues and neural atrophy in all areas associated with venous congestion.
Conclusions:
Contrary to traditional understanding, signs and symptoms in the Sturge-Weber and related syndromes, including both congenital and acquired port-wine stains, are shown to arise from effects of localized primary venous dysplasia or acquired venous obstruction rather than neural dysfunction, differentiating these syndromes from actual phacomatoses. Effects of focal venous hypertension are transmitted to nearby areas via compensatory collateral venous channels in the above conditions, as in the Parkes Weber syndrome. A novel underlying etiology—prenatal venous thrombo-occlusion—is proposed to be responsible for the absence of veins with persistence and enlargement of collateral circulatory pathways with data in the literature backing this offshoot hypothesis. The mechanism for isolated pathologic tissue hypertrophy in these syndromes clarifies physiologic mechanisms for exercise-induced muscle hypertrophy to occur via venous compression and increased capillary transudation.
INTRODUCTION
Several years ago, a new pathophysiologic mechanism was hypothesized by the author to explain findings noted in the Sturge-Weber and related syndromes (Parsa CF. Sturge-Weber syndrome and the phacomatoses: odd man found out. Transactions of the 31st North American Neuro-Ophthalmology Society Annual Meeting, February 17, 2005:339–343).1 This mechanism emphasized that with the congenital absence of portions of veins, diverted blood flow could cause responses in different tissues that could explain various aspects of these syndromes. This prediction was based on anatomical features of venous drainage in normal individuals and the absence of veins noted in affected individuals,2 with presumed hemodynamic consequences that could be inferred.
The purpose of this thesis is to provide new evidence and proof of concept for the validity of the proposed hypothesis that focal venous hypertension resulting from venous dysplasia is a cause of tissue hypertrophy. The first evidentiary part consists of an extensive and in-depth review of the literature with inductive analysis of findings accumulated to date. The second evidentiary part is the directed prospective investigation and collection of new data from the author’s specialty practice. The findings and new data obtained confirm predictions of the author’s proposed pathophysiologic mechanism, and bring forth additional implications. A deductive analysis is made, and a new underlying etiology for the Sturge-Weber and related syndromes is hypothesized, in which congenital venous dysplasia itself is a result of in utero venous thrombosis. What may be considered a third level of evidentiary data was found in the extant literature and supports this novel offshoot hypothesis. A methodology for the screening and prevention of these entities is proposed, and direction for future confirmatory studies is provided. Novel optimized medical and surgical treatment strategies for currently afflicted individuals are also proposed.
METHODS
IN-DEPTH LITERATURE REVIEW
An extensive and in-depth review and analysis of the literature was performed for findings accumulated to date, to support or refute the proposed pathophysiological mechanism. The literature was searched using MEDLINE, and articles and textbooks were obtained from the bibliographies of these publications without any language restrictions.
CLINICAL INVESTIGATIONS
New patient data with novel, directed investigational methods (dermatologic observations of natural evolution of port-wine stains, corneal pachymetry, qualitative fluorometry, dynamic and static ocular ultrasonography, orbital venous Doppler ultrasonography, magnetic resonance neuroimaging, and other methods) was collected prospectively by the author in an institutional referral-based practice on an ad hoc basis in accordance with all institutional guidelines, including HIPAA compliance. Johns Hopkins Medicine Institutional Review Board approval was obtained prospectively before the study began and prior to patient data collection. The Johns Hopkins University approved this multidisciplinary protocol for the prospective collection and analysis of patient data to address the pathophysiology of Sturge-Weber syndrome. Prior to each examination, informed consent was obtained from either the patient or the patient’s parents. Data presented includes all 20 patients with port-wine stains or the Sturge-Weber syndrome and with corneal pachymetry readings.
RESULTS AND NEW IMPLICATONS
HISTORY AND DESCRIPTION OF “NORMAL” AND “ABERRANT” FINDINGS ASSOCIATED WITH THE STURGE-WEBER SYNDROME
Definition of the Syndrome and Early History of Classification of Findings
The Sturge-Weber syndrome has been defined by a combination of at least two of various signs—cephalic port-wine stains, increased intraocular pressure, and various central nervous system (CNS) effects, notably leptomeningeal enlargement, cortical atrophy, and seizures.
In 1923, van der Hoeve3 coined the term phacomatoses (from the Greek phakos for “mother spot”) initially to describe two inherited disorders, tuberous sclerosis (ie, Bourneville disease) and neurofibromatosis (ie, von Recklinghausen disease). These two disorders are both characterized by tumefactions (hamartomas) termed phakomata occurring anywhere in the body, but predominantly affecting the skin, eye, CNS, and viscera.
Years later, van der Hoeve4,5 enlarged the concept to include other conditions characterized by skin, ocular, and CNS findings such as the von Hippel-Lindau syndrome. Eventually, after noting an apparent forme fruste inherited case,6 van der Hoeve along with other colleagues proposed the Sturge-Weber syndrome as a fourth phacomatosis. Others would go on to include the Klippel-Trénaunay syndrome in the category of phacomatoses,7 distinguishing between the cerebral atrophy noted in the Sturge-Weber syndrome and the hypertrophy of other tissues8 in the Klippel-Trénaunay syndrome.
However, in neither the Sturge-Weber nor the Klippel-Trénaunay syndrome, has heredity or predisposition been confirmed. This lack of confirmation makes these disorders difficult to retain in the so-defined phacomatoses group (Borchert M. Neurocutaneous disorders: five important things to ponder about their clinical manifestations. Transactions of the 25th Annual Meeting of the North American Neuro-Ophthalmology Society, March 16, 1999:91–92). While some investigators9 have proposed that a sine qua non for phacomatoses might be regional overgrowth of tissues, it is hypertrophy rather than hyperplasia that is noted in the Sturge-Weber and Klippel-Trénaunay syndromes. Some investigators have thus considered these later additions to the phacomatoses group to represent “odd men out.”10
Indeed, more than merely odd men out, both disorders, as well as the Cobb syndrome, can be distinguished from phacomatoses— as originally defined—by their underlying etiology. A reexamination of objective findings leads to a new, straightforward pathophysiologic mechanism. Such a reexamination leads also to a mechanism for port-wine stain development, both congenital and acquired (as in Fegeler syndrome), and for enhanced tissue hypertrophy in the Parkes Weber syndrome as well as in general physiologically. Most of the work in this article is a synthesis of previous research supported by original and published data with new, different, and relevant conclusions.
Traditional Interpretation of Cutaneous Findings and Challenges to Trigeminal Nerve Involvement and Angiomatous Nature
Originally referred to as encephalofacial angiomatosis, and later as the Sturge-Weber syndrome, this entity was also promulgated as encephalotrigeminal angiomatosis to avoid contested eponymous designations.11–13 This attempt at descriptive terminology, however, is particularly unfortunate,14 as it seems to have prevented investigators in various disciplines from reaching the full conclusions of their research. The notion of associating cutaneous lesions to nerve distributions in general was first proposed by von Bärensprung in 1863 for zoster dermatitis15 and was later evoked for port-wine stains and the trigeminal nerve.16–21 Cushing19 reinforced and emphasized this putative link with the trigeminal nerve to highlight concurrent meningeal involvement.
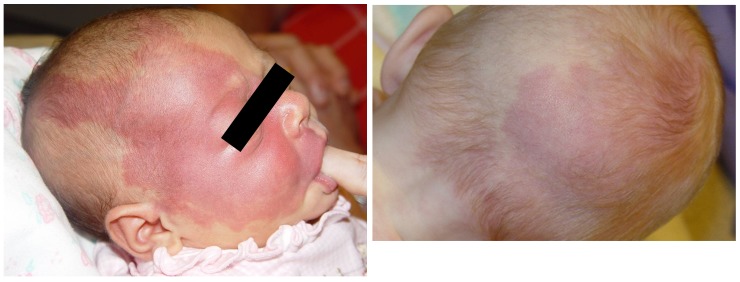
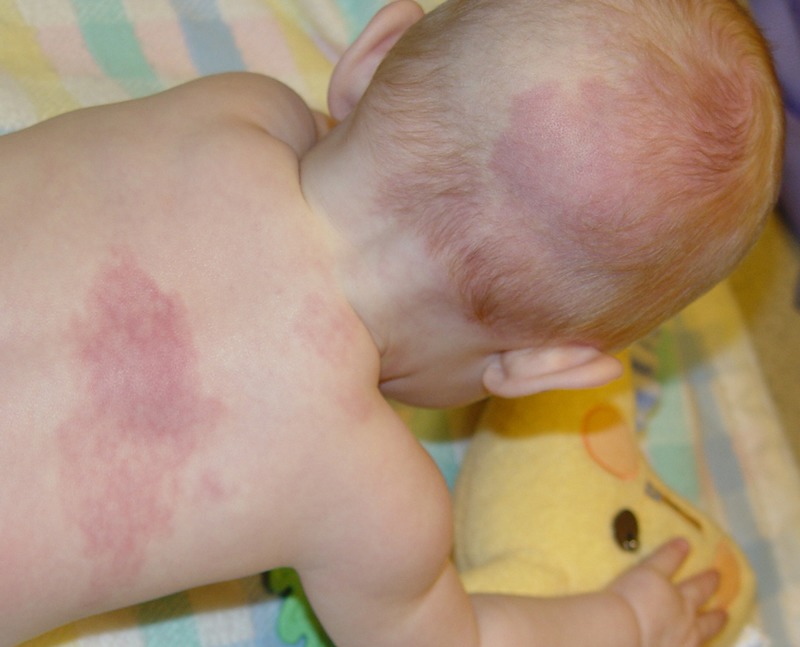
Despite their supposed neurological causation, port-wine stains’ dermatomal association has been shown to be purely coincidental.6,22–24 Port-wine stains often fall short of, or cross over, the facial midline, and are frequently found over portions of the scalp not innervated by the trigeminal nerve (Figure 1).24,25 Nor is the presence of facial port-wine stains a necessary or ubiquitous finding in the Sturge-Weber syndrome.12,26–31 Cases lacking the stains have, at times, been regarded as a subtype or forme fruste of the syndrome.
FIGURE 1.
Baby with the Sturge-Weber syndrome and right-sided facial, periorbital, and occipital port-wine stains. Left, Although involvement of the right V1 and V2 dermatomes might first appear implicated, closer examination reveals extension into the C1 dermatomal area. Right, Posteriorly, the C2 dermatome appears involved in the occipital area. (Photograph courtesy of Bernard A. Cohen, MD, The Johns Hopkins Hospital, Baltimore, Maryland.)
While port-wine stains themselves are sometimes referred to as nevus flammeus, no nevus cells are present within these lesions, whether congenital or acquired, isolated or associated with other findings. In fact, port-wine stains consist of ectatic, dilated veins. The port-wine coloration is due to the presence of deoxygenated blood within enlarged vascular spaces; the lesions blanch with pressure. Though the cutaneous lesions may thicken over the years,32,33 as the vascular dilation progresses34 they do not change in their extent. While actual superficial hemangiomas have proliferative characteristics, enlarging and then eventually always regressing,33,35,36 port-wine stains show normal endothelial mitotic activity, never show malignant growth, and thus do not constitute true angiomas.32,34,35 Indeed, various investigators have shown by histological examination that these lesions have no vascular wall abnormalities.34,37–39
Histopathological evidence cited as supportive for neural mechanisms of venous ectasia has been the documented decreased nerve densities within cutaneous biopsy specimens.32,40 Nonetheless, the failure of port-wine stains to follow dermatomal patterns, including the trigeminal branch distribution in Sturge-Weber syndrome, and the initial lack of expected nerve dysfunctions (ie, secretory or motor) do not support these hypotheses. Moreover, since the parasympathetic activity of cranial nerves serves to dilate vessels in the blush area of the face,41 it is difficult to foresee how a putative dysfunction of these nerves could be expected to produce overaction and a dilation rather than underaction and a constriction of blood vessels. Loss of sympathetic vasoconstricting activity, on the other hand, could initially lead to vasodilation in nontrigeminal, spinal nerve dermatomal patterns. However, any such denervation effects should be expected to last only weeks or months, as intrinsic tone of the smooth muscle of the vessels eventually increases, restoring almost normal vasoconstriction41 in the absence of any neural control. Such inconsistencies in proposed neural theories for the etiology of port-wine stains have not been fully addressed in the literature.
Ocular Findings That Challenge Traditional Thoughts: The “Tomato-catsup” Fundus Not Due to Angiomatous Growth
As in the cutaneous findings, angiomatous growth is also absent from the eye. Histopathological examination demonstrates the diffuse choroidal changes present in the Sturge-Weber syndrome to have characteristics altogether different from isolated and discrete choroidal hemangiomas; the choroid show no evidence of vessel, endothelial cell, or pericyte proliferation.42 Neither do they show malignant growth. Though the appearance of the so-called tomato-catsup fundus (Figure 2) is created by the diffuse thickening of the choroidal vasculature,43 only simple engorgement of pre-existing vessels is noted histopathologically. There is no distinct edge of a tumor mass.26,42 Post mortem, when drained of blood, the choroidal thickening may be noted to have diminished or to have entirely disappeared.26 Identical findings, generally bilateral, may be seen in patients who do not have the Sturge-Weber syndrome, but who have elevated ocular venous pressure secondary to congenital heart disease,44 cloverleaf osseous malformations of the head,42,45 nanophthalmos,46 or carotid-cavernous sinus fistulas.46,47 These vascular findings in the affected ocular choroid are analogous to those seen above in cutaneous port-wine stains. In the absence of actual overgrowth or disorganization of tissues, neither should be considered to represent hamartomas in the Sturge-Weber syndrome.
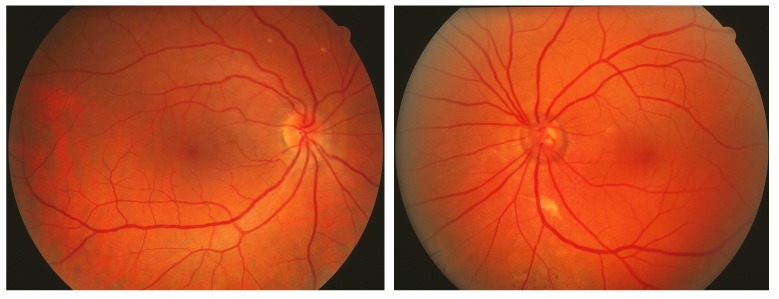
FIGURE 2.
Patient with port-wine stain involving the entire left side of face with left ocular fundus darker red, without normal choroidal vascular markings (patient 20, Table, also seen in Figures 10 and 16). Left, Normal right ocular fundus. Right, The left ocular fundus has a deeper red appearance due to the thickened blood-filled choroidal layer. Expansion of the choriocapillaris often dissimulates underlying choroidal channels and details otherwise visible. Such subtle changes, which give rise to the so-called tomato-catsup fundus, are best noted when there is a normal contralateral eye (left photo) for comparison and are often overlooked whenever venous hypertension is present bilaterally. The cup-disc ratio is increased in the left eye, indicative of the patient’s glaucoma with nasal visual field loss.
Traditional Impressions and Descriptions of the Leptomeningeal Venous Mass as Angiomatous
Decreased arterial perfusion and increased venous drainage times were first noted in 1935 via angiography by Moniz and Lima48 and confirmed shortly thereafter by other investigators.13,49 At the time, these findings were attributed to the noted leptomeningeal venous mass, which was presumed to increase resistance to venous outflow. Hudelo similarly believed reduced orbital drainage could account for many of the ocular findings, attributing this to venous angioma proliferation, localized either within the orbit or in such a location as to impede flow to the cavernous sinus.49
Although the leptomeningeal venous changes were initially described as “angiomatous”11 or angioma-like,49 they do not reflect the characteristics of true or circumscribed angiomas. Once again, no separate tumors arise from the blood vessels,6 no vascular proliferation is noted,27,50 and there is no malignant growth. Although the leptomeninges may thicken in early infancy, often peaking at 6 to 8 months of age (when blood flow to the brain is at its greatest and seizures often commence), they may thin again later,51,52 sometimes with concomitant brain atrophy.24 The leptomeningeal mass does not change in its surface area of involvement over time. The ectatic veins that compose the leptomeningeal mass exhibit a cyanotic blue discoloration both on the pial surface and on the inner surface of the dura mater, analogous to the port-wine stains noted on the scalp surface (Figure 3).
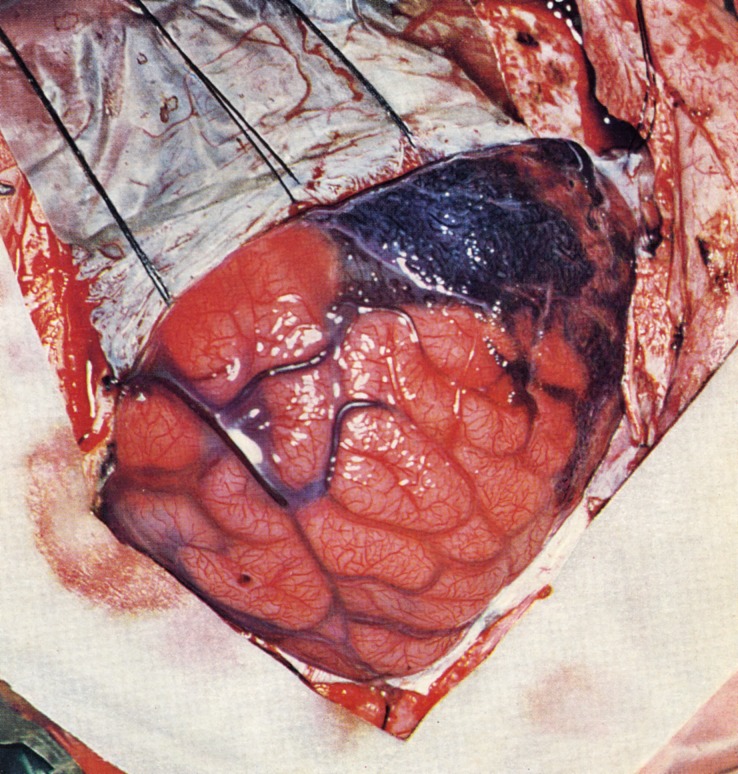
FIGURE 3.
Appearance of thickened leptomeninges overlying brain at surgery. The dura is reflected, revealing the cyanotic leptomeningeal mass with engorged superficial cortical veins. (Reprinted from Alexander GL, Norman RM.22 This book represents an orphan work with no known or traceable copyright owner.)
Findings That Refute Leptomeningeal Mass as Angiomatous, and Demonstrate That It Is Due to Lack of Bridging Veins and Resulting Venous Drainage
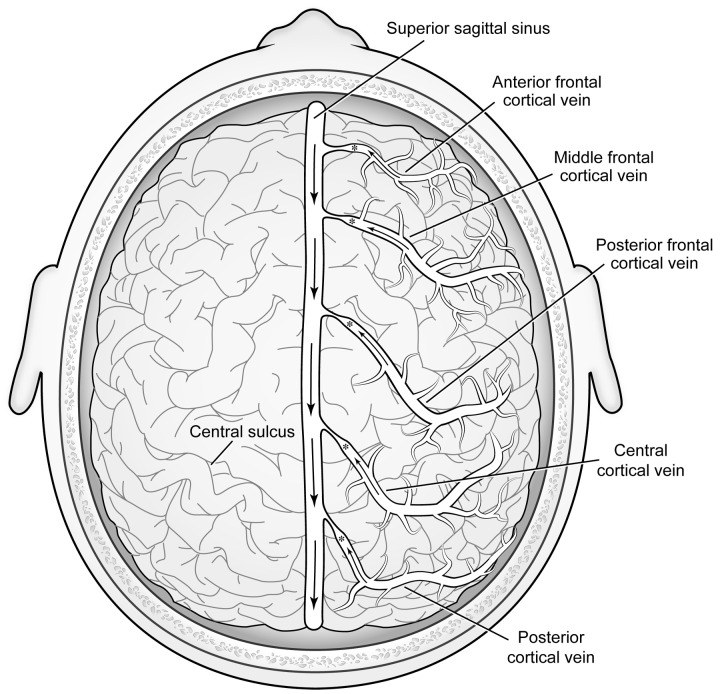
In 1971, Bentson and associates,2 via simultaneous bilateral carotid angiography, identified a relative paucity of functioning superficial cortical bridging veins draining into the dural sinus system as the cause of impeded venous flow (Figure 4). These findings contradicted the prior identification of venous leptomeningeal mass as the cause. The reduced number of bridging veins causes the leptomeninges to expand due to venous blood pooling. In the absence of a patent centrifugal venous blood passageway, cortical drainage must consequently proceed centripetally. The collateral venous development caused by the centripetal drainage is seen on angiography as enlarged, tortuous deep medullary (Figure 5) and subependymal cerebral veins.
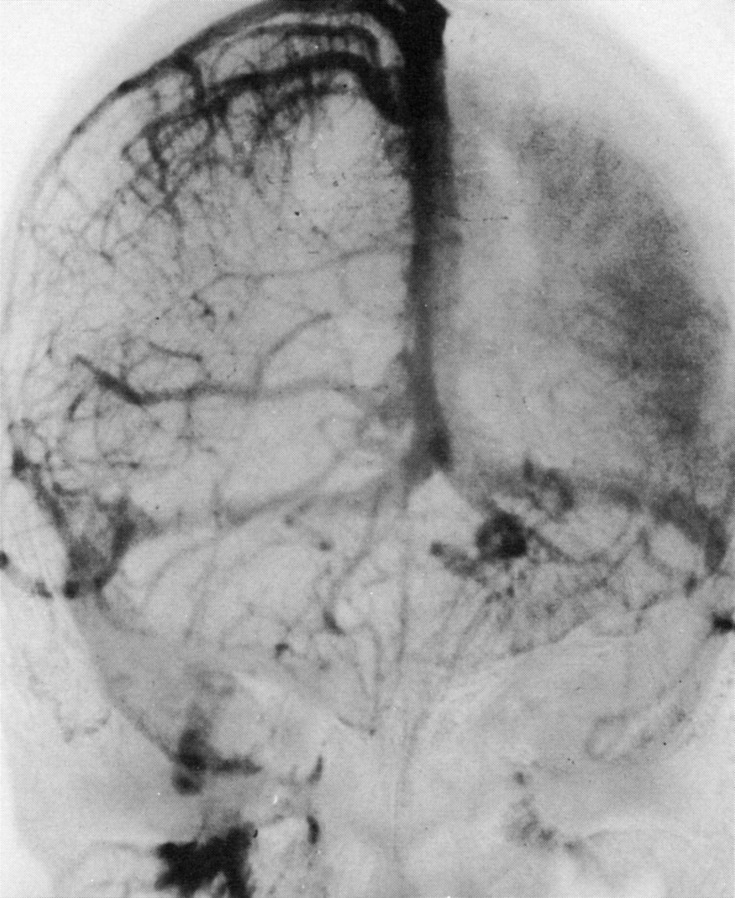
FIGURE 4.
Bilateral simultaneous internal carotid artery injection angiogram, mid-venous phase, anteroposterior projection in patient with Sturge-Weber syndrome. An 8-year-old girl with port-wine stain affecting the left forehead, right facial seizures, and right hemiparesis. The right superficial cerebral veins and the superior sagittal sinus are well filled. A striking lack of cortical veins over the left hemisphere can be noted. A prominent “brain stain” of the left cerebral hemisphere is related to delayed venous drainage. Enlargement of the internal cerebral and basal veins and their tributaries is evident. (Reprinted with permission from the Radiological Society of North America.2)
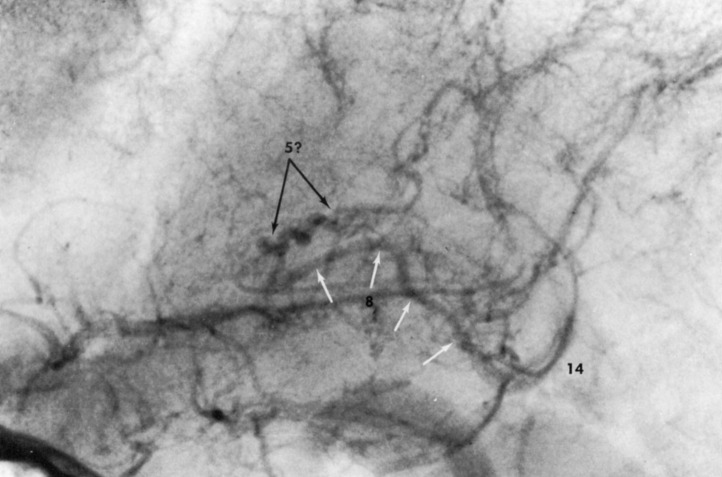
FIGURE 5.
