Abstract
Introduction
The scale-up of highly active antiretroviral therapy (HAART) has led to a significant improvement in survival of the HIV-positive patient but its effects on health-related quality of life (HRQOL) are less known and context-dependent. Our aim was to assess the temporal changes and factors associated with HRQOL among HIV-positive adults initiating HAART in Burkina Faso.
Methods
HIV-positive people initiating HAART were prospectively included and followed over a one-year period in three HIV clinics of Ouagadougou. HRQOL was assessed at baseline and at each follow-up visit using physical (PHS) and mental (MHS) summary scores derived from the Medical Outcome Study 36-Item short-form health survey (MOS SF-36) questionnaire. Toxicity related to HAART modification and self-reported symptoms were recorded during follow-up visits. Determinants associated with baseline and changes in both scores over a one-year period were assessed using a mixed linear model.
Results
A total of 344 patients were included. Their median age at baseline was 37 years [interquartile range (IQR) 30–44] and their median CD4 count was 181 cells/mm3 (IQR 97–269). The mean [standard deviation (SD)] PHS score increased from 45.4 (11.1) at baseline to 60.0 (3.1) at 12 months (p<10−4) and the mean (SD) MHS score from 42.2 (8.7) to 43.9 (3.4) (p<10−2). After one year of treatment, patients that experienced on average two symptoms during follow-up presented with significantly lower PHS (63.9) and MHS (43.8) scores compared to patients that presented no symptoms with PHS and MHS of 68.2 (p<10−4) and 45.3 (p<10−3), respectively.
Discussion
The use of HAART was associated with a significant increase in both physical and mental aspects of the HRQOL over a 12-month period in this urban African population. Perceived symptoms experienced during follow-up visits were associated with a significant impairment in HRQOL. The appropriate and timely management of reported symptoms during the follow-up of HAART-treated patients is a key component to restore HRQOL.
Keywords: quality of life, HIV/AIDS, antiretroviral treatment, Burkina Faso, sub-Saharan Africa
Introduction
The rapid and high uptake of highly active antiretroviral therapy (HAART) over the past decade has led to a significant improvement in survival of the HIV-positive patient in West Africa [1–3]. As life expectancy of HIV-positive people continues to increase, their health-related quality of life (HRQOL) is now also becoming an important issue for the sustainability of HAART programs. Indeed, to be sustainable, HAART needs to be accepted by HIV-positive patients over a long period of time. Thus, HRQOL could be a key indicator for tracking the long-term acceptability and efficiency of HAART in the context of a chronic condition such as HIV infection. In this perspective, HAART programs in West Africa need now to incorporate monitoring systems that measure both the effects of HAART on clinical markers and on overall wellbeing, including HRQOL.
HRQOL is a multidimensional construct that includes global health perspectives, functional status, biological and physical variables, individual and environmental characteristics and general perceptions [4]. Many instruments have been developed to quantify HRQOL. The Medical Outcome Study 36-Item short-form health survey (MOS SF-36) questionnaire is characterized by the fact that it explores two dimensions. The first dimension measures the disease consequences on physical quality of life by assessing physical and functional limitations. The second dimension addresses changes in the emotional state of patients, with an emphasis on mood, depression and life satisfaction [5, 6].
Since the advent of HAART, a significant improvement of HRQOL has been reported over time in treatment-naïve patients initiating HAART [7, 8]. However, these reports mainly conducted in controlled clinical trials focused on HIV-positive patients from high-income settings. Early reports from Southern and Eastern African countries have prospectively explored the impact of HAART on HRQOL of HIV-positive people over a 12-month period, showing an overall positive effect [9, 10]. Authors have also highlighted a potential negative effect of adverse drug reactions on HRQOL evolution after HAART initiation but their assessment of adverse events relied only on toxicity associated with treatment modification. None of them have taken into account patient's perceived symptoms or effective adherence to HAART [11, 12]. Moreover, these findings reported at the beginning of the HAART era are hardly extendable to West African countries with a very different socioeconomic and cultural context. Burkina Faso is a country with a generalized HIV epidemic, which has one of the highest HAART coverage in West Africa. Indeed, by the end of 2010, more than 31,000 (49%) of the 64,000 estimated people living with HIV were receiving HAART in this country [13]. This report aims at assessing the temporal changes and factors associated with HRQOL evolution over a 12-month period in HIV-positive adults initiating HAART in the urban area of Ouagadougou, Burkina Faso.
Methods
Study design and population
From April to December 2010, three HIV clinics located in the urban area of Ouagadougou, one in the university hospital, one in a general public hospital and one run by a non-governmental organization, systematically and consecutively proposed their participation to all HIV-positive patients initiating HAART. Patients were eligible for the present study if they were HAART-naïve, aged ≥18 years and if they gave their informed consent to participate in the present study.
Baseline visit
In the three participating clinical sites, healthcare providers were in charge to administer a baseline standardized questionnaire to patients initiating HAART during face-to-face interviews. This questionnaire assessed patient's characteristics including gender, economic resources defined by their monthly income and level of formal education (no school versus primary school or secondary school and over). Alcohol use was reported using the Alcohol Use Disorder Identification Test (AUDIT) that measured alcohol use and its impact on health during the past 12 months [14]. Tobacco use was defined according to three categories: current smoker, past smoker and ever smoker as described in a previous report from West Africa [15]. Disclosure was defined as having revealed their HIV status or not to relatives or friends. Discrimination was defined as a binary variable in response to the following formulation: did you felt neglect, contempt or did you experience other behaviours that have taken away relatives, friends or healthcare providers because of your HIV status. After completing these interviews, healthcare providers were in charge to report from their medical charts information related to patient's baseline clinical characteristics including CD4 cell count (cells/mm3) and clinical stage according to World Health Organization (WHO) classification at HAART initiation.
Health-related quality of life
To assess the HRQOL, the MOS SF-36 questionnaire was administered. The choice of this tool relied on several arguments. First, a validated French version, the official language in Burkina Faso, is available [16]. Second, it is a widely used generic questionnaire, which has been already administered in the specific context of HIV infection in France and also in sub-Saharan Africa [17–19]. The MOS SF-36 is composed of 36 items assessing eight health concepts: limitations in physical activities because of health problems, limitations in social activities because of physical or emotional problems, limitations in usual role activities because of physical health problems, bodily pain, general mental health (psychological distress and wellbeing), limitations in usual role activities because of emotional problems, vitality (energy and fatigue) and general health perceptions.
