Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disorder that involves multiple organ systems and typically presents as a chronic inflammatory disease. Antibodies to double-stranded (ds) DNA are present in approximately 70% of patients and form nucleic acid containing immune complexes which activate dendritic cells through engagement of toll-like receptors, leading to a pro-inflammatory, pro-immunogenic milieu. In addition, anti-dsDNA antibodies deposit in kidneys to initiate glomerulonephritis. Antibodies to C1q have also been implicated in lupus nephritis and are found in 30–50% of patients. C1q is a known suppressor of immune activation and C1q deficiency is the strongest risk factor for SLE.
We previously identified a subset of anti-DNA antibodies that binds the N-methyl-d-aspartate receptor. We now show that both mouse and human anti-DNA antibodies with this specificity bind C1q. These antibodies bind to Clq in glomeruli and exhibit decreased glomerular deposition in the absence of C1q. We propose that this subset of anti-DNA antibodies participates in lupus pathogenesis through direct targeting of C1q on glomeruli and also through removal of soluble C1q thereby limiting the ability of C1q to mediate immune homeostasis.
Keywords: Lupus, Complement C1q, Anti-DNA antibody
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disorder primarily affecting women during their reproductive years. It is characterized by activation of autoreactive B cells with ensuing elevation in serum autoantibody titers. Autoantibodies against nuclear antigens are found in 95% or more of lupus patients; antibodies to double-stranded (ds) DNA are present in approximately 70% of patients [1]. High titers of anti-dsDNA antibodies correlate with disease activity, are most common in patients with renal disease and can be isolated from glomeruli of patients with lupus nephritis [2,3]. Indeed, many anti-dsDNA antibodies cross-react with glomerular antigens [4]. Clinical involvement of the kidneys occurs in 50–80% of lupus patients during the course of their disease and renal pathology is found in as many as 90% of patients at autopsy [5].
More recently, it has been demonstrated that lupus patients with anti-DNA or anti-RNP antibodies experience systemic inflammation as well as discrete target organ injury, with increased expression of type I interferon (IFN) inducible genes in peripheral blood mononuclear cells [6]. This appears to result from activation of plasmacytoid dendritic cells (pDCs) and secretion of IFN, mediated in part by nucleic acid-containing immune complexes (IC) that are internalized by activating Fc receptors (FcRs) and subsequently engage toll-like receptors (TLRs) that recognize nucleic acid ligands or even solely by engaging activating FcRs [7,8].
C1q is a 460 KDa protein formed by 6 homotrimeric subunits containing an N-terminal collagen-like sequence and a C-terminal globular region. It functions in the innate immune response to clear pathogens by activation of the classical complement cascade [9]. Moreover, it contributes to the clearance of IC and apoptotic cells from the circulation, an activity which is important for maintenance of immune tolerance to self antigens [10]. C1q has also been found to inhibit monocyte to DC differentiation, DC activation and interferon production by plasmacytoid DCs (pDCs) and therefore may also play a central role in preventing aberrant innate and adaptive immune responses [11–13]. Although C1q deficiency is a rare phenomenon, it provides the strongest genetic risk for lupus [14]. Several receptors binding C1q have been identified in various cell types including C1qRp (CD93); cC1qR (calreticulin), LAIR-1, CR1 and CD35 which bind the collagen region of C1q; gC1qR (multi-ligand binding receptor) which binds to the globular domain of C1q; and C1qR02 [13,15]. Engagement of each of these receptors appears to initiate distinct cellular functions; for example, engagement of C1qRp enhances phagocytosis while engagement of C1qR02 triggers a superoxide burst in neutrophils. Most importantly for an understanding of SLE, absence of C1q has been shown to lead to enhance IFNα production by both human and murine pDCs [17,18].
Antibody to C1q has also been implicated in lupus nephritis, and is found in 30–50% of lupus patients [19]. Indeed, antibody to C1q correlates more strongly with renal disease than antibody to dsDNA and increased serum levels of anti-C1q antibodies correlate with flares [20]. Since C1q together with natural IgM autoantibodies plays a major role in maintenance of self-tolerance through opsonization of apoptotic material and through engaging the inhibitory LAIR-1 receptor on monocytes and DCs, it has been postulated that anti-C1q antibodies might decrease the availability of C1q for this tolerogenic function [21]. Anti-C1q antibodies may also contribute to lupus pathogenesis by binding to IC in target organs. In support of this model are data that monoclonal anti-C1q antibodies administered to mice exacerbate glomerular immunoglobulin deposition by anti-glomerular basement membrane antibodies [22], although they do not induce disease by themselves.
