Abstract
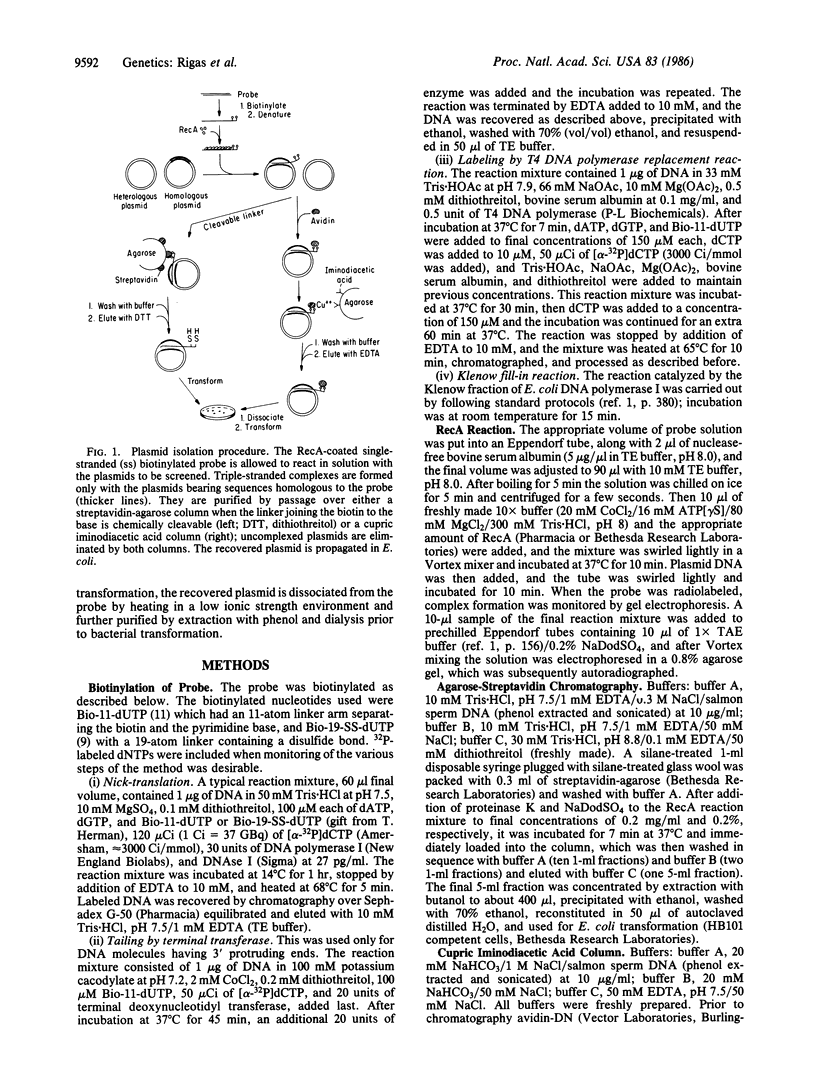
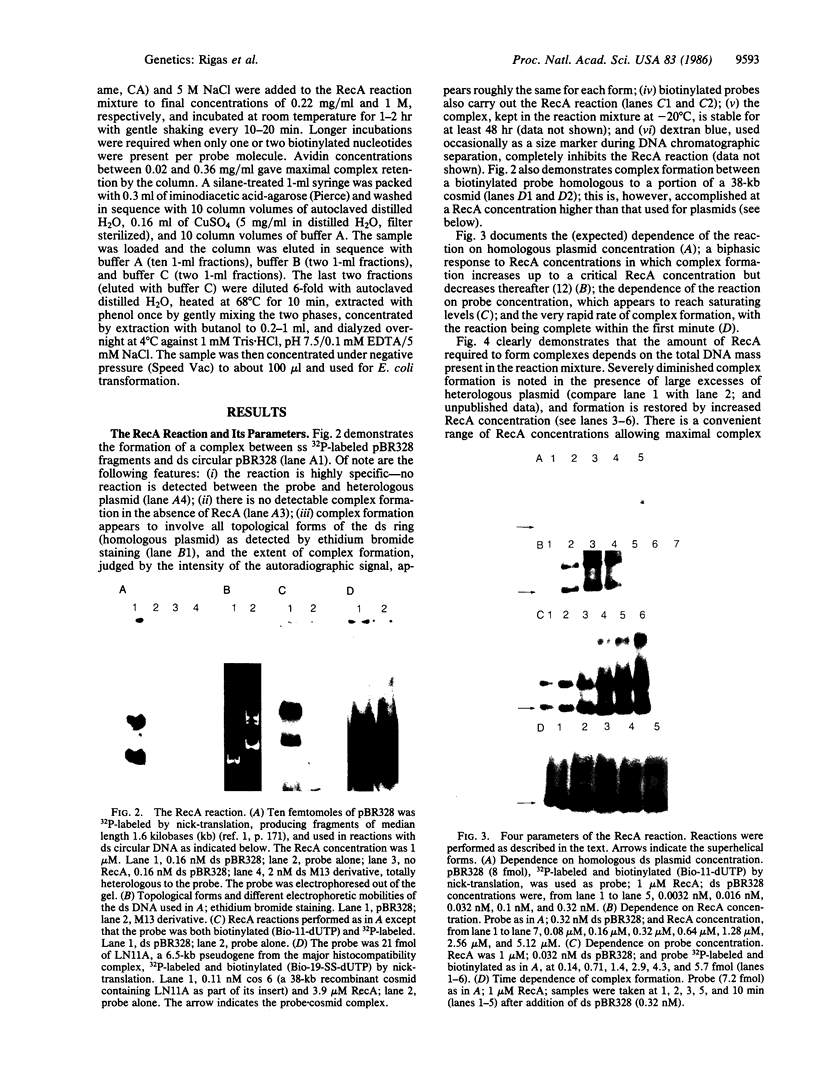
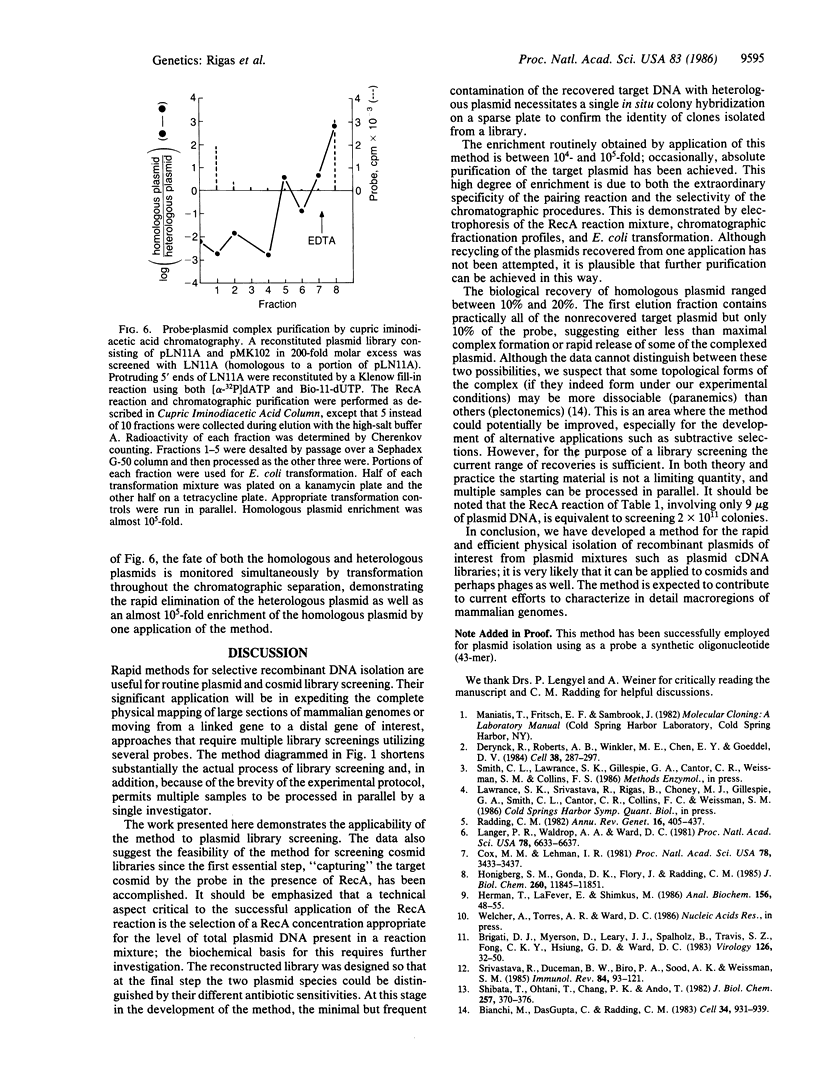
A method for the rapid physical isolation of recombinant plasmids of interest from a mixture of plasmids such as a plasmid cDNA library is presented. This method utilizes the ability of RecA protein to form stable complexes between linear single-stranded and circular double-stranded DNA molecules sharing sequence homology, and procedures allowing isolation of biotinylated nucleic acid. Biotinylated linear DNA probes coated with RecA have been used to screen reconstituted plasmid libraries consisting of two plasmid species, one homologous and the other heterologous to the probe. When the link between biotin and the nucleotide base could be cleaved by reducing agents, the complex was purified by streptavidin-agarose chromatography and the recovered plasmid was propagated in Escherichia coli. When the link was not cleavable the complex was bound to avidin in solution and purified by cupric iminodiacetic acid-agarose chromatography. The