Angiogram of internal cerebral vein in another patient with Sturge-Weber syndrome, lateral projection. The internal cerebral vein (8, white arrows) is dilated and tortuous. A corkscrew-like vein (5?) is seen in the position of the thalamostriate vein, but may represent an abnormally filled superior choroid vein. These changes are secondary to increased flow in the deep venous system when this flow serves as a collateral pathway of drainage for the involved cortical veins. Number 14 represents the great cerebral vein in this example where the deep medullary veins are not visible. (Reprinted with permission from the Radiological Society of North America.2)
The dilated, ectatic veins composing the leptomeningeal mass noted during surgery and on magnetic resonance imaging (MRI), reflect the development of enlarged collateral venous drainage channels.53 These findings are analogous to optociliary collateral vessel formation directly observed within the eye following central retinal vein occlusion. Congenital dysplasia of the central retinal vein results in multiple visible compensatory cilioretinal veins. These cilioretinal veins have no relationship whatsoever to angioma formation.54,55 As with the thickened tomato-catsup ocular choroid above, the leptomeningeal venous mass appears much larger in vivo (ie, 2 to 3 mm on contrast MRI) than when emptied of blood in biopsy specimens or at postmortem examination.51
Neuroimaging discloses a direct proportion between a correspondingly thickened leptomeninges and cerebral choroidal plexus and the lack, or dysplasia, of cortical veins.2,24,51,56–59 However, rather than functioning as a site of angiomatosis,6,11,27,57 the choroidal plexus, with the thin lining of its walls and its sponge-like characteristics, allows for an expansion (Figure 6), reflecting the increased deep venous pressure.24,51,58
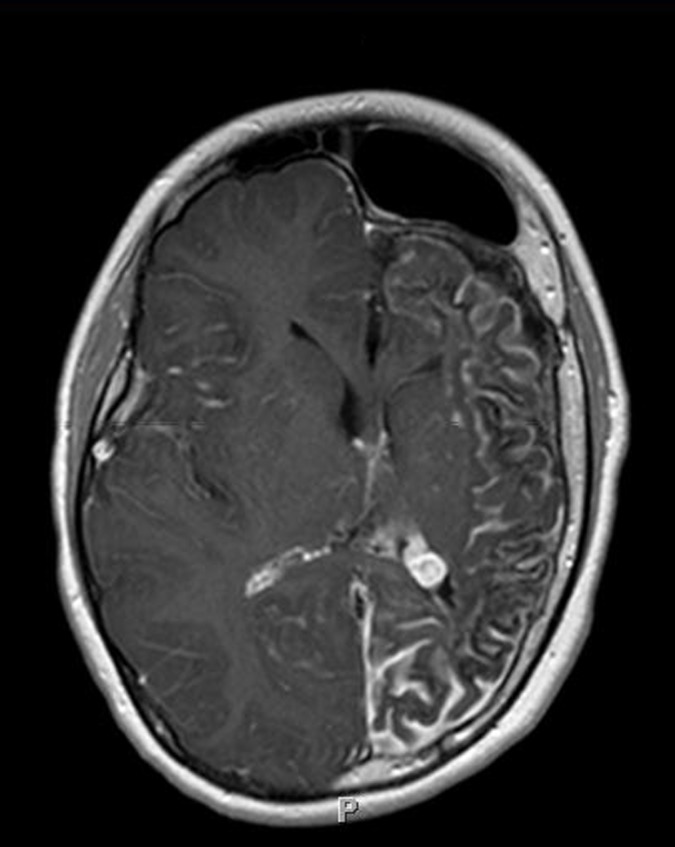
FIGURE 6.
Leptomeningeal enhancement with associated brain atrophy in the Sturge-Weber syndrome. Axial T2-weighted post-gadolinium contrast-enhanced MRI scan of patient with port-wine stain involving the left forehead and upper lid, and dense right homonymous hemianopia (patient 12, Table), reveals marked progressive volume loss involving the grey and white matter of the left cerebral hemisphere most pronounced in the occipital and parietal lobes. The left choroid plexus demonstrates avid contrast enhancement and is much larger compared to the right.
As the deeper veins expand and become more prominent, with centripetal drainage facilitated months and years after birth, surface pial and intradural leptomeningeal venous engorgement may decrease.51 In some cases, this alternative deep drainage pathway for cortical blood51,58 is insufficient, and venous stasis damage and obliteration of vessels,12,13,27,50 with secondarily reduced arterial perfusion causing hypoxia, may occur.56,58–62 Such slowed flow can also lead to arterial thrombi formation.63 Cortical metabolic activity becomes increasingly impaired,61,64 while the increased venous pressure also raises parenchymal pressure. Focal, nonuniform pressure along axons,65–67 in turn, can lead to additional neuronal degeneration and atrophy. Indeed, brain atrophy in various patients has been correlated with the degree of pial enhancement.51 Neuronal death and calcification occur in the middle layers of the cerebral cortex, in the subcortical white matter, and in local blood vessels.11,13,50 Seizures develop. If, on the other hand, extreme metabolic disturbances occur very early in infancy, there may be a complete loss of nerve cells with reactive gliosis and little calcification,27 while the phenomenon of transynaptic degeneration allows for further neuronal loss and reduction of brain volume (Figure 6).
A REEVALUATION OF THE PATHOPHYSIOLOGIC MECHANISM OF STURGE-WEBER SYNDROME
Traditional Proposed Pathophysiologic Mechanism for Sturge-Weber Syndrome: Insult to the Neural Crest
The major signs of the Sturge-Weber syndrome have been considered to be the triad of cephalic port-wine stains, ocular involvement, and leptomeningeal thickening. Concurrent involvement of these involved organs was first reconciled and linked by Cushing and Bailey in 1928.68 They postulated an insult to the neural crest occurring when these three structures are in proximity during the third period of primordial vascular plexus development,69 between the 5th and 8th week of gestation. Some have proposed that a malformation of an embryonic vascular plexus occurs within the prosencephalic and mesencephalic neural crests between the epidermis (neuroectoderm) and the telencephalic vesicle.70 However, since neither skin, eye, nor brain possesses actual proliferative angiomas, this time frame, level of involvement, and locus can be reevaluated.
An Alternative Pathophysiologic Mechanism: An Insult Affecting Brain Cortical Venous Drainage
All veins related to the CNS, including scalp and orbital veins as well as spinal veins, are valveless, permitting bidirectional blood flow, and often emissary in nature.71 Thus, the origin of the cutaneous and ocular abnormalities in the Sturge-Weber syndrome can be traced to a primary insult affecting brain cortical venous drainage.
As already discussed, lack of cortical bridging veins impairs venous flow from cerebral cortex to the dural sinuses and causes venous stasis, pressure elevation, and leptomeningeal thickening. Such elevated dural vein or sinus pressure will also impede the normal drainage of scalp, meningeal, and diploic emissary vein blood into the dural veins and sinuses.71–75 Cutaneous and calvarial bone drainage will also be diminished. Dilation ectasia of both scalp meningeal and diploic emissary veins can occur on account of elevated pressure.75,76 Additionally, the direction of venous flow may reverse, with cortical venous flow exiting through cutaneous channels, eventually draining through the jugular veins. Where remaining bridging cortical veins are still patent with supranormal flow in adjoining areas,77 segments of the dural sinus may have pressures above typical levels, especially distal to where the sinus might also be affected by hypoplasia, atresia, or other forms of relative or total blockage, such as thromboses. Calvarial port-wine stains are simply manifestations of elevated dural vein or sinus pressures, with diminished or reversed cutaneous drainage serving as an alternative route for cortical blood flow.
It is important to note here that when the constellation of cutaneous, ocular, and brain findings seen in Sturge-Weber syndrome are attributed to increased venous pressure, they need not be congenital. Insults to the cerebral veins or sinuses in children and adults76,78,79 can produce similar blood flow patterns and symptoms80 as pointed out by Bentson and associates2 regarding the cerebral findings and noted by others.81,82 If venous sinuses are occluded or involved,83–85 intracranial pressure can also become elevated, producing what was occasionally referred to as a “facial nevi associated with anomalous venous return and hydrocephalus” syndrome. Conversely, an acquired Sturge-Weber syndrome consisting of facial port-wine stains and glaucoma without intracranial involvement86 could occur with occlusion of the orbital veins. Fegeler syndrome, isolated acquired port-wine stains produced by trauma,87–90 results from localized venous occlusion and collateral formation, rather than as a result of the previously hypothesized traumatic nerve damage. Fegeler syndrome may thus be thought of simply as a post-thrombotic syndrome, with endophlebectomy procedures potentially curative.
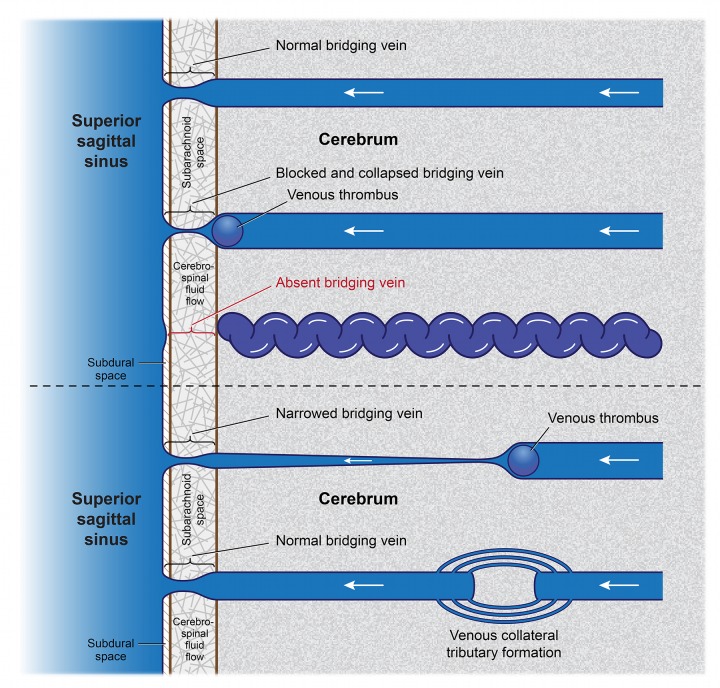
The hypothesis of an embryologic insult, either common to three tissues or organs or occurring within a set moment in embryologic development when these tissues are in proximity, no longer mirrors the findings. Cortical venous or dural sinus dysplasia or venous occlusion occurring any time during or after embryogenesis can secondarily affect these various organs via disruption of normal cephalic hemodynamics. In the mildest cases, there may be leptomeningeal thickening alone, without notable cutaneous findings.80 More extensive involvement from in utero or acquired causes will generate port-wine stains (Figure 7) and, in still more severe instances, symptoms producing the full Sturge-Weber syndrome (Figure 8).
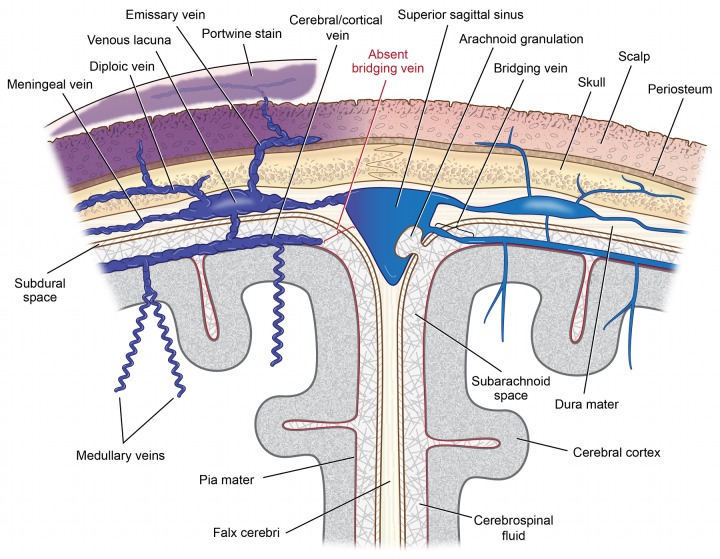
FIGURE 7.
Drainage of blood from superficial cortical veins into the superior sagittal sinus with and without presence of a bridging vein. On the left, absence of the bridging portion of the superficial cerebral/cortical vein crossing the subarachnoid cerebrospinal fluid and subdural space to the superior sagittal sinus leads to impaired drainage causing cyanotic engorgement and enhancement of vessels within the leptomeninges. Redirection of cortical venous blood into the deep venous drainage system occurs via engorged, corkscrew medullary veins. The increased venous pressure reduces arterial perfusion, causing eventual brain atrophy. Higher leptomeningeal venous pressure is also transmitted via variable emissary veins to venous lacunae within the dura mater. This, in turn, reduces venous and lymphatic drainage from the scalp. Such impaired drainage produces visible port-wine stains, with variable lymphedema, as well as hypertrophy of both bone and skin. The right side of the schema depicts normal venous structure and blood flow when bridging veins are present.
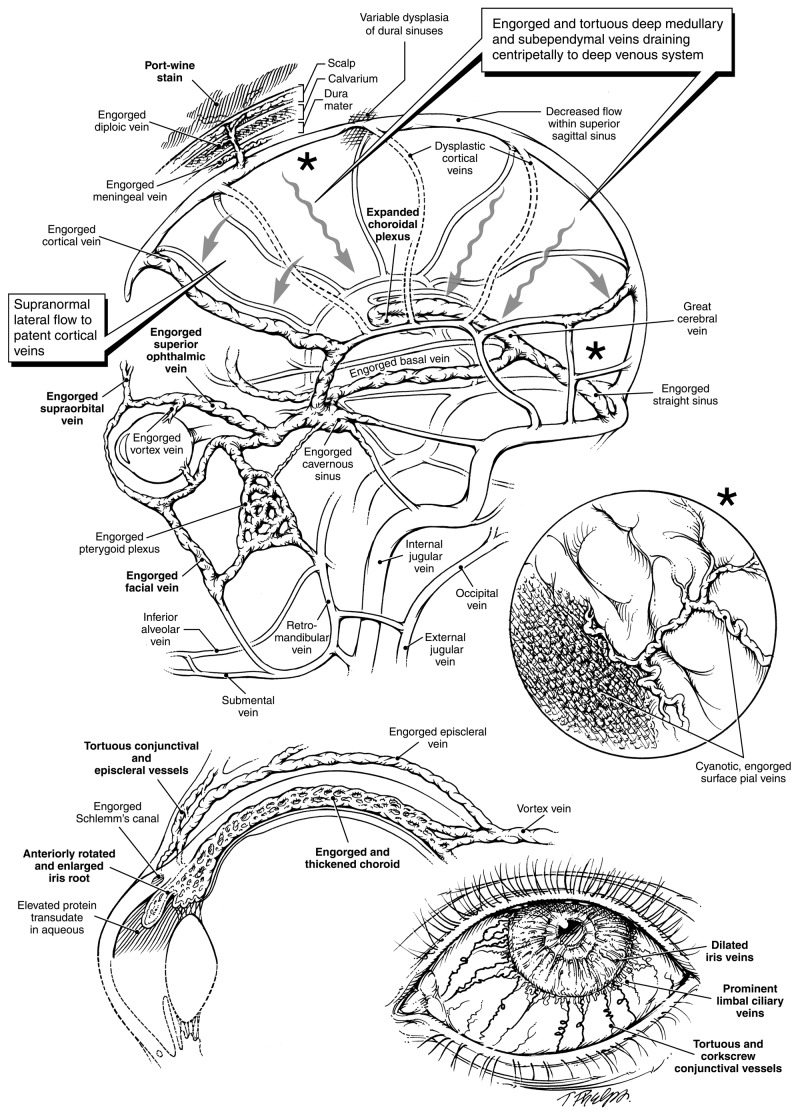
FIGURE 8.
The Sturge-Weber syndrome: dysplasia of cortical veins creates alternative cerebral venous outflow passageways. (Boldfaced letters indicate engorged vessels clinically visible or readily detectable by MRI.) Top left, Superficial cortical veins normally drain blood from brain cortex via bridging segments into the dural sinuses. Absence or dysplasia of these veins will obstruct the flow of venous blood and cause engorgement of surface pial vessels (see insert top right). Rather than draining centrifugally, cortical venous blood will be forced to flow centripetally through deep brain tissue. Remaining cortical veins will conduct supranormal bloodflow. Particularly if there is any dysplasia of the dural venous sinuses, dural sinus pressures may be raised segmentally, also impeding venous drainage from the scalp, consequently also producing port-wine stains visible within the skin. Cortical blood will be forced into the deep venous system via expanded medullary and subependymal veins. The resulting higher pressure in the deep system considerably expands the choroidal plexus, which is linked to the great cerebral vein and the straight sinus, and ultimately joins the cavernous sinus via an engorged basal vein. High cavernous sinus pressures, in turn, impede the normal drainage of orbital and ocular blood, with consequences shown at bottom left and bottom right. Since normal drainage of blood from the face into the orbit is also impeded, this produces the periocular and upper facial port-wine stains characteristic of the Sturge-Weber syndrome. Venous drainage of the mandible occurs via the inferior alveolar and submental veins which do not have any direct connection to the brain. Unless neck veins are also dysplastic, mandibular port-wine stains alone do not occur as part of the syndrome. Middle right insert, Engorgement of surface pial vessels. The obstructed flow of deoxygenated blood with pressure elevation renders a cyanotic appearance to expanded vessels with “angiomatosis” appearance. Bottom left, Restricted outflow from an expansile ocular choroid causes diffuse thickening, much as for the choroidal plexus in the brain. The thickened choroid, in turn, may rotate the iris root forward. In a geometrically smaller eye, this may give the appearance of goniodysgenesis and cause obstruction of the trabecular meshwork and aqueous outflow passages. Elevated intraluminal venous pressures will also cause transudation of protein into the aqueous fluid, which can also block outflow passages. Bottom right, Engorged and tortuous conjunctival and episcleral vessels are often apparent, as may be engorged iris vessels. Corkscrewing venous tortuosity, often visible in older affected individuals, is pathognomonic for an elevated transluminal venous pressure gradient.
PROOF OF CONCEPT SUPPORTED BY NEW DATA
The Proposed Alternative Pathophysiologic Mechanism Accounts for Blood Flow and Venous Findings, Formerly Documented But Not Adequately Explained, and Is Also Now Supported by New Data
As early as 1906, Cushing noted large dural veins present in areas corresponding to cutaneous port-wine stains,13,19 with many of the large dural veins noted to be in emissary communication with the diploë. Obviously, the nature of the cerebral vascular flow abnormalities was not yet recognized; rather than an embryonic malformation, these vascular dilatations represent physiologic adaptations to disease nearby.
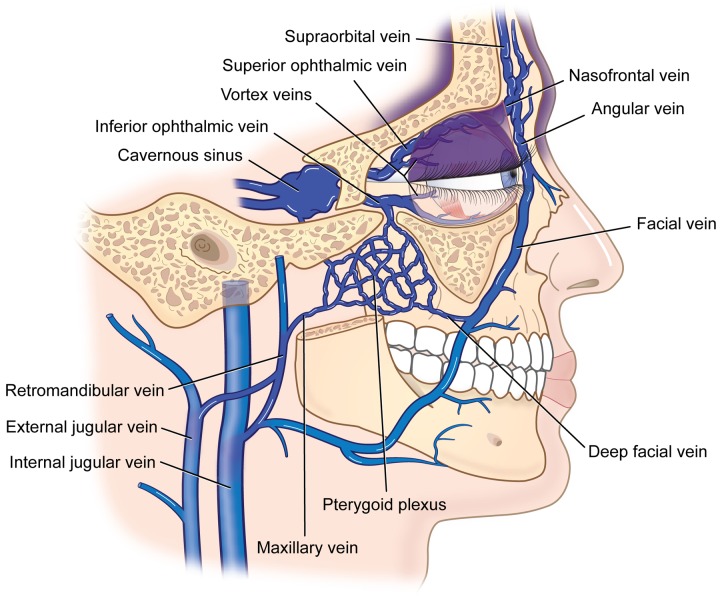
Deeper in the brain, centripetal drainage takes place via deep cerebral veins in communication not only with the choroidal plexus but consequently with the cavernous sinus53 and the straight sinus.60 The resulting increased venous flow to the cavernous sinus reduces and can reverse the pressure gradient that exists from orbital veins to the cavernous sinus. This is particularly true if the sphenoparietal or superior petrosal sinuses are also atretic (with enhancement of the coupled leptomeninges visible on MRI). As previously described by the author (Parsa CF. Sturge-Weber syndrome and the phacomatoses: Odd man found out. Transactions of the 31st North American Neuro-Ophthalmology Society Annual Meeting, Copper Mountain, Colorado, February 17, 2005:339–343),1,10 the reduced flow and increased orbital venous pressure is then transmitted via ophthalmic to facial veins (and eventually to the jugular veins), resulting in orbital and periorbital venous ectasia and upper facial port-wine stains (Figure 9).
FIGURE 9.
Periocular port-wine stain development and distribution. Cavernous sinus venous hypertension transmitted to the superior ophthalmic vein and its anastomoses with the nasofrontal and angular veins will cause port-wine stain involvement of the upper eyelid. Since the inferior ophthalmic vein, however, also drains into the pterygoid plexus proximal to the globe, venous pressure in the inferior ophthalmic vein distal to this juncture is much reduced, and port-wine stain involvement of the lower eyelid is less common.
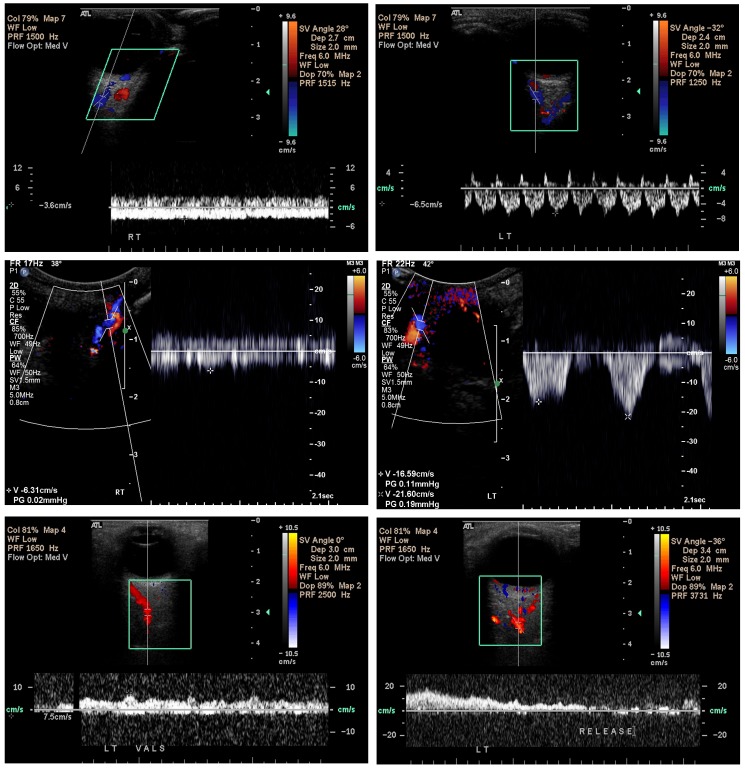
Orbital Doppler scans (Figure 10) confirm the slowed and occasionally reversed venous drainage within affected orbits both in patients with isolated periorbital port-wine stains (Patients 3 and 7, Table) and in those with the Sturge-Weber syndrome (Patients 1, 10, 18, and 20, Table). Reduced pulsatility of orbital venous blood flow attests to increased intraluminal venous pressure, as does the enlarged caliber of the veins. In 1957, Wohlwill11 had also noted enlarged orbital veins in the Sturge-Weber syndrome. In what was thought to be an atypical Sturge-Weber syndrome patient, Yallapragada and colleagues91 recently described reversed flow on MRI. Other investigators have reported similar findings of reversed blood flow secondary to increased intracranial venous pressure with dural carotid-cavernous sinus fistulas92 as well as in achondroplastic dwarfs.93
FIGURE 10.
Orbital Doppler ultrasonography in the Sturge-Weber syndrome. Scans confirm the slowed and occasionally reversed venous drainage within affected orbits both in patients with isolated facial port-wine stains and in those with the Sturge-Weber syndrome. The color red is assigned to blood flowing away from the L 12-5 transducer (assumed by the device to be arterial), and blue is assigned to blood flowing toward the transducer (assumed by the device to be venous). Top left, blood flow is measured by the cursor of the Doppler ultrasound device (Phillips 5000, Bothell, Washington) placed over the superior ophthalmic vein of the affected right eye and is found to be −3.6 cm/sec, compared to −6.5 cm/sec for the unaffected left eye (top right). The flat waveform for the right eye, moreover, indicates elevated intraluminal pressure preventing a normal pulsatility as noted in the left eye (top right) produced by variations in orbital pressure caused by increased arterial blood flow during systole. Similar findings are noted in other patients (middle left) with nonpulsatile superior ophthalmic vein flow of −6.31 cm/sec in the affected right orbit, but highly pulsatile with peak flow velocities up to −21.6 cm/sec in the unaffected left orbit (middle right). Bottom (patient 20, Table, also seen in Figures 2 and 16), blood flow within the left superior ophthalmic vein could not initially be detected. Once the patient performed a Valsalva maneuver, however, the blood flow within the vessel suddenly became apparent (bottom left), but with blood flowing away from the cavernous sinus toward the face, with a velocity of +7.5 cm/sec and erroneously encoded as arterial (red) by the device (anterograde venous blood flow). After the patient released her breath (bottom right), venous blood flow quickly ceased and could no longer be visualized, with neither red nor blue coding assigned, indicating venous stasis. (Captured images courtesy of M. Robert De Jong, RDCS, RDMS, RVT, The Johns Hopkins Hospital, Baltimore, MD.)
TABLE.