Our laboratory has previously generated a murine monoclonal antibody R4A which binds to dsDNA [23]. By screening a peptide library, we showed that R4A binds a consensus pentapeptide sequence D/EWD/EYS/G. Immunization of BALB/c mice with a multimeric configuration of the DWEYS peptide induces the formation of antibodies that cross-react with dsDNA, deposit in glomeruli and induce proteinuria [24]. The consensus sequence is present in the extracellular region of the NR2A and NR2B subunits of the N-methyl-d-aspartate receptor (NMDAR) in both mice and humans. In mice, antibodies to dsDNA and DWEYS bind neuronal NMDAR, induce apoptosis and cause some neuropsychiatric manifestations of lupus if they gain access to brain tissue [25]. Antibodies cross-reactive with both dsDNA and DWEYS peptide are present in approximately 40% of lupus patients [25].
R4A was also found to bind the decapeptide WCEADYGRCP in the screen of the peptide library. Because R4A bound the consensus pentapeptide D/EWD/EYS/G, we presumed it bound the EADYG sequence which shares 4 of 5 amino acids with the consensus sequence [26]. A peptide blast query using the EADYG peptide sequence through the National Center for Biotechnology information (NLM-NIH) search engine, identified a homologous sequence present in the globular domain (GSEADSV) of the C1q A chain (NP 001002259.1, NCBI, NLM, NIH). We now demonstrate for the first time that antibodies to dsDNA cross-react with C1q and play a potential role in the pathogenesis of lupus through deposition in glomeruli.
2. Material and methods
2.1. Mice
Female C1q−/− C57BL/6J mice were obtained from Dr. Keith Elkon, University of Washington and female C57BL/6J from Jackson Laboratories (Bar Harbor, ME, USA). Mice were 8–16 weeks of age.
2.2. ELISAs
Costar half-volume 96-well plates (Corning, NY) were used for C1q NR2 A and B, and peptide ELISAs and Immulon 2HB 96-well plates (Milford, MA) were used for DNA ELISAs. C1q was purchased from Comptech (Tyler, TX) and adsorbed to 96-well plate (50 μg/ml). NR2A and NR2B were expressed in a cell line and extracted from an electrophoresis gel of cell lysate. Peptides were purchased from AnaSpec Inc, San Jose, CA. DNA, C1q, the collagen tail of C1q obtained by digestion of intact C1q, peptides or NR2A and NR2B were added to plates and incubated overnight at 4 °C then blocked with 2% BSA in PBS for 1 h at RT. R4A antibody, IgG2b control immunoglobulin (MPC-11 hybridoma, ATCC, Manassas, VA), was added for 1 h at RT at various concentrations. C1q collagen-tail of C1q was prepared as described in Ref. [27]. Reagents used in vivo experiments were tested for LPS content by LAL (limulus amebocyte lysate) assay and were found to have less than 0.05 EU/ml.
2.3. Glomerular binding assay
Glomeruli were isolated from C1q−/− or wild type C57BL/6J mice [28,29]. Ten to 20 glomeruli were attached per slide and acetone fixed. Some slides were treated with DNAse (Sigma) at 100 μg/ml in 5 mM CaCl and 0.9% NaCl for 45 min at 37 °C or with PBS alone. After blocking with 10% goat serum, R4A mouse monoclonal anti-DNA antibody mouse IgG2b control antibody (MCP-11) G11 human antibody to DNA anti-peptide antibody human IgG control immunoglobulin (B1) or anti-C1q antibody (JL1, Pierce biotechnology, Rockford, IL) was added for 1 h at RT at 20 μg/ml. Secondary antibodies were added at a 1:200 dilution for FITC-labeled anti-mouse IgG (BD) or a 1:500 dilution for FITC-labeled anti-human IgG (Inova diagnostics). DAPI was used at a 1:200 dilution from a stock solution of 200 μg/ml. Purified C1q at 250 μg/ml was incubated with G11 or anti-C1q for 30 min at RT prior to being added to glomeruli.