Scores for each concept of the HRQOL were derived from responses to the 36 items of the MOS SF-36 and transformed into a scale ranging from 0 to 100, with higher score values corresponding to better HRQOL. Two summary scores were then derived from these scales, the physical health summary (PHS) score and the mental health summary (MHS) score. These summary scales were obtained by using a method that standardizes the scores so that the mean is 50 and the standard deviation is 10 [20]. We adapted this version of the questionnaire by piloting the administration of the original form and adding some minor explanations in specific items. After this pilot phase, all health workers involved in the study were trained together on the administration of the locally adapted MOS SF-36 during a one-day session.
Follow-up visits
As part of their usual follow-up, HIV-positive patients initiating HAART were scheduled for follow-up visits at month three, six and twelve. During these follow-up visits, healthcare providers were in charge to administer the dedicated questionnaire assessing HRQOL using the same MOS SF-36 survey form.
Drug-related toxicity and number of self-reported symptoms
Any HAART modification, as well as its causes including drug-related toxicity that occurred during the follow-up period, was documented at each follow-up visits. In addition, patients were also asked about the occurrence of 18 listed disease-related symptoms during the previous four weeks. This list was developed by Justice et al. and included symptoms such as dizziness, memory loss, nausea/vomiting, muscle/joint pain, diarrhoea, abdominal pain, headaches, sleep trouble as described elsewhere [21]. Symptoms of this list have been previously used as a proxy to capture undiagnosed drug-related toxicity in clinical trials conducted in industrialized countries [22, 23]. Indeed, relying only upon physician's assessment to capture treatment-related adverse events may underestimate toxicity perceived by patients [23, 24]. The presence of any of these symptoms during follow-up visits was summed for each patient in order to provide a quantitative assessment of the total number of perceived symptoms.
Adherence to HAART
The questionnaire also included a four-day recall on adherence to HAART based on the AIDS Clinical Trials Group (ACTG) follow-up questionnaire for adherence to antiretroviral medications [25]. Adherence was coded as a dichotomous variable, where patients <95% adherent during the previous four days were considered non-adherent (based on previous studies suggesting that ≥5% missed doses was associated with poorer virological outcomes) [26]. For the present analysis, patients were defined as non-adherent to HAART in the model if they were identified non-adherent according to the previous definition during at least one of the three follow-up visits.
Statistical analysis
We used a Chi2 test to compare baseline qualitative characteristics and non-parametric test (Kruskal-Wallis) to compare baseline quantitative characteristics according to gender. Matched paired t-tests were used to compare the observed PHS and MHS change as well as their respective eight domains from baseline to month 12.
HRQOL modelling
To examine factors associated with HRQOL, we chose to assess changes over a 12-month period of the two summary scores (PHS and MHS). We used two separate linear mixed models (LMM) to determine factors associated with a PHS or MHS change over a 12-month period. Both PHS and MHS scores were normally distributed.
We used LMM with one slope and an intercept. The variance of random effects on the intercept and on the slope was tested and was not significantly different to 0 (unstructured and diagonal covariance matrix was both tested for random effects). Thus, only fixed effects were included in the two LMMs. The following adjustment variables were investigated for both models: age, education, disclosure, discrimination, perceived symptoms, drug-related treatment modification, baseline WHO clinical stage, baseline CD4 cell count, HIV clinic, antiretroviral regimen as well as adherence to antiretroviral treatment. Variables that were statistically associated with HRQOL with a p-value ≤0.25 in the univariable analysis were selected in the multivariable analysis. The two final LMM were performed by using a manual descending method and potential confounders were investigated. A p-value≤0.05 was considered as statistically significant in the final models. We reported the mean PHS and MHS scores at baseline as well as the mean PHS and MHS changes for a one-month period with their 95% confidence intervals (CIs). Finally, we used the parameters of the multivariable LMM to estimate mean PHS and MHS scores at 12 months according to the variables selected in the models. Residual homoscedasticity and residual normality were graphically verified. All statistical analyses were performed with SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 364 HIV-positive patients initiated HAART during the study period in the three participating clinics. Of these, 344 (94.5%) were included and assessed at baseline, 13 (3.6%) patients refused to participate in the present study and seven (1.9%) patients were excluded as they had taken mono or dual-therapy prior to HAART initiation. Of the 344 patients included with a baseline HRQOL assessment, 299 (86.9%) were reassessed for HRQOL during their follow-up visits at three months, 274 (79.6%) at six months and 265 (77.0%) at 12 months. Six (1.7%) patients were transferred to another clinic during the first year of follow-up and 13 (3.8%) patients were reported as dead.
Baseline characteristics
Patients with a baseline HRQOL assessment had a median age of 37 years [interquartile range (IQR) 30–44] and 73.8% were women. Their median CD4 count at HAART initiation was 181 cells/mm3 (IQR 97–269) and 138 (40.0%) were diagnosed with a stage III or IV classifying disease according to WHO classification. Of the 344 participants that initiated HAART, 316 (91.9%) were below the treatment initiation threshold defined by a CD4 count ≤350 cells/mm3 and/or the presence of a severe HIV infection defined by a stage 3 or 4 clinical condition according to the WHO recommendations. Additional socio-demographic and clinical characteristics of this sample of HAART initiators are summarized in Table 1.
Table 1.
