Abstract
Rhes, Ras homolog enriched in striatum (Rhes), is a highly conserved small GTP binding protein belonging to the Ras superfamily. Rhes is involved in the dopamine receptor-mediated signaling and behavior though adenylyl cyclase. The striatum-specific GTPase shares a close homology with Dexras1, which regulates iron trafficking in the neurons when activated though the post-translational modification called s-nitrosylation by nitric oxide (NO). We report that Rhes physiologically interacted with PAP7 and participated in iron uptake via DMT1 similar to Dexras1. Interestingly, Rhes is not S-nitrosylated by NO-treatment, however phosphorylated by Protein Kinase A (PKA) at the site of serine-239. Two Rhes mutants - the phosphomimetic form (serine 239 to aspartic acid) and constitutively active form (alanine 173 to valine) - displayed an increase in iron uptake compared to the wild type Rhes. These findings suggest that Rhes may play a crucial role in striatal iron homeostasis.
Keywords: Rhes, Iron uptake, Striatum, Phosphorylation, G protein
1. Introduction
Iron is an essential metal ion critical for basic cellular processes, such as mitochondrial ATP generation and DNA replication (Hentze and Kuhn, 1996; McCord, 1998; Todorich et al., 2009)(Todorich, Pasquini et al. 2009). Iron deficiency studies show that iron plays an important role in normal division of cells, including neuronal precursor cells, astrocytes and oligodendrocytes (Ke and Qian, 2007; Moos and Morgan, 1998). In addition, iron is required for several neuronal specific functions, such as dopaminergic neurotransmitter synthesis and myelination (Moos and Morgan, 1998; Rouault and Cooperman, 2006; Todorich et al., 2009). Due to its unique chemical nature, however, iron can have deleterious effects by generating reactive oxygen species through Fenton Reaction when in excess (McCord, 1998). The precise mechanism and participating molecules are not clearly understood, but iron accumulation and its pathological implications have been reported in numerous neurodegenerative diseases such as multiple sclerosis, Alzheimer's disease and Parkinson's disease (Barnham and Bush, 2008; Berg and Hochstrasser, 2006; Khalil et al., 2011; Nunez et al., 2012; Schneider and Bhatia, 2012; Todorich et al., 2009).
Rhes, a novel Ras Homolog Enriched in Striatum, is a GTP binding protein. Like all G proteins, Rhes contains the conserved domain including the GTP binding domain, a C-terminal prenylation site, and the magnesium binding domain. The physiological role of Rhes is not fully understood, however, it has been reported to involve in PI3K activation (Todorich et al., 2009; Vargiu et al., 2004) and regulatory actions on AKT pathway (Bang et al., 2012; Harrison et al., 2013) at a cellular level and modulation of dopamine receptor-mediated behavior with Rhes mutant mice (Errico et al., 2008; Harrison and Lahoste, 2006; Quintero et al., 2008). Recently, it has been shown that interaction of Rhes with a huntingtin protein gives rise to selective vulnerability to striatal pathology in Huntington's disease (Subramaniam and Snyder, 2011)(Subramaniam and Snyder 2011).
Rhes amino acids shares 67 % identity in the open reading frames with Dexras 1, a brain-enriched member of the Ras family of small G proteins (Falk et al., 1999; Vargiu et al., 2004)(Falk, Vargiu et al. 1999). Moreover, these two have a common feature of an extended C-terminal tail which differentiates them from the conventional Ras family members (Falk et al., 1999; Graham et al., 2001)(Graham, Key et al. 2001). Dexras1 has been shown to activate G protein signaling via selectively binding to Gαi2, increasing GTPγS binding to Gi and Go and activating extracellular signal-regulated kinases 1 and 2 (Erk 1 and 2)(Cismowski et al., 2000; Graham et al., 2002). Particularly, we discovered that Dexras1 participates in iron uptake through NMDA receptor mediated signaling. Specifically, stimulation of NMDA receptors activates nNOS, leading to S-nitrosylation and activation of Dexras1 which, via PAP7 (Peripheral benzodiazepine Receptor-associated Protein7) and DMT1, physiologically induces iron uptake (Cheah, Kim et al. 2006). More recently, we found that Dexras1 is required for NMDA-elicited neuronal toxicity via NO and iron influx (Chen et al., 2013).
Since Rhes is highly expressed in the striatum where the level of iron is the highest and shares a close homology with Dexras1 which controls neuronal iron trafficking (Cheah et al., 2006; Falk et al., 1999), we wondered whether Rhes is involved in the neuronal iron uptake in striatum. We found that wild type Rhes interacts with PAP7, a scaffolding protein between Dexras1 and DMT1, as an iron transporter and an active form of Rhes enhances iron uptake compared to a native form. Our in vitro phosphorylation assay revealed that PKA specifically phosphorylates at the residue of 239 in Rhes. Surprisingly, the phosphomimetic mutant of serine-239 to aspartic acid (S239D) induced an increase of iron uptake while the phosphodead mutant of serine-239 to alanine (S239A) did not. These observations indicate that PKA-mediated phosphorylation of Rhes activates Rhes GTPase and regulates the intracellualr iron influx.