2.4. Isolation of human anti-DWEYS antibodies
Lupus sera known to have antibodies reactive to DWEYS peptide were incubated with a sepharose 4B resin coupled to DWEYS peptide (AnaSpec Inc.) for 12 h at 4 °C. The resin was washed with PBS prior to elution of bound antibodies with a 0.2 M glycine buffer pH 3.
2.5. Generation of human monoclonal antibody
The monoclonal G11 and B1antibodies were obtained from blood B cells of a lupus patient as described [27].
2.6. In vivo administration of R4A
R4A and MPC-11 (IgG2b control) were labeled with Alexa Fluor 488 (AF488) according to manufacturer (Invitrogen, Grand Island, NY). Two hundred micrograms of labeled R4A or MPC-11 were injected intravenously in C57BL6/J mice. Kidneys were harvested 8 h later and frozen. Kidneys were processed in a cryostat and 10 micron sections were mounted on slides for microscopy.
Moreover, R4A or MPC-11 antibodies were labeled with infrared Dye (Licor, Lincoln, NE) and 200 μg injected intravenously in C57BL6/J or C1q−/− mice. Kidneys were harvested after perfusion with normal saline to remove intravascular blood before analysis on an infrared imaging system (Odyssey – Licor). Integrated density values (IDV) were obtained for each kidney as the sum of pixel intensities after background corrections.
3. Results
3.1. R4A antibody binds to human complement C1q
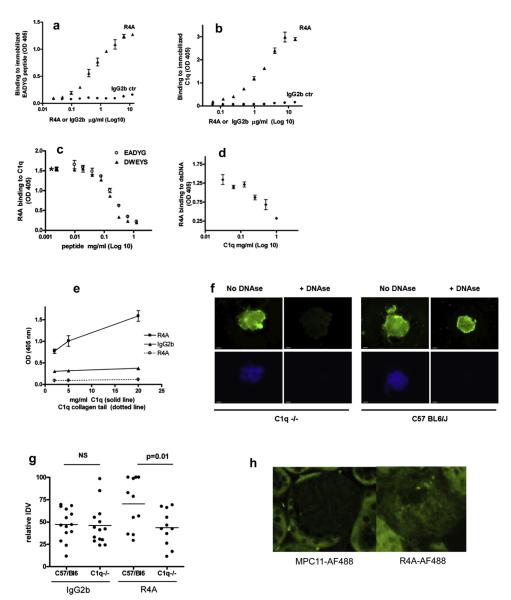
The anti-dsDNA antibody R4A bound to the EADYG peptide and to C1q in a dose-dependent manner (Fig. 1a and b). In addition, peptides EADYG and DWEYS both inhibited R4A binding to C1q (Fig. 1c) while C1q inhibited R4A binding to dsDNA (Fig. 1d). These results demonstrate that R4A binds dsDNA, C1q and the two peptides. In an attempt to identify the region of C1q that is bound by R4A, we performed binding experiments with purified collagen tail of C1q. R4A did not bind C1q collagen tail suggesting that the binding site is present in the globular region of C1q (Fig. 1e), which possesses the GSEADSV sequence.
Fig. 1.
Cross reactivity of R4A. a. R4A and an isotype control antibody were assayed by ELISA for binding to EADYG peptide. b. R4A and an isotype control antibody were assayed by ELISA for binding to C1q. c. R4A was assayed by ELISA for binding to C1q in the presence of varying concentrations of EADYG or DWEYS peptide. d. R4A was assayed by ELISA for binding to DNA in the presence of varying concentrations of C1q. e. R4A was assayed by ELISA for binding to C1q (solid line) and to the collagen tail of C1q (dotted line). f. R4A was incubated with isolated glomeruli from wild type and C1q−/− mice and binding was detected with a labeled antibody to mouse IgG. DAPI was used to visualize DNA in the glomeruli. Results are representative of three different experiments. g. IgG binding to wild type or C1q−/− kidney was quantitated after intravenous injection of infra-red-labeled R4A or MPC-11 isotype control. Data from three separate experiments are represented as percent IDV signal within each experiment. h. R4A-AF488 binding to glomeruli following intravenous administration to wild type and C1q−/− mice, was assessed by microscopy and compared to control immunoglobulin MPC11-AF488.