Baseline characteristics of patients initiating HAART according to gender in Ouagadougou, Burkina Faso. The IeDEA West Africa collaboration, 2010–2011
| Women (N=254) | Men (N=90) | p | Total (N=344) | |
|---|---|---|---|---|
| Age, median (IQR) | 35 (30–42) | 41 (34–49) | <10−4 | 37 (30–44) |
| Monthly income (USD), n (%) | <10−4 | |||
| <80 | 173 (68.1) | 42 (46.7) | 215 (62.5) | |
| ≥80–160 | 38 (15.0) | 32 (35.6) | 70 (20.3) | |
| ≥160 | 43 (16.9) | 16 (17.8) | 59 (17.2) | |
| Formal education, n (%) | 0.23 | |||
| No school | 108 (42.5) | 30 (33.3) | 38 (40.1) | |
| Primary school | 69 (27.2) | 25 (27.8) | 94 (27.3) | |
| Secondary and over | 77 (30.3) | 35 (38.9) | 112 (32.6) | |
| Smoking status, n (%) | <10−4 | |||
| No smoker | 242 (95.3) | 59 (65.6) | 301 (87.5) | |
| Present/past smoker | 12 (4.7) | 31 (34.4) | 43 (12.5) | |
| Alcohol use,a n (%) | 0.01 | |||
| No | 192 (75.6) | 56 (62.2) | 248 (72.1) | |
| Yes | 62 (24.4) | 34 (37.8) | 96 (27.9) | |
| Disclosure,b n (%) | 0.65 | |||
| No | 77 (30.3) | 25 (27.8) | 102 (29.7) | |
| Yes | 177 (69.7) | 65 (72.2) | 242 (70.3) | |
| Discrimination,c n (%) | 0.63 | |||
| No | 230 (90.6) | 83 (92.2) | 313 (91.0) | |
| Yes | 24 (9.4) | 7 (7.8) | 31 (9.0) | |
| HIV clinic, n (%) | 0.55 | |||
| University hospital | 102 (40.2) | 38 (42.2) | 140 (40.7) | |
| General hospital | 56 (22.0) | 15 (16.7) | 71 (20.6) | |
| NGO | 96 (37.8) | 37 (41.1) | 133 (38.7) | |
| Body mass index (kg/m2), median (IQR) | 20.7 (18.4–23.0) | 19.6 (17.3–22.1) | 0.02 | 20.5 (18.0–22.7) |
| CD4 count (cells/mm3), median (IQR) | 193 (98–280) | 162 (96–213) | 0.02 | 181 (97–270) |
| Clinical stage,d n (%) | 0.05 | |||
| I or II | 160 (63.0) | 45 (50.0) | 205 (59.6) | |
| III or IV | 91 (35.8) | 42 (46.7) | 133 (38.7) | |
| Missing values | 3 (1.2) | 3 (3.3) | 6 (1.7) | |
| First-line regimen, n (%) | 0.12 | |||
| AZT+3TC+NVP | 86 (33.9) | 29 (32.2) | 115 (33.4) | |
| D4T+3TC+NVP | 76 (29.9) | 15 (16.7) | 91 (26.5) | |
| AZT+3TC+EFV | 47 (18.5) | 26 (28.9) | 73 (21.2) | |
| TDF-based regimens | 23 (9.1) | 11 (12.2) | 34 (9.9) | |
| D4T+3TC+EFV | 13 (5.1) | 6 (6.7) | 19 (5.5) | |
| Other regimens | 9 (3.5) | 3 (3.3) | 12 (3.5) |
Any alcohol use declared during the past 12 months
to relatives or friends
from relatives, friends or healthcare providers
clinical stage according to World Health Organization classification. IQR=interquartile range; NGO=non-governmental organization; AZT=zidovudine; D4T=stavudine 3TC=lamivudine; NVP=nevirapine; EFV=efavirenz; TDF=tenofovir.
Follow-up characteristics
During the first 12 months of follow-up, 73 treatment modifications were reported in 71 patients. A total of 30 drug-related toxicities were reported and represented the main (41.1%) reason of treatment modification: 12 (41.4%) cutaneous reactions, nine (31.0%) peripheral neuropathy, three (10.3%) gastrointestinal disorders, two (9.9%) lipodystrophy, two (6.9%) elevated transaminases, one (3.4%) central nervous disorders and one (3.4%) renal toxicity.
A median number of 2 (IQR 0–6) self-reported symptoms were declared by patients during their follow-up. The distribution of reported symptoms among patients that attended at least one follow-up visit was as follows: 149 (11.9%) changes in the body image/fat accumulates, 137 (11.0%) headache, 128 (10.2%) fatigue/loss of energy, 100 (8.0%) fever/chills or sweats, 95 (7.6%) cough/breathlessness, 89 (7.1%) pain/numbness/tingling in the hands or feet, 87 (7.0%) muscle/joint pain, 72 (5.8%) nausea/vomiting, 59 (4.7%) diarrhoea, 57 (4.6%) abdominal pain, 52 (4.2%) dizziness, 48 (3.8%) sexual dysfunction, 45 (3.6%) loss of appetite/change in food taste, 44 (3.5%) weight loss, 38 (3.0%) sleep disorders, 33 (2.6%) skin problems, 14 (1.1%) memory loss and three (0.2%) hair loss. None of the reported symptoms showed any difference according to gender, even for sexual dysfunction (4.6% in men versus 3.6% in women, p=0.41). The frequency of participants reporting at least one self-reported symptom according to follow-up visit was as follows: 244/299 (81.6%) at three months, 19/274 (6.9%) at six months and 18/265 (6.8%) at 12 months. Non-adherence to HAART was reported in 16 (5.1%) of the 344 patients who attended at least one follow-up visit.
HRQOL
A significant improvement was noted in patients initiating HAART for both physical and mental aspects of HRQOL after one year. Overall, the mean [standard deviation (SD)] PHS score increased from 45.4 (11.1) at baseline to 60.0 (3.1) at 12 months (p<10−4) and the mean (SD) MHS score increased from 42.2 (8.7) to 43.9 (3.4) (p<10−2). Table 2 presents the PHS and MHS scores as well as the eight dimensions of the MOS SF-36 questionnaire at HAART initiation and after the 12-month follow-up visit according to gender. All dimensions of the HRQOL assessed by the MOS SF-36 increased significantly in men and women after 12 months on HAART except for the vitality and general mental health dimensions. Both men and women presented a significant increase in the PHS score over this 12-month period (p<10−4). The increase in the MHS score was significant in women (p<10−2) but not in men (p= 0.41).
Table 2.