complex was then dissociated and the plasmids were propagated in E. coli. With either protocol, homologous plasmid recovery was between 10% and 20%, and enrichment was between 10(4)- and 10(5)-fold. Potential applications and extensions of this method, such as plasmid, cosmid, and phage library screening and facilitation of physical mapping of macroregions of mammalian genomes are presented and discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchi M., DasGupta C., Radding C. M. Synapsis and the formation of paranemic joints by E. coli RecA protein. Cell. 1983 Oct;34(3):931–939. doi: 10.1016/0092-8674(83)90550-0. [DOI] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. recA protein of Escherichia coli promotes branch migration, a kinetically distinct phase of DNA strand exchange. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3433–3437. doi: 10.1073/pnas.78.6.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Roberts A. B., Winkler M. E., Chen E. Y., Goeddel D. V. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell. 1984 Aug;38(1):287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- Herman T. M., Lefever E., Shimkus M. Affinity chromatography of DNA labeled with chemically cleavable biotinylated nucleotide analogs. Anal Biochem. 1986 Jul;156(1):48–55. doi: 10.1016/0003-2697(86)90152-1. [DOI] [PubMed] [Google Scholar]
- Honigberg S. M., Gonda D. K., Flory J., Radding C. M. The pairing activity of stable nucleoprotein filaments made from recA protein, single-stranded DNA, and adenosine 5'-(gamma-thio)triphosphate. J Biol Chem. 1985 Sep 25;260(21):11845–11851. [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Shibata T., Ohtani T., Chang P. K., Ando T. Role of superhelicity in homologous pairing of DNA molecules promoted by Escherichia coli recA protein. J Biol Chem. 1982 Jan 10;257(1):370–376. [PubMed] [Google Scholar]
- Srivastava R., Duceman B. W., Biro P. A., Sood A. K., Weissman S. M. Molecular organization of the class I genes of human major histocompatibility complex. Immunol Rev. 1985 Jul;84:93–121. doi: 10.1111/j.1600-065x.1985.tb01127.x. [DOI] [PubMed] [Google Scholar]