We next tested whether R4A bound isolated glomeruli from wild type and C1q−/−C57BL6/J mice. R4A antibody strongly stained glomeruli from wild type mice both before and after DNAse treatment (Fig. 1f). In contrast, R4A bound glomeruli from C1q−/− mice prior to DNAse treatment but not after DNAse treatment. C1q−/− mice injected intravenously with R4A had lower levels of antibody deposition in their kidney than wild type mice (Fig. 1g). AF488-labeled R4A was shown to deposit in glomeruli after intravenous injection of C57BL6/J mice (Fig. 1h).
3.2. Anti-DWEYS antibody purified from serum of lupus patients binds to C1q
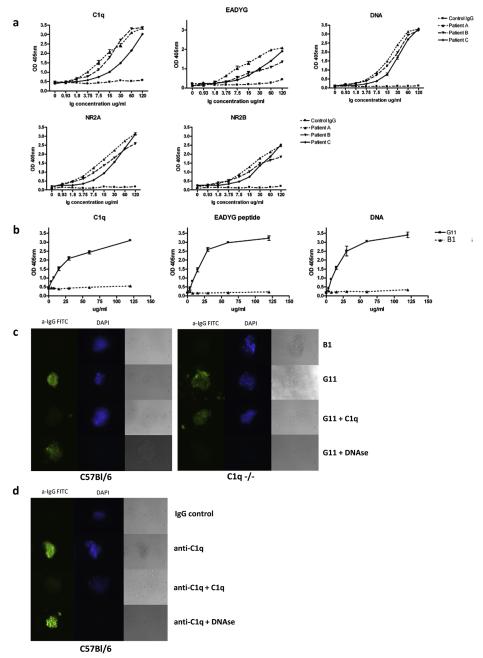
Affinity purified DWEYS-reactive antibodies from serum of three lupus patients were shown to bind C1q, EADYG peptide, dsDNA, NR2A and NR2B (Fig. 2a).
Fig. 2.
Reactivity of human anti-DWEYS and anti-C1q antibodies. a. Human affinity purified anti-DWEYS antibodies and control IgG were assayed by ELISA for binding to C1q, EADYG peptide, dsDNA, NR2A and NR2B. b. Human monoclonal anti-DNA antibody G11 and a control monoclonal antibody B1 were assayed by ELISA for binding to C1q, EADYG peptide and DNA. c. G11 and B1 antibody were assayed for binding to glomeruli from wild type or C1q−/− mice in the presence or absence of C1q or DNAse. DAPI was used to visualize DNA. d. Anti-C1q and B1 antibody were assayed for binding to glomeruli from wild type mice in the presence or absence of C1q or DNAse.
3.3. A monoclonal antibody obtained from lupus patients bind DNA, C1q and EADYG peptide and bind glomeruli ex vivo
G11, a human monoclonal antibody obtained by cloning of Ig genes from a peripheral blood B cell of a lupus patient and previously shown to bind to dsDNA and DWEYS peptide, was demonstrated to bind both C1q and EADYG as well (Fig. 2b). Moreover, like R4A, G11 bound glomeruli from wild type mice before and after DNAse treatment but bound glomeruli from C1q−/− mice prior to, but considerably less, after DNAse treatment (Fig. 2c). B1, a human monoclonal antibody with no reactivity to DNA or DWEYS failed to bind glomeruli from either mouse strain. A commercial anti-C1q monoclonal antibody was shown to bind glomeruli (Fig. 2d); this binding was not affected by DNAse treatment. Soluble C1q partially decreased the binding of G11 or anti-C1q to glomeruli from C57BL6/J mice but failed to decrease binding of these antibodies to glomeruli of C1q−/− mice.