The Medical Outcome Study 36-Item short-form health scores at HAART initiation and at the 12-month follow-up visit. The IeDEA West Africa collaboration, 2010–2011
| HAART initiation n=344 Mean (SD) |
12-month visit n=265 Mean (SD) |
Score change n=265 Differencea (CI 95%) |
p* | |
|---|---|---|---|---|
| MOS SF-36 dimensions | ||||
| Physical functioning | ||||
| Women | 73.5 (27.2) | 98.9 (5.9) | +23.7 (26.6) | <10−4 |
| Men | 75.4 (25.7) | 99.0 (4.8) | +22.7 (26.0) | <10−4 |
| Physical-related role limitations | ||||
| Women | 53.5 (45.7) | 99.1 (5.9) | +43.8 (45.5) | <10−4 |
| Men | 51.9 (45.5) | 98.6 (9.5) | +44.9 (47.9) | <10−4 |
| Bodily pain | ||||
| Women | 62.7 (28.9) | 97.0 (10.0) | +32.6 (29.2) | <10−4 |
| Men | 59.1 (26.5) | 96.2 (11.3) | +36.3 (25.3) | <10−4 |
| General health perception | ||||
| Women | 60.6 (25.6) | 86.0 (12.3) | +24.4 (28.8) | <10−4 |
| Men | 59.9 (27.4) | 88.1 (8.9) | +26.2 (26.9) | <10−4 |
| Vitality | ||||
| Women | 47.6 (17.6) | 48.5 (9.4) | +0.5 (19.6) | 0.74 |
| Men | 47.6 (16.8) | 48.0 (8.6) | −0.4 (18.0) | 0.87 |
| Social functioning | ||||
| Women | 71.1 (25.9) | 96.4 (8.9) | +23.1 (25.4) | <10−4 |
| Men | 70.8 (26.4) | 96.2 (9.9) | +23.4 (25.3) | <10−4 |
| Emotional-related role limitations | ||||
| Women | 62.7 (43.6) | 99.5 (5.3) | +35.7 (43.7) | <10−4 |
| Men | 62.6 (44.7) | 98.6 (12.0) | +33.3 (45.4) | <10−4 |
| General mental health | ||||
| Women | 55.6 (15.5) | 54.5 (9.7) | −0.8 (18.4) | 0.57 |
| Men | 57.9 (12.8) | 54.5 (9.0) | −2.7 (15.2) | 0.14 |
| MOS SF-36 composite scores | ||||
| Physical health summary score | ||||
| Women | 45.6 (11.2) | 60.0 (3.1) | +13.6 (11.4) | <10−4 |
| Men | 44.9 (10.7) | 60.1 (3.2) | +14.6 (11.0) | <10−4 |
| Mental health summary score | ||||
| Women | 42.1 (8.9) | 43.9 (3.4) | +1.8 (9.2) | <10−2 |
| Men | 42.7 (8.0) | 43.8 (3.3) | +0.8 (7.9) | 0.41 |
Matched paired t-test
mean observed scores differences between 12-month visit and HAART initiation. SD = standard deviation; CI=confidence intervals; MOS SF-36 = Medical Outcome Study 36-Item short form; HAART = highly active antiretroviral therapy.
Determinants of HRQOL and their temporal changes
In univariable analysis, a baseline history of discrimination, having disclosed his/her HIV status as well as other baseline measured characteristics, was not significantly associated with MHS or PHS score evolution over 12 months. The type of HAART regimen, experiencing a treatment modification associated with drug toxicity or being identified as non-adherent during follow-up visits, was also not significantly associated with PHS or MHS score evolution in univariable analysis (data not shown). These variables were thus not included in the multivariate model.
Table 3 shows the PHS and MHS mean score change estimated by the multivariable LMM. Although not associated with the PHS score, gender was associated with the MHS score in multivariate analysis. Women presented with a lower baseline MHS score (41.8) compared to men (42.9) (p=0.04). Due to a steeper increase in the MHS score in women over time, the 12-month MHS score estimate in women (45.4) was not significantly different compared to the MHS score estimate in men (45.1) (p=0.69). The number of perceived symptoms was the main characteristic associated with both PHS and MHS score evolution with an estimated mean decrease for any additional reported symptom of −0.2 (p<10−4) and −0.1 (p<10−2) unit/month, respectively.
Table 3.
Baseline mean physical health summary and mental health summary scores and their mean changes (in units/month) after HAART initiation, estimated by multivariable linear mixed models, the IeDEA West Africa Collaboration, 2010–2011 (344 patients; 1,182 observations)
| Initial PHS scorea | PHS score changea | Initial MHS scoreb | MHS score changeb | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Variables | Mean (95% CI) | p | Mean (95% CI) | p | Mean (95% CI) | p | Mean (95% CI) | p | |
| Gender | 0.04 | 0.15 | |||||||
| Men | Not estimatedc | Not estimatedc | 42.9 (41.1;44.8) | +0.2 (−0.1; + 0.5) | |||||
| Women | – | – | 41.8 (40.1;43.4) | +0.3 (+0.0; + 0.6) | |||||
| Age at HAART initiation (years) | <10−2 | 0.10 | |||||||
| < 35 | Not estimatedc | Not estimatedc | 42.9 (41.1;44.8) | +0.2 (−0.1; + 0.5) | |||||
| ≥ 35 | – | – | 44.5 (42.7;46.3) | +0.0 (−0.2; + 0.3) | |||||
| Missing | – | – | 41.9 (38.4;45.4) | +0.3 (−0.2; + 0.9) | |||||