2. Experimental Procedures
2.1. Cells and generation of mutant constructs
HEK 293T cells were maintained in DMEM with 10% FBS, 2 mM L-glutamine and 100 U/ml penicillin-streptomycin at 37°C with 5% CO2 atmosphere in a humidified incubator. Wild type Rhes was cloned into pCMV-Myc (Clonotech) and subsequently S293A and S293D mutants were created with QuickChange (Stratagene) method according to manufacturer's instruction.
2.2. Iron uptake assay
Non-transferrin-bound iron (NTBI) uptake assays were performed as previously described (Cheah, Kim et al. 2006). In brief, HEK293T cells were transfected with Rhes-Myc or mutants using Polyfect reagent (Qiagen). After 48 hr, the cells were washed with phosphate-buffered saline (PBS) then resuspended into iron uptake buffer (25 mM Tris, 25 mM MES, 140 mM NaCl, 5.4 mM KCl, 5 mM glucose, 1.8 mM CaCl2 [pH 5.5]) and transferred to glass test tubes. Ascorbic acid was added to 1 mM FeSO4 at a 44:1 ratio. 55FeCl3 (PerkinElmer Life Science) was added to the iron/ascorbic acid mixture, which was then added to the cells in iron uptake buffer to a final concentration of 20 μM. Cells were incubated at 37°C with shaking for 15 min. The cells were washed twice with cold PBS plus 0.5 mM EDTA and harvested. An aliquot of resuspended cells was taken for protein assay using the Bio-Rad Protein Assay Reagent; the protein concentrations of individual samples were used to quantitate 55Fe incorporation (cpm/μg protein). Samples were normalized to control. Statistical comparisons of iron uptake were performed by student's t-test. All NBTI uptake experiments were repeated at least three times, each sample in triplicate.
2.3 GST Pull-down assay
GST or GST-tagged PAP7 constructs were cotransfected with Rhes-Myc constructs into HEK293T cells using PolyFect (Qiagen), with a transfection efficiency of greater than 90%. Cells were lysed 48 hr after transfection in buffer A (100 mM Tris [pH 7.4], 150 mM NaCl, 1% Triton X-100, 15% glycerol, 1 mM PMSF, 25 mg/ml antipain, 50 mg/ml leupeptin, 50 mg/ml aprotinin, 25 mg/ml chymostatin, and 25 mg/ml pepstatin). Lysates were precleared with pansorbin cells (Calbiochem), then 1 mg of total protein was incubated with Glutathione-Sepharose beads overnight at 4 C. Beads were washed with wash buffer (50 mM Tris [pH 7.4], 500 mM NaCl, 10 mM b-glycerophosphate) twice, then once with buffer A. Beads were quenched in sample buffer (100 mM Tris [pH 6.8], 10% glycerol, 250 mM b-mercaptoethanol, 2% sodium dodecyl sulfate, and bromophenol blue). Total protein (50 mg) was loaded as input. Rhes-Myc binding was examined using an anti-myc antibody (Roche) followed by incubation with anti-mouse secondary conjugated to horseradish peroxidase (HRP) (Jackson Immunoresearch); blots were then stripped and probed with an anti-GST antibody conjugated to HRP to detect PAP7. Chemiluminescence (Pierce) was used to detect bands on the Western blot.
2.4. in vitro phosphorylation
Immunoprecipitation and in vitro kinase assay were performed as previously described (Faul, Dhume et al. 2007). Cells transfected with Rhes-Myc or Myc were lysed in buffer A, and then centrifuged at 12,000 ×g for 10 min at 4 °C. After preclearing with 125 μl of Protein A beads prepared as a 20% (v/v) suspension for 1 h at 4 °C, supernatants were incubated with anti-Myc antibody and the immunocomplexes were precipitated by addition of Protein A bead suspensions. The immunoprecipitates were collected by centrifugation and washed twice with buffer A and twice with PBS. The kinase assay was performed by incubating cell lysates in phosphatase buffer containing 20 mM MgCl2 with or without λ phosphatase (λ PPase) for 3 hours at 30°C. After thoroughly washing with PBS three times, protein bound beads were incubated with kinase buffer containing 1 mM MgATP, λ PPase inhibitors, and trace mount of [γ-32P] ATP for 30 min at 30°C. When indicated, protein kinases, such as PKA, protein kinase C (PKC), and casein kinase 2 (CK2) were added to the reaction mixture. The kinase reaction was stopped by adding 4 × SDS loading buffer and the samples were subjected to SDS-PAGE. Protein transferred blot was exposed to X-ray film for autoradiography. The blot was incubated with blocking buffer of 10% skim milk then proved with anti-Myc antibody for detection of input signal. To block PKA activities in cells, 1 μM H89 was added to the kinase buffer.
2.5. Immunofluorescence staining
Stable cell line of HEK293 was seeded on glass coverslips and transfected with iron responsive element (IRE)-GFP and Rhes-Myc (or mutants). After 24 hours, cells were treated with 50 ug/ml ferric ammonium citrate (FAC) or 100 μM iron chelator, deferoxamine (DFO). Another 24 hours after, cells were fixed with 4% PFA, washed, permeabilized, and blocked with 1% BSA, 2% normal goat serum in PBS. Cells were then incubated with a mouse anti-Myc epitope primary antibody and then visualized by an appropriate Alexa Fluor 596 secondary antibody (Invitrogen). Confocal microscopy was performed under oil immersion on Leica DMI6000 (Leica Microsystems) with a 63x objective.