COLLECTED DATA FROM 20 PATIENTS WITH PORT-WINE STAINS OR THE STURGE-WEBER SYNDROME
| Pt |
AGE (YEARS) |
PORT-WINE STAIN CONJUNCTIVA |
EYE | DROPS |
REFRACTIVE ERROR (AXIAL LENGTH [mm]) |
CCT [μm] (DIAMETER Ø [mm]) |
IOP (mm Hg)h |
C/D |
FUNDUS CHOROID BY US |
ORBITAL ULTRASOUND (DOPPLER WHEN AVAILABLE) |
MRI (SEIZURES) |
VISUAL FIELD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.9 | OD | - | −1.50 +1.00 × 120 | 561 | 9 | 0.2 | Normal 1 mm thick |
Normal (Doppler: SOV pulsatile) | Left LH, atrophy & enlarged choroidal plexus (Szs) | RHH | |
| Entire left face & parietal area (fading since birth) Injected conjunctiva |
OS | + | −1.50 +1.00 × 60 | 611 | 14 | 0.35 | “Tomato-catsup” with slightly tortuous veins 1.3 mm thick |
Orbit congested (Doppler: SOV nonpulsatile; high resistance to arterial flow) | ||||
| 2 | 1.3 | OD | - | +1.50 | 548 | 14 | 0.1 | Normal | Normal | Normal at age 5 months & 5 | Full | |
| Left forehead & upper lid nasally | OS | - | +1.50 | 545 | 16 | 0.15 | Normal | Normal | years (Ø Szs) | |||
| 3 | 2 | Upper & lower lid, mild | OD | - | +0.50 (AL =21.54) | 520 | 13 | 0.2 | Trace “tomato-catsup” 1.2 mm thick |
Orbit congested (Doppler: SOV flow 11 cm/sec; nonpulsatile) | No MRI (Ø Szs) | N/A |
| OS | - | +0.50 (AL =21.74) | 504 | 10 | 0.1 | Normal 1 mm thick |
(Doppler: SOV flow19 cm/sec; pulsatile) | |||||
| 4 | 2.3 | OD | - | −2.50 +2.50 × 90 | 592 (Ø = 12) | 17 | 0.25 | Normal 1 mm thick |
N/A | No MRI (Ø Szs) | Full | |
| Left face (fading since birth) Diffusely injected conjunctiva |
OS | + | −3.50 +3.00 × 90 | 620 (Ø = 12.4) | 22 | 0.55 | Slight “tomato-catsup” 1.5 mm thick, compressible to 1.3 mm |
|||||
| 5 | 2.5 | OD | - | +1.00 | 577 | 18 | 0.1 | Normal | Normal | Left fronto-parietal LH (Szs) | Full | |
| Upper nasal lid & minimal lower lid Conjunctiva normal |
OS | - | +1.00 | 581 | 20 | 0.1 | Normal | Normal | ||||
| 6 | 2.7 | OD | - | +1.50 | 548 | 14 | 0 | Normal | Normal | MRI normal (Ø Szs) | Full | |
| Fading, left frontal & parietal, trace upper lid; seen in cold weather & when crying | OS | - | +1.50 | 549 | 15 | 0 | Normal | Normal | ||||
| 7 | 3 | Right face with both lids nearly fully affected | OD | - | +0.75 | 548 | 14 | 0.1 | Possible trace “tomato-catsup” | Grossly normal, but limited cooperation (Doppler: SOV flow 17.3 cm/sec; nonpulsatile) | No MRI (Ø Szs) | Full |
| OS | - | +0.75 | 550 | 13 | 0.1 | Normal | Grossly normal, but limited cooperation (Doppler: SOV flow 18.2 cm/sec; pulsatile) | |||||
| 8 | 3.3 | Entire right face (spontaneous resolution by age 3) Injected conjunctiva |
OD | + | −0.25 +1.00 × 171 (AL = 23.17) | 609 (Ø OD>OS) Haab’s striae |
31 | 0.4 | Thickening OD>OS | Orbit congested | Bilateral frontal lobe LH, R>L (Szs ceased as port-wine stains faded) | Full |
| Lateral aspect of lid (essentially absent by age 3) | OS | - | +2.00 (AL = 21.65) | 595 | 21 | 0.1 | Thickening OS<OD | Normal | ||||
| 9 | 5 | OD | - | Plano +2.50 × 101 | 587 | 16 | 0 | Normal | Grossly normal with limited cooperation | Left occipital LH (One Sz) | RHH | |
| Faint, left face, affecting upper lid | OS | - | Plano +2.75 × 85 | 629 | 20 | 0.1 | Possible “tomato-catsup” | Grossly normal with limited cooperation | ||||
| 10 | 5 | Upper & lower lid, mild Conjunctival vessel tortuosity |
OD | + | +3.00 +2.50 × 90 | 603 (Ø = 11.9) | 28 | 0.6 | “Tomato-catsup” Thick choroid (RD later) |
Orbit congested (Doppler: SOV flow 6 cm/sec; nonpulsatile) | Hypoplastic right transverse sinus & jugular vein Occipitoparietal LH R>L & marked atrophy R>>L |
Possible LHH (difficult exam) |
| Partial upper lid only | OS | + | +2.75 +1.25 × 90 | 551 (Ø = 11.6) | 11 | 0.1 | Normal | Normal (Doppler: SOV flow 20 cm/sec; pulsatile) | GH deficiency | |||
| 11 | 6 | OD | - | +2.00 | 494 | 16 | 0.3 | Normal | Normal | Left LH (Szs) | Full | |
| Upper & lower lid | OS | + | +1.00 | 488 Fluorescein dye + |
21 | 0.5 | “Tomato-catsup” Mild choroidal thickening |
Orbit congested | ||||
| 12 | 9.6 | OD | - | −2.00 + 1.00 × 180 | 581 | 10 | 0.1 | Normal | N/A | Left occipital & parietal LH with atrophy & enlarged choroidal plexus | Dense RHH | |
| Left forehead & upper lid; hypertrophy | OS | + Trab age 4 |
−1.75 | 600 | 14 | 0.4 | “Tomato-catsup” Thickened choroid |
|||||
| 13 | 10.8 | OD | - | +1.00 | 533 | 17 | 0.1 | Normal | N/A | Left occipital lobe LH | Full | |
| Entire left face Injected conjunctiva |
OS | - | +2.75 +2.75 × 180 | 581 | 23 | 0.5 | “Tomato-catsup” | No AVM by carotid angiography (Szs) | ||||
| 14 | 18 | None (and never) | OD | - | −0.75 | 572 | 17 | 0.1 | Normal | N/A | Left frontal & parietal lobe LH (Sz age 13) | Full on repeated testing |
| OS | - | +0.25 | 569 | 17 | 0.1 | Normal | ||||||
| 15 | 19 | Right side of head & upper lid Corkscrewing conjunctival vessels Decreased corneal sensation |
OD | - | -0.50 | 555 | 17 | 0.3 | Thickened choroid | Orbit congested | Diffuse, right LH & enlarged choroidal plexus No stenosis of brain vasculature by CT |
LHH, denser inferiorly |
| OS | - | −1.50 | 535 | 11 | 0.1 | Normal | Normal | |||||
| 16 | 20 | Much of right face; hypertrophy | OD | + | Plano | 646 | 29 | 0.6 | Red “tomato-catsup” | Orbit congested | Normal MRI (Ø Szs) | Infero-nasal step OD |
| Corkscrewing conjunctival vessels Darker iris |
Thickened choroid | |||||||||||
| OS | - | Plano | 607 | 19 | 0.2 | Normal | Normal | Full OS | ||||
| 17 | 20 | Right lower face; hypertrophy | OD | + | −0.50 + 0.75 × 145 | 570 | 17 | 0.8 | “Tomato-catsup” OD<<OS | Orbit congested | (Ø Szs) | Full to counting fingers |
| Corkscrewing conjunctival vessels | PAS temporally | Thickened choroid | OD<OS | |||||||||
| 2 trabs Cryo | Slow choroidal filling with IVFA | |||||||||||
| Conjunctival bleb superiorly | ||||||||||||
| Entire left face & left parietal & occipital; hypertrophy | OS | + | −0.50 +1.00 × 35 | 560 | 18 | 0.8 | “Tomato-catsup” OS>>OD | Orbit congested | ||||
| PAS nasally | Retinal vein tortuosity OS>OD | OS>OD | ||||||||||
| Corkscrewing conjunctival vessels | 2 trabs | Thickened choroid | ||||||||||
| Macular folds with fluorescein dye leakage OS | ||||||||||||
| Inf chemosis | ||||||||||||
| Sup conjunctival bleb | Slow choroidal filling with IVFA | |||||||||||
| 18 | 25 | Blue iris | OD | - | −0.50 +2.00 × 156 | 529 | 18 | 0 | Normal | Normal (Doppler: pulsatile) | Left temporo- occipital LH with atrophy GH deficiency (Szs) | Dense RHH |
| Upper left side of face & lid, trace lower lid nasally; hypertrophy Corkscrewing conjunctival vessels & boxcarring Inf chemosis Hazel iris |
OS | - | +0.25 +4.25 × 4 | 578 | 19 | 0 | “Tomato-catsup” Thickened choroid |
Orbit congested (Doppler: nonpulsatile) | ||||
| 19 | 37 | OD | - | Plano | 575 | 14 | 0.2 | Normal | N/A | Left parieto-occipital LH (Szs) | RHH, denser inferiorly | |
| Left face, upper lid & nasal edge of lower lid; hypertrophy Corkscrewing conjunctival vessels |
OS | - | Plano | 622 | 18 | 0.25 | Trace “tomato-catsup” | |||||
| 20 | 45 | OD | - | +1.75 +0.25 × 85 | 544 | 13 | 0.1 | Normal | Normal | No LH, no atrophy; small left frontal transmedullary vein in left frontal centrum semiovale | Normal OD | |
| Entire left face; hypertrophy | OS | + | +5.25 +2.25 × 101 | 559 | 31 | 0.5 | “Tomato-catsup” | Orbit congested | Nasal field loss OS | |||
| Corkscrewing conjunctival vessels | Thickened choroid Macular folds developed once pressure normalized via surgery | (Doppler: SOV enlarged; nonpulsatile venous flow stasis & slowed arterial flow) | No AVM by carotid angiography (Ø Szs) | |||||||||
| Tenon hypertrophy noted during surgery |
Transient reversal of emissary venous blood flow occurs in normal individuals as a brain cooling mechanism94 and in response to Valsalva maneuvers resulting in facial flushing.95 Such maneuvers, including external compression of the jugular vein, can accentuate the visibility of extant port-wine stains.19,96 However, when cephalic circulation is completely and uniformly affected (such as in achondroplasia, pulmonary arterial hypertension, or the tetralogy of Fallot), effects associated with focal or local venous hypertension—including escape collaterals, which port-wine stains represent—may not develop. In achondroplasia, the bilateral and sustained high dural venous pressure may result instead in megalencephaly. One also notes elevation of intracranial pressure along with epicranial edema due to the generalized diminished return of cephalic venous blood flow to the heart from the absence of gravitational assist during space travel.97,98
Cutaneous Findings, Including New Data, More Adequately Explained by the New Pathophysiologic Mechanism
The persistence of the orbital emissary veins accounts for the high frequency of facial port-wine stains in the Sturge-Weber syndrome (all except Patient 14, Table). The vast majority of port-wine stains occur in the cephalic area99,100 followed by the lumbosacral area,11,101 where dependent emissary veins are also present. The myriad patterns of cephalic port-wine stains described by Kautzky,21 and attributed to metameric units of involvement in relation to the three sensory branches of the trigeminal nerve supplying innervation of the mesodermal derivates of the corresponding branchial arches,11,21 indeed correlate far better with the anatomy of brain sinus drainage passageways instead (Figures 7 through 9).
The preference for venous correlation can be seen throughout eyelid and other cephalic locations. In upper eyelid involvement, cavernous sinus and superior ophthalmic vein hypertension are indicated, not involvement of the first branch of the trigeminal nerve (V1) or hypothesized associated parasympathetic nerves. Lower eyelid and midfacial involvement indicates sufficient impedance of blood flow from the orbit via the inferior ophthalmic vein to involve the pterygoid plexus and from there, the deep facial vein—not involvement of the second trigeminal nerve branch (V2) or hypothesized associated parasympathetic nerves (Figure 9). When both upper and lower lids are affected, orbital pressure is obligatorily elevated (Parsa CF. Sturge-Weber syndrome and the phacomatoses: odd man found out. Transactions of the 31st North American Neuro-Ophthalmology Society Annual Meeting, Colorado, February 17, 2005:339–343) as is the intraocular pressure. Other cephalic port-wine stain locations depend on nearby dural sinus pressures and the varying persistence of emissary veins. Any increase of intracranial pressure or an increase of pressure within the dural sinuses causes reversal of flow within the emissary veins, which causes prominence of the scalp veins.73
It is important to note that the trigeminal neuronal dysfunction hypothesis19,21 would suggest the potential for exclusive involvement of the mandible by port-wine stains to correspond to an involvement of the third trigeminal nerve branch (V3); this is not seen in the Sturge-Weber syndrome. In fact, mandibular port-wine stains in Sturge-Weber syndrome are noted only with an associated maxillary area affected, often including most of the face and part of the neck. Once again, noting venous drainage patterns (Figures 8 and 9), rather than forcing an overfunction of parasympathetic nerves, better explains clinical findings in Sturge-Weber. The submental and alveolar veins, both tributaries of the retromandibular vein, drain the mandible. The presence of a mandibular port-wine stain thus implicates venous dysplasia downstream, in the area of the retromandibular vein by the ramus, which also affects venous drainage of the maxillary area. A port-wine stain affecting the mandible alone could develop with focal dysplasia affecting alveolar or submental veins before entry into the submandibular vein, but this would not produce any intracranial or ocular involvement to constitute the Sturge-Weber syndrome.
Ocular Findings, Including New Data, More Adequately Explained by the New Pathophysiologic Mechanism
Decreased orbital venous flow necessarily also reduces vortex vein drainage, causing dilation and expansion of the ocular choroid and choriocapillaris.102 Choroidal expansion (Patients 1, 3, 4, 8–13,15–20, Table), sometimes better appreciated by ultrasonography (Figures 11 and 12) than by ophthalmoscopy, gives rise to the “tomato-catsup” fundus appearance and correlates with the degree of both cerebral choroidal plexus expansion and leptomeningeal expansion.103 Since vortex vein and choroidal pressures are elevated, the propensity for expulsive hemorrhage during decompressive surgery in the Sturge-Weber syndrome is high. Anterior ciliary and conjunctival venous pressure is also high, so vessels become not only dilated but tortuous, occasionally displaying corkscrew turns, as is especially notable in older patients (Figure 13; Patients 15–20, Table). Such findings are also seen in pulmonary arterial hypertension,104 tetralogy of Fallot,42,44,83,104 cloverleaf skull syndrome,42,45 carotid-cavernous sinus fistula, and cavernous sinus thrombosis46 and are pathognomonic for elevation of venous pressure. Choroidal expansion also occurs in the so-called microgravity environment of space97 and more commonly is elicited during head-down positioning.105
FIGURE 11.
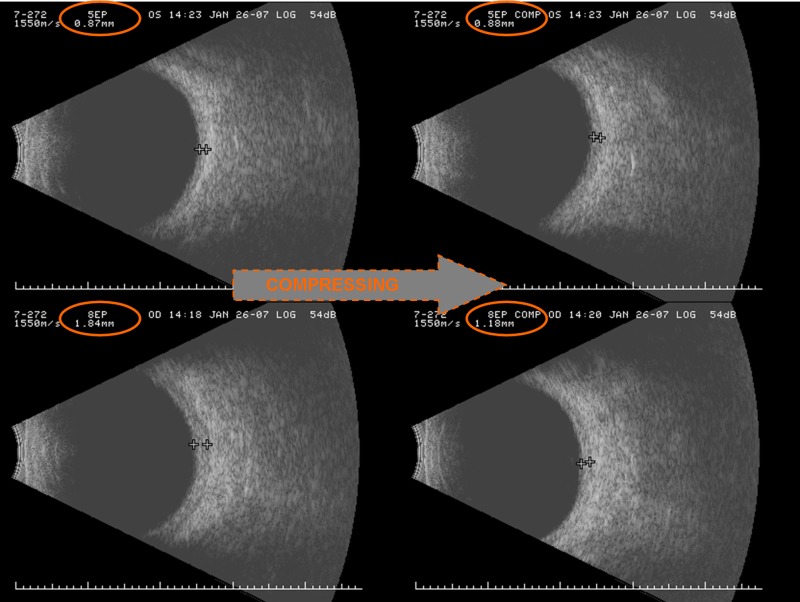
B-scan ultrasonography of unaffected and affected eyes in a patient with Sturge-Weber syndrome. The retinal-choroidal complex in the unaffected left eye (top) measures a normal 0.87 mm in thickness, which remains unchanged by digital compression of the globe. In the affected right eye (bottom), the retinal-choroidal complex is markedly thickened to 1.84 mm. It is also compressible to a thickness of only 1.18 mm by the application of simple digital pressure to the globe, indicating the expansion to be due to filling by blood, and not by proliferation of solid tissue. (Captured images and photomontage courtesy of Maria Bernadete Ayres, MD, The Johns Hopkins Hospital, Baltimore, MD.)
FIGURE 12.
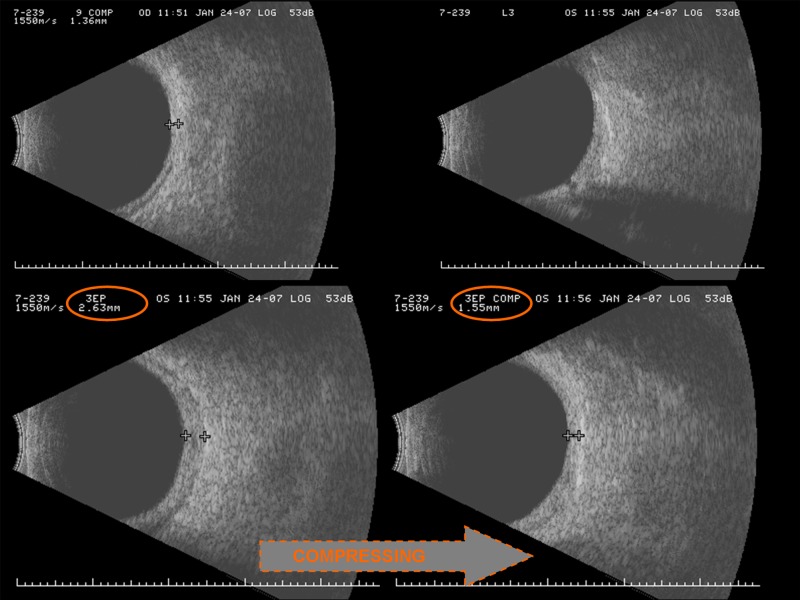
B-scan ultrasonography of unaffected and affected eyes in another patient with the Sturge-Weber syndrome. The normal right eye (top left) has an incompressible retinal-choroidal complex measuring 1.36 mm in thickness, but which is nearly twice as thick at 2.63 mm in the affected left eye (bottom left), and compresses easily to 1.55 mm by the application of simple digital pressure to the globe (bottom right). (Captured images and photomontage courtesy of Maria Bernadete Ayres, MD, The Johns Hopkins Hospital, Baltimore, MD.)
FIGURE 13.
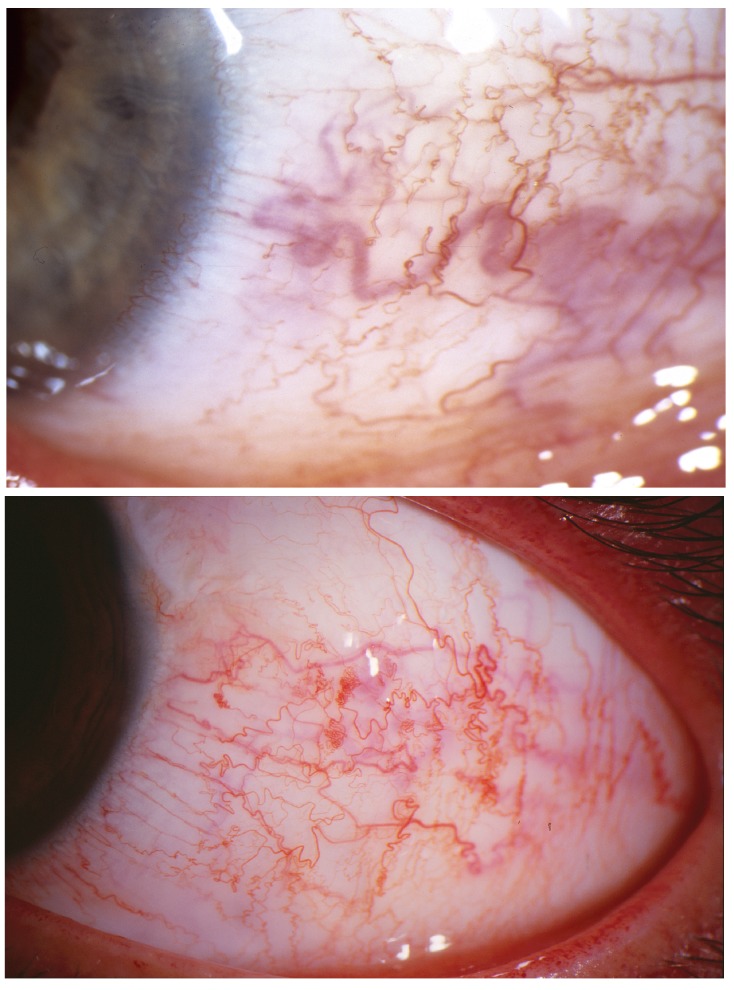
Conjunctival findings in patients with the Sturge-Weber syndrome. Dilatation and tortuosity of conjunctival and episcleral vessels become increasingly evident over time. Top (patient 18, Table), a background episcleral blue hue is evident in the left eye of 20-year-old. Conjunctival vessel tortuosity reveals frank corkscrewing, a sign pathognomonic for elevated transluminal venous pressure gradient. Slowed venous blood flow is also evidenced by boxcarring phenomenon noted on slit-lamp examination. Slight chemosis is present inferiorly, indicative of venous transudate taxing lymphatic drainage capacities. Bottom (patient 17, Table), Similar tell-tale corkscrewing of conjunctival vessels in another affected young adult with the Sturge-Weber syndrome and severe glaucoma.
Slowed venous blood flow is also evidenced by boxcarring phenomenon within the conjunctival vessels noted on slit-lamp examination. Patients with a variety of cerebral venous drainage anomalies, including carotid-cavernous sinus fistulas,83,85 or dural sinus atresia,84,85 may also develop “facial nevi”83 with similar conjunctival and choroidal findings.47 Retinal vein tortuosity and even retinal venous collaterals may develop in the fundus106 in the Sturge-Weber syndrome (Patient 17, Table), also with anastomoses107,108 if venous pressure differentials exist across the globe within the orbit. As opposed to exposed conjunctiva or orbital tissues, the elevated intraocular pressure that develops within the relatively rigid scleral walls of a closed ocular system counteracts the transluminal vascular pressure gradient and reduces the degree of retinal vessel tortuosity that one might otherwise expect from elevated retinal venous pressures alone.
Cerebral Findings Including Frequent Occipital Lobe Involvement, Associated Hemianopsias, and New Data, More Adequately Explained by the New Pathophysiologic Mechanism
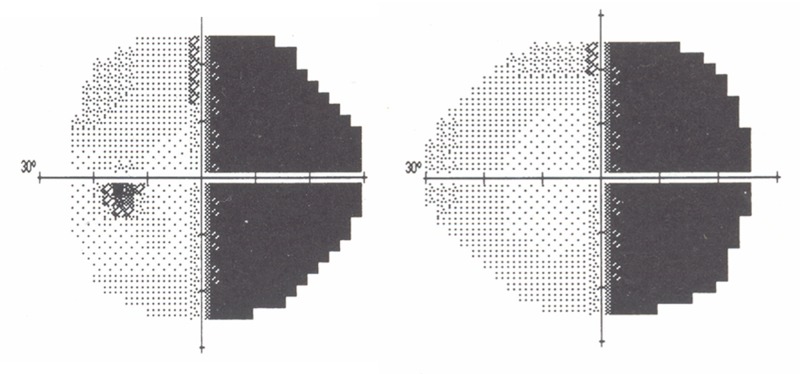
The persistence of orbital emissary veins explains the ocular and cutaneous findings discussed above. However, much of the increase in deep cerebral venous drainage is also routed posteriorly via the straight sinus toward the confluence of sinuses helping to explain, in part, the regularity of hemispheric occipital lobe involvement (Patients 1, 5, 9, 12, 13, 15, 18, 19, Table). Increased intraluminal pressure within the straight sinus can compromise venous drainage from surrounding neural tissue, particularly in the hemisphere already affected by reduced cortical venous drainage. This will, in turn, reduce cortical perfusion within the calcarine fissure, contributing to frequent hemianopic visual field defects (Figure 14; Patients 1, 9, 10, 12, 15, 18, 19, Table). More important, however—for reasons discussed later—associated leptomeningeal enhancement is also found more frequently in the posterior cortex than elsewhere in the brain.109
FIGURE 14.