| Education | <10−3 | 0.02 | |||||||
| No education | 41.8 (39.5;44.2) | +2.2 (+1.8; + 2.6) | Not estimatedd | Not estimatedd | |||||
| Primary | 43.8 (41.3;46.4) | +2.0 (+1.6; + 2.4) | – | – | |||||
| Secondary and more | 44.9 (42.5;47.2) | +1.9 (+1.5; + 2.2) | – | – | |||||
| Perceived symptoms | – | – | <10−4 | – | <10−2 | ||||
| For each additional symptome | – | −0.2 (−0.2. −0.1) | – | −0.1 (−0.1; −0.0) | |||||
| Baseline WHO clinical stage | <10−4 | <10−3 | <10−2 | 0.04 | |||||
| Stage I, II | 46.0 (43.2;48.7) | +1.7 (+1.3; + 2.2) | 44.8 (42.7;46.9) | −0.0 (−0.3; + 0.3) | |||||
| Stage III, IV | 41.8 (39.5;44.2) | +2.2 (+1.8; + 2.6) | 42.9 (41.1;44.8) | +0.2 (−0.1; + 0.5) | |||||
| Missing | 43.1 (37.2;49.1) | +2.0 (+1.1; + 3.0) | 43.3 (39.1;47.6) | +0.3 (−0.4; + 1.0) | |||||
| Baseline CD4 count (cells/mm3) | <10−3 | 0.03 | <10−2 | <10−2 | |||||
| < 50 | 41.8 (39.5;44.2) | +2.2 (+1.8; + 2.6) | 42.9 (41.1;44.8) | +0.2 (−0.1; + 0.5) | |||||
| ≥ 50–200 | 45.5 (43.6;47.3) | +1.7 (+1.5; + 2.0) | 43.9 (42.3;45.5) | +0.1 (−0.1; + 0.3) | |||||
| ≥ 200 | 47.0 (44.9;49.1) | +1.7 (+1.4; + 2.0) | 45.7 (44.0;47.4) | −0.1 (−0.4; + 0.1) | |||||
| Missing | 48.1 (44.9;51.4) | +1.6 (+1.1; + 2.0) | 45.9 (43.4;48.5) | −0.3 (−0.7; + 0.1) | |||||
| HIV clinic | <10−4 | <10−4 | <10−4 | <10−4 | |||||
| University hospital | 41.8 (39.5;44.2) | +2.2 (+1.8; + 2.6) | 42.9 (41.1;44.8) | +0.2 (−0.1; + 0.5) | |||||
| General public hospital | 46.7 (44.2;49.2) | +1.5 (+1.1; + 1.8) | 43.0 (41.0;45.0) | +0.2 (−0.1; + 0.5) | |||||
| Non-governmental organization | 33.6 (31.1;36.0) | +2.9 (+2.5; + 3.3) | 35.9 (33.9;37.8) | +0.9 (+0.6; + 1.2) | |||||
Reference group: no symptoms reported, no education, followed at university hospital, initial CD4 < 50 cells/µl and clinical stage III or IV
reference group: no symptoms reported, male aged < 35, followed at university hospital, initial CD4 < 50 cells/µl and clinical stage III or IV
not estimated as the variable was not significantly associated with baseline mean PHS score and/or its mean change in univariate analysis
not estimated as the variable was not significantly associated with baseline mean MHS score and/or its mean change in univariate analysis
reported symptoms during follow-up visits were summed for each patient in order to provide a quantitative assessment of the total number of perceived symptoms. CI = confidence interval; HAART = highly active antiretroviral treatment; PHS = physical health summary; MHS = mental health summary; WHO = World Health Organization.
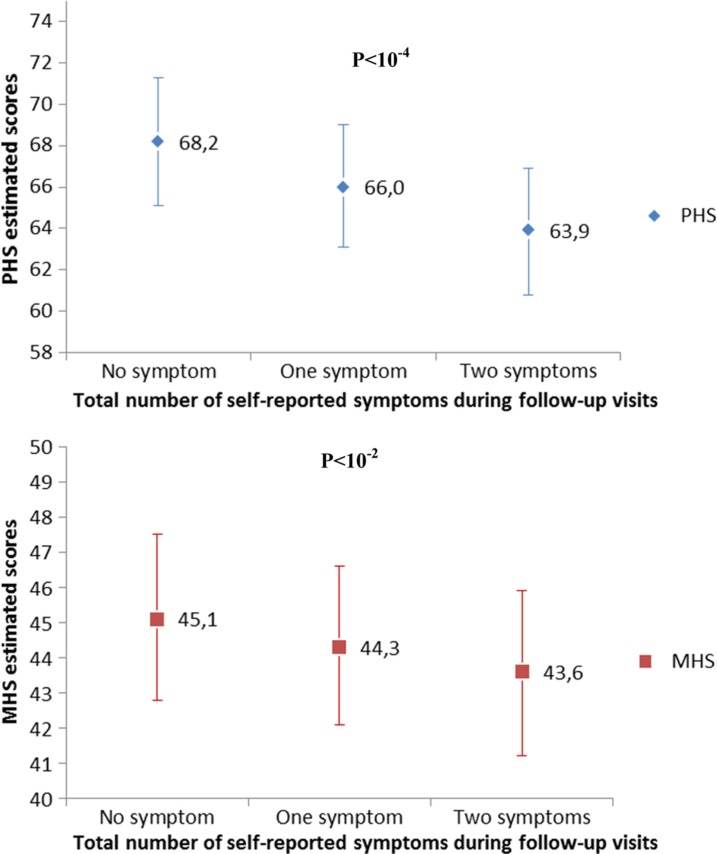
At 12 months, patients that experienced on average two symptoms per visit presented with significantly lower PHS (63.9) and MHS (43.6) scores compared to patients that presented no symptoms during follow-up with respective PHS and MHS of 68.2 (p<10−4) and 45.1 (p<10−2) (Figure 1). Baseline clinical stage and immunological status were also the main determinants of HRQOL changes over a 12-month period. Patients with a WHO stage III/IV presented with a significantly lower PHS (41.8) and MHS (42.9) scores compared to patients with stage I/II with respective PHS (46.0) (p<10−4) and MHS (44.8) (p<10−3) scores prior to HAART initiation. However, patients experienced a significant improvement of both PHS and MHS scores irrespective of their baseline WHO clinical stage. At 12 months, PHS and MHS scores were not significantly different between patients that experienced a stage I/II or a stage III/IV clinical outcome prior to HAART initiation with respective p-values of 0.14 and 0.48.
Figure 1.
Mean PHS and MHS scores at 12 month after HAART initiation according to the number of perceived symptoms estimated with the multivariable linear mixed models. The IeDEA West Africa collaboration, 2010–2011.
PHS = physical health summary; MHS = mental health summary; HAART = highly active antiretroviral treatment.
The type of HIV clinic was also associated with a significant difference in baseline HRQOL. Patients attending the NGO had lower PHS (33.6) and MHS (35.0) scores compared to patients attending the general public hospital with PHS of 46.7 (p<10−4) and MHS of 42.1 (p<10−4). However, the gain in PHS (+2.9 units/month) and MHS (+1.0 units/month) was greater for the patients in care in the NGO-type clinic compared to those followed in the general public hospital who experienced a PHS gain of 1.5 unit/month (p<10−4) and a MHS gain of +0.3 unit/month (p<10−4).