3. Results
3.1 Rhes increases iron uptake via DMT1
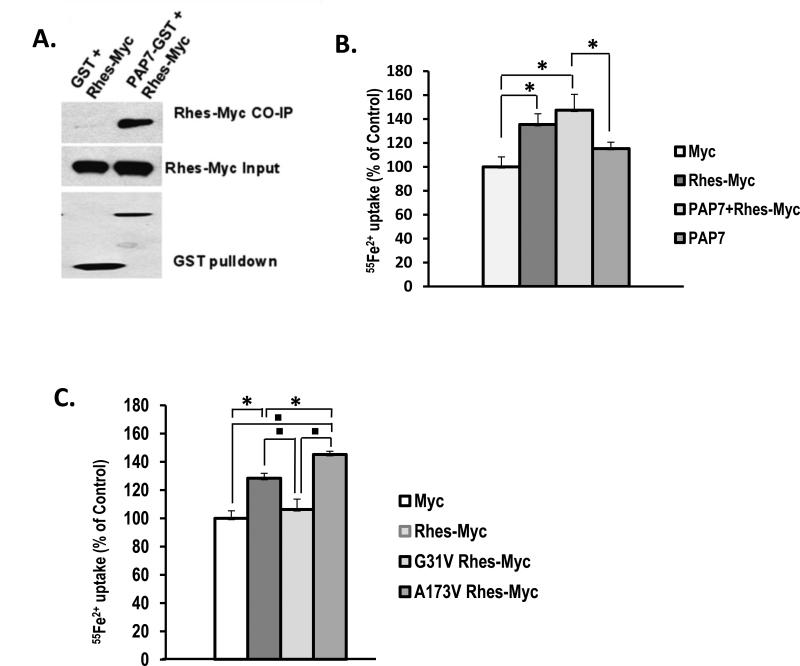
Since Rhes shares a close homology with Dexras 1, which is known to involve neuronal iron trafficking through an interaction of DMT1 via PAP7, we first examined whether Rhes can also interact with PAP7 using the GST Pull down assay. HEK293T cells transiently expressing Rhes-Myc and GST-PAP7 revealed selective binding of Rhes to PAP7 (Fig. 1A). To test whether Rhes-PAP7 complex is involved in the iron uptake via DMT1, we performed a non-transferrin bound iron (NTBI) uptake assay. We found an increase of iron uptake in HEK293T cells overexpressed with Rhes and PAP7 compared to the control cells (Fig. 1B). We wondered if the action of Rhes in iron uptake might be derived from its GTPase activity. From the comparison between constitutively inactive Rhes, with a mutation of glycine-31 to valine (G31V) and active Rhes, with a mutation of alanine-173 to valine (A173V), we observed that A173V increased iron uptake more than the native Rhes whereas G31V transfected cells showed no effect on iron uptake as similar to those transfected with a Myc control vector (Fig. 1C). This indicates Rhes GTPase activity drives iron uptake.
Figure 1. Involvement of Rhes in iron uptake.
(A) HEK293T cells were transfected with Rhes-myc, and GST or GST-PAP7. Cell lysates were subjected to GST-pull down assay. (B) NBTI uptake was measured with cells transfected with either wild type, PAP7, or wild type and PAP7 (* P<0.05). (C) HEK293T cells were transfected with Rhes, G31V-, or A173V-Rhes were subjected to NBTI uptake assay as described in the Experimental Procedures (* P<0.05, P<0.005).