Dense right hemianopic visual field defect in patient with left-sided Sturge-Weber syndrome. Patient with upper left facial port-wine stains and left occipital and parietal leptomeningeal enhancement noted on MRI (patient 18, Table 1). Much of the compensatory increase in deep cerebral venous drainage is routed posteriorly via the straight sinus toward the confluence of sinuses. The occipital lobe and visual cortex are more frequently overwhelmed by effects of transmitted venous hypertension with ensuing visual field defects (patients 1, 9, 10, 12, 15, 18 and 19, Table 1). Due to the increasing countercurrent drainage of the more posterior cerebral veins into the sagittal sinus (see Figure 19) increasing venous stasis, thrombosis with venous dysplasia is likely to be more common in veins subserving the posterior cortex.
Inconsistent Neurological Deterioration More Adequately Explained by the New Pathophysiologic Mechanism
Despite venous impedance, breakdown of the blood-brain barrier with vasogenic edema and abnormal parenchymal enhancement on MR imaging may not always occur with venous occlusion.110,111 Moreover, a measure of brain swelling can persist for years without producing abnormal T2-weighted images.112 When the “tipping point” of elevated venous pressure is reached, however, it begins to cause vessel degeneration. This degeneration, as part of a vicious cycle, engenders further elevation of pressure and further venous deterioration.111–113 Such effects are noted with chronic venous insufficiency in the skin,114 where the number of perfused capillaries may still remain normal in mild chronic venous insufficiency but is reduced via obliterations and thrombosis with more severe insufficiency.115 The existence of such a tipping point or threshold level for venous integrity explains why some individuals with the Sturge-Weber syndrome remain stable from a neurological standpoint, whereas others deteriorate. It should be noted that blood flow to the brain must increase as a response to increasing metabolic demands, particularly during the first few months of life during myelination and the establishment of higher cortical functions,60 and that this may exacerbate backup of venous flow. Hence the appreciation of leptomeningeal enhancement via MRI often peaks at 6 to 8 months, prior to the adoption of the sitting position which, via gravitational assist, improves cephalic venous drainage. With elevations of venous pressure, perfusion will also become relatively diminished. Finally, should seizures begin, they will increase the demand for oxygen and glucose, precipitating further brain damage.
Findings Neglected by Earlier Mechanism Are the Result of Impeded Venous Flow, Demonstrated by New Data: Tissue Hypertrophy
While not always commented upon, progressive thickening of calvarial bones (Figure 15), understood by some investigators as a compensatory reaction to brain atrophy,63 and associated soft tissue hypertrophy (Figure 16 ) are often features of the Sturge-Weber syndrome. Although diploic enlargement of calvarial bone accompanied by compensatory enlargement of the bony sinuses can occur secondary to brain atrophy as a result of arterial insufficiency, as in the Dyke-Davidoff-Masson syndrome, in such instances it is unaccompanied by any changes in the scalp—a central feature of the Sturge-Weber syndrome. Focal venous hypertension itself explains this process. Though normally seen only in venous capillaries in areas with high metabolic tissue demands, bridged fenestrations can be detected via electron microscopy also in the postcapillary venules in the Sturge-Weber syndrome.116 The effects of high intraluminal pressure can account for the development of such widespread venular fenestrations. The resulting increased venous and capillary transudate, in turn, allows for an increase in the availability of plasma-borne nutrients and growth factors causing hypertrophy of muscle and other soft tissues in addition to bone.
FIGURE 15.
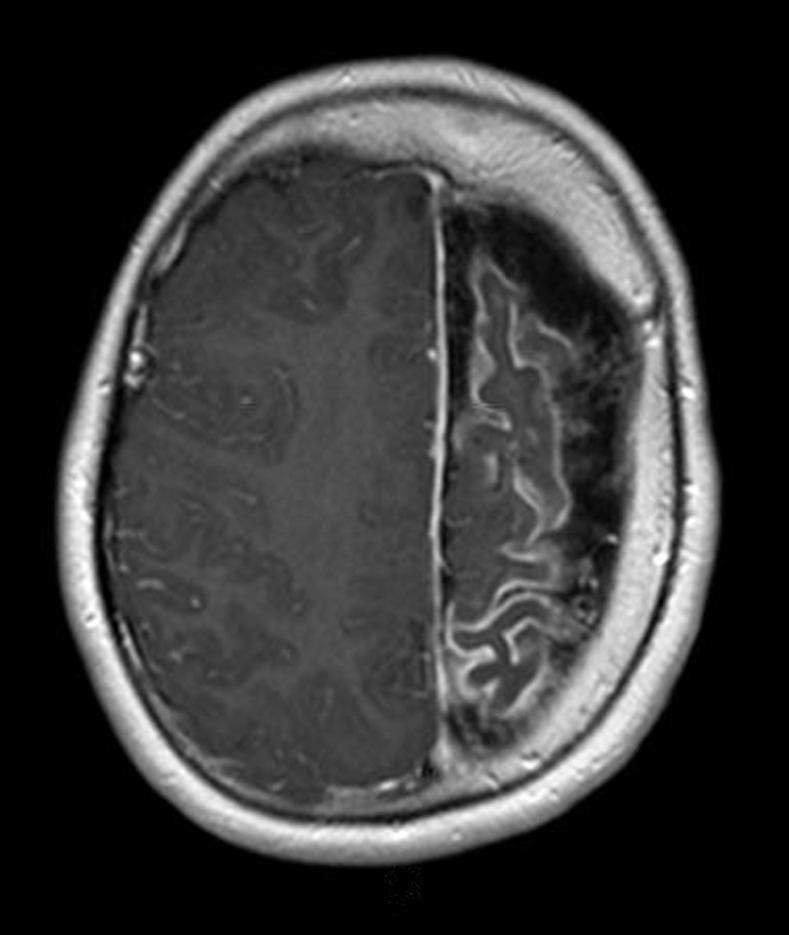
Leptomeningeal enhancement with associated brain atrophy and calvarial bone thickening in the Sturge-Weber syndrome. Axial T2-weighted post-gadolinium contrast-enhanced scans of patient 12, Table again reveals marked left cerebral atrophy (see Figure 6). While the left hemicranium is asymmetrically smaller than the right, left calvarial hypertrophy is also noted in correspondence with the thickened leptomeninges.
FIGURE 16.
Port-wine stain tissue hypertrophy in the Sturge-Weber syndrome associated with elevated venous pressure. Facial port-wine stain with progressive tissue hypertrophy (patient 20, Table) and glaucoma (see Figure 2). Although spontaneous fading is often noted in the first years of life, port-wine stains that persist thereafter often thicken with significant hypertrophy possible. Carotid angiography ruled out any arteriovenous shunt or feeder vessel to the face or lip. High-resolution MRI did not detect any leptomeningeal enhancement or brain atrophy in this woman without seizures, but showed a small left frontal linear transmedullary vein in the centrum semi-ovale. Doppler orbital ultrasonography revealed lack of orbital venous drainage to the cavernous sinus, with instead anterograde flow, draining from brain to periorbital skin, during Valsalva maneuvers (see Figure 10 bottom, left and right). (Photograph and permission to print provided courtesy of patient Dolores Reaves.)
Hence, accelerated brain myelination in affected brain in those with the Sturge-Weber syndrome117 is also explained. Any venous constriction, whether it be from the Sturge-Weber syndrome or other cause, has been noted to cause focal accelerated myelination.118 The proposed pathophysiological mechanism helps to thus clarify physiological processes, heretofore unexplained,119 by which normal muscle and tissues, as a rule, undergo hypertrophy following forceful muscle contractions and isometric exercise; such activities physiologically increase venous pressures to produce plasma transudate, allowing for increased metabolic activity within the tissues permeated. Normally functioning lymphatics prevent extracellular fluid accumulation, which in other diseases would diminish the diffusion of nutrients to cells and instead allow for tissue atrophy. It should be noted that impaired venous blood flow alone will elevate interstitial fluid pressure to slightly higher levels,120–123 particularly if in the skin or soft tissues there is lymphatic drainage that later becomes impaired.120–124 There is no need, however, to invoke or postulate neural mechanisms for tissue hypertrophy either under physiologic conditions or in the Sturge-Weber and other related syndromes. Identifying variably present mutant alleles within port-wine stains or leptomeningeal masses as putative causative somatic mutations cannot be reconciled with the fact that the same mutant alleles are also found more frequently in the general population within germline DNA from blood cells.125 It is instead more plausible that pathologically increased capillary and venous transudate and tissue metabolism initiated focally in utero has the effect of increasing frequency of mutant alleles within those tissues. This may be verified via comparison studies of port-wine stains produced by well-defined arteriovenous fistulas, such as in the Parkes Weber syndrome.
To better evaluate the hypothesis of tissue hypertrophy as a result of increased capillary and venous transudate, the unique properties of corneal tissue were utilized. As an avascular, transparent, noncontractile and immobile tissue receiving tissue receiving nutrients via capillary and venous transudate (aqueous humor), the cornea affords unique opportunities to examine and test the proposed pathophysiologic mechanism in the absence of other confounding elements. Central corneal thickness, within the measurement accuracy limits, was higher in nearly all affected eyes associated with periocular port-wine stains, compared to eyes unassociated with port-wine stains in the same individual (Table). An elevation of intraocular pressures was similarly associated, though not always sufficient to produce glaucoma. B-scan and Doppler ultrasonography of the globes and orbits associated with periocular port-wine stains uniformly disclosed associated venous congestion of the ocular choroid and of the contents of the orbits. When Doppler flow measurements were performed, they revealed slowed venous flow in the affected orbit with raised intraluminal venous pressures. Magnetic resonance imaging scans generally revealed corresponding leptomeningeal thickening and choroidal plexus expansion, with visual field defects if the occipital lobe was involved. No substantial differences were noted in corneal thickness measurements between the two eyes if periocular port-wine stains were not present, and the greatest differences existed when periocular stains affected both the upper and lower lid of an eye.
Thus, despite the mechanical stretching and thinning of corneas that occurs with infantile glaucoma, the affected eyes of those with the Sturge-Weber syndrome tended to have thicker corneas, without loss of transparency. Such transparent tissue hypertrophy, in a noncontractile and nonvascularized tissue without other potentially contributory factors (eg, excess innervation), provides a notable demonstration how the capillary and venous protein transudate that is present at the limbus and in the anterior chamber must also contain the growth factors responsible for the variegated hypertrophy of tissues noted in the Sturge-Weber syndrome. Capillary and venous transudate is known to increase during isometric muscular contractions where venous constriction occurs, and can explain the heretofore enigmatic physiologic mechanism for muscle hypertrophy that occurs as a result of such load-bearing exercises.
Geometrical stretching of the globe by higher pressures, use of topical medications affecting venous transudate to control intraocular pressures, young age, and differences in baseline corneal thicknesses at birth can account for the few instances (Patients 2, 7, and 11) where corneal thickness was not distinctly greater in the eye with periocular port-wine staining.
Thicker corneas will render applanation readings of intraocular pressure somewhat higher than actual pressures in the affected eyes of those with the Sturge-Weber syndrome, and this should be kept in mind in the interpretation of these readings. The effect, however, is quite mild and less related to disc laminar diameter size and deformability, as is the case otherwise126 and for which such differences can be quite revelatory.
Findings Neglected by Earlier Mechanism Are the Result of Impeded Venous Flow Explained by the New Pathophysiologic Mechanism: Decreased Peripheral Nerve Densities
One must also explain the observed decreased cutaneous nerve densities, since they do not, in fact, follow a dermatomal pattern. Ochoa and colleagues,66 and subsequently other investigators,65,67 demonstrated that nonuniform elevations of tissue and interstitial fluid pressure with focal compression of axons, over time, will cause demyelination and eventual neuronal atrophy. With sustained focal elevations of tissue pressure due to venous impedance such as we have seen to be present in the Sturge-Weber syndrome, one should indeed expect eventual atrophy of nerves within tissues, particularly if high venous pressure reduces arterial perfusion. Reduced neural densities noted within port-wine stains, peripheral and unrelated to atrophic portions of the brain, are therefore simply explained through pathophysiology. As is the case within the brain, cutaneous neuronal atrophy is an effect rather than a cause of focal venous hypertension and ectasia.
RELATED SYNDROMES EXPLAINED BY THE NEW PATHOPHYSIOLOGIC MECHANISM
The Cobb Syndrome
The findings usually associated with the Sturge-Weber syndrome are not restricted to the cephalic portion of the CNS. Berenbruch in 1890127 and Cobb in 1915101 described vascular skin “nevi” in combination with “angiomas” within the same metamere in the spinal canal. This combination, more frequently found in the lower spine,101 is referred to as cutaneo-meningospinal angiomatosis, or the Cobb syndrome (Figure 17). We can now understand this to be a simple extension of the same process of venous dysplasia noted in the Sturge-Weber syndrome, but affecting the spinal, in lieu of cortical, veins.
FIGURE 17.
Patient with both the Sturge-Weber and the Cobb syndrome. Cephalic port-wine stains in this patient do not respect trigeminal dermatomal distributions as shown in Figure 1, and port-wine stains over the spinal canal correspond to the Cobb syndrome. (Photograph courtesy of Bernard A. Cohen, MD, The Johns Hopkins Hospital, Baltimore, MD.)
The Klippel-Trénaunay Syndrome
The Klippel-Trénaunay syndrome explained pathophysiologically
If we further pursue the extension of the process of venous dysplasia below the level of the heart, and particularly in the lower extremities where gravity cannot assist in venous drainage from tissues, reduction of venous flow ought to cause the greatest tissue pressure elevation and cellular hypertrophy. This expected outcome is the case in the Klippel-Trénaunay syndrome.
Venous dysplasia in the trunk or extremities will cause alternative collateral venous drainage passageways to develop, analogous to what we saw above in the head and neck. Since most veins not associated with the CNS possess valves and are not originally bidirectional in nature, superficial port-wine stain development is less common. However, when pressure changes are severe enough, valvular incompetence may ensue. This further exacerbates stasis and flow reversal and often produces visible varicosities in addition to superficial port-wine stains. Calcifications representing phleboliths are considered pathognomonic for venous malformations representing organized and calcified thromboses from slow venous flow.33 Such calcifications are often noted in this syndrome.
Lymphangiopathy involving the small and large lymph vessels is nearly always present following chronic venous insufficiency.120–122,128 Thus, one can explain the greater lower limb compared to upper limb symptoms in the Klippel-Trénaunay syndrome. Additionally, as might be expected, the varicosities become more prominent as the child begins to ambulate, walking upright.129–132 There is no need to invoke an underlying mesodermal defect of microscopic arteriovenous communications133 to explain these findings.
Valvular incompetence will exacerbate ambulatory venous hypertension, worsening wall deterioration. As a result, treatment with external elastic compression can improve both venous and lymphatic return,128,129,134 whereas sclerosing therapies or surgically removing varicosities will only worsen venous return.130–132,135–137 Atresia of the popliteal, femoral, or other deep venous channels in the Klippel-Trénaunay syndrome was recognized by Servelle in some instances138–140 and by other investigators.130–132,136,137,139,141–143 In two patients who underwent venous grafting procedures,134,144 both experienced alleviated symptoms.
Vascular findings in the Klippel-Trénaunay syndrome: alternative terminology proposed
Many investigators depict the so-called hemangiomas, venous aneurysms, and associated port-wine stains of the Klippel-Trénaunay syndrome as representing vascular “malformations” ascribed to faulty autogenesis.141,145 Rather than “malformations” or “pathologic lesions,”143 these cutaneous manifestations should be viewed as functional compensatory channels linked to the site of actual pathology. Their physiologic role should be better understood and appreciated.
The appellation “collateralization,” rather than “malformation,” would be more appropriate and would help to discourage the inappropriate surgical excision or sclerosing therapy146 still applied to these lesions. Appreciation of this underlying pathophysiology allows one to better understand how and why venous stasis and thrombosis occur.114,124
The Klippel-Trénaunay syndrome: limb elongation and increased bone growth explained
Horton and others147,148 felt increased blood flow by the epiphyseal bone line with arteriovenous fistulas caused increased bone growth. Servelle and others, on the other hand, believed the underlying basis to be delayed epiphyseal plate fusion due to decreased arterial perfusion from venous stasis.138,140,149,150 This could occur with venous thrombosis138 or ligation138,140,149,150 and was used to restore limb symmetry in children.140 This mechanism, however, fails to explain the hypertrophy of surrounding soft tissues that is also noted. Instead, one may look at focal venous hypertension itself, with the transudation of plasma-borne nutrients and growth factors, as responsible for not only hypertrophy of long bones in the Klippel-Trénaunay syndrome, but also the thickening of flat calvarial bones in the Sturge-Weber syndrome, as well as of the surrounding soft tissues. A variable balance of the effects of focally increased venous pressure (causing increased transudation and tissue hypertrophy) vs secondarily decreased arterial perfusion (causing arrested growth and atrophy) may produce different outcomes. In some patients, one may note limb hypertrophy, in others normal limb growth, and in yet others limb hypotrophy.132,143,151–154 Neurological lesions in the brain in the Sturge-Weber syndrome have subsequent neural degeneration further away along the course of axons secondarily also affecting target tissues. In the Klippel-Trénaunay syndrome, focal areas of tissue atrophy secondary to hypoperfusion can be masked by nearby nonneural tissue hypertrophy occurring secondary to venous hypertension.
The Klippel-Trénaunay syndrome: summary and conclusions
The Klippel-Trénaunay syndrome shares the same underlying pathophysiology as the Sturge-Weber and the Cobb syndromes, but with dysplasia of the non-CNS associated veins. Given the common embryologic origin of both lymphatics and veins,139–141 this may also be variably associated with secondary lymphatic dysfunction and, in some cases, be coupled with primary lymphatic malformations,155 including arteries.141,156,157 It is the nature of the reaction of tissues to focally elevated venous pressure—eventual atrophy of neural tissue and potential hypertrophy of nonneural tissue—which primarily distinguishes the Sturge-Weber from the Klippel-Trénaunay syndrome, rather than any difference in underlying pathophysiology.
Parkes Weber Syndrome
The Parkes Weber syndrome158 shares so many features with the Klippel-Trénaunay syndrome that it is occasionally considered a variant.159 Its main distinction has been the presence of arteriovenous fistulas. Much as carotid-cavernous sinus fistulas may produce port-wine stains and other symptoms akin to the Sturge-Weber syndrome, arteriovenous fistulas in the limbs raise venous pressures to mimic the Klippel-Trénaunay syndrome. The Klippel-Trénaunay syndrome and the Parkes Weber syndrome share a final common pathway of focally increased venous pressure. In the Klippel-Trénaunay syndrome, this is due to venous outflow obstruction, whereas in the Parkes Weber syndrome, it is due to arterial shunting into the venous system. Treatment modalities can thus differ, with embolization strategies indicated to treat the arterial fistula in the Parkes Weber syndrome.160
While this distinct underlying cause of Parkes Weber syndrome has long been understood, its relationship to tissue hypertrophy has remained obscure; underlying generalized mesodermal dysgeneses were often proposed as a distinct abnormality.161 Some investigators have described arterially produced elevations of venous pressures producing phenotypes otherwise identical to the Cobb syndrome162,163 or similar to the Sturge-Weber syndrome.164–169 One may suspect that it is similar focal insults to vascular development that lead to a spectrum of angiodysplasias, from isolated venous aplasia, as in the Klippel-Trénaunay syndrome, to the formation of arteriovenous shunts, as in the Parkes Weber syndrome. Angiography may be indicated in selected patients with phenotypic Sturge-Weber, Cobb, and Klippel-Trénaunay syndromes (particularly if indicated by clinical signs such as “thrill,” or by Doppler studies) to determine if more easily treatable arteriovenous fistulas are causative, rather than assuming an underlying venous dysplasia for all instances.
ACCOUNTING FOR THE NEW PATHOPHYSIOLOGIC MECHANISM IN THE TREATMENT OF THE STURGE-WEBER AND RELATED SYNDROMES
With the new understanding of the pathophysiology of Sturge-Weber and related syndromes, we should consider improved strategies for treatment until we learn how best to improve cortical venous drainage, perhaps in some cases via neurosurgical vascular diversion surgery with venous or synthetic grafts or shunts.
Traditional Treatments: Port-wine Stains and Surgery
If port-wine stains are obliterated (as could potentially be achieved by deep laser photocoagulation, and particularly by surgical debulking of the more hypertrophic lesions), the potential for cerebral venous escape through these alternative venous outflow channels is reduced to some extent. By doing so, cerebral (including ocular) blood flow anomalies may be exacerbated. Exacerbation may manifest as choroidal expansion, increased intraocular pressure, or exudative retinal detachment in addition to progression of neurologic venous deterioration. Such risks should henceforth be taken into account when considering treatment of these lesions. For current laser therapies, which treat only the most superficial aspect of port-wine stains, leaving the bulk of the collateral passageways unperturbed, the effects may not be clinically significant.
Postponing therapy, particularly during the first months or years of life, poses no hazards and does not render eventual cutaneous treatment less effective.100 Delays in intervention, moreover, allow time for improved collateral flow pathways to develop within the brain. Since the development of enlarged deep cerebral veins facilitates cortical drainage, some regression of pial51 as well as ocular170 and cutaneous abnormalities may be expected. Indeed, it is the author’s not infrequent clinical observation (Patients 1, 3, 4, 6, and 8, Table), as well as being mentioned in sporadic comments in the literature,13,19,36,38,129 that cephalic port-wine stains may spontaneously fade in coloration during the first few years of life, with re-reddening noted during a fever or during crying and Valsalva maneuvers. Other potential contributing factors to spontaneous improvement of port-wine stains could be continued calvarial bone growth with narrowing of venous foramina (as occurs with normal subjects71) or emissary vein thrombosis and closure.11 A most important overlooked cause, however, is almost certainly the erect posture of the ambulatory child, facilitating venous drainage not only from scalp emissary veins, but from leptomeninges, brain, and upper spine as well, where veins are all valveless in nature. This serves as a corollary to the well-described worsening of the port-wine stains and varicosities in the lower limbs noted following ambulation in the Klippel-Trénaunay syndrome, with normally unidirectional veins also becoming incompetent.
It is critical to maintain maximal alternative venous blood flow pathways, notably during the critical period of brain maturation, to avoid reaching a “tipping point” for progressive neurological deterioration. With leptomeningeal dilatation and thickening noted to progress over the first year of life in MRI studies, conjunctival vessel tortuosity and iris vessel prominence may diminish.170 At a minimum, cutaneous laser therapy should be deferred during this critical brain maturation period, after which spontaneous regression of cephalic port-wine stains may also occur. Although laser therapy may only obliterate the visible and most superficial aspect of the port-wine stain, and not generally cause clinically significant effects in adults,171 the thinner skin of infants compared to older children leads the laser’s relative depth penetration to be greater and therefore more likely to cause a clinically significant reduction in venous shunt flow from the brain. Surgical resection and debulking of cutaneous lesions at any age is more likely to produce consequences of hemodynamic significance to the eye or brain, and, similarly, any surgical resection of thickened leptomeninges170 will certainly worsen perfusion anomalies and potentially precipitate strokes and seizures.
Traditional Treatments: Port-wine Stains and Pharmacology
Medications that decrease cerebrospinal fluid (CSF) pressure, such as systemic carbonic anhydrase inhibitors, should be avoided. By decreasing interstitial and extravascular parenchymal pressure, such medications raise the gradient of the intraluminal venous vs extraluminal parenchymal pressure and accelerate venous wall degeneration and obliteration. The diuretic action of carbonic anhydrase inhibitors, moreover, may induce additional venous thrombosis within the cerebral veins, worsening perfusion anomalies.
Port-wine Stains: Minimizing Deleterious Outcomes
With slowed venous flow, stasis and thromboembolic events become more common.11,13,30,63 Yet thromboembolism can only further exacerbate perfusion anomalies and symptoms.112 Aspirin thromboprophylaxis, as suggested in the past, should be encouraged (Roach ES, et al. Aspirin therapy for Sturge-Weber syndrome [abstract]. Ann Neurol 1985;18:387).64,77,112,172–176 which reduces the number of strokes and seizures. In some instances, thrombolytic treatment as well could prove warranted to minimize the effects of an acute stroke, and such treatment could also be indicated for Fegeler syndrome, especially prior to the onset of any brain or other sequelae.
Nevertheless, when venous pressure is above the threshold for producing vessel wall degeneration, aspirin or thrombolytic treatment alone cannot prevent their eventual deterioration and obliteration.11,13,113,177 To diminish the transmural pressure gradient, either CSF pressure must be raised to increase extraluminal pressure, or intraluminal venous pressure must be lowered, potentially with interventional shunting procedures to unaffected veins. Although the use of pharmacologic venous antihypertensive afterload reducers such as sodium nitroprusside might initially come to mind, such agents would be unlikely to have the desired effect upstream from veins that are absent or constricted due to malformation.
