Abstract
Women with lobular carcinoma in situ (LCIS) have an elevated breast cancer risk, yet the benefit of MRI screening is unclear. We examined cancer detection rates with mammography alone versus mammography plus MRI in this high-risk population. From a prospectively maintained, single-institution database, we identified 776 patients diagnosed with LCIS after the adoption of screening MRI in April 1999. In addition to annual mammography and breast exam, MRI was used at the discretion of the physician and patient. Kaplan–Meier methods and landmark analyses at 1, 2, and 3 years following LCIS diagnosis were performed to compare rates of cancer detection with or without MRI. MRI screening was performed in 455 (59 %) patients (median, 3/patient). Median time from LCIS diagnosis to first MRI was 9 months (range 0.3–137 months). Patients undergoing MRI were younger (p < 0.0001), premenopausal (p < 0.0001), and more likely to have ≥1 first-degree relative with breast cancer (p = 0.009). At a median follow-up of 58 months, 98/776 (13 %) patients developed cancer. The crude cancer detection rate in both screening groups was 13 %. MRI was not associated with earlier stage, smaller size, or node negativity. Landmark analyses at 1, 2, and 3 years after LCIS diagnosis failed to demonstrate increased cancer detection rates among women having MRI (p = 0.23, 0.26, and 0.13, respectively). Although a diagnosis of LCIS remains a significant risk factor for breast cancer, the routine use of MRI does not result in increased cancer detection rates (short-term), nor does it result in earlier stage at diagnosis, illustrating the importance of defining optimal screening strategies for high-risk patients based on tumor biology rather than numerical risk.
Keywords: LCIS, MRI, Screening, High risk, Breast cancer
Introduction
A diagnosis of lobular carcinoma in situ (LCIS) is a major risk factor for the development of breast cancer (BC) [1–3]. Compared to the general population, women with LCIS have an eightfold to tenfold increased risk of BC [4], with a probability of developing intraductal or invasive cancer of 13 % at 10 years after diagnosis, 26 % after 20 years, and 35 % by 35 years, approximately 1 % per year [2]. The absolute risk of BC for an individual is therefore impacted by age at LCIS diagnosis, and clinical management strategies should be tailored appropriately.
Enhanced surveillance strategies that include breast MRI are commonly recommended for women at high risk. In 2007, based on expert consensus opinion, the American Cancer Society recommended annual MRI as an adjunct to mammography in women whose lifetime BC risk exceeded 20 %; however, this recommendation was