3.2 Protein Kinase A (PKA) phosphorylates Rhes
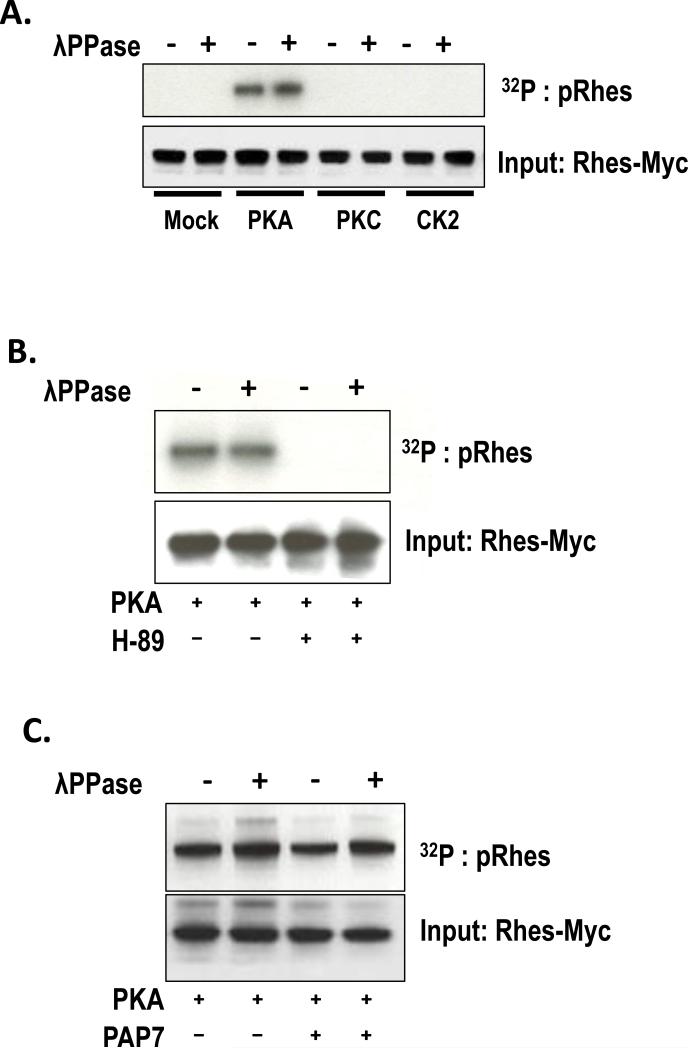
It has been shown before that S-nitrosylation, a nitric oxide (NO)-mediated post-translational modification, acts as a guanidine exchange factor (GEF) for Dexras1 and activates its GTPase activity, leading to an increase of iron influx in neurons. Therefore, we examined if Rhes is a target for NO-mediated S-nitrosylation. HEK293T cells were transfected with a Rhes-myc plasmid and treated with either glutathione (GSH) or nistroso-S-glutathione (GSNO) for 2 hours. Unlike Dextras 1, we found that Rhes was not S-nitrosylated by NO treatment, suggesting that NO does not activate Rhes GTPase activity. Alternatively, the primary sequence analysis by ScanSite (http://scansite.mit.edu) revealed that Rhes contains putative PKA phosphorylation sites. To test whether PKA indeed phosphorylates Rhes, we performed an in vitro phosphorylation assay to identify a putative modulator of Rhes. Recombinant Rhes was incubated with various kinases and we found that PKA was the only kinase that can phosphorylate the Rhes protein (Fig. 2A). Moreover, treatment of H-89, a well-known PKA inhibitor, abrogated PKA mediated phosphorylation of Rhes (Fig. 2B), supporting that PKA mediates phosphorylation of Rhes. PAP7 is known to bind to a regulatory subunit of PKA, so we wondered whether PAP7 had any effect on PKA-mediated phosphorylation of Rhes. We found co-overexpression of PAP7 with Rhes does not affect PKA-mediated phosphorylation of Rhes indicating that PAP7 is not involved in phosphorylation of Rhes by PKA (Fig. 2C).
Figure 2. Phosphorylation of Rhes by PKA.
Rhes-myc was transfected into HEK293T and was immunoprecipitated by anti-myc antibody. (A) In vitro phosphorylation assay was performed in the presence of various kinases with or without pretreatment of λ phosphotase. (B) PKA-mediated phosphorylation assay was performed in the presence or absence of PKA inhibitor, H-89. (C) PKA-mediated phosphorylation assay was performed in the presence or absence of PAP7.
3.3 PKA phosphorylates only serine-239 amino acid on Rhes
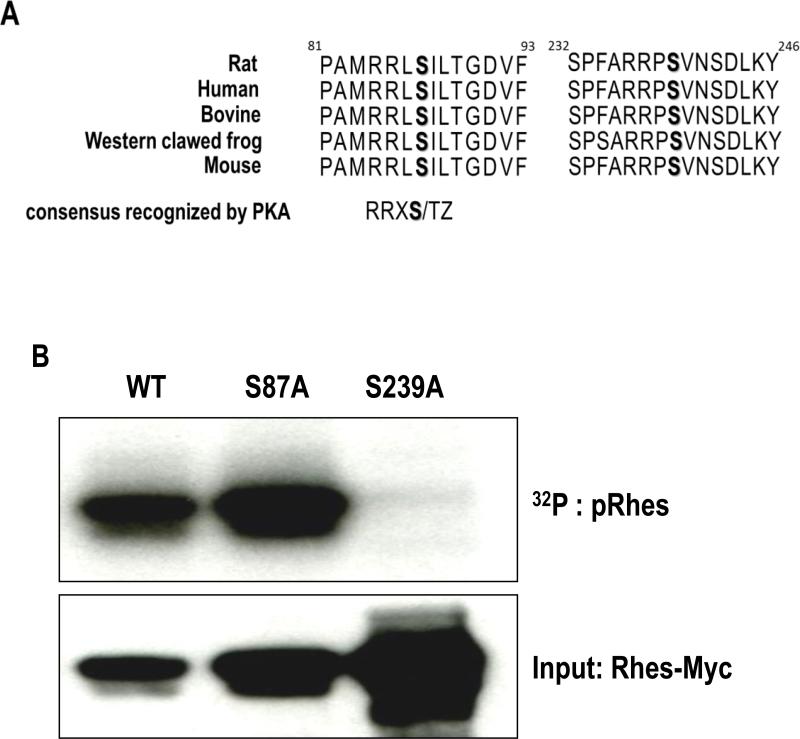
We performed primary sequence analysis of Rhes and identified two putative substrate motifs between residues 84 and 88 (RRLSI) and 236 and 240 (RRPSV) for the PKA (the consensus motif for PKA substrates is R-R- pS/pT -Z, where X represents any amino acid residue, pS or pT represents the phosphorylated serine or threonine, and Z represents the small size of the amino acid residue) (Pinna and Ruzzene, 1996; Songyang et al., 1994). The motif is highly conserved in Rhes protein from all species examined, suggesting these two may be potential target for PKA-mediated phosphorylation of Rhes (Fig. 3A). To directly examine which amino acid is modified by PKA, we mutated each serine (S87, and S239) into nonphosphorylatable alanine. From the in vitro phosphorylation assay with HEK293T cells overexpressed with phosphodead mutants of S87A and S239A we generated, PKA mediated phosphorylation was completely eliminated from S239A and not S87A (Fig. 3B), demonstrating that serine-239 is the single amino acid phosphorylated by PKA.