Treating Sturge-Weber Syndrome: Interventional Approaches
Some have advocated various forms of ionizing radiation178–180 to the ocular choroid for treatment of serous retinal detachments. The intended sclerosis of the ocular choroid can reduce transudate, though concomitant sclerosis of orbital veins and episcleral vessels could potentially further elevate intraocular pressure. However, such intraocular pressure elevation, exacerbating glaucomatous damage to the optic nerve, may itself help to counter choroidal effusion and assist in reattachment of the retina. Conversely, the lowering of intraocular pressure, even by medical means, has the potential to cause ciliochoroidal effusion181 and exudative retinal detachment. Selective methods to increase intraocular pressure, such as the use of topical or intraocularly injected steroids, could provide an alternative temporary treatment modality. Medications commonly used to treat glaucoma may have secondary pharmacologic effects and consequences, either alternatively increasing vascular permeability182 in some instances, or reducing such permeability, as steroids may also do, in others.183
Moreover, the deleterious effects of ionizing radiation on the retina, particularly by decreasing retinal capillary perfusion and causing ischemia, can cause additional complications. Laser photodynamic therapy, which can more selectively reduce subretinal choroidal exudation with less generalized effect on retinal vasculature, while sparing orbital vessels, is one alternative.184–187 Lowering of orbital venous pressures via vascular diversion procedures to an unaffected contralateral orbital vein or to other uninvolved venous systems of the head or neck may prove feasible and warranted in some instances.
With limited options available to improve venous outflow, one may consider other possibilities to reduce ocular choroidal plexus transudate. For this high-flow expansile plexus of vessels specifically, reducing arterial pressure could potentially decrease the production of plexus transudate to help resolve exudative detachments. The use of propanolol should not be presumed to affect the choroidal tissue as an anti-angiogenic agent188–190 since there is no hemangioma present. However, when no distal venous obstruction is present nearby, the lowering of arterial perfusion pressure, as with other antihypertensive agents, may potentially decrease the production of choroidal transudate and permit retinal reattachment.
Systemic antihypertensives would have a similar effect in the cerebral choroidal plexus; however, exudate there is generally of little concern since cerebral transudate is more easily carried away within the CSF passageways. An exception would be to lower CSF production in the setting of raised intracranial pressure.
Glaucoma in the Sturge-Weber Syndrome
Glaucoma affects approximately two-thirds of individuals with the Sturge-Weber syndrome, many of whom are diagnosed before the age of 2 years. In the past, this was noted to affect the majority of those in whom both upper and lower eyelids were fully affected by port-wine stains. It becomes clear when looking at orbital vascular anatomy (Figure 9) that impaired posterior venous flow from the upper lid via the superior ophthalmic vein implies poor drainage from the cavernous sinus. More significantly, port-wine stain involvement of the entire lower lid implicates impeded flow, not only from the inferior ophthalmic vein to the cavernous sinus, but also from the inferior ophthalmic vein to the pterygoid plexus. Orbital vein pressure will almost necessarily be elevated, often producing glaucomatous damage to the optic nerve. Contralateral intraocular pressures in unilateral Sturge-Weber syndrome may serve as effective target pressures for the affected eye.
Contesting Goniodysgenesis in Sturge-Weber Patients
In younger patients, the iridocorneal angle has been described as suggestive of goniodysgenesis.191–194 These depictions are more accurately interpreted as a simple reflection of choroidal expansion42 and iris vascular dilation with forward projection in a geometrically small eye. This expansion, dilation, and projection alter anterior chamber angle anatomy and at times produce partial angle closure (Figure 8, bottom left). The same anomalous gonioscopic appearance is again noted in other congenital syndromes such as the tetralogy of Fallot and occasionally ascribed to neural crest anomalies.195 However, it develops whenever orbital venous pressure is elevated,196 including in the cloverleaf skull syndrome,42,45 with pulmonary arterial hypertension,104 and with acquired carotid-cavernous sinus fistulas.47,197
While goniotomy procedures in these patients have been reported to provide good results in infancy, a high incidence of relapse of elevated intraocular pressure also occurs.191,198 Since goniodysgenesis is, in effect, not present, in lieu of goniotomy, secondary angle closure can be minimized in infants through the use of miotics such as pilocarpine. Conversely, the use of mydriatic agents would increase the chances for trabecular meshwork obstruction. It is important to note that most ocular growth occurs in the first 2 years of life,199,200 during which time the trabecular meshwork gradually becomes more fully exposed. Indeed, in older children and in adults with the Sturge-Weber syndrome, the illusion of goniodysgenesis recedes, and the gonioscopic appearance is entirely normal.
The Role of Plasma Protein Build-up in Glaucoma
Nonetheless, episcleral pressure193,201,202 and outflow resistance remain elevated201 as long as orbital venous outflow is subnormal, accounting for a second peak of detected glaucoma later in childhood. Therapy should focus on decreasing aqueous production. Topical carbonic anhydrase inhibitors and beta blockers should be mainstays of initial treatment. Systemic carbonic anhydrase inhibitors again must be avoided. Given their intracranial CSF and thus interstitial fluid pressure-lowering actions, these systemic agents would exacerbate the transmural venous pressure gradient. Venous obliteration and disease progression would thereby be accelerated. More pointedly, at the level of the optic disc, any decrease of intraocular pressure would be canceled by the concomitant decrease in intracranial pressure at the opposing face of the lamina cribrosa via the simultaneous decrease in CSF production.203
In addition to high venous pressures limiting aqueous outflow, there are other mechanisms that can limit egress. As some investigators have pointed out,204–207 outflow can also potentially be limited at the level of the trabecular meshwork by the presence of soluble aqueous proteins. The higher venous pressures in Sturge-Weber syndrome, also present in carotid-cavernous fistulas,208 greatly increase the transudation of plasma protein exudates from ciliary body intraocular capillaries into the iris root. The plasma protein exudates can be swept almost immediately into the trabecular meshwork,209 as well as into the aqueous fluid, potentially obstructing or clogging trabecular meshwork and decreasing aqueous outflow capabilities.108,210–212 Indeed, in 1932 Tyson211 demonstrated this potential when he showed increased aqueous fluorescein excretion in the Sturge-Weber syndrome, repeated and verified by the author in one patient (Patient 11, Table). This mechanism appears to have soon thereafter been entirely overlooked. More recent work213 confirms that aqueous humor “spiked” with various plasma proteins does exhibit greater blocking potential. Histopathological and ultrastructural examination of the trabecular meshwork in Sturge-Weber syndrome has, in effect, revealed increased extracellular deposits of granulo-amorphous and fine fibrillar material, with flocculent material intracellularly.214,215
In analogous manner and supporting this mechanism, spinal fluid protein may also be elevated in the Sturge-Weber syndrome30,216–218 and in anomalous venous return,83,84 indicating significant breakdown of the blood-brain barrier in the brain and, possibly, a poorer neurological prognosis. Though some cases of hydrocephalus have been reported,91,112 high intracranial pressure does not generally result, since the process is often restricted to only one hemisphere. Additionally, unlike the ocular situation where aqueous-draining episcleral venous pressure is elevated, the absence of cortical bridging veins does not elevate the pressure within CSF-draining channels in the dural sinuses to impede CSF outflow capacity. In some situations, eventual brain atrophy may also lead to decreased CSF production. The fact that excesses of plasma protein molecules in the CSF can lead to elevated CSF pressure in other diseases is well appreciated219; this mechanism must also be at play intraocularly. It is plausible that the bimodal presentation of glaucoma in the Sturge-Weber syndrome may, in part, be due to the gradual buildup of proteins blocking the outflow passageways. Empiric trials with a prostaglandin derivate have shown some effect in occasional patients with later-onset glaucoma in the Sturge-Weber syndrome.220–222 This may be due to the pharmacologic clearance of such protein deposits. The presence of extracellular deposits of amorphous material has also been noted within port-wine stains116 and attests to the general phenomenon of protein transudation secondary to high venous pressure.
As discussed above, this venous protein transudate is likely to contain the growth factors responsible for the accompanying tissue hypertrophy (Table).
Surgical Treatment of Glaucoma
Should medical therapy fail, one may proceed to more invasive interventions. Filtering surgery can help bypass elevated pressure in the aqueous venous channels, draining aqueous directly into conjunctival lymphatics. To limit the intraoperative risk of expulsive hemorrhage, one may consider staging penetrating procedures. Such procedures could include initially performing nonpenetrating external trabeculectomy223,224 or placing a shunt device first sealed. Both procedures may require supplementation later with YAG-laser goniotomy or suture lysis. To further minimize intraoperative risks of expulsive choroidal hemorrhage, temperature lowering and pharmacologic means may be considered to temporarily reduce cerebral arterial perfusion while at the same time maintaining maximal venous dilation and blood flow.
Ultimately, trabeculectomy and other filtering procedures typically have a lower success rate in patients with the Sturge-Weber syndrome. The distended episcleral veins may leak both aqueous and plasma into surrounding conjunctiva to be reabsorbed via the lymphatic channels, and though lymph flow can increase several fold120,121 to prevent edema formation, tissue pressure will be more elevated and reduce shunted aqueous absorptive capabilities. If conjunctival chemosis is present preoperatively, the episcleral venous leakage is already in excess of lymph drainage capabilities. Such conjunctival edema may augur a reduced intraocular pressure-lowering effect for any form of filtering procedure. In such situations, cyclodestructive procedures to reduce aqueous production may play a greater role. One must keep in mind, however, that baseline aqueous production, as for CSF production in the brain, might be abnormal. Aqueous fluid component produced by diffusion might be greater due to higher venous pressures, but the secreted component might be reduced due to diminished arterial perfusion. Particular caution must be exercised in selecting treatment parameters to avoid producing ocular hypotony.
Analogous to decreased arterial perfusion in the brain noted via cerebral angiography, slowed ocular choroidal perfusion secondary to high venous pressure (Patient 17, Table) has been observed using indocyanine green angiography.225 Similar angiographic findings have been noted in the tetralogy of Fallot,103 pulmonary arterial hypertension,104 as well as nanophthalmos.226 Despite the reduced ocular perfusion, however, neovascular glaucoma is rare. Presumably, reduced perfusion throughout the period of development modulates demand and tissue growth accordingly. Even so, should thrombotic events occur, leading to superimposed acquired drops in perfusion, ischemia can ensue, triggering a cascade of signaling factors for neovascularization. The presence of neovascular glaucoma in the Sturge-Weber syndrome may eventually prove to be an indicator of new perfusion defects from venous thrombi or obliteration, as the sudden development of a ciliochoroidal effusion227 could possibly indicate worsening venous outflow capacity.
DISCUSSION
When venous dysplasia affects cortical drainage to a sufficient degree to cause two or more recognized clinical signs, it gives rise to what has been called the Sturge-Weber syndrome. Dysplasia of deeper cerebral veins produces other symptoms, with or without visible port-wine stain production, including venous angiomas, caput medusae, and “cavernomas”—and even various “subtypes” of the Sturge-Weber syndrome.30,91,157,228,229 When dural sinuses distal to the arachnoid granulations are involved primarily, rather than the bridging cortical or deeper intracranial veins, CSF absorption will be reduced from the resulting elevation in venous sinus pressure. This scenario, occasionally termed by some investigators83–85 “facial nevi associated with anomalous venous return and hydrocephalus syndrome,” need not be viewed as a distinct syndrome.230,231
Dysplasia of deep cerebral veins, on the other hand, will cause centrifugal rather than centripetal shunting of blood to the superficial cortical system. Such shunting of venous blood flow to the dural sinuses instead of to the deep venous system not only produces cutaneous port-wine stains, but can inhibit CSF absorption from the arachnoid villi into the dural sinus system, also leading to hydrocephalus. When venous dysplasia occurs within the extremities, the consequent effects of nonneural tissue hypertrophy may cause it to be termed the Klippel-Trénaunay syndrome. But when venous dysplasia and collateral formation occur only to an extent to produce visible superficial cutaneous lesions (over 90% of all instances), we may call them isolated port-wine stains. There are even instances where superficial CNS-associated port-wine stains are not visible clinically (though perhaps elicitable by Valsalva maneuvers or detectable by other means such as thermography), but where deeper leptomeningeal thickening and enhancement may be noted on MRI scans.28,29,31 Such cases have been noted histopathologically, reported as formes frustes of the Sturge-Weber syndrome.12 Acquired port-wine stains99 with associated symptoms, sometimes referred to as the Fegeler syndrome, simply represent postnatal venous occlusions or a postthrombotic syndrome from trauma or other causes.
In many instances, MRI may be unable to detect the associated intervening leptomeningeal thickening. Some have speculated that this could be due to thrombosis of vessels diminishing perfusion.232 However, just as the port-wine stain may be subtle, flat, and difficult to perceive, in most instances the associated pial “mass” will also be difficult to detect by MRI. Focused attention,233 as well as newer imaging techniques and modalities,234 should be able to eventually detect many “absent” lesions. It should also be remembered that as deeper cerebral veins become prominent over time, the leptomeninges themselves may thin51 and become more difficult to detect. Additionally, not all cephalic port-wine stains will be associated with leptomeningeal thickening. Individuals with port-wine stains affecting only the lower part of the face may have primarily retromandibular vein dysplasia without intracranial involvement. Isolated orbital and ocular involvement constituting the Sturge-Weber syndrome can also occur without any leptomeningeal or intracranial involvement from dysplasia of ophthalmic veins, similar to the situation of ophthalmic vein or cavernous sinus thrombosis.
Elsewhere in the body, deep vein dysplasia can occur with collaterals noted on phlebography, other imaging techniques,160 or Doppler ultrasonography. Internal organs such as the lung and gastrointestinal system can also be affected,11 with or without externally visible signs of either the Sturge-Weber or Klippel-Trénaunay syndromes. Conversely, more extensive cases of vascular dysplasia involving veins such as the subclavian, renal, and other11,129,140,157,235 arteries,160 and lymphatics will cause even greater symptoms than is traditionally associated with these eponymous syndromes. Patients with both cephalic and noncephalic involvement, along with glaucoma or seizures, have sometimes been referred to as having both the Sturge-Weber and the Klippel-Trénaunay syndromes, or, as some have proposed,236,237 the Sturge-Weber-Klippel-Trénaunay syndrome, or yet other variations such as Klippel-Trénaunay-Weber syndrome,238 but these will not encompass all possible effects of various angiodysplasias. Syndromes related to the focal effects of venous hypertension are not limited to humans, and at least one instance of the Sturge-Weber syndrome has been recorded in an animal.239 As achieved for other studies, induced thromboses,82 surgical ligation of veins, or creation of arteriovenous fistulas can be performed to create animal models of the Sturge-Weber, Klippel-Trénaunay, Cobb, and other focal venous hypertension syndromes.
Heretofore unexplained, it should now also be understood through the elucidation of this special and exaggerated pathologic model, that focal venous hypertension more generally, whether pathologic in the vascular dysplasia syndromes or physiologic as during forced muscle contraction exercises, is the “engine” that drives hypertrophy of tissues. This occurs via pressure-induced capillary and venous transudation of plasma-borne nutrients and growth factors into surrounding tissues. Neural signaling need not be invoked. When constant, as in pathologic situations, the localized nonuniform tissue pressure imbalances that result can cause localized axonal compressions and distortions as well as reduced perfusion producing eventual neural atrophy within the affected tissues.
Other associated signs, symptoms, and findings can now also be better understood and anticipated. For example, in a patient with extreme bilateral ophthalmic vein dilation reported by Baráth and associates,157 hormone deficiency was coincidentally noted. We can now understand that via the cavernous sinus and hypophyseal veins, increased venous plexus pressure within the pituitary gland would decrease arterial perfusion, causing hormonal hyposecretion leading to dwarfism27,157 and amenorrhea. Indeed, thrombosed veins within the hypophysis in a patient with the Sturge-Weber syndrome were documented by Wohlwill.11 As recently collected data now demonstrates,240–242 pituitary hyposecretion is likely to be an underdiagnosed aspect of cases of the Sturge-Weber syndrome in general and in particular for individuals with bilateral disease.
CONCLUSIONS
NEED FOR LINGUISTIC CLARITY
It would appear that the incorrect use of the words angioma and trigeminal in the attempted descriptions of the Sturge-Weber syndrome, and angioma and venous “malformations” in the Klippel-Trénaunay and Cobb syndromes, prevented investigators in various disciplines from drawing the full conclusions of their work.
A variety of erroneous attempts at descriptive terminology such as encephalotrigeminal angiomatosis, or designations such as venous angioma, caput medusae, or cavernoma, along with a plethora of eponymous appellations including Fegeler, Cobb, and others, served to confuse thinking in the face of evidenced findings. These entities may all be thought of as simply manifestations of venous disorders resulting in focal venous hypertension occurring in different regions of the body.
For the sake of additional linguistic clarity, it may also be beneficial, in the absence of any hamartomatous growth or pattern of inheritance, to remove the Sturge-Weber, Klippel-Trénaunay, and Cobb syndromes from the rubric of phacomatoses. These focal venous hypertension syndromes, along with the Fegeler syndrome, port-wine stains in general, and various associated arteriovenous malformations such as the Parkes Weber syndrome, previously considered “odd men out” within the category of phacomatoses, may now be considered found out.
NOVEL INDICATION OF UNDERLYING ETIOLOGICAL CAUSE
One may speculate as to the cause for the primary venous dysplasia leading to these various syndromes. As initially reported by Bentson, Wilson, and Newton in 1971,2 angiographic findings alone could not determine whether cerebral vein thrombosis or aplasia was causative for the noted cortical venous drainage abnormalities of the Sturge-Weber syndrome. With today’s understanding of apoptosis, it is easy to understand how both possibilities may occur sequentially: an initial thrombus formation in utero leading to subsequent venous regression and dysplasia. Short-lasting fibrin emboli obstructing blood flow can precipitate the rapid apoptosis of vascular structures.243,244 Indeed, persistence of arterial fetal vasculature has been associated in neonates with endocardial thrombosis245 as well as when venous emboli paradoxically migrate into the arterial circulation, as recently proposed by Parsa and Robert.246 With regard to the venous circulatory system, persistence of fetal vasculature in the setting of Klippel-Trénaunay syndrome has been noted since the entity was first described by Klippel and by Trénaunay247 and confirmed by others.140,248,249
The sporadic and usually unilateral findings argue against systemic disorders of vasculogenesis or angiogenesis, and instead argue for causation by the more erratic development and effects of venous thrombus formation. We now understand how similar findings occur postnatally in adults: obstruction of postthrombotic veins may occur from endovascular scar tissue creating synechiae and septae that variably block the lumen,250 in some instances producing the Fegeler syndrome.
Parrot251 in 1873 systematically examined the brains of newborn infants in whom the cerebral sinuses or veins were thrombosed, and Ford252 later described additional cases associated with seizures and atrophy of the brain, and thickening of the meninges. As recently described by Kalbag,253 in the absence of any of any lesions of the vessel walls themselves, Parrot postulated the cause of the thrombosis must lie in the blood itself. Hutinel254 in 1877 pointed out the particular vulnerability for thrombosis to develop in the renal veins, dural venous sinuses, and cerebral veins, attributing the vulnerability of the latter to the dependent position of the head. One may also suppose that sustained maternal abdominal compression and accompanying elevation in fetal intracranial pressures would further narrow the outlet of the bridging veins and promote conditions for thrombosis within the cerebral veins. Such conditions may occur during pre-eclampsia.255,256 As written by Kalbag, “The most striking clinical indication of venous thrombosis in the infant is probably dilatation of the scalp veins, which seems to be the result of diploic veins transmitting and reflecting the intracranial venous stasis.”253 Kalbag later writes, “The dilatation of the scalp veins may be striking enough to be the caput medusa described by Lermoyez in 1897 as a sign of thrombosis of the superior sagittal sinus”257 while “Edema of the scalp . . . and over the line of the affected sinus . . . with or without edema of the upper eyelids, may be valuable additional signs.253 The dramatic onset of convulsions is consistent with thrombosis of a cortical vein.253,258–260 As could be expected, angiography findings reveal anastomic channels circumventing the occluded segment of a sinus with small, corkscrew vessels.253 In cases of infantile cerebral venous thrombosis most often related to acute dehydration, Aicardi and Goutières261 in 1973 notably also described the finding of ipsilateral ocular fundus venous dilation.
In at least one report, prenatal diagnosis of a dural sinus thrombosis was made via Doppler ultrasonography and pregnancy terminated. Significantly, necropsy confirmed the presence of a posterior dural sinus thrombus associated with an occipital “hemangioma” over the occipital lobe.262
A variable response of individuals with the Sturge-Weber syndrome to aspirin prophylaxis for seizure control may also, in part, be predicated by such inherited thromboembolic susceptibilities. Although such thrombi could develop or lodge anywhere within the venous system, the widespread presence of microscopic venous anastomoses throughout the majority of solid and fixed tissues could easily allow collaterals to enlarge and compensate for many smaller venous obstructions (Figure 18). Larger thrombi would cause abnormalities for which it is more difficult to compensate and which could come to clinical attention. Given the relatively immobile position of the fetus, it is not unreasonable to presume that venous thrombi within limbs might preferentially form or lodge within flexion points (ie, elbows and knees) to cause the Klippel-Trénaunay syndrome.
FIGURE 18.
Specificity of venous response according to site of cerebral occlusive thrombus. Top vein: Normal unoccluded superficial cerebral vein passes through the leptomeninges (pia mater and arachnoid) to bridge the space from brain to the dural sinuses. Variable “pinching” of the bridging portion of the vein occurs in response to the surrounding varying cerebrospinal fluid pressure as part of a Starling resistor mechanism to regulate intraluminal venous pressure. Second vein: A venous thrombus that develops and lodges outside the cerebral tissue can obstruct venous flow. Decreased venous flow can cause a collapse of the thin-walled bridging portion of the vein. Third vein: During embryological development, an absence of blood flow can quickly precipitate apoptosis of the bridging portion of the vein along with its disappearance. Since there are no microscopic anastomotic venous channels in the subarachnoid space filled with moving cerebrospinal fluid, collateral venous channels to bypass the obstruction cannot form. Over time, the vein distal to the occlusion, along with its leptomeningeal portion, becomes engorged and tortuous, with higher pressure and deoxygenated cyanotic blood within. Fourth vein: Fibrin clots that may block the venous lumen more distally within cerebral tissue may also cause narrowing of the immediate proximal portion of the vein and cause apoptosis of a venous segment. However, smaller tributary channels continue to feed into the vein more proximally, preserving venous integrity and maintaining a reduced flow into the dural sinus. Bottom vein: Over time, enlargement of otherwise microscopic channels that exist within fixed cerebral substrate, and/or persistence of fetal vasculature can allow for distributory and tributary venous collateral formation to bypass the site of the original thrombus and renormalize blood flow in the proximal vein.
Most instances of the Klippel-Trénaunay syndrome do occur as a result of anomalies in the popliteal veins,248 where venous diameter is known to decrease with knee flexion. However, even smaller thrombi, should they form or lodge at the interface of mobile tissues relatively devoid of available microanastomotic channels for compensatory enlargement, pose a greater risk for producing clinical symptoms. Such collateral-poor areas include the cerebrospinal-filled space between spinal cord and dura, and between brain and dura through which pass the narrowed cerebral cortical bridging veins (Figure 18). Significantly, too, the bridging veins drain into the superior sagittal sinus in the direction opposite of blood flow in the sinus.263,264 The angle at which the cortical veins enter into the superior sagittal sinus becomes more acute the more posterior one is in the brain, and hence the degree of opposing venous blood flow with increase in blood stasis (Figure 19). Hence, these are known as a common site for cerebral venous thrombosis to occur in adults,264 while it is also known that a shift of the brain265 or of unfused calvarial bones in any direction with traction on the bridging veins may deform the venous wall, inducing thrombosis.252,266,267 The thin walls of cerebral veins just prior to their entry into the superior sagittal sinus and their sphincter-like termination268 make it ever more likely for thrombi to form or lodge at such sites. The thinning of venous walls at this juncture allows for improved responsiveness to alterations in CSF pressure in order to regulate venous intraluminal pressure via a Starling resistor model.269 That such mechanical conditions could predispose to thrombus formation was noted by Virchow in 1855270 and by von Recklinghausen.271 Moreover, Bergeat in 1891272 pointed out the lack of surrounding contractile musculature and the valveless nature of the cerebral vasculature, which makes them particularly dependent upon the strength of the cardiac output for normal flow. Hence, any decrease in cardiac output would reduce flow. The bidirectional flow potential and dependence on gravity in an erect position for enhanced cerebral venous drainage was also recently emphasized by Kim and Parsa.97,98 In at least one patient with the Sturge-Weber syndrome, venous clot formation from cerebral vein into dural sinus has been observed.262 The fact that many more individuals with the Sturge-Weber syndrome have been reported to have leptomeningeal enhancement more posteriorly109 where cortical veins insert at the most acute angles into the superior sagittal sinus with countercurrent flow (Figure 19) is supportive of this mechanism, and would provide the primary explanation for why occipital lobe defects with hemianopsias, alluded to previously, are more frequently noted in this syndrome. Congenital scalp defects273–276 will likely develop when larger sinus thrombosis occurs.
FIGURE 19.
Axial view of the normal anatomy of the superficial cortical and bridging veins draining into the superior sagittal sinus. Variable narrowing of the terminal portion of the thin-walled and emptying bridging vein occurs in response to variable cerebrospinal fluid pressure as part of a Starling resistor mechanism to regulate intraluminal venous pressure and prevent collapse. The more posterior cortical veins enter into the dural sagittal sinus at more acute angles, with cortical venous blood draining against the direction of sinus blood flow. This unique countercurrent venous blood flow, particularly with the more posterior veins, predisposes to stasis and venous thrombus formation, with “capture” more likely at or near the narrowed dural sinus opening. Such thrombus formation obstructing blood flow in utero can trigger vascular apoptosis with disappearance of the bridging portion of the vein, giving rise to the Sturge-Weber syndrome.