Discussion
A significant increase in both physical and mental aspects of the HRQOL in a population of 344 HIV-positive persons after one year of HAART was measured in Ouagadougou, Burkina Faso. Results of this prospective observational cohort are consistent with previous reports from sub-Saharan Africa and highlight that the use of HAART in real conditions is associated with an improved quality of life in Burkina Faso [9–12]. Moreover, this is the first report from sub-Saharan Africa that includes both perceived symptoms and adherence to HAART in the prospective assessment of HRQOL of HIV-positive persons.
One year of HAART significantly improved most dimensions of HRQOL. All physical dimensions of HRQOL improved, but the impact of treatment was less striking on the mental dimensions, particularly in men for whom the increase in the MHS score was not significant. Women presented with a significantly lower MHS score at baseline compared to men, but a steeper increase was observed after HAART initiation. These results are in accordance with previous findings from high-income settings and emphasize the particular benefit of HAART initiation on women's mental HRQOL [27]. However, such a gender difference has not been reported in previous reports from sub-Saharan Africa so far [10, 11]. Additional studies are needed to confirm these results and explore potential factors associated with gender difference in mental dimensions of HRQOL. Our results also highlight the high level of physical disability and depleted functional autonomy at HAART initiation in sub-Saharan Africa compared to Northern countries. Indeed, results from a French cohort of HIV-positive patients reporting HRQOL evolution one year after HAART initiation showed that physical dimensions of the MOS SF-36 were high at enrolment compared to our results, with mean (SD) physical functioning, bodily pain and physical-related role limitations of 84.5 (21.6) 76.7 (25.2) and 67.8 (38.9), respectively [18]. The relatively low scores in physical dimensions found in our study probably reflect the delay in HAART initiation in Ouagadougou, with patients presenting with more advanced and severe HIV-related diseases.
As reported elsewhere, drug-related toxicity accounts for the majority of treatment modification in HIV-positive patients treated with HAART in sub-Saharan Africa [28, 29]. Previous studies have documented the potential impact of drug-related toxicity on HRQOL. In 2009, Pitt et al. reported factors associated with a negative evolution of PHS or MHS scores administrating the MOS SF-36 to 295 HIV-positive patients prior to HAART initiation and 48 weeks thereafter [11]. They found no association between drug-related toxicity and negative PHS or MHS scores. However, they only measured toxicity severe enough to prompt a change in HAART regimen. This could have led to an underestimation of the true negative impact of drug-related toxicity, especially as the treatment withdrawal tends to restore patient's health. In our study, drug-related toxicity confirmed by treatment modification did not have a significant impact on HRQOL evolution in the first year of HAART. However, the number of patient's self-reported symptoms recorded at each follow-up visit was associated with a significant decrease in both physical and mental dimensions of HRQOL over one year. This finding is consistent with previous reports from high-income countries where the number of self-reported symptoms experienced during the first year of treatment among HIV-positive patients initiating HAART was highly predictive of a reduced HRQOL [30, 31]. An appropriate management of reported symptoms during the follow-up of HIV-positive patients initiated on HAART is thus a key component to restore HRQOL. As no causal relation could be formally established between perceived symptoms and drug-related toxicity among HAART-treated patients, there is a clear need to enhance pharmacovigilance activities in a context of HAART scale-up in sub-Saharan Africa. Indeed, previous reports have highlighted the lack of knowledge concerning drug-related adverse events identification and staging in our study environment [28, 32] and elsewhere in Africa [33]. With this respect, appropriate training in the assessment and management of drug-related toxicity is a priority to improve HRQOL.
Despite official recommendations of withdrawal with regards to its known toxicity, stavudine was still largely prescribed as a first-line drug in 2010 in Ouagadougou. Indeed, reported symptoms potentially linked to mitochondrial toxicity (i.e. change in the body image/fat accumulation and pain/numbness/tingling in the hands or feet) were frequently reported by patients during their follow-up visits. Withdrawal of stavudine and sustained access to less toxic recommended first-line and second-line antiretroviral drug regimens are indeed key components for the management of drug-related toxicity, thus maximizing a sustained adherence to HAART and retention in healthcare programs.
After controlling for baseline HRQOL, a baseline-impaired immunological status and the presence of serious HIV-associated disease (stage III or IV WHO classifying disease) were associated with a lower HRQOL prior to HAART initiation but with a subsequent higher increase of HRQOL over 12 months. These results are consistent with prior reports from high-income countries and other settings in sub-Saharan Africa [11, 18, 34]. Some authors have advocated that patients with a less severe baseline immunological and clinical status were more sensitive to the constraints and inconvenience of HAART than to its benefits. This question will require close monitoring as the general tendency is a shift towards a higher CD4 count at HAART initiation.
The type of healthcare facility delivering HAART and HIV services was also a major determinant of baseline HRQOL and its evolution. Indeed, the NGO facility was associated with a significantly lower baseline HRQOL and a subsequent higher increase over 12 months compared to the public sector facilities for both PHS and MHS scores. These differences could be attributed to unmeasured sites characteristics. Many African countries have eliminated user fees for HAART as it was identified as an important barrier to universal access [35]. However, until 2010, Burkina Faso was an exception to this trend of providing HAART for free. Nonetheless, NGO's provided free access to HAART and other healthcare services to selected HIV-positive persons based on the severity of their health condition [36]. This might have selected a specific population attending the NGO site with a particularly low reported HRQOL prior to HAART initiation.
Limitations
Our study population might not be representative of all HIV-positive patients initiating HAART in Burkina Faso as we essentially included patients living in the urban area of Ouagadougou. However, they might be quite representative of those living in the urban area of Ouagadougou as these three HIV clinics follow the great majority of HIV-positive people of this district. Some patients initially included in the study did not attend the subsequent follow-up visits. These patients might have presented a quite different of HRQOL during their follow-up, introducing potential biases in our PHS and MHS estimates. However, the comparisons of baseline characteristics of patients that attended the three following visits versus the ones that missed at least one visit did not show any significant differences in terms of age, baseline CD4 count and clinical stage (data not shown). Our modelling approach that allowed the inclusion of all patients as long as they had been seen at a baseline provided more accurate estimates of HRQOL. Like previous reports assessing HRQOL in HIV-positive persons, the positive effect of HAART was only documented for the first 12-month period. Further studies are now needed to explore the sustainability of HRQOL after one year of HAART exposure. Finally, as there is currently no MOS SF-36 form that has been validated in the general population of Burkina Faso, we do not have any “normal level” score of the PHS and MHS to refer to. There is now a need to develop culturally adapted and validate tools to assess HRQOL in countries of sub-Saharan Africa.