Figure 3. Phosphorylation of serine-239 amino acid by PKA.
(A) Amino acid sequence alignment of Rhes (B) Either WT Rhes-myc or phosphodead mutants (S87A or S239A) were transfected into HEK293T cells and were immunoprecipitated by anti-myc antibody. In vitro phosphorylation assay was performed and the results were visualized by autoradiography (top). Western blot image showed the loading of Myc-tagged Rhes protein in the reactions (bottom).
3.4 Phosphomimetic Rhes mutant induces an increase in iron uptake
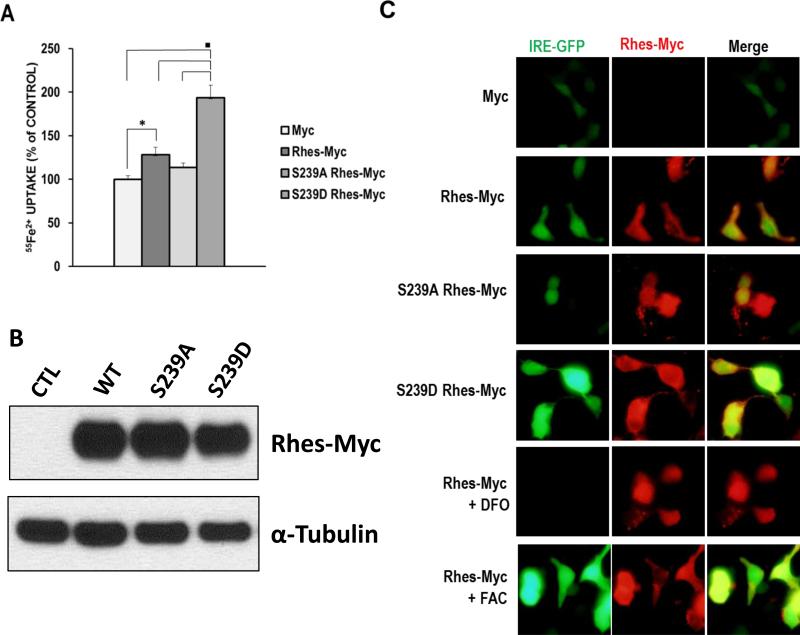
We wondered if PKA-mediated phosphorylation of Rhes is involved in iron trafficking in the cells. To test this, we created a phosphomimetic mutant by changing serine 239 to aspartic acid (S293D) and examined its effect on iron uptake. In cells transfected with the S239D Rhes mutant, a more than 50% increase in iron uptake was observed compared to the native Rhes (Fig. 4A). However, the phosphodead mutant of S239A showed no effect on iron uptake. We have also confirmed that all the forms of Rhes (WT, S239D and S239A) are expressed at comparable levels, demonstrating that the differences in iron uptake between Rhes mutants are due to the phosphorylation status rather than the protein expression levels (Fig 4B). To visualize intracellular iron, HEK293T cells were co-transfected with Rhes (or mutants) and the iron reporter construct IRE-GFP, whose expression is positively correlated with intracellular iron levels. This reporter construct has been used previously to measure changes in cytosolic iron (Li et al., 2004). S239D consistently displayed stronger GFP intensity than the native Rhes and S239A showed weak GFP signal (Fig. 4C and 4A).
Figure 4. Increase in Iron uptake with phosphomimetic S239D mutant Rhes.
(A) HEK293T cells transfected with Rhes or mutants (S239A or S239D) Rhes were subjected to NBTI uptake assay. (* P<0.05, ■ P<0.005). (B) HEK293T cell that were transfected with Rhes or Rhes mutants (from A) were detected by using anti-myc antibody and α–tubulin as a loading control. (C) The levels of intracellular iron were visualized by using IRE-GFP, an iron reporter GFP system. HEK293T cells transfected with Myc or Rhes-Myc (or mutants) and IRE-GFP were further incubated with in the absence or presence of either FAC or DFO. Merge presents an overlay of Rhes in red and IRE-GFP in green.
4. Discussion
We provide evidence that Rhes is phosphorylated by PKA at the site of serine 239 and consequently modulates iron trafficking via the iron importer channel DMT1. We observed that native Rhes is involved in the cellular iron uptake. However, the degree of increase in iron entry from the cells with wild type Rhes was less than the mutants of either constitutively active A178V or phosphomimetic S239D. This might be due to the possibility that not all of native Rhes is participating in the modulation of iron uptake. Indeed, it has been shown that only a small portion of Rhes in its native status is bound to GTP (Vargiu et al., 2004).