4. Discussion
In this study we demonstrate for the first time that a subset of anti-DNA antibodies cross-reacts with C1q as well as the N-methyl-d-aspartate receptor (NMDAR). The presence of anti-C1q antibodies in lupus is well established, however the possibility of anti-DNA antibodies cross-reactive with C1q had not been proposed until now. Our laboratory has previously shown that the R4A monoclonal antibody which binds both DNA and the DWEYS pentapeptide was found to bind the WCEADYGRCP decapeptide. Moreover, this sequence contains the sequence EADYG that is homologous to a sequence that is present in C1q. Therefore we carried out binding experiments showing that R4A bound both EADYG and C1q. Since EADYG differs from the consensus pentapeptide D/EWD/EYS/G by 1 amino acid, we next demonstrated that R4A binding to C1q could be inhibited by both the DWEYS and EADYG peptides. It is also important to note that R4A binding to C1q is not limited to solid phase but we were also able to show that R4A binds fluid phase C1q through inhibition of binding to immobilized dsDNA. The proposed binding site of R4A in C1q is in the globular region since it did not bind isolated collagen tail. This would be in agreement with the observation that the majority of anti-C1q antibodies found in lupus patients are against the globular region of C1q [30–33].
Lupus nephritis can be initiated by the deposition of anti-dsDNA antibody in glomeruli. R4A has been shown to bind glomeruli even after removal of exposed DNA binding sites through DNAse treatment. We considered, therefore, that R4A might bind C1q present in glomeruli. The study of antibody binding to isolated glomeruli has been used as a surrogate model for anti-DNA antibody binding in vivo and may identify nephritogenic potential of these antibodies. Our results demonstrate that C1q is a major target antigen in wild type glomeruli. These results were in agreement with data from in vivo experiments of R4A binding to kidney of wild type but not C1q−/− mice.
In an attempt to investigate whether similar cross-reactive antibodies could be found in patients with lupus, we demonstrated that anti-DWEYS antibodies from lupus patients also showed cross-reactivity to DNA, C1q, EADYG peptide, NR2A and NR2B. In addition, a human monoclonal antibody to DNA (G11) was also found to bind to isolated glomeruli and C1q was a major target antigen as well, similar to what we observed for R4A. Soluble C1q was able to partially inhibit binding of G11 and anti-C1q to glomeruli from C57BL6/J mice; however, this inhibition was not observed in glomeruli from C1q−/− mice. We interpret this result to suggest that C1q binds DNA in glomeruli of wild type mice; soluble C1q inhibits the binding of R4A to either DNA or C1q. In contrast, when soluble C1q is added to C1q−/− glomeruli, it binds to DNA and is a target antigen for G11, thereby enhancing binding of G11.
The normal plasma concentrations of C1q range between 100 and 200 μg/ml; thus, the concentration of C1q in plasma is sufficient for in vivo antibody binding. This cross-reactivity appears crucial in the binding of this subset of anti-DNA antibodies to glomeruli. This observation helps explain the multiple studies showing that anti-C1q antibodies are present in approximately 50% of lupus patients, almost exclusively in patients who also harbor anti-dsDNA antibodies, and are highly predictive of renal disease.
There are a number of studies that show the critical contribution of DCs to glomerulonephritis [34]. Anti-C1q antibodies could contribute to IC-mediated glomerulonephritis by enhancing IC deposition in glomeruli. We postulate R4A-like antibodies may also bind and remove soluble C1q, thus cause a functional deficiency of C1q and enhanced systemic inflammation and DC activation [35].
Overall, these studies demonstrate a novel cross-reactivity between DNA and C1q and provide evidence supporting a role for cross-reactive antibodies in SLE.
5. Conclusions
Our data demonstrate that a subset of anti-dsDNA antibodies cross-react with C1q and deposit in the kidney. We propose that anti-C1q antibodies could contribute to the development of IC-mediated glomerulonephritis by direct binding to glomeruli or by removal of soluble C1q.
Acknowledgments
Grant support Giovanni Franchin was funded by NIH NIAID F32 AI058520-01, a Career Development Award from the SLE Foundation and is currently funded by NIH K08DK083818. Betty Diamond was funded by grants from the NIH. Sun Jung Kim was funded by a fellowship from the SLE Foundation and is currently funded by NIH K01AR059378. Myoungsun Son is a recipient of an Arthritis Foundation Fellowship.