Conclusions
The initiation of HAART was associated with a significant improvement in both physical and mental dimensions of HRQOL in HIV-positive patients over a one-year period in Burkina Faso. Despite a lower baseline HRQOL, patients with a low immunological status and more advanced clinical stage presented a steeper HRQOL increase showing that HIV-positive patients presenting with a severe clinical condition can achieve similar HRQOL recovery compared to patients with less severe condition at HAART initiation. Although no association was found between HRQOL and drug-related toxicity inducing treatment modification, the number of symptoms reported by patients taking HAART was associated with a lower HRQOL increase over 12 months. Particular attention must be paid to the identification and management of self-reported symptoms to enhance HRQOL.
Acknowledgements
We are indebted to all of the HIV-positive people in Ouagadougou who agreed to participate in this present study as well as to the health workers who performed the HRQOL assessments and data collection. We are also indebted to officials from the participating HIV clinics in Ouagadougou [Hopital de jour, Médecine interne, Centre Hospitalier Universitaire de Yalgado Ouedraogo (CHU-YO), Centre médical associatif African Association Solidarity (AAS), Centre médical avec antenne chirurgicale de Pisssy] for their positive implication in the present research project. We would also like to thank Mrs Valérie Journot and Dr Bruno Spire for their helpful advises during the study initiation.
Funding
This work was funded by the following institutes: the National Cancer Institute (NCI), the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), the National Institute of Allergy and Infectious Diseases (NIAID) (grant no 5U01AI069919).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
To access the supplementary material to this article please see Supplementary Files under Article Tools online.
Competing interests
The authors declare that they have no conflict of interest.
Authors' contributions
A.J., D.K.E., F.D. and J.D. designed the study; A.J. and F.G. supervised and conducted the study. F.G., R.B., E.D. and J.C.K supervised and/or performed the data acquisition; F.G., A.J., E.B. and J.C.A. supervised and/or performed the data management and data entry; Statistical analysis was done by E.B. and interpretation of data was done by E.B. and A.J. The manuscript was drafted by A.J. and critical revision of the manuscript for important intellectual content was provided by D.K.E, E.B. and F.D.; All authors read and commented on the original manuscript and all agreed on the version finalized by A.J. for submission.
References
- 1.Sow PS, Otieno LF, Bissagnene E, Kityo C, Bennink R, Clevenbergh P, et al. Implementation of an antiretroviral access program for HIV-1-infected individuals in resource-limited settings: clinical results from 4 African countries. J Acquir Immune Defic Syndr. 2007;44(3):262–7. doi: 10.1097/QAI.0b013e31802bf109. [DOI] [PubMed] [Google Scholar]
- 2.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d'Ivoire: 2-year outcomes and determinants. AIDS. 2008;22(7):873–82. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekouevi DK, Balestre E, Ba-Gomis FO, Eholie SP, Maiga M, Amani-Bosse C, et al. Low retention of HIV-infected patients on antiretroviral therapy in 11 clinical centres in West Africa. Trop Med Int Health. 2010;15(Suppl 1):34–42. doi: 10.1111/j.1365-3156.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. [PubMed] [Google Scholar]
- 5.Revicki DA, Sorensen S, Wu AW. Reliability and validity of physical and mental health summary scores from the Medical Outcomes Study HIV Health Survey. Med Care. 1998;36(2):126–37. doi: 10.1097/00005650-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Ware JE, Jr., Kosinski M, Gandek B, Aaronson NK, Apolone G, Bech P, et al. The factor structure of the SF-36 Health Survey in 10 countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1159–65. doi: 10.1016/s0895-4356(98)00107-3. [DOI] [PubMed] [Google Scholar]
- 7.Wu AW, Hanson KA, Harding G, Haider S, Tawadrous M, Khachatryan A, et al. Responsiveness of the MOS-HIV and EQ-5D in HIV-infected adults receiving antiretroviral therapies. Health Qual Life Outcomes. 2013;11:42. doi: 10.1186/1477-7525-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayson DJ, Wild DJ, Quarterman P, Duprat-Lomon I, Kubin M, Coons SJ. A comparative review of health-related quality-of-life measures for use in HIV/AIDS clinical trials. Pharmacoeconomics. 2006;24(8):751–65. doi: 10.2165/00019053-200624080-00003. [DOI] [PubMed] [Google Scholar]
- 9.Jelsma J, Maclean E, Hughes J, Tinise X, Darder M. An investigation into the health-related quality of life of individuals living with HIV who are receiving HAART. AIDS Care. 2005;17(5):579–88. doi: 10.1080/09540120412331319714. [DOI] [PubMed] [Google Scholar]
- 10.Stangl AL, Wamai N, Mermin J, Awor AC, Bunnell RE. Trends and predictors of quality of life among HIV-infected adults taking highly active antiretroviral therapy in rural Uganda. AIDS Care. 2007;19(5):626–36. doi: 10.1080/09540120701203915. [DOI] [PubMed] [Google Scholar]
- 11.Pitt J, Myer L, Wood R. Quality of life and the impact of drug toxicities in a South African community antiretroviral programme. J Int AIDS Soc. 2009;12(1):5. doi: 10.1186/1758-2652-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wouters E, Heunis C, van Rensburg D, Meulemans H. Physical and emotional health outcomes after 12 months of public-sector antiretroviral treatment in the Free State Province of South Africa: a longitudinal study using structural equation modelling. BMC Public Health. 2009;9:103. doi: 10.1186/1471-2458-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNAIDS. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011; UNAIDS: Geneva; 2011. [cited 03/05/13]. Available from: http://www.who.int/hiv/pub/progress_report2011/en/index.html. [Google Scholar]