SCREENING AND PREVENTION
Both maternal and fetal genetic and environmental risk factors may be responsible for venous thrombi to form, such as Factor V Leiden or FII G20210A genetic mutations,277,278 and more than one factor may be involved. Malnutrition, dehydration, alcohol, infections, trauma, ionizing radiation, antiphospholipid syndrome and other autoimmune disorders, pre-eclampsia, and thrombogenic medications may act synergistically with maternal or fetal inborn predispositions to produce focal vaso-occlusion and thus the focal and sporadic effects noted in these syndromes. Though thrombophilic tendencies have so far not been generally appreciated in prenatal sinus thrombosis or in the Sturge-Weber syndrome, there are indications that it may be overlooked. As Govaert states: “An association with thrombophilia has been suggested for prenatal sinus thrombosis, but not documented.”279 Yet in one series of 30 children with sinovenous thrombosis, four of seven who were tested for thrombophilia were found to be positive280 with one instance of hemocoagulative aspects in a case of Sturge-Weber syndrome reported decades ago.281 Thrombophilic tendencies, have frequently been noted in the Klippel-Trénaunay syndrome249 and, though the etiology is often unclear, have been attributed to consumptive coagulopathy as a result of the venous malformation. However, several studies have shown abnormal thrombin activity and other factors that have clearly indicated an underlying predisposition for thrombophilia that cannot be attributed to vascular malformation.282,283 In pregnant women with the Klippel-Trénaunay syndrome, a high proportion were diagnosed with prothrombin genetic mutations.284 It does appear that many predisposing factors for coagulopathies may be overlooked in these patients,285,286 as well as in those with the Sturge-Weber syndrome.
Pregnant women so predisposed may benefit, as they currently do for those suffering from antiphospholipid syndrome, from aspirin prophylaxis during pregnancy.287,288 Given the extremely high rate of thromboembolism in the Klippel-Trénaunay syndrome, often causing pulmonary emboli or triggering a consumptive coagulopathy, aspirin prophylaxis may be as critical for those with this syndrome as for those with the Sturge-Weber syndrome, where it has been found to reduce microinfarcts and seizures (Roach ES, et al. Aspirin therapy for Sturge-Weber syndrome [abstract]. Ann Neurol 1985;18:387).64,77,112,172–176 Overlap between the two syndromes may thus not be so surprising.289 The fact, moreover, that prenatal venous emboli may paradoxically migrate into the arterial circulation290 should also allow one to infer that a greater number of other congenital malformations might be associated with these entities,246 a likelihood noted by at least one recent publication.291
It appears quite likely that rather than simply an effect of venous malformations, underlying predispositions for venous coagulopathies might be a contributory cause.
HOPE FOR SURGICAL CURES FOR THOSE ALREADY AFFECTED
Though the compartments and spaces are often restricted and procedures can be very technically challenging,292 where possible, dilatation of dural sinuses, vascular diversion surgery, endophlebectomy, or venous grafts may be considered in the treatment of the vascular dysplasia disorders such as the Sturge-Weber, Klippel-Trénaunay, and related syndromes. Rapidly improving capabilities with robotically assisted surgery engender further hope for such attempts where spaces are confined and where vascular reconstruction may otherwise not be technically feasible.
Acknowledgments
Funding/Support: The author received partial salary support through The Hunter Nelson-Sturge-Weber Center at Kennedy Krieger Institute, Baltimore, Maryland.
Financial Disclosures: None.
Author Contributions: Design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript were all performed by the author.
REFERENCES
- 1.Parsa CF. Sturge-Weber syndrome: a unified pathophysiologic mechanism. Curr Treat Options Neurol. 2008;10(1):47–54. doi: 10.1007/s11940-008-0006-0. [DOI] [PubMed] [Google Scholar]
- 2.Bentson JR, Wilson GH, Newton TH. Cerebral venous drainage pattern of the Sturge-Weber syndrome. Radiology. 1971;101(1):111–118. doi: 10.1148/101.1.111. [DOI] [PubMed] [Google Scholar]
- 3.Van der Hoeve J. Eye diseases in tuberose sclerosis of the brain and in Recklinghausen’s disease. Trans Ophthalmol Soc U K. 1923;43:534–541. [Google Scholar]
- 4.Van der Hoeve J. The Doyne Memorial Lecture: Eye symptoms in phakomatoses. Trans Ophthalmol Soc U K. 1932;52:380–401. [Google Scholar]
- 5.Van der Hoeve J. Les phakomatoses de Bourneville, de Recklinghausen et de von Hippel-Lindau. J Belg Neurol Psych. 1933;11:752–762. [Google Scholar]
- 6.Brouwer B, Van der Hoeve J, Mahoney W. A fourth type of phakomatosis: Sturge-Weber syndrome. Verh K Akad Wet Amst. 1937;36(4):1–33. [Google Scholar]
- 7.Louis-Bar D, Legros J. Les hypertrophies partielles avec angiome (syndrome de Klippel-Trénaunay) et leurs rapports avec les phacomatoses. Confin Neurol. 1947;7(5):245–263. [PubMed] [Google Scholar]
- 8.Trélat U, Monod A. De l’hypertrophie unilatérale partielle ou totale du corps. Arch Gen Med. 1869;180:536–558. [Google Scholar]
- 9.Good WV, Hoyt CS. Optic nerve shadow enlargement in the Klippel-Trenaunay-Weber syndrome. J Pedr Ophthalmol Strabismus. 1989;26(6):288–289. doi: 10.3928/0191-3913-19891101-10. [DOI] [PubMed] [Google Scholar]
- 10.Parsa CF. Taylor DSI, Hoyt CS. Pediatric Ophthalmology and Strabismus. 3rd ed. London: Elsevier Saunders; 2005. Conjunctiva and Subconjunctival tissue. Subsection: Sturge-Weber/Klippel-Trenaunay-Weber syndromes; pp. 235–243. [Google Scholar]
- 11.Wohlwill FJ, Yakovlev PI. Histopathology of meningo-facial angiomatosis (Sturge-Weber’s disease). Report of four cases. J Neuropathol Exp Neurol. 1957;16(3):341–364. doi: 10.1097/00005072-195707000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein BW, Rosenberg C. Sturge-Weber-Dimitri’s disease. Report of an abortive case with observation on the form, chemical nature, and pathogenesis of the cerebral cortical concretions. J Neuropathol Exp Neurol. 1947;6(4):369–382. [PubMed] [Google Scholar]
- 13.Green JR. Encephalo-trigeminal angiomatosis. J Neuropathol Exp Neurol. 1945;4:27–42. [PubMed] [Google Scholar]
- 14.Brodsky MC, Baker RS, Hamed LM. Pediatric Neuro-ophthalmology. New York: Springer-Verlag; 1996. Neuro-ophthalmic manifestations of systemic and intracranial disease; pp. 407–410. [Google Scholar]
- 15.von Baerensprung Naevus unius lateris. Ann Charite-Krankenhaus (Berl) 1863;11:91–95. [Google Scholar]
- 16.Etienne G. Des nævi dans leur rapports avec les territories nerveux. Essai de pathogénie et d’étiologie. Nouv Iconogr Salpêtriere. 1897;10(4):263–281. [Google Scholar]
- 17.Kalischer S. Demonstration des Gehirns eines Kindes mit Teleangiectasie der linksseitigen Gesicht-Kopfhaut und Hirnoberfläche. Berl Klin Wochenschr. 1897;29:1059. [Google Scholar]
- 18.Lannois Bernoud. Énorme nævus angiomateux de la face, avec hémiplegie spasmodique et épilepsie. Nouv Iconogr Salpetriere. 1898;11:446–451. [Google Scholar]
- 19.Cushing H. Cases of spontaneous intracranial hemorrhage associated with trigeminal nevi. JAMA. 1906;47(3):176–183. [Google Scholar]
- 20.Yakovlev PI, Guthrie RH. Congenital ectodermoses (neuro-cutaneous syndromes) in epileptic patients. Arch Neurol Psychiatry. 1931;26(6):1145–1194. [Google Scholar]
- 21.Kautzky R. Die Bedeutung der Hirnhaut-Innervation und ihrer Entwicklung für die Pathogenese der Sturge-Weber’schen Krankheit. Dtsch Z Nervenheilkd. 1949;161(5–6):506–525. [Google Scholar]
- 22.Alexander GL, Norman RM. The Sturge-Weber syndrome. Vol. 2. Bristol: John Wright & Sons, Ltd; 1960. The history and definition of the syndrome. The distribution of the facial nævus and its relation to the cerebral angiomatosis; pp. 71–76. [Google Scholar]
- 23.Selhorst JB. Phacomatoses. In: Miller NR, Newman NJ, editors. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 5th ed. Baltimore: Williams & Wilkins; 1998. p. 2709. [Google Scholar]
- 24.Smirniotopoulos JG, Murphy FM. The phakomatoses. AJNR. 1992;13(2):725–746. [PMC free article] [PubMed] [Google Scholar]
- 25.Uram M, Zubillaga C. The cutaneous manifestations of Sturge-Weber syndrome. J Clin Neuro-ophthalmol. 1982;2(4):245–248. [PubMed] [Google Scholar]
- 26.Rosen E. Hemangioma of the choroid. Ophthalmologica. 1950;120(3):127–149. doi: 10.1159/000300885. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenstein BW. Sturge-Weber-Dimitri syndrome. Cephalic form of neurocutaneous hemagiomatosis. Arch Neurol Psychiatry. 1954;71(3):291–301. doi: 10.1001/archneurpsyc.1954.02320390021002. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Bermejo A, Tendero A, López-Martín V, et al. Angiomatosis leptomeníngea occipital sin angioma facial ¿Debe considerarse como variante del síndrome de Sturge-Weber? Rev Neurol. 2000;30:837–841. [PubMed] [Google Scholar]
- 29.Gorman RJ, Snead OC. Sturge-Weber syndrome without port-wine nevus. Pediatrics. 1977;60(5):785. [PubMed] [Google Scholar]
- 30.Poser CM, Taveras JM. Cerebral angiography in encephalo-trigeminal angiomatosis. Radiology. 1957;68(3):327–336. doi: 10.1148/68.3.327. [DOI] [PubMed] [Google Scholar]
- 31.Gomez MR, Bebin EM. Sturge-Weber syndrome. In: Gomez ER, editor. Neurocutaneous Diseases: A Practical Approach. 5th ed. Stoneham, MA: Butterworth; 1987. pp. 356–375. [Google Scholar]
- 32.Smoller B, Rosen S. Port-wine stains: A disease of altered neuromodulation of blood vessels? Arch Dermatol. 1986;122(2):177–179. doi: 10.1001/archderm.122.2.177. [DOI] [PubMed] [Google Scholar]
- 33.Enjolras O, Riche MC, Merland JJ. Malformations vasculaires superficielles (artérielles et veineuses): aspects cliniques et examens complémentaires. Ann Chir Plast Esthet. 1991;36(4):271–278. [PubMed] [Google Scholar]
- 34.Schnyder UW. Zur Klinik und Histologie der Angiome. 2. Mitteilung: Die Feuermäler (Naevi telangiectatici) Arch Dermatol Syph. 1954;198(1):51–74. [PubMed] [Google Scholar]
- 35.Mulliken J, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69(3):412–420. doi: 10.1097/00006534-198203000-00002. discussion 421–422. [DOI] [PubMed] [Google Scholar]
- 36.Burns AJ, Kaplan LC, Mulliken JB. Is there an association between hemangioma and syndromes with dysmorphic features? Pediatrics. 1991;88(6):1257–1267. [PubMed] [Google Scholar]
- 37.Finley JL, Clark RAF, Colvin RB, et al. Immunofluorescent staining with antibodies to factor VIII, fibronectin, and collagenous basement membrane protein in normal human skin and port-wine stains. Arch Dermatol. 1982;118(12):971–975. [PubMed] [Google Scholar]
- 38.Miescher G. Über plane Angiome (Naevi hyperaemici) Dermatologica. 1953;106(3–5):176–183. [PubMed] [Google Scholar]
- 39.Mitsuhashi Y, Odermatt BF, Schneider BV, Schnyder UW. Immunohistological evaluation of endothelial markers and basement membrane components in port-wine stains. Dermatologica. 1988;176(5):243–250. doi: 10.1159/000248712. [DOI] [PubMed] [Google Scholar]
- 40.Rydh M, Malm M, Jernbeck J, Dalsgaard CJ. Ectatic blood vessels in port-wine stains lack innervation: possible role in pathogenesis. Plast Reconstr Surg. 1991;87(3):419–421. doi: 10.1097/00006534-199103000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Guyton AC, Hall JE. Texbook of Medical Physiology. 10th ed. Philadelphia: WB Saunders; 2000. The autonomic nervous system and the adrenal medulla; pp. 697–708. [Google Scholar]
- 42.Witschel H, Font RL. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv Ophthalmol. 1976;20(6):415–431. doi: 10.1016/0039-6257(76)90067-9. [DOI] [PubMed] [Google Scholar]
- 43.Susac JO, Smith JL, Scelfo RJ. The “tomato-catsup” fundus in Sturge-Weber syndrome. Arch Ophthalmol. 1974;92:69–70. doi: 10.1001/archopht.1974.01010010073018. [DOI] [PubMed] [Google Scholar]
- 44.Heine L. Hämangiome des Ziliarkörpers mit Bemerkungen über Hypertrophie der Ziliarfortsätze. Zeitschr Augenheilkd. 1926;58(3):191–193. [Google Scholar]
- 45.Watters EC, Hiles DA, Johnson BL. Cloverleaf skull syndrome. Am J Ophthalmol. 1973;76(5):716–720. doi: 10.1016/0002-9394(73)90567-9. [DOI] [PubMed] [Google Scholar]
- 46.Phelps CD, Thompson HS, Ossoining KC. The diagnosis and prognosis of atypical carotid-cavernous fistula (red-eyed shunt syndrome) Am J Ophthalmol. 1982;93(4):423–426. doi: 10.1016/0002-9394(82)90132-5. [DOI] [PubMed] [Google Scholar]
- 47.Harris GJ, Rice PR. Angle closure in carotid-cavernous fistula. Ophthalmology. 1979;86(8):1521–1529. doi: 10.1016/s0161-6420(79)35367-2. [DOI] [PubMed] [Google Scholar]
- 48.Moniz E, Lima A. Pseudo-angiomes calcifiés du cerveau. Angiome de la face et calcifications corticales du cerveau (maladie de Knud H. Krabbe) Rev Neurol. 1935;63:743–750. [Google Scholar]
- 49.Peters G. Zur Pathogenese der Sturge-Weberschen Krankheit. Z Gesamte Neurol Psychiatr. 1939;164(1):365–379. [Google Scholar]
- 50.Henkes H, Bittner R, Huber G, et al. Die Sturge-Weber-Erkrankung. Bildgebende Diagnostik in Bezug zur Neuropathologie. Radiologe. 1991;31(6):289–296. [PubMed] [Google Scholar]
- 51.Benedikt RA, Brown DC, Walker R, et al. Sturge-Weber syndrome: cranial MR imaging with Gd-DTPA. AJNR. 1993;14(2):409–415. [PMC free article] [PubMed] [Google Scholar]
- 52.Aguglia U, Latella MA, Cafarelli F, et al. Spontaneous obliteration of MRI-silent cerebral angiomatosis revealed by CT angiography in a patient with Sturge-Weber syndrome. J Neurol Sci. 2008;264(1–2):168–172. doi: 10.1016/j.jns.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 53.Padget DH. The cranial venous system in man in reference to development, adult configuration, and relation to the arteries. Am J Anat. 1956;98(3):307–355. doi: 10.1002/aja.1000980302. [DOI] [PubMed] [Google Scholar]
- 54.Parsa CF, Cheeseman EW, Jr, Maumenee IH. Demonstration of exclusive cilioretinal vascular system supplying the retina in man: vacant discs. Trans Am Ophthalmol Soc. 1998;96:95–106. discussion 106–109. [PMC free article] [PubMed] [Google Scholar]
- 55.Parsa CF, Silva ED, Sundin OH, et al. Redefining papillorenal syndrome: an underdiagnosed cause of ocular and renal morbidity. Ophthalmology. 2001;108(4):738–749. doi: 10.1016/s0161-6420(00)00661-8. [DOI] [PubMed] [Google Scholar]
- 56.Vogl TJ, Stemmler J, Bergman C, Pfluger T, Egger E, Lissner J. MR and MR angiography of Sturge-Weber syndrome. AJNR. 1993;14(2):417–425. [PMC free article] [PubMed] [Google Scholar]
- 57.Stimac GK, Solomon MA, Newton TH. CT and MR of angiomatous malformations of the choroid plexus in patients with Sturge-Weber disease. AJNR. 1986;7(4):623–627. [PMC free article] [PubMed] [Google Scholar]
- 58.Griffiths PD, Blaser S, Boodram MB, et al. Choroid plexus size in young children with Sturge-Weber syndrome. AJNR. 1996;17(1):175–180. [PMC free article] [PubMed] [Google Scholar]
- 59.Newton TH, Potts DG. Radiology of the skull and brain. In: Newton TH, Potts DG, editors. Angiography. II. St Louis: CV Mosby; 1974. pp. 1886–1887.pp. 2551pp. 2749–2751. [Google Scholar]
- 60.Andeweg J. The anatomy of collateral venous flow from the brain and its value in aetiological interpretation of intracranial pathology. Neuroradiology. 1996;38(7):621–628. doi: 10.1007/s002340050321. [DOI] [PubMed] [Google Scholar]
- 61.Ton-That QT, Picard D, Bissoon-Doyal D, et al. Technectium-99 m HMPAO imaging in Sturge-Weber syndrome. Clin Nucl Med. 1990;15(3):178–180. doi: 10.1097/00003072-199003000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Riela AR, Stump DA, Roach ES, McLean WT, Jr, Garcia JC. Regional cerebral blood flow characteristics of the Sturge-Weber syndrome. Pediatr Neurol. 1985;1(2):85–90. doi: 10.1016/0887-8994(85)90042-6. [DOI] [PubMed] [Google Scholar]
- 63.Sager WD, Schmidberger H. Enzephalo-Trigeminal Angiomatosis. Sturge-Weber-Krabbe. Radiologe. 1977;17(10):428–431. [PubMed] [Google Scholar]
- 64.Maria BL, Neufeld JA, Rosainz LC, et al. Central nervous system structure and function in Sturge-Weber syndrome: evidence of neurologic and radiologic progression. J Child Neurol. 1998;13(12):606–618. doi: 10.1177/088307389801301204. [DOI] [PubMed] [Google Scholar]
- 65.Gupta R, Rowshan K, Chao T, et al. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol. 2004;187(2):500–508. doi: 10.1016/j.expneurol.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 66.Ochoa J, Fowler TJ, Gilliatt RW. Anatomical changes in peripheral nerves compressed by a pneumatic tourniquet. J Anat. 1972;113(Pt 3):433–455. [PMC free article] [PubMed] [Google Scholar]
- 67.Dyck PJ, Lais AC, Giannini C, Engelstad JK. Structural alterations of nerve during cuff compression. Proc Natl Acad Sci U S A. 1990;87(24):9828–9832. doi: 10.1073/pnas.87.24.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cushing H, Bailey P, Part I. Tumors Arising From the Blood Vessels of the Brain: Angiomatous Malformations and Hemangioblastomas. Springfield, IL: Charles C Thomas; 1928. The angiomatous malformations; pp. 9–10. [Google Scholar]
- 69.Streeter GL. The development of the venous sinuses of the dura mater in the human embryo. Am J Anat. 1915;18(2):145–178. [Google Scholar]
- 70.Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev Biol. 1987;120(1):198–214. doi: 10.1016/0012-1606(87)90118-7. [DOI] [PubMed] [Google Scholar]
- 71.Matson DD. Neurosurgery of Infancy and Childhood. 2nd ed. Springfield, IL: Charles C Thomas; 1969. p. 777. [Google Scholar]
- 72.Browder J, Browder A, Kaplan HA. Anatomical relationships of the cerebral and dural venous systems in the parasagittal area. Anat Rec. 1973;176(3):329–332. doi: 10.1002/ar.1091760308. [DOI] [PubMed] [Google Scholar]
- 73.Heinz ER. Section II. Pathology involving the supratentorial veins and dural sinuses, radiology of the skull and brain. In: Newton TH, Potts DG, editors. Angiography. Vol. 2. St Louis: CV Mosby; 1974. p. 1878. [Google Scholar]
- 74.Rhoton AL., Jr The cerebral veins. Neurosurgery. 2002;51(4 Suppl):S159–205. [PubMed] [Google Scholar]
- 75.Johnston KD, Walji AH, Fox RJ, Pugh JA, Aronyk KE. Access to cerebrospinal fluid absorption sites by infusion into vascular channels of the skull diploë. J Neurosurg. 2007;107(4):841–843. doi: 10.3171/JNS-07/10/0841. [DOI] [PubMed] [Google Scholar]
- 76.Tech KE, Becker CJ, Lazo A, et al. Anomalous intracranial venous drainage mimicking orbital or cavernous arteriovenous fistula. AJNR. 1995;16(1):171–174. [PMC free article] [PubMed] [Google Scholar]
- 77.Cambon H, Truelle JL, Baron JC, et al. Ischémie chronique focale et migraine accompagnée: forme atypique d’une angiomatose de Sturge-Weber? Rev Neurol (Paris) 1987;143(8–9):588–594. [PubMed] [Google Scholar]
- 78.Hadjikoutis S, Carroll C, Plant GT. Raised intracranial pressure presenting with spontaneous periorbital bruising: two case reports. J Neurol Neurosurg Psychiatry. 2004;75(8):1192–1193. doi: 10.1136/jnnp.2003.016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brodsky MC, Hoyt WF. Spontaneous involution of retinal and intracranial arteriovenous malformation in Bonnet-Dechaume-Blanc syndrome. Br J Ophthalmol. 2002;86(3):360–361. doi: 10.1136/bjo.86.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacobs K, Moulin T, Bogousslavsky J, et al. The stroke syndrome of cortical vein thrombosis. Neurology. 1996;47(2):376–382. doi: 10.1212/wnl.47.2.376. [DOI] [PubMed] [Google Scholar]
- 81.Gabrielsen TO, Heinz ER. Spontaneous aseptic thrombosis of the superior sagittal sinus and cerebral veins. Am J Roentgenol Radium Ther Nucl Med. 1969;107(3):579–588. doi: 10.2214/ajr.107.3.579. [DOI] [PubMed] [Google Scholar]
- 82.Heinz ER, Geeter D, Gabrielsen TO. Cortical vein thrombosis in the dog with a review of aseptic intracranial venous thrombosis in man. Acta Radiol Diagn (Stockh) 1972;13(1):105–114. doi: 10.1177/02841851720130p116. [DOI] [PubMed] [Google Scholar]
- 83.Orr LS, Osher RH, Savino PJ. The syndrome of facial nevi, anomalous cerebral venous return, and hydrocephalus. Ann Neurol. 1978;3(4):316–318. doi: 10.1002/ana.410030407. [DOI] [PubMed] [Google Scholar]
- 84.Shapiro K, Shulman K. Facial nevi associated with anomalous venous return and hydrocephalus. J Neurosurg. 1976;45(1):20–25. doi: 10.3171/jns.1976.45.1.0020. [DOI] [PubMed] [Google Scholar]
- 85.Prats Vinas JM, Suinaga Errasti I, Blanco Lago R. Shapiro Shulman syndrome: a forgotten condition. Pediatr Neurol. 2011;44(4):308–310. doi: 10.1016/j.pediatrneurol.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 86.Salim A, Kurwa H, Turner R. Acquired port-wine stain associated with glaucoma. Clin Exp Dermatol. 2003;28(2):230–231. doi: 10.1046/j.1365-2230.2003.01232_8.x. [DOI] [PubMed] [Google Scholar]
- 87.Fegeler F. Naevus flammeus im Trigeminusgebiet nach Trauma im Rahmen eines posttraumatisch-vegetativen Syndroms. Arch Dermatol Syph. 1949;188(3):416–422. [Google Scholar]