Iron is taken into the cell via two methods: the classical Transferrin (Tf)-mediated iron uptake pathway and the Non-Tf Bound Iron (NTBI) uptake from the plasma membrane. Physiologically, the majority of cells in the organism acquire iron from a well-characterized plasma glycoprotein, Tf. Iron uptake from Tf is mediated by receptor-mediated endocytosis upon its binding to Tf receptor (TfR). After endocytosis, the acidic environment of the early endosomes triggers the release of trivalent iron from the Tf–TfR complex, which is recycled to the plasma membrane. Members of the Steap family of ferric reductases localized in the endosome reduce ferric iron to its ferrous form before releasing it into the cytosol via DMT1. Moreover, DMT1 in the plasma membrane directly mediates iron transport for NTBI uptake (Fleming et al., 1997; Gunshin et al., 1997). NTBI uptake can occur when iron overload produces fully saturated Tf, yet physiological roles for NTBI have not been well characterized. Therefore, iron has to go through DMT1 to enter into cytoplasm regardless of iron uptake path. Moreover, it has been shown that DMT1 expression levels or its activity are not limiting factors for TfR-mediated iron uptake, suggesting that results from NTBI may provide useful information about overall iron trafficking status. Indeed, our previous study showed that Dexras1 equally regulated TfR-mediated iron uptake as well as NTBI uptake (Cheah et al., 2006). Surprisingly, the amount of Tf made in the brain is about 100-fold lower than that of serum Tf. Unlike the systemic circulation, brain iron levels are in a molar excess relative to that of Tf iron binding capacity and thus, brain Tf is often saturated (Burdo and Connor, 2003; Sorond and Ratan, 2000). Therefore, under normal conditions, there may be substantial non-Tf bound iron-uptake into cells.
Several studies have examined the activity of intracellular enzymes that are affected by Rhes (Harrison and He, 2011; Vargiu et al., 2004). However, this is the first time to how Rhes is regualted by other enzyme. Since Dexras 1 is activated via S-nitrosylation, failure to detect S-nitrosylat of Rhes with NO treatment was an unexpected outcome. We found that PKA is the only tentative enzyme that regulates Rhes activity by phosphorylation at the site of serine-239 (Fig. 2A). The phosphomimetic mutant induced a considerable increase in iron entry (Fig. 4A and C), implying that conformational change by phosphorylation on serine 239 might lead to an active state evidenced from the increase in iron uptake with the constitutively active form of Rhes (Fig. 1C). Hence, it is tempting to speculate that PKA-mediated phosphorylation is the guanidine exchange factor (GEF) for Rhes analogous to NO-mediated S-nitrosylation for Dexras1.
Even though Rhes participates in iron uptake like Dexras1, a relative GTPase sharing the closest homology with Rhes, their precise mechanisms are not likely identical. For Dexras1, glutamate-NMDA receptor stimulation-induced nNOS activation results in S-nitrosylation of Dexras1 (Cheah et al., 2006; Fang et al., 2000). However, PKA-mediated phosphorylation on serine 293 is required for Rhes to induce iron uptake. In addition, the striatal localization is a primary characteristics of Rhes distinguished from Dexras1, which is widely distributed in the brain (Falk et al., 1999; Fang et al., 2000)(Falk, Vargiu et al. 1999; Fang, Jaffrey et al. 2000). The striatum is full of dopaminergic synapses projected from the substantia nigra and is responsible for balanced movement. Dopamine binding to the D1 receptor leads to production of cAMP, which activates PKA (Borgkvist and Fisone, 2007; Nishi et al., 2011). Moreover, PKA is known to regulate signaling pathways that are involved in learning and memory (Heyser et al., 2000), addiction (Hiroi et al., 1999), and synaptic plasticity (Lee et al., 2000). Furthermore, striatal PKA also participates in motor behavior from the study of the RIIβ PKA mutant mouse model (Brandon et al., 1998). However, upon aging, PKA activity seems perturbed from the evidence that RIIβ PKA disruption promoted anti-aging phenotype (Enns et al., 2009). Since the striatum is a physiologically iron-rich area (Dexter et al., 1993) and imbalance of iron metabolism is aggravated with aging (Xu et al., 2012), striatal neurons are prone to degeneration by iron-associated oxidative stress, leading to aging-related decline in motor function (Cass et al., 2007; Xu et al., 2012). Its precise mechanism of region-dependent iron dysregulation remains to be clarified. However, our study suggests that striatal specific Rhes, when its activity is perturbed, might contribute to dysregulation of dopaminergic function in the striatum.
Highlights.
Rhes interacts with an iron importer, DMT1 via PAP7.
Rhes is not S-nitrosylated by nitric oxide.
PKA phosphorylates Rhes at the site of serine 239.
PKA-mediated phosphorylation of Rhes modulates iron trafficking.
Acknowledgement
This work was supported by HD026979, MH079614, DK084336 and the generous gift from the Byrd family, (SFK).