- 14.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 15.Jaquet A, Ekouevi DK, Aboubakrine M, Bashi J, Messou E, Maiga M, et al. Tobacco use and its determinants in HIV-infected patients on antiretroviral therapy in West African countries. Int J Tuberc Lung Dis. 2009;13(11):1433–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Leplege A, Ecosse E, Verdier A, Perneger TV. The French SF-36 Health Survey: translation, cultural adaptation and preliminary psychometric evaluation. J Clin Epidemiol. 1998;51(11):1013–23. doi: 10.1016/s0895-4356(98)00093-6. [DOI] [PubMed] [Google Scholar]
- 17.Preau M, Marcellin F, Carrieri MP, Lert F, Obadia Y, Spire B. Health-related quality of life in French people living with HIV in 2003: results from the national ANRS-EN12-VESPA Study. AIDS. 2007;21(Suppl 1):S19–27. doi: 10.1097/01.aids.0000255081.24105.d7. [DOI] [PubMed] [Google Scholar]
- 18.Carrieri P, Spire B, Duran S, Katlama C, Peyramond D, Francois C, et al. Health-related quality of life after 1 year of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;32(1):38–47. doi: 10.1097/00126334-200301010-00006. [DOI] [PubMed] [Google Scholar]
- 19.O'Keefe EA, Wood R. The impact of human immunodeficiency virus (HIV) infection on quality of life in a multiracial South African population. Qual Life Res. 1996;5(2):275–80. doi: 10.1007/BF00434749. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Jr., Gandek B, Kosinski M, Aaronson NK, Apolone G, Brazier J, et al. The equivalence of SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1167–70. doi: 10.1016/s0895-4356(98)00108-5. [DOI] [PubMed] [Google Scholar]
- 21.Justice AC, Holmes W, Gifford AL, Rabeneck L, Zackin R, Sinclair G, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54(Suppl 1):S77–90. doi: 10.1016/s0895-4356(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 22.Duran S, Spire B, Raffi F, Walter V, Bouhour D, Journot V, et al. Self-reported symptoms after initiation of a protease inhibitor in HIV-infected patients and their impact on adherence to HAART. HIV Clin Trials. 2001;2(1):38–45. doi: 10.1310/R8M7-EQ0M-CNPW-39FC. [DOI] [PubMed] [Google Scholar]
- 23.Spire B, Duran S, Souville M, Leport C, Raffi F, Moatti JP. Adherence to highly active antiretroviral therapies (HAART) in HIV-infected patients: from a predictive to a dynamic approach. Soc Sci Med. 2002;54(10):1481–96. doi: 10.1016/s0277-9536(01)00125-3. [DOI] [PubMed] [Google Scholar]
- 24.Fontaine A, Larue F, Lassauniere JM. Physicians’ recognition of the symptoms experienced by HIV patients: how reliable? J Pain Symptom Manage. 1999;18(4):263–70. doi: 10.1016/s0885-3924(99)00078-0. [DOI] [PubMed] [Google Scholar]
- 25.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 26.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 27.Mrus JM, Williams PL, Tsevat J, Cohn SE, Wu AW. Gender differences in health-related quality of life in patients with HIV/AIDS. Qual Life Res. 2005;14(2):479–91. doi: 10.1007/s11136-004-4693-z. [DOI] [PubMed] [Google Scholar]
- 28.Jaquet A, Djima MM, Coffie P, Kacou HD, Eholie SP, Messou E, et al. Pharmacovigilance for antiretroviral drugs in Africa: lessons from a study in Abidjan, Cote d'Ivoire. Pharmacoepidemiol Drug Saf. 2011;20(12):1303–10. doi: 10.1002/pds.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braitstein P, Ayuo P, Mwangi A, Wools-Kaloustian K, Musick B, Siika A, et al. Sustainability of first-line antiretroviral regimens: findings from a large HIV treatment program in western Kenya. J Acquir Immune Defic Syndr 2010. 2010;53(2):254–9. doi: 10.1097/QAI.0b013e3181b8f26e. [DOI] [PubMed] [Google Scholar]
- 30.Herrmann S, McKinnon E, Hyland NB, Lalanne C, Mallal S, Nolan D, et al. HIV-related stigma and physical symptoms have a persistent influence on health-related quality of life in Australians with HIV infection. Health Qual Life Outcomes. 2013;11:56. doi: 10.1186/1477-7525-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mannheimer SB, Wold N, Gardner EM, Telzak EE, Huppler Hullsiek K, Chesney M, et al. Mild-to-moderate symptoms during the first year of antiretroviral therapy worsen quality of life in HIV-infected individuals. Clin Infect Dis. 2008;46(6):941–5. doi: 10.1086/528859. [DOI] [PubMed] [Google Scholar]
- 32.Oshikoya KA, Awobusuyi JO. Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Clin Pharmacol. 2009;9(11):14. doi: 10.1186/1472-6904-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruud KW, Srinivas SC, Toverud EL. Healthcare providers’ experiences with adverse drug reactions and adherence challenges in antiretroviral therapy of HIV patients in the Eastern Cape Province, South Africa. Eur J Clin Pharmacol. 2012;68(9):1321–8. doi: 10.1007/s00228-012-1254-1. [DOI] [PubMed] [Google Scholar]
- 34.Jia H, Uphold CR, Wu S, Chen GJ, Duncan PW. Predictors of changes in health-related quality of life among men with HIV infection in the HAART era. AIDS Patient Care STDS. 2005;19(6):395–405. doi: 10.1089/apc.2005.19.395. [DOI] [PubMed] [Google Scholar]
- 35.Souteyrand YP, Collard V, Moatti JP, Grubb I, Guerma T. Free care at the point of service delivery: a key component for reaching universal access to HIV/AIDS treatment in developing countries. AIDS. 2008;22(Suppl 1):S161–8. doi: 10.1097/01.aids.0000327637.59672.02. [DOI] [PubMed] [Google Scholar]
- 36.Ridde V, Some PA, Pirkle CM. NGO-provided free HIV treatment and services in Burkina Faso: scarcity, therapeutic rationality and unfair process. Int J Equity Health. 2012;11:11. doi: 10.1186/1475-9276-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]