- 88.Piaserico S, Belloni Fortina A. Posttraumatic port-wine stain in a 4-year-old-girl: Fegeler syndrome. Pediatr Dermatol. 2004;21(2):131–133. doi: 10.1111/j.0736-8046.2004.21209.x. [DOI] [PubMed] [Google Scholar]
- 89.Senti G, Trüeb RM. Erworbener Naevus flammeus (Fegeler syndrom) Vasa. 2000;29(3):225–228. doi: 10.1024/0301-1526.29.3.225. [DOI] [PubMed] [Google Scholar]
- 90.Adams BB, Lucky AW. Acquired port-wine stains and antecedent trauma: case report and review of the literature. Arch Dermatol. 2000;136(7):897–899. doi: 10.1001/archderm.136.7.897. [DOI] [PubMed] [Google Scholar]
- 91.Yallapragada AV, Cure JK, Holden KR. Sturge-Weber syndrome variant with atypical intracranial findings: case report. J Child Neurol. 2006;21(2):155–157. doi: 10.1177/08830738060210020801. [DOI] [PubMed] [Google Scholar]
- 92.Kawaguchi S, Sakaki T, Uranishi R. Color Doppler flow imaging of the superior ophthalmic vein in dural arteriovenous fistulas. Stroke. 2002;33(8):2009–2013. doi: 10.1161/01.str.0000023578.25318.b9. [DOI] [PubMed] [Google Scholar]
- 93.Mueller SM, Reinertson JE. Reversal of emissary vein blood flow in achondroplastic dwarfs. Neurology. 1980;30(7 Pt 1):769–772. doi: 10.1212/wnl.30.7.769. [DOI] [PubMed] [Google Scholar]
- 94.Caputa M, Perrin G, Cabanac M. Écoulement sanguin réversible dans la veine ophtalmique méchanisme de refroidissement sélectif du cerveau humain. C R Acad Sci Hebd Seances Acad Sci D. 1978;287(11):1011–1014. [PubMed] [Google Scholar]
- 95.Erickson SJ, Hendrix LE, Massaro BM, et al. Color Doppler flow imaging of the normal and abnormal orbit. Radiology. 1989;173(2):511–516. doi: 10.1148/radiology.173.2.2678264. [DOI] [PubMed] [Google Scholar]
- 96.Stokes JJ. The ocular manifestations of the Sturge-Weber syndrome. Southern Med J. 1957;50(1):82–89. [PubMed] [Google Scholar]
- 97.Kim DH, Parsa CF. Space flight and disc edema. Ophthalmology. 2012;119(11):2420–2421. doi: 10.1016/j.ophtha.2012.07.016. author reply 2421–2422. [DOI] [PubMed] [Google Scholar]
- 98.Kim DH, Parsa CF. Emissary veins and neuroanatomic changes in space. Radiology. 2013;266(1):362–363. doi: 10.1148/radiol.12121272. [DOI] [PubMed] [Google Scholar]
- 99.Traub EF. Naevus flammeus appearing at the age of twenty-three. Arch Dermatol Syph. 1939;39:752. [Google Scholar]
- 100.Orten SS, Waner M, Flock S, Roberson PK, Kincannon J. Port-wine stains. An assessment of 5 years of treatment. Arch Otolaryngol Head Neck Surg. 1996;122(11):1174–1179. doi: 10.1001/archotol.1996.01890230022005. [DOI] [PubMed] [Google Scholar]
- 101.Cobb S. Hæmangioma of the spinal cord associated with skin nævi of the same metamere. Ann Surg. 1915;62(6):641–649. doi: 10.1097/00000658-191512000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Griffiths PD, Boodram MB, Blaser S, et al. Abnormal ocular enhancement in Sturge-Weber syndrome: correlation of ocular MR and CT findings with clinical and intracranial imaging findings. AJNR. 1996;17(4):749–754. [PMC free article] [PubMed] [Google Scholar]
- 103.Wu WC, Lai CC, Su WJ, et al. Ischemic retinopathy and uveitis in a patient with Tetrology of Fallot. Ophthalmology. 2005;112(11):1936–1940. doi: 10.1016/j.ophtha.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 104.Faure C, Miocque S, Fleury L, Robert MP. Primary pulmonary arterial hypertension diagnosed via its ophthalmic features in an adult: diagnosis and therapeutic challenges. Br J Ophthalmol. 2012;96(3):459–460. 469. doi: 10.1136/bjo.2010.187468. [DOI] [PubMed] [Google Scholar]
- 105.Shinojima A, Iwasaki K, Aoki K, Ogawa Y, Yanagida R, Yuzawa M. Subfoveal choroidal thickness and foveal retinal thickness during head-down tilt. Aviat Space Environ Med. 2012;83(4):388–393. doi: 10.3357/asem.3191.2012. [DOI] [PubMed] [Google Scholar]
- 106.Schirmer R. Ein Fall von Teleangiektasie. Albrecht von Graefes Arch Ophthalmol. 1860;7(1):119–121. [Google Scholar]
- 107.Bär C. Ein bemerkenswerter Fall von Feuermal und Glaukom. Z Augenheilkd. 1925;57(1–6):628–632. [Google Scholar]
- 108.Mehney GH. Naevus flammeus associated with glaucoma. Report of a case. Arch Ophthalmol. 1937;17(6):1018–1023. [Google Scholar]
- 109.Thomas-Sohl KA, Vaslow DF, Maria BL. Sturge-Weber syndrome: a review. Pediatr Neurol. 2004;30(5):303–310. doi: 10.1016/j.pediatrneurol.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 110.Tsai FY, Wang AM, Matovich VB, et al. MR staging of acute dural sinus thrombosis: correlation with venous pressure measurements and implications for treatment and prognosis. AJNR. 1995;16(5):1021–1029. [PMC free article] [PubMed] [Google Scholar]
- 111.Yoshimoto Y, Endo M, Mori T, Wakai S. Correlation between venous stump pressure and brain damage after cortical vein occlusion: an experimental study. J Neurosurg. 1997;86(4):694–698. doi: 10.3171/jns.1997.86.4.0694. [DOI] [PubMed] [Google Scholar]
- 112.Kiley MA, Oxbury JM, Coley SC. Intracranial hypertension in Sturge-Weber/Klippel-Trenaunay-Weber overlap syndrome due to impairment of cerebral venous outflow. J Clin Neurosci. 2002;9(3):330–333. doi: 10.1054/jocn.2001.1041. [DOI] [PubMed] [Google Scholar]
- 113.Curé J, Holden KR, Van Tassel P. Progressive venous occlusion in a neonate with Sturge-Weber syndrome: demonstration with MR venography. AJNR. 1995;16(7):1539–1542. [PMC free article] [PubMed] [Google Scholar]
- 114.Bollinger A, Leu AJ, Hoffmann U, Franzeck UK. Microvascular changes in venous disease: an update. Angiology. 1997;48(1):27–32. doi: 10.1177/000331979704800105. [DOI] [PubMed] [Google Scholar]
- 115.Speiser DE, Bollinger A. Microangiopathy in mild chronic venous incompetence (CVI): morphological alterations and increased transcapillary diffusion detected by fluorescence videomicroscopy. Int J Microcirc Clin Exp. 1991;10(1):55–66. [PubMed] [Google Scholar]
- 116.Schneider BV, Mitsuhashi Y, Schnyder UW. Ultrastructural observations in port wine stains. Arch Dermatol Res. 1988;280(6):338–345. doi: 10.1007/BF00426611. [DOI] [PubMed] [Google Scholar]
- 117.Moritani T, Kim J, Sato Y, Bonthius D, Smoker WR. Abnormal hypermyelination in a neonate with Sturge-Weber syndrome demonstrated on diffusion-tensor imaging. J Magn Reson Imaging. 2008;27(3):617–620. doi: 10.1002/jmri.21248. [DOI] [PubMed] [Google Scholar]
- 118.Porto L, Kieslich M, Yan B, Zanella FE, Lanfermann H. Accelerated myelination associated with venous congestion. Eur Radiol. 2006;16(4):922–926. doi: 10.1007/s00330-005-0044-x. [DOI] [PubMed] [Google Scholar]
- 119.Guyton AC, Hall JE. Texbook of Medical Physiology. 10th ed. Philadelphia: WB Saunders; 2000. Contraction of skeletal muscle; p. 77. [Google Scholar]
- 120.Mortimer PS. Implications of the lymphatic system in CVI-associated edema. Angiology. 2000;51(1):3–7. doi: 10.1177/000331970005100102. [DOI] [PubMed] [Google Scholar]
- 121.Stemmer R, Marescaux J, Furderer CR. Lymphatique et paroi veineuse clinique. Phlebologie. 1984;37(2):195–200. [PubMed] [Google Scholar]
- 122.Bollinger A, Isenring G, Franzeck UK. Lymphatic microangiopathy: a complication of severe chronic venous incompetence (CVI) Lymphology. 1982;15(2):60–65. [PubMed] [Google Scholar]
- 123.Partsch H, Kahn P, Worell P. Lymphovenöse Anastomosen im Bereich post-thrombotischer Unterschenkelgeschwüre. Vasa. 1982;11(3):188. [PubMed] [Google Scholar]
- 124.Franzeck UK, Haselbach P, Speiser D, Bollinger A. Microangiopathy of cutaneous blood and lymphatic capillaries in chronic venous insufficiency. Yale J Biol Med. 1993;66(1):37–46. [PMC free article] [PubMed] [Google Scholar]
- 125.Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368(21):1971–1979. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pakravan M, Parsa A, Sanagou M, Parsa CF. Central corneal thickness and correlation to optic disc size: a potential link for susceptibility to glaucoma. Br J Ophthalmol. 2007;91(1):26–28. doi: 10.1136/bjo.2006.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berenbruch K. Ein Fall von multiplen Angio-Lipomen kombiniert mit einem Angiom des Rückenmarks. Universität Tübingen; 1890. [doctoral thesis]. [Google Scholar]
- 128.Peschen M, Petter O, Vanscheidt W. Chronic venous insufficiency—from pathophysiology to therapy. 1:Pathophysiology-compression treatment-systemic pharmacotherapy. Fortschr Med. 1996;114(26):315–318. [PubMed] [Google Scholar]
- 129.Jacob AG, Driscoll DJ, Shaughnessy WJ, et al. Klippel-Trénaunay syndrome: spectrum and management. Mayo Clin Proc. 1998;73(1):28–36. doi: 10.1016/S0025-6196(11)63615-X. [DOI] [PubMed] [Google Scholar]
- 130.Phillips GN, Gordon DH, Martin EC, Haller JO, Casarella W. The Klippel-Trénaunay syndrome: clinical and radiological aspects. Pediatr Radiol. 1978;128(2):429–434. doi: 10.1148/128.2.429. [DOI] [PubMed] [Google Scholar]
- 131.Lindenauer SM. The Klippel-Trenaunay syndrome: varicosity, hypertrophy and hemangioma with no arteriovenous fistula. Ann Surg. 1965;162(2):303–314. doi: 10.1097/00000658-196508000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lindenauer SM. Congenital arterivenous fistula and the Klippel-Trenaunay syndrome. Ann Surg. 1971;174(2):248–263. doi: 10.1097/00000658-197108000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baskerville PA, Akroyd JS, Browse NL. The etiology of the Klippel-Trenaunay syndrome. Ann Surg. 1985;202(5):624–627. doi: 10.1097/00000658-198511000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gloviczki P, Stanson AW, Stickler GB, et al. Klippel-Trenaunay syndrome: the risks and benefits of vascular interventions. Surgery. 1991;110(3):469–479. [PubMed] [Google Scholar]
- 135.Vollmar J, Voss E. Vena marginalis lateralis persistens—die vergessene Vene der Angiologen. Vasa. 1979;8(3):192–202. [PubMed] [Google Scholar]
- 136.Lea Thomas M, Macfie GB. Phlebography in the Klippel-Trenaunay syndrome. Acta Radiol. 1974;15(1):43–56. doi: 10.1177/028418517401500105. [DOI] [PubMed] [Google Scholar]
- 137.Gorenstein A, Shifrin E, Gordon RL, et al. Congenital aplasia of the deep veins of lower extremeties in children: the role of ascending functional phlebography. Surgery. 1986;99(4):414–419. [PubMed] [Google Scholar]
- 138.Servelle M. Stase veineuse et croissance osseuse. Bull Acad Natl Med. 1948;132:471–474. [Google Scholar]
- 139.Servelle M, Albeaux-Fernet M, Laborde S, Chabot J, Rougeulle J. Lésions des vasseaux lymphatiques dans les malformations congénitales des veines profondes. Presse Med. 1957;65(23):531–534. [PubMed] [Google Scholar]
- 140.Servelle M. Klippel and Trénaunay’s syndrome. 768 operated cases. Ann Surg. 1985;201(3):365–373. doi: 10.1097/00000658-198503000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Malan E, Puglionisi A. Congenital angiodysplasias of the extremities. I. Generalities and classification; venous dysplasias. J Cardiovasc Surg. 1964;5:87–130. [PubMed] [Google Scholar]
- 142.Olivier C. Les varices symptomatiques de malformations vaculaires. Essai de classification. Presse Med. 1955;63(86):1822–1825. [PubMed] [Google Scholar]
- 143.Bircher AJ, Koo JYM, Frieden IJ, Berger TG. Angiodysplastic syndrome with capillary and venous malformation associated with soft tissue hypotrophy. Dermatology. 1994;189(3):292–296. doi: 10.1159/000246865. [DOI] [PubMed] [Google Scholar]
- 144.Taheri SA, Williams J, Boman L, Pisano S. Superficial femoral vein transposition in Klippel-Trénaunay syndrome. J Pediatr Surg. 1989;24(5):494–496. doi: 10.1016/s0022-3468(89)80411-7. [DOI] [PubMed] [Google Scholar]
- 145.Gloviczki P, Hollier LH, Telander RL, et al. Surgical implications of Klippel-Trénaunay syndrome. Ann Surg. 1983;197(3):353–362. doi: 10.1097/00000658-198303000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Noel AA, Gloviczki P, Cherry KJ, Jr, et al. Surgical treatment of venous malformations in Klippel-Trénaunay syndrome. J Vasc Surg. 2000;32(5):840–847. doi: 10.1067/mva.2000.110343. [DOI] [PubMed] [Google Scholar]
- 147.Horton BT. Some medical aspects of congenital arteriovenous fistula:report of 38 cases. Staff Meet Mayo Clin. 1934;9:460–463. [Google Scholar]
- 148.Janes JM, Musgrove JE. Effect of arteriovenous fistula on growth of bone: preliminary report. Mayo Clin Proc. 1949;24(16):405–408. [PubMed] [Google Scholar]
- 149.Hutchison WJ, Burdeaux BD. The influence of stasis on bone growth. Surg Gynecol Obstet. 1954;99(4):413–420. [PubMed] [Google Scholar]
- 150.Kishikawa E. Studien über einige lokale Reize, welche das Längenwachstum des Langröhrenknochens steigern. Fukuoka Acta Med. 1936;29:4. [Google Scholar]
- 151.Ippen H. Systematisierte Angiektasie mit Gliedmaβenatrophie (ein Beitrag zum “Klippel-Trenaunay-syndrom”) Hautarzt. 1957;8(7):317–320. [PubMed] [Google Scholar]
- 152.Nafday SM, Heiden RA, Rai DB. Unusual case of Klippel-Trenaunay syndrome. A case report and review of the literature. Int J Angiol. 2000;9(4):250–253. doi: 10.1007/BF01623905. [DOI] [PubMed] [Google Scholar]
- 153.Korting GW, Dölcher G. Ungewohnliches Klippel-Trenaunay-und Parkes-Weber-Syndrom mit homolateraler Muskelatrophie. Med Welt. 1969;46:2543–2544. [PubMed] [Google Scholar]
- 154.Kresbach H, Röckl H. Klippel-Trénaunay-Syndrom mit homolateraler Atrophie. Hautarzt. 1958;9(9):417–419. [PubMed] [Google Scholar]
- 155.Liu NF, Lu Q, Yan ZX. Lymphatic malformation is a common component of Klippel-Trenaunay syndrome. J Vasc Surg. 2010;52(6):1557–1563. doi: 10.1016/j.jvs.2010.06.166. [DOI] [PubMed] [Google Scholar]
- 156.Poulet J, Ruff F. Les dysplasies veineuses congénitales des membres. Presse Med. 1969;77(5):163–166. [PubMed] [Google Scholar]
- 157.Baráth B, Vörös E, Bak Z, Bodosi M. Cerebral venous drainage via the ophthalmic veins in the Sturge-Weber syndrome. Neuroradiology. 1994;36(4):318–320. doi: 10.1007/BF00593271. [DOI] [PubMed] [Google Scholar]
- 158.Parkes Weber F. Angioma-formation in connection with hypertrophy of limbs and hemi-hypertrophy. Br J Dermatol. 1907;19:231–235. [Google Scholar]
- 159.Mullins JF, Naylor D, Redetski J. The Klippel-Trenaunay-Weber syndrome: naevus vasculosus osteohypertrophicus. Arch Dermatol. 1962;86(2):202–206. doi: 10.1001/archderm.1962.01590080072008. [DOI] [PubMed] [Google Scholar]
- 160.Ziyeh S, Spreer J, Rössler J, et al. Parkes Weber or Klippel-Trenauney syndrome? Non-invasive diagnosis with MR projection angiography. Eur Radiol. 2004;14(11):2025–2029. doi: 10.1007/s00330-004-2274-8. [DOI] [PubMed] [Google Scholar]
- 161.Young AE. Congenital mixed vascular deformities of the limbs and their associated lesions. Birth Defects Orig Artic Ser. 1978;14(6B):289–296. [PubMed] [Google Scholar]
- 162.Maramattom BV, Cohen-Gadol AA, Wijdicks EF, Kallmes D. Segmental cutaneous hemangioma and spinal arteriovenous malformation (Cobb syndrome). Case report and historical perspective. J Neurosurg Spine. 2005;3(3):249–252. doi: 10.3171/spi.2005.3.3.0249. [DOI] [PubMed] [Google Scholar]
- 163.Soeda A, Sakai N, Iihara K, Nagata I. Cobb Syndrome in an infant: treatment with endovascular embolization and corticosteroid therapy: case report. Neurosurgery. 2003;52(3):711–714. doi: 10.1227/01.neu.0000048483.21777.b7. [DOI] [PubMed] [Google Scholar]
- 164.Kojima Y, Kuwana N. Progressive diffuse arteriovenous malformation. Case report. Neurol Med Chir (Tokyo) 1993;33(4):242–245. doi: 10.2176/nmc.33.242. [DOI] [PubMed] [Google Scholar]
- 165.Brodsky MC, Hoyt WF, Higashida RT, et al. Bonnet-Dechaume-Blanc syndrome with large facial angioma. Arch Ophthalmol. 1987;105(6):854–855. doi: 10.1001/archopht.1987.01060060144050. [DOI] [PubMed] [Google Scholar]
- 166.Fukushima T, Hamano K, Shin K, et al. A case of multiple ateriovenous malformations and diffuse venous abnormalities with facial port-wine stain. Childs Nerv Syst. 1989;5(2):114–117. doi: 10.1007/BF00571122. [DOI] [PubMed] [Google Scholar]
- 167.Kurozumi K, Onoda K, Tsuchimoto S. Large cerebral arteriovenous malformation presenting with venous ischemia in the contralateral hemisphere. Case report. J Neurosurg. 2002;97(4):995–997. doi: 10.3171/jns.2002.97.4.0995. [DOI] [PubMed] [Google Scholar]
- 168.Anderson FH, Duncan GW. Sturge-Weber disease with subarachnoid hemorrhage. Stroke. 1974;5(4):509–511. doi: 10.1161/01.str.5.4.509. [DOI] [PubMed] [Google Scholar]
- 169.Laur A. Cerebrales Riesenangiom mit Anomalie der groβen Arterien und multiplen sackförmigen Aneurysmen. Dtsch Arch Klin Med. 1953;200(2):236–249. [PubMed] [Google Scholar]
- 170.Sperner J, Schmauser I, Bittner R, et al. MR-imaging findings in children with Sturge-Weber syndrome. Neuropediatrics. 1990;21(3):146–152. doi: 10.1055/s-2008-1071483. [DOI] [PubMed] [Google Scholar]
- 171.Quan SY, Comi AM, Parsa CF, Irving ND, Krakowski AC, Cohen BA. Effect of a single application of pulsed dye laser treatment of port-wine birthmarks on intraocular pressure. Arch Dermatol. 2010;146(9):1015–1018. doi: 10.1001/archdermatol.2010.223. [DOI] [PubMed] [Google Scholar]
- 172.Garcia JC, Roach ES, McLean WT. Recurrent thrombotic deterioration in the Sturge-Weber syndrome. Childs Brain. 1981;8(6):427–433. doi: 10.1159/000120011. [DOI] [PubMed] [Google Scholar]
- 173.McCaughan B, Ouvrier RA, De Silva K, McLaughlin A. The value of the brain scan and cerebral arteriogram in Sturge-Weber syndrome. Proc Aust Assoc Neurol. 1975;12:185–190. [PubMed] [Google Scholar]
- 174.Jansen FE, van der Worp HB, van Huffelen A, van Nieuwenhuizen O. Sturge-Weber syndrome and paroxysmal hemiparesis: epilepsy or ischaemia? Dev Med Child Neurol. 2004;46(11):783–786. [PubMed] [Google Scholar]
- 175.Bay MJ, Kossoff EH, Lehmann CU, Zabel TA, Comi AM. Survey of aspirin use in Sturge-Weber syndrome. J Child Neurol. 2011;26(6):692–702. doi: 10.1177/0883073810388646. [DOI] [PubMed] [Google Scholar]
- 176.Lance EI, Sreenivasan AK, Zabel TA, Kossoff EH, Comi AM. Aspirin use in Sturge-Weber syndrome: side effects and clinical outcomes. J Child Neurol. 2013;28(2):213–218. doi: 10.1177/0883073812463607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Coley SC, Britton J, Clarke A. Status epilepticus and venous infarction in Sturge-Weber syndrome. Childs Nerv Syst. 1998;14(12):693–696. doi: 10.1007/s003810050299. [DOI] [PubMed] [Google Scholar]
- 178.Scott TA, Ausburger JJ, Brady LW, Hernandez C, Woodleigh R. Low dose ocular irradiation for diffuse choroidal hemangiomas associated with bullous nonrhegmatogenous retinal detachment. Retina. 1991;11(4):389–393. doi: 10.1097/00006982-199111040-00004. [DOI] [PubMed] [Google Scholar]
- 179.Zografos L, Bercher L, Chamot L, Gailloud C, Raimondi S, Egger E. Cobalt 60 treatment of choroidal hemangiomas. Am J Ophthalmol. 1996;121(2):190–199. doi: 10.1016/s0002-9394(14)70584-7. [DOI] [PubMed] [Google Scholar]
- 180.Zografos L, Egger E, Bercher L, Chamot L, Munkel G. Proton beam irradiation of choroidal hemangiomas. Am J Ophthalmol. 1998;126(2):261–268. doi: 10.1016/s0002-9394(98)00150-0. [DOI] [PubMed] [Google Scholar]
- 181.Sakai H, Sakima N, Nakamura Y, Nakamura Y, Hayakawa K, Sawaguchi S. Ciliochoroidal effusion induced by topical latanoprost in a patient with Sturge-Weber syndrome. Jpn J Ophthalmol. 2002;46(5):553–555. doi: 10.1016/s0021-5155(02)00542-7. [DOI] [PubMed] [Google Scholar]
- 182.Gambrelle J, Denis P, Kocaba V, Grange JD. Uveal effusion induced by topical travoprost in a patient with Sturge-Weber-Krabbe syndrome. J Fr Ophtalmol. 2008;31(9):e19. [PubMed] [Google Scholar]
- 183.Paulus YM, Jain A, Moshfeghi DM. Resolution of persistent exudative retinal detachment in a case of Sturge-Weber syndrome with anti-VEGF administration. Ocul Immunol Inflamm. 2009;17(4):292–294. doi: 10.1080/09273940902989357. [DOI] [PubMed] [Google Scholar]
- 184.Anand R. Photodynamic therapy for diffuse choroidal hemangioma associated with Sturge Weber syndrome. Am J Ophthalmol. 2003;136(4):758–760. doi: 10.1016/s0002-9394(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 185.Bains HS, Cirino AC, Ticho BH, Jampol LM. Photodynamic therapy using verteporfin for a diffuse choroidal hemangioma in Sturge-Weber syndrome. Retina. 2004;24(1):152–155. doi: 10.1097/00006982-200402000-00022. [DOI] [PubMed] [Google Scholar]
- 186.Huiskamp EA, Müskens RPHM, Ballast A, Hooymans JMM. Diffuse choroidal haemangioma in Sturge-Weber syndrome treated with photodynamic therapy under general anaesthesia. Graefes Arch Clin Exp Ophthalmol. 2005;243(7):727–730. doi: 10.1007/s00417-004-1102-9. [DOI] [PubMed] [Google Scholar]
- 187.Singh AD, Rundle PA, Vardy SJ, Rennie IG. Photodynamic therapy of choroidal haemangioma associated with Sturge-Weber syndrome. Eye. 2005;19(3):365–367. doi: 10.1038/sj.eye.6701474. [DOI] [PubMed] [Google Scholar]
- 188.Arevalo JF, Arias JD, Serrano MA. Oral propranolol for exudative retinal detachment in diffuse choroidal hemangioma. Arch Ophthalmol. 2011;129(10):1373–1375. doi: 10.1001/archophthalmol.2011.294. [DOI] [PubMed] [Google Scholar]