References
- [1].Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–68. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- [2].Paul E, Manheimer-Lory A, Livneh A, Solomon A, Aranow C, Ghossein C, et al. Pathogenic anti-DNA antibodies in SLE: idiotypic families and genetic origins. Int Rev Immunol. 1990;5:295–313. doi: 10.3109/08830189009056736. [DOI] [PubMed] [Google Scholar]
- [3].Sasaki T, Hatakeyama A, Shibata S, Osaki H, Suzuki M, Horie K, et al. Heterogeneity of immune complex-derived anti-DNA antibodies associated with lupus nephritis. Kidney Int. 1991;39:746–53. doi: 10.1038/ki.1991.91. [DOI] [PubMed] [Google Scholar]
- [4].Du H, Chen M, Zhang Y, Zhao MH, Wang HY. Cross-reaction of anti-DNA autoantibodies with membrane proteins of human glomerular mesangial cells in sera from patients with lupus nephritis. Clin Exp Immunol. 2006;145:21–7. doi: 10.1111/j.1365-2249.2006.03102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bruijn J. Fundamentals of renal pathology. Springer; New York: 2007. New York. [Google Scholar]
- [6].Ronnblom L, Alm GV, Eloranta ML. Type I interferon and lupus. Curr Opin Rheumatol. 2009;21:471–7. doi: 10.1097/BOR.0b013e32832e089e. [DOI] [PubMed] [Google Scholar]
- [7].Finke D, Eloranta ML, Ronnblom L. Endogenous type I interferon inducers in autoimmune diseases. Autoimmunity. 2009;42:349–52. doi: 10.1080/08916930902831829. [DOI] [PubMed] [Google Scholar]
- [8].Yasuda K, Richez C, Uccellini MB, Richards RJ, Bonegio RG, Akira S, et al. Requirement for DNA CpG content in TLR9-dependent dendritic cell activation induced by DNA-containing immune complexes. J Immunol. 2009;183:3109–17. doi: 10.4049/jimmunol.0900399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kishore U, Reid KB. C1q: structure, function, and receptors. Immunopharmacology. 2000;49:159–70. doi: 10.1016/s0162-3109(00)80301-x. [DOI] [PubMed] [Google Scholar]
- [10].van KC, Fiore N, Trouw LA, Csomor E, Xu W, Castellano G, et al. Complement production and regulation by dendritic cells: molecular switches between tolerance and immunity. Mol Immunol. 2008;45:4064–72. doi: 10.1016/j.molimm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- [11].Csomor E, Bajtay Z, Sandor N, Kristof K, Arlaud GJ, Thiel S, et al. Complement protein C1q induces maturation of human dendritic cells. Mol Immunol. 2007;44:3389–97. doi: 10.1016/j.molimm.2007.02.014. [DOI] [PubMed] [Google Scholar]
- [12].Liu S, Wu J, Zhang T, Qian B, Wu P, Li L, et al. Complement C1q chemoattracts human dendritic cells and enhances migration of mature dendritic cells to CCL19 via activation of AKT and MAPK pathways. Mol Immunol. 2008 doi: 10.1016/j.molimm.2008.08.279. [DOI] [PubMed] [Google Scholar]
- [13].Son M, Santiago-Schwarz F, Al-Abed Y, Diamond B. Clq limits dendritic cell differentiation and activation by engaging LAIR-1. PNAS. 2012;109:3160–7. doi: 10.1073/pnas.1212753109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol. 2004;22:431–56. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- [15].Nicholson-Weller A, Klickstein LB. C1q-binding proteins and C1q receptors. Curr Opin Immunol. 1999;11:42–6. doi: 10.1016/s0952-7915(99)80008-9. [DOI] [PubMed] [Google Scholar]
- [17].Santer DM, Hall BE, George TC, Tangsombatvisit S, Liu CL, Arkwright PD, et al. C1q deficiency leads to the defective suppression of IFN-alpha in response to nucleoprotein containing immune complexes. J Immunol. 2010;185:4738–49. doi: 10.4049/jimmunol.1001731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lood C, Gullstrand B, Truedsson L, Olin AI, Alm GV, Ronnblom L, et al. C1q inhibits immune complex-induced interferon-alpha production in plasmacytoid dendritic cells: a novel link between C1q deficiency and systemic lupus erythematosus pathogenesis. Arthritis Rheum. 2009;60:3081–90. doi: 10.1002/art.24852. [DOI] [PubMed] [Google Scholar]
- [19].Trouw LA, Daha MR. Role of anti-C1q autoantibodies in the pathogenesis of lupus nephritis. Expert Opin Biol Ther. 2005;5:243–51. doi: 10.1517/14712598.5.2.243. [DOI] [PubMed] [Google Scholar]