Abbreviations
- Rhes
ras homolog enriched in striatum
- nNOS
neuronal nitric oxide synthase
- DMT1
divalent metal transporter 1
- PAP7
Peripheral benzodiazepine Receptor-associated Protein7
- S239D
serine-239 aspartic acid
- PKA
protein kinase A
- S239A
serine-239 alanine
- NTBI
nontransferrin-bound iron
- PBS
phosphate-buffered saline
- HRP
horseradish peroxidase
- IRE
iron responsive element
- FAC
ferric ammonium citrate
- DFO
deferoxamine
- G31V
glycine-31 valine
- A173V
alanine-173 valine
- Tf
transferrin
- TrR
transferrin receptor
- PD
Parkinson's disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Bang S, Steenstra C, Kim SF. Striatum specific protein, Rhes regulates AKT pathway. Neurosci Lett. 2012;521:142–147. doi: 10.1016/j.neulet.2012.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham KJ, Bush AI. Metals in Alzheimer's and Parkinson's diseases. Curr Opin Chem Biol. 2008;12:222–228. doi: 10.1016/j.cbpa.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Berg D, Hochstrasser H. Iron metabolism in Parkinsonian syndromes. Mov Disord. 2006;21:1299–1310. doi: 10.1002/mds.21020. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Fisone G. Psychoactive drugs and regulation of the cAMP/PKA/DARPP-32 cascade in striatal medium spiny neurons. Neurosci Biobehav Rev. 2007;31:79–88. doi: 10.1016/j.neubiorev.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Brandon EP, Logue SF, Adams MR, Qi M, Sullivan SP, Matsumoto AM, Dorsa DM, Wehner JM, McKnight GS, Idzerda RL. Defective motor behavior and neural gene expression in RIIbeta-protein kinase A mutant mice. J Neurosci. 1998;18:3639–3649. doi: 10.1523/JNEUROSCI.18-10-03639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo JR, Connor JR. Brain iron uptake and homeostatic mechanisms: an overview. Biometals. 2003;16:63–75. doi: 10.1023/a:1020718718550. [DOI] [PubMed] [Google Scholar]
- Cass WA, Grondin R, Andersen AH, Zhang Z, Hardy PA, Hussey-Andersen LK, Rayens WS, Gerhardt GA, Gash DM. Iron accumulation in the striatum predicts aging-related decline in motor function in rhesus monkeys. Neurobiol Aging. 2007;28:258–271. doi: 10.1016/j.neurobiolaging.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Cheah JH, Kim SF, Hester LD, Clancy KW, Patterson SE, III, Papadopoulos V, Snyder SH. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron. 2006;51:431–440. doi: 10.1016/j.neuron.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Khan RS, Cwanger A, Song Y, Steenstra C, Bang S, Cheah JH, Dunaief J, Shindler KS, Snyder SH, Kim SF. Dexras1, a Small GTPase, Is Required for Glutamate-NMDA Neurotoxicity. J Neurosci. 2013;33:3582–3587. doi: 10.1523/JNEUROSCI.1497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cismowski MJ, Ma C, Ribas C, Xie X, Spruyt M, Lizano JS, Lanier SM, Duzic E. Activation of heterotrimeric G-protein signaling by a ras-related protein. Implications for signal integration. J Biol Chem. 2000;275:23421–23424. doi: 10.1074/jbc.C000322200. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Sian J, Jenner P, Marsden CD. Implications of alterations in trace element levels in brain in Parkinson's disease and other neurological disorders affecting the basal ganglia. Adv Neurol. 1993;60:273–281. [PubMed] [Google Scholar]
- Enns LC, Morton JF, Treuting PR, Emond MJ, Wolf NS, Dai DF, McKnight GS, Rabinovitch PS, Ladiges WC. Disruption of protein kinase A in mice enhances healthy aging. PLoS One. 2009;4:e5963. doi: 10.1371/journal.pone.0005963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico F, Santini E, Migliarini S, Borgkvist A, Centonze D, Nasti V, Carta M, De C, V, Prosperetti C, Spano D, Herve D, Pasqualetti M, Di LR, Fisone G, Usiello A. The GTP-binding protein Rhes modulates dopamine signalling in striatal medium spiny neurons. Mol Cell Neurosci. 2008;37:335–345. doi: 10.1016/j.mcn.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Falk JD, Vargiu P, Foye PE, Usui H, Perez J, Danielson PE, Lerner DL, Bernal J, Sutcliffe JG. Rhes: A striatal-specific Ras homolog related to Dexras1. J Neurosci Res. 1999;57:782–788. [PubMed] [Google Scholar]
- Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH. Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Fleming MD, Trenor CC, III, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- Graham TE, Key TA, Kilpatrick K, Dorin RI. Dexras1/AGS-1, a steroid hormone-induced guanosine triphosphate-binding protein, inhibits 3',5'-cyclic adenosine monophosphate- stimulated secretion in AtT-20 corticotroph cells. Endocrinology. 2001;142:2631–2640. doi: 10.1210/endo.142.6.8209. [DOI] [PubMed] [Google Scholar]