- 189.Krema H, Yousef YA, Durairaj P, Santiago R. Failure of systemic propranolol therapy for choroidal hemangioma of Sturge-Weber syndrome: a report of 2 cases. JAMA Ophthalmol. 2013;131(5):681–683. doi: 10.1001/jamaophthalmol.2013.2879. [DOI] [PubMed] [Google Scholar]
- 190.Thapa R, Shields CL. Oral propranolol therapy for management of exudative retinal detachment from diffuse choroidal hemangioma in Sturge-Weber syndrome. Eur J Ophthalmol. 2013 Jun 2; doi: 10.5301/ejo.5000322. 0 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 191.Sullivan TJ, Clarke MP, Morin JD. The ocular manifestations of the Sturge-Weber syndrome. J Pediatr Ophthalmol Strabismus. 1992;29(6):349–356. doi: 10.3928/0191-3913-19921101-05. [DOI] [PubMed] [Google Scholar]
- 192.Hoskins HD, Jr, Shaffer RN, Hetherington J. Goniotomy vs trabeculotomy. J Pediatr Ophthalmol Strabismus. 1984;21(4):153–158. doi: 10.3928/0191-3913-19840701-06. [DOI] [PubMed] [Google Scholar]
- 193.Weiss DI. Dual origin of glaucoma in encephalotrigeminal hæmangiomatosis. A pathogenetic concept based upon histopathologic and hæmodynamic considerations. Trans Ophthalmol Soc U K. 1973;93:477–493. [PubMed] [Google Scholar]
- 194.Aggarwal NK, Gandham SB, Weinstein R, Saltzmann R, Walton DS. Heterochromia iridis and pertinent clinical findings in patients with glaucoma associated with Sturge-Weber syndrome. J Pediatr Ophthalmol Strabismus. 2010;47(6):361–365. doi: 10.3928/01913913-20100218-01. [DOI] [PubMed] [Google Scholar]
- 195.Chen SC, D’Souza N. Familial tetrology of Fallot and glaucoma. Am J Med Genet. 1990;37(1):40–41. doi: 10.1002/ajmg.1320370111. [DOI] [PubMed] [Google Scholar]
- 196.Shaeffer RN. Stereoscopic Manual of Gonioscopy. St Louis: CV Mosby; 1962. pp. 74–77. [Google Scholar]
- 197.Buus DR, Tse DT, Parrish RK. Spontaneous carotid cavernous fistula presenting with acute angle closure glaucoma. Arch Ophthalmol. 1989;107(4):596–597. doi: 10.1001/archopht.1989.01070010610039. [DOI] [PubMed] [Google Scholar]
- 198.Iwach AG, Hoskins HD, Jr, Hetherington JJR, Shaffer RN. Analysis of surgical and medical management of glaucoma in Sturge-Weber syndrome. Ophthalmology. 1990;97(7):904–909. doi: 10.1016/s0161-6420(90)32483-1. [DOI] [PubMed] [Google Scholar]
- 199.Gordon RA, Donzis PB. Refractive development of the human eye. Arch Ophthalmol. 1985;103(6):785–789. doi: 10.1001/archopht.1985.01050060045020. [DOI] [PubMed] [Google Scholar]
- 200.Weiss L. Über das Wachstum des menschlichen Auges und über die Veränderung der Muskelinsertionen am wachsenden Auge. Anat Hefte. 1897;8:193–248. [Google Scholar]
- 201.Jørgensen JS, Guthoff R. Sturge-Weber-Syndrom: Glaukom mit erhöhtem episkleralen Venendruck. Klin Monatsbl Augenheilkd. 1987;191(4):275–278. doi: 10.1055/s-2008-1050508. [DOI] [PubMed] [Google Scholar]
- 202.Phelps CD. The pathogenesis of glaucoma in Sturge-Weber syndrome. Ophthalmology. 1978;85(3):276–286. doi: 10.1016/s0161-6420(78)35667-0. [DOI] [PubMed] [Google Scholar]
- 203.Parsa CF. Spontaneous venous pulsations should be monitored during glaucoma therapy. Br J Ophthalmol. 2002;86(10):1187. doi: 10.1136/bjo.86.10.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kee C, Gabelt BT, Croft MA, et al. Serum effects on aqueous outflow during anterior chamber perfusion in monkeys. Invest Ophthalmol Vis Sci. 1996;37(9):1840–1848. [PubMed] [Google Scholar]
- 205.Russell P, Koretz J, Epstein DL. Is primary open angle glaucoma caused by small proteins? Med Hypotheses. 1993;41(5):455–458. doi: 10.1016/0306-9877(93)90125-a. [DOI] [PubMed] [Google Scholar]
- 206.Moreau PG. L’électrophorèse de l’humeur aqueuse au cours du glaucome. Bull Soc Ophtalmol Fr. 1957;7–8:444–447. [PubMed] [Google Scholar]
- 207.Renard G, Sergent P, Magis C. Données fournies par l’examen électrophorétique de glaucoma chronique simple a angle large. Bull Mem Soc Fr Ophtalmol. 1961;74:62–71. [PubMed] [Google Scholar]
- 208.Sokolova ON, Serbinenko FA, Zakhidov B. Ocular hydro- and hemodynamics in patients with carotid-cavernous fistulas. Vopr Neirokhir. 1973;37(5):36–41. [PubMed] [Google Scholar]
- 209.Bert RJ, Caruthers SD, Jara H, et al. Demonstration of an anterior diffusional pathway for solutes in the normal human eye with high spatial resolution contrast-enhanced dynamic MR imaging. Invest Ophthalmol Vis Sci. 2006;47(12):5153–5162. doi: 10.1167/iovs.05-0372. [DOI] [PubMed] [Google Scholar]
- 210.Beltman J. Über angeborene Teleangiektasien des Auges als Ursache von Glaucoma simplex. Albrecht Von Græfes Arch Ophthalmol. 1904;59(3):502–519. [Google Scholar]
- 211.Tyson HH. Nevus flammeus of the face and globe. Arch Ophthalmol. 1932;8:365–371. [Google Scholar]
- 212.Dunphy EB. Glaucoma accompanying nevus flammeus. Trans Am Ophthalmol Soc. 1934;32:143–152. [PMC free article] [PubMed] [Google Scholar]
- 213.Ethier CR, Kamm RD, Johnson M, et al. Further studies on the flow of aqueous humor through microporous filters. Invest Ophthalmol Vis Sci. 1989;30(4):739–746. [PubMed] [Google Scholar]
- 214.Cibis GW, Tripathi RC, Tripathi BJ. Glaucoma in Sturge-Weber syndrome. Ophthalmology. 1984;91(9):1061–1071. doi: 10.1016/s0161-6420(84)34194-x. [DOI] [PubMed] [Google Scholar]
- 215.Mwinula JH, Sagawa T, Tawara A, Inomata H. Anterior chamber angle vascularization in Sturge-Weber syndrome. Report of a case. Graefes Arch Clin Exp Ophthalmol. 1994;232(7):387–391. doi: 10.1007/BF00186578. [DOI] [PubMed] [Google Scholar]
- 216.Skoglund RR, Paa D, Lewis WJ. Elevated spinal-fluid protein in Sturge-Weber syndrome. Dev Med Child Neurol. 1978;20(1):99–102. doi: 10.1111/j.1469-8749.1978.tb15235.x. [DOI] [PubMed] [Google Scholar]
- 217.Boltshauser E, Wilson J. Elevated spinal-fluid protein in Sturge-Weber syndrome. Dev Med Child Neurol. 1978;29(3):392–393. doi: 10.1111/j.1469-8749.1978.tb15235.x. [DOI] [PubMed] [Google Scholar]
- 218.Bock RH. A case of bilateral Sturge-Weber syndrome. Am J Ophthalmol. 1950;33(7):1121–1127. doi: 10.1016/0002-9394(50)91730-2. [DOI] [PubMed] [Google Scholar]
- 219.Guyton AC, Hall JE. Texbook of Medical Physiology. 10th ed. Philadelphia: WB Saunders; 2000. Cerebral blood flow; the cerebrospinal fluid; and brain metabolism; pp. 697–708. [Google Scholar]
- 220.Yang CB, Freedman SF, Myers JS, Buckley EG, Herndon LW, Allingham RR. Use of latanoprost in the treatment of glaucoma associated with Sturge-Weber syndrome. Am J Ophthalmol. 1998;126(4):600–602. doi: 10.1016/s0002-9394(98)00129-9. [DOI] [PubMed] [Google Scholar]
- 221.Altuna JC, Greenfield DS, Wand M, et al. Latanoprost in glaucoma associated with Sturge-Weber syndrome: benefits and side-effects. J Glaucoma. 1999;8(3):199–203. [PubMed] [Google Scholar]
- 222.Ong T, Chia A, Nischal KK. Latanoprost in port wine stain related paediatric glaucoma. Br J Ophthalmol. 2003;87(9):1091–1093. doi: 10.1136/bjo.87.9.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Audren F, Abitbol O, Dureau P, et al. Non-penetrating deep sclerectomy for glaucoma associated with Sturge-Weber syndrome. Acta Ophthalmol Scand. 2006;84(5):646–660. doi: 10.1111/j.1600-0420.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 224.Roche O, Beby F, Parsa A, Orssaud C, Dufier JL, Parsa CF. Nonpenetrating external trabeculectomy for congenital glaucoma: a retrospective study. Ophthalmology. 2007;114(11):1994–1999. doi: 10.1016/j.ophtha.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 225.Wen F, Wu D. Indocyanine green angiographic findings in diffuse choroidal hemangioma associated with Sturge-Weber syndrome. Graefes Arch Clin Exp Ophthalmol. 2000;238(7):625–627. doi: 10.1007/s004170000146. [DOI] [PubMed] [Google Scholar]
- 226.Cross HE, Yoder F. Familial nanophthalmos. Am J Ophthalmol. 1976;81(3):300–306. doi: 10.1016/0002-9394(76)90244-0. [DOI] [PubMed] [Google Scholar]
- 227.Maruyama I, Ohguro H, Nakazawa M. A case of acute angle-closure glaucoma secondary to posterior scleritis in patient with Sturge-Weber syndrome. Jpn J Ophthalmol. 2002;46(1):74–77. doi: 10.1016/s0021-5155(01)00463-4. [DOI] [PubMed] [Google Scholar]
- 228.Fishman MA, Baram TZ. Megalencephaly due to impaired cerebral venous return in a Sturge-Weber variant syndrome. J Child Neurol. 1986;1(2):115–118. doi: 10.1177/088307388600100204. [DOI] [PubMed] [Google Scholar]
- 229.Slasky SE, Shinnar S, Bello JA. Sturge-Weber syndrome: deep venous occlusion and the radiologic spectrum. Pediatr Neurol. 2006;35(5):343–347. doi: 10.1016/j.pediatrneurol.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 230.Azar GG, Robert MP. Shapiro-Shulman and Sturge-Weber syndromes. Pediatr Neurol. 2012;47(3):227. doi: 10.1016/j.pediatrneurol.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 231.Prats Vinas JM. Shapiro-Shulman and Sturge-Weber syndromes: response. Pediatr Neurol. 2012;47(3):227–228. doi: 10.1016/j.pediatrneurol.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 232.Fishbein NJ, Barkovich AJ, Wu J, Berg BO. Sturge-Weber syndrome with no leptomeningeal enhancement on MRI. Neuroradiology. 1998;40(3):177–180. doi: 10.1007/s002340050563. [DOI] [PubMed] [Google Scholar]
- 233.Juhasz C, Chugani HT. An almost missed leptomeningeal angioma in Sturge-Weber syndrome. Neurology. 2007;68(3):243. doi: 10.1212/01.wnl.0000242581.43024.0a. [DOI] [PubMed] [Google Scholar]
- 234.Martí-Bonmatí L, Menor F, Poyatos C, Cortina H. Diagnosis of Sturge-Weber syndrome: comparison of the efficacy of CT and MR imaging in 14 cases. AJR. 1992;158(4):867–871. doi: 10.2214/ajr.158.4.1546607. [DOI] [PubMed] [Google Scholar]
- 235.Stewart G, Farmer G. Sturge-Weber and Klippel-Trenaunay syndromes with absence of inferior vena cava. Arch Dis Child. 1990;65(5):546–547. doi: 10.1136/adc.65.5.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Happle R. Sturge-Weber-Klippel-Trenaunay syndrome: What’s in a name? Eur J Dermatol. 2003;13(3):223. [PubMed] [Google Scholar]
- 237.Vissers WHPM, van Steensel MAM, Steijlen PM, Renier W, Van De Kerkhof P, Van Der Vleuten C. Klippel-Trenaunay syndrome and Sturge-Weber syndrome: Variations on a theme? Eur J Dermatol. 2003;13(3):238–241. [PubMed] [Google Scholar]
- 238.Williams DW, 3rd, Elster AD. Cranial CT and MR in the Klippel-Trenaunay-Weber syndrome. AJNR. 1992;13(1):291–294. [PMC free article] [PubMed] [Google Scholar]
- 239.McEntee M, Summers BA, de Lahunta A, Cummings J. Mengingocerebral hemangiomatosis resembling Sturge-Weber disease in a horse. Acta Neuopathol (Berl) 1987;74(4):405–410. doi: 10.1007/BF00687221. [DOI] [PubMed] [Google Scholar]
- 240.Comi AM, Bellamkonda S, Ferenc LM, Cohen BA, Germain-Lee EL. Central hypothyroidism and Sturge-Weber syndrome. Pediatr Neurol. 2008;39(1):58–62. doi: 10.1016/j.pediatrneurol.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 241.Miller RS, Ball KL, Comi AM, Germain-Lee EL. Growth hormone deficiency in Sturge-Weber syndrome. Arch Dis Child. 2006;91(4):340–341. doi: 10.1136/adc.2005.082578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kota SK, Meher LK, Kota SK, Jammula S, Krishna SV, Modi KD. Sturge-Weber syndrome: presentation with partial hypopituitarism. J Pediatr Endocrinol Metab. 2012;25(7–8):785–789. doi: 10.1515/jpem-2012-0056. [DOI] [PubMed] [Google Scholar]
- 243.Zhu M, Madigan MC, van Driel D, et al. The human hyaloid system: cell death and vascular regression. Exp Eye Res. 2000;70(6):767–776. doi: 10.1006/exer.2000.0844. [DOI] [PubMed] [Google Scholar]
- 244.Meeson A, Palmer M, Calfon M, Lang R. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development. 1996;122(12):3929–3938. doi: 10.1242/dev.122.12.3929. [DOI] [PubMed] [Google Scholar]
- 245.Morrow WR, Haas JE, Benjamin DR. Nonbacterial endocardial thrombosis in neonates: relationship to persistent fetal circulation. J Pediatr. 1982;100(1):117–122. doi: 10.1016/s0022-3476(82)80250-3. [DOI] [PubMed] [Google Scholar]
- 246.Parsa CF, Robert MP. Thromboembolism and congenital malformations: from Duane syndrome to thalidomide embryopathy. JAMA Ophthalmol. 2013;131(4):439–447. doi: 10.1001/jamaophthalmol.2013.1111. [DOI] [PubMed] [Google Scholar]
- 247.Klippel M, Trénaunay P. Du naevus variqueux ostéohypertrophique. Arch Général Méd. 1900:641–672. [Google Scholar]
- 248.Bastarrika G, Redondo P, Sierra A, et al. New techniques for the evaluation and therapeutic planning of patients with Klippel-Trenaunay syndrome. J Am Acad Dermatol. 2007;56(2):242–249. doi: 10.1016/j.jaad.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 249.Baskerville PA, Ackroyd JS, Lea Thomas M, Browse NL. The Klippel-Trenaunay syndrome: clinical, radiological and haemodynamic features and management. Br J Surg. 1985;72(3):232–236. doi: 10.1002/bjs.1800720331. [DOI] [PubMed] [Google Scholar]
- 250.Puggioni A, Kistner RL, Eklof B, Lurie F. Surgical disobliteration of postthrombotic deep veins—endophlebectomy—is feasible. J Vasc Surg. 2004;39(5):1048–1052. doi: 10.1016/j.jvs.2003.12.036. discussion 1052. [DOI] [PubMed] [Google Scholar]
- 251.Parrot MJ. Étude sur les ram-ollissements de l’encéphale chez le nouveau-né. Arch Physiol Norm. 1873;5:59–73. 176–195, 285–303. [Google Scholar]
- 252.Ford FR, Part I. Birth Injuries of the Central Nervous System. Baltimore: Williams & Wilkins; 1927. Cerebral birth Injuries; pp. 12–13.pp. 62–71. [Google Scholar]
- 253.Kalbag RM. Cerebral venous thrombosis. In: Kapp JP, Schmidek HH, editors. The Cerebral Venous System and Its Disorders. Orlando, FL: Grune & Stratton; 1984. pp. 505–536. [Google Scholar]
- 254.Hutinel V. Une contribution à l’étude des troubles de la circulation veineuse chez l’enfant et en particulier le nouveau-né. Paris: 1877. [thèse]. [Google Scholar]
- 255.Sugerman HJ. Hypothesis: preeclampsia is a venous disease secondary to an increased intra-abdominal pressure. Med Hypotheses. 2011;77(5):841–849. doi: 10.1016/j.mehy.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 256.Hunt RW, Badawi N, Laing S, Lam A. Pre-eclampsia: A predisposing factor for neonatal venous sinus thrombosis? Pediatr Neurol. 2001;25(3):242–246. doi: 10.1016/s0887-8994(01)00291-0. [DOI] [PubMed] [Google Scholar]
- 257.Lermoyez M. A sign of thrombosis of the superior longitudinal sinus. Arch Internat Laryngol Otol Rhinol. 1897;13(4):198. [Google Scholar]
- 258.Byers RK, Hass GM. Thrombosis of dural venous sinuses in infancy and childhood. Am J Dis Child. 1933;45:1161–1183. [Google Scholar]
- 259.Mitchell RG. Venous thrombosis in acute infantile hemiplegia. Arch Dis Child. 1952;27(131):95–104. doi: 10.1136/adc.27.131.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gowers WR. A Manual of Diseases of the Nervous System. Vol. 2. London: J & A Churchill; 1888. Thrombosis in the cerebral sinuses and veins; p. 416. [Google Scholar]
- 261.Aicardi J, Goutieres F. Les thromboses veineuses intra-craniennes. Complication des deshydratations aigues du nourrisson. Arch Fr Pediatr. 1973;30(8):809–829. [PubMed] [Google Scholar]
- 262.Laurichesse Delmas H, Winer N, Gallot D, et al. Prenatal diagnosis of thrombosis of the dural sinuses: report of six cases, review of the literature and suggested management. Ultrasound Obstet Gynecol. 2008;32(2):188–198. doi: 10.1002/uog.5348. [DOI] [PubMed] [Google Scholar]
- 263.Kedzia A. Cerebral bridge veins in man. Preliminary report. Folia Morphol (Warsz) 1984;43(4):295–301. [PubMed] [Google Scholar]
- 264.O’Connell JEA. Some observations on the cerebral veins. Brain. 1934;57:484–503. [Google Scholar]
- 265.Han H, Tao W, Zhang M. The dural entrance of cerebral bridging veins into the superior sagittal sinus: an anatomical comparison between cadavers and digital subtraction angiography. Neuroradiology. 2007;49(2):169–175. doi: 10.1007/s00234-006-0175-z. [DOI] [PubMed] [Google Scholar]
- 266.Cushing H. Concerning surgical intervention for the intracranial hemorrhages of the new-born. Am J Med Sci. 1905;130:563–581. doi: 10.1007/s003810000255. [DOI] [PubMed] [Google Scholar]
- 267.Ehrenfest H. The causation of intracranial hemorrhages in the new-born. Am J Dis Child. 1923;26:503. [Google Scholar]
- 268.Vignes JR, Dagain A, Guerin J, Liguoro D. A hypothesis of cerebral venous system regulation based on a study of the junction between the cortical bridging veins and the superior sagittal sinus. Laboratory investigation. J Neurosurg. 2007;107(6):1205–1210. doi: 10.3171/JNS-07/12/1205. [DOI] [PubMed] [Google Scholar]
- 269.Stopford JS. The functional significance of the arrangement of the cerebral and cerebellar veins. J Anat. 1930;64(Pt 3):257–261. [PMC free article] [PubMed] [Google Scholar]
- 270.Virchow R. Ueber Perlgeschwulste. Arch Pathol Anat. 1855;8:371–418. [Google Scholar]
- 271.Von Recklinghausen F. Handbuch der Allgemeinen Pathologie des Kreislaufs und der Ernährung. Stuttgart: Enke; 1883. Die Thrombose; p. 129. [Google Scholar]
- 272.Bergeat H. Über mehrere Fälle von autochthoner Sinusthrombose. München: 1891. [Thesis Med]. [Google Scholar]
- 273.Conway H, Johnson G., Jr Congenital absence of the scalp and skull. Ann Surg. 1956;144(6):1035–1044. doi: 10.1097/00000658-195612000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fowler GW, Dumars KW. Cutis aplasia and cerebral malformation. Pediatrics. 1973;52(6):861–864. [PubMed] [Google Scholar]
- 275.Mannino FL, Jones KL, Benirschke K. Congenital skin defects and fetus papyraceus. J Pediatr. 1977;91(4):559–564. doi: 10.1016/s0022-3476(77)80502-7. [DOI] [PubMed] [Google Scholar]
- 276.Ruiz-Maldonado R, Tamayo L. Aplasia cutis congenita, spastic paralysis, and mental retardation. Am J Dis Child. 1974;128(5):699–701. doi: 10.1001/archpedi.1974.02110300109016. [DOI] [PubMed] [Google Scholar]
- 277.Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med. 2000;342(6):374–380. doi: 10.1056/NEJM200002103420602. [DOI] [PubMed] [Google Scholar]
- 278.Gohil R, Peck G, Sharma P. The genetics of venous thromboembolism. A meta-analysis involving approximately 120,000 cases and 180,000 controls. Thromb Haemost. 2009;102(2):360–370. doi: 10.1160/TH09-01-0013. [DOI] [PubMed] [Google Scholar]
- 279.Govaert P. Prenatal stroke. Semin Fetal Neonatal Med. 2009;14(5):250–266. doi: 10.1016/j.siny.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 280.Wu YW, Miller SP, Chin K, et al. Multiple risk factors in neonatal sinovenous thrombosis. Neurology. 2002;59(3):438–440. doi: 10.1212/wnl.59.3.438. [DOI] [PubMed] [Google Scholar]
- 281.Esposito L, Rossi A, Indolfi P, Pezzolla F. Su alcuni aspetti emocoagulativi e terapeutici di un caso di sindrome di Sturge-Weber-Krabbe. Pediatria (Napoli) 1977;85(4):613–624. [PubMed] [Google Scholar]
- 282.Baskerville PA. Maladie thrombo-embolique et anomalie veineuses congénitales. Phlebologie. 1987;40(2):531–536. [PubMed] [Google Scholar]
- 283.Gianlupi A, Harper RW, Dwyre DM, Marelich GP. Recurrent pulmonary embolism associated with Klippel-Trenaunay-Weber syndrome. Chest. 1999;115(4):1199–1201. doi: 10.1378/chest.115.4.1199. [DOI] [PubMed] [Google Scholar]
- 284.Rebarber A, Roman AS, Roshan D, Blei F. Obstetric management of Klippel-Trenaunay syndrome. Obstet Gynecol. 2004;104(5 Pt 2):1205–1208. doi: 10.1097/01.AOG.0000141649.11305.4b. [DOI] [PubMed] [Google Scholar]
- 285.Grira M, Ben-Jemaa H, Lammouchi T, Benammou S. Syndrome de Klippel-Trenaunay et déficit en antithrombine III: À propos d’une observation. Rev Neurol (Paris) 2008;164(10):855–858. doi: 10.1016/j.neurol.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 286.Endo Y, Takahashi K, Mamiya S, Satoh M, Matsuda M. Factor XIII deficiency associated with Klippel-Weber disease, platelet dysfunction and cryofibrinogenemia. Acta Haematol. 1983;69(6):398–403. doi: 10.1159/000206928. [DOI] [PubMed] [Google Scholar]
- 287.Coulam CB. Hypothesis: antiphospholipid antibodies associated with congenital anomalies? Early Pregnancy. 1997;3(2):109–112. [PubMed] [Google Scholar]
- 288.Heilmann L, von Tempelhoff GF, Pollow K. Antiphospholipid syndrome in obstetrics. Clin Appl Thromb Hemost. 2003;9(2):143–150. doi: 10.1177/107602960300900209. [DOI] [PubMed] [Google Scholar]
- 289.Heller C, Schobess R, Kurnik K, et al. Abdominal venous thrombosis in neonates and infants: role of prothrombotic risk factors—a multicentre case-control study. For the Childhood Thrombophilia Study Group. Br J Haematol. 2000;111(2):534–539. doi: 10.1046/j.1365-2141.2000.02349.x. [DOI] [PubMed] [Google Scholar]
- 290.Heller C, Nowak-Göttl U. Maternal thrombophilia and neonatal thrombosis. Best Pract Res Clin Haematol. 2003;16(2):333–345. doi: 10.1016/s1521-6926(02)00096-8. [DOI] [PubMed] [Google Scholar]
- 291.Comi AM, Mehta P, Hatfield LA, Dowling MM. Sturge-Weber syndrome associated with other abnormalities: a medical record and literature review. Arch Neurol. 2005;62(12):1924–1927. doi: 10.1001/archneur.62.12.1924. [DOI] [PubMed] [Google Scholar]
- 292.Sindou M, Auque J, Jouanneau E. Neurosurgery and the intracranial venous system. Acta Neurochir Suppl. 2005;94:167–175. doi: 10.1007/3-211-27911-3_27. [DOI] [PubMed] [Google Scholar]