- [20].Marto N, Bertolaccini ML, Calabuig E, Hughes GR, Khamashta MA. Anti-C1q antibodies in nephritis: correlation between titres and renal disease activity and positive predictive value in systemic lupus erythematosus. Ann Rheum Dis. 2005;64:444–8. doi: 10.1136/ard.2004.024943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wu FQ, Zhao Q, Cui XD, Zhang W. C1q and anti-C1q antibody levels are correlated with disease severity in Chinese pediatric systemic lupus erythematosus. Rheumatol Int. 2011;31:501–5. doi: 10.1007/s00296-009-1257-0. [DOI] [PubMed] [Google Scholar]
- [22].Trouw LA, Groeneveld TW, Seelen MA, Duijs JM, Bajema IM, Prins FA, et al. Anti-C1q autoantibodies deposit in glomeruli but are only pathogenic in combination with glomerular C1q-containing immune complexes. J Clin Invest. 2004;114:679–88. doi: 10.1172/JCI21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shefner R, Kleiner G, Turken A, Papazian L, Diamond B. A novel class of anti-DNA antibodies identified in BALB/c mice. J Exp Med. 1991;173:287–96. doi: 10.1084/jem.173.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med. 1998;188:29–38. doi: 10.1084/jem.188.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kowal C, Degiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci U S A. 2006;103:19854–9. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gaynor B, Putterman C, Valadon P, Spatz L, Scharff MD, Diamond B. Peptide inhibition of glomerular deposition of an anti-DNA antibody. Proc Natl Acad Sci U S A. 1997;94:1955–60. doi: 10.1073/pnas.94.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Reid KB. Isolation, by partial pepsin digestion, of the three collagen-like regions present in subcomponent Clq of the first component of human complement. Biochem J. 1976;155:5–17. doi: 10.1042/bj1550005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Budhai L, Oh K, Davidson A. An in vitro assay for detection of glomerular binding IgG autoantibodies in patients with systemic lupus erythematosus. J Clin Invest. 1996;98:1585–93. doi: 10.1172/JCI118952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang J, Jacobi AM, Mackay M, Aranow C, Wang T, Chinnasamy P, et al. Identification of DNA-reactive B cells in patients with systemic lupus erythematosus. J Immunol Methods. 2008;338:79–84. doi: 10.1016/j.jim.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang J, Jacobi AM, Wang T, Diamond B. Pathogenic autoantibodies in systemic lupus erythematosus are derived from both self-reactive and non-self-reactive B cells. Mol Med. 2008;14:675–81. doi: 10.2119/2008-00066.Zhang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kojouharvoa MS, Tsacheva IG, Tchorbadjieva MI, Reid KBM, Kishore U. Localization of ligand-binding sites on human C1q globular head region using recombinant globular head fragments and single-chain antibodies. Biochim Biophys Acta. 2003;1652:64–74. doi: 10.1016/j.bbapap.2003.08.003. [DOI] [PubMed] [Google Scholar]
- [32].Tan Y, Zhou W, Yu F, Fang Q, Yang HZ, Zhao MH. Detection of anti-C1q antibodies and antiC1q globular head domain antibodies in sera from Chinese patients with lupus nephritis. Mol Immunol. 2009;46:2178–82. doi: 10.1016/j.molimm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- [33].Deliyska B, Tsacheva I, Radanova M, Stoianova V, Tchorbadjieva M, Dobreva N. Lupus nephritis sera contain autoantibodies that recognize epitopes within the globular fragments of C1q. Med Pregl. 2007;2:25–7. [PubMed] [Google Scholar]
- [34].Castellano G, Trouw LA, Fiore N, Daha MR, Schena FP, van Kooten C. Infiltrating dendritic cells contribute to local synthesis of C1q in murine and human lupus nephritis. Mol Immunol. 2010;47:2129–37. doi: 10.1016/j.molimm.2010.02.006. [DOI] [PubMed] [Google Scholar]
- [35].Sontheimer RD, Racila E, Racila DM. C1q: its functions within the innate and adaptive immune responses and its role in lupus autoimmunity. J Invest Dermatol. 2005;125:14–23. doi: 10.1111/j.0022-202X.2005.23673.x. [DOI] [PubMed] [Google Scholar]