- Graham TE, Prossnitz ER, Dorin RI. Dexras1/AGS-1 inhibits signal transduction from the Gi-coupled formyl peptide receptor to Erk-1/2 MAP kinases. J Biol Chem. 2002;277:10876–10882. doi: 10.1074/jbc.M110397200. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Harrison LM, He Y. Rhes and AGS1/Dexras1 affect signaling by dopamine D1 receptors through adenylyl cyclase. J Neurosci Res. 2011;89:874–882. doi: 10.1002/jnr.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison LM, Lahoste GJ. Rhes, the Ras homolog enriched in striatum, is reduced under conditions of dopamine supersensitivity. Neuroscience. 2006;137:483–492. doi: 10.1016/j.neuroscience.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Harrison LM, Muller SH, Spano D. Effects of the Ras homolog Rhes on Akt/protein kinase B and glycogen synthase kinase 3 phosphorylation in striatum. Neuroscience. 2013;236:21–30. doi: 10.1016/j.neuroscience.2012.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze MW, Kuhn LC. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci U S A. 1996;93:8175–8182. doi: 10.1073/pnas.93.16.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Fienberg AA, Greengard P, Gold LH. DARPP-32 knockout mice exhibit impaired reversal learning in a discriminated operant task. Brain Res. 2000;867:122–130. doi: 10.1016/s0006-8993(00)02272-1. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Fienberg AA, Haile CN, Alburges M, Hanson GR, Greengard P, Nestler EJ. Neuronal and behavioural abnormalities in striatal function in DARPP-32-mutant mice. Eur J Neurosci. 1999;11:1114–1118. doi: 10.1046/j.1460-9568.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- Ke Y, Qian ZM. Brain iron metabolism: neurobiology and neurochemistry. Prog Neurobiol. 2007;83:149–173. doi: 10.1016/j.pneurobio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Khalil M, Teunissen C, Langkammer C. Iron and neurodegeneration in multiple sclerosis. Mult Scler Int. 2011;2011:606807. doi: 10.1155/2011/606807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Li JY, Ram G, Gast K, Chen X, Barasch K, Mori K, Schmidt-Ott K, Wang J, Kuo HC, Savage-Dunn C, Garrick MD, Barasch J. Detection of intracellular iron by its regulatory effect. Am J Physiol Cell Physiol. 2004;287:C1547–C1559. doi: 10.1152/ajpcell.00260.2004. [DOI] [PubMed] [Google Scholar]
- McCord JM. Iron, free radicals, and oxidative injury. Semin Hematol. 1998;35:5–12. [PubMed] [Google Scholar]
- Moos T, Morgan EH. Evidence for low molecular weight, non-transferrin-bound iron in rat brain and cerebrospinal fluid. J Neurosci Res. 1998;54:486–494. doi: 10.1002/(SICI)1097-4547(19981115)54:4<486::AID-JNR6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Nishi A, Kuroiwa M, Shuto T. Mechanisms for the modulation of dopamine d(1) receptor signaling in striatal neurons. Front Neuroanat. 2011;5:43. doi: 10.3389/fnana.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez MT, Urrutia P, Mena N, Aguirre P, Tapia V, Salazar J. Iron toxicity in neurodegeneration. Biometals. 2012;25:761–776. doi: 10.1007/s10534-012-9523-0. [DOI] [PubMed] [Google Scholar]
- Pinna LA, Ruzzene M. How do protein kinases recognize their substrates? Biochim Biophys Acta. 1996;1314:191–225. doi: 10.1016/s0167-4889(96)00083-3. [DOI] [PubMed] [Google Scholar]
- Quintero GC, Spano D, Lahoste GJ, Harrison LM. The Ras homolog Rhes affects dopamine D1 and D2 receptor-mediated behavior in mice. Neuroreport. 2008;19:1563–1566. doi: 10.1097/WNR.0b013e3283118434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA, Cooperman S. Brain iron metabolism. Semin Pediatr Neurol. 2006;13:142–148. doi: 10.1016/j.spen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Schneider SA, Bhatia KP. Syndromes of neurodegeneration with brain iron accumulation. Semin Pediatr Neurol. 2012;19:57–66. doi: 10.1016/j.spen.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Blechner S, Hoagland N, Hoekstra MF, Piwnica-Worms H, Cantley LC. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr Biol. 1994;4:973–982. doi: 10.1016/s0960-9822(00)00221-9. [DOI] [PubMed] [Google Scholar]
- Sorond FA, Ratan RR. Ironing-out mechanisms of neuronal injury under hypoxic-ischemic conditions and potential role of iron chelators as neuroprotective agents. Antioxid Redox Signal. 2000;2:421–436. doi: 10.1089/15230860050192206. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Snyder SH. Huntington's disease is a disorder of the corpus striatum: focus on Rhes (Ras homologue enriched in the striatum). Neuropharmacology. 2011;60:1187–1192. doi: 10.1016/j.neuropharm.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57:467–478. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- Vargiu P, De AR, Garcia-Ranea JA, Valencia A, Santisteban P, Crespo P, Bernal J. The small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptors. Oncogene. 2004;23:559–568. doi: 10.1038/sj.onc.1207161. [DOI] [PubMed] [Google Scholar]
- Xu J, Jia Z, Knutson MD, Leeuwenburgh C. Impaired iron status in aging research. Int J Mol Sci. 2012;13:2368–2386. doi: 10.3390/ijms13022368. [DOI] [PMC free article] [PubMed] [Google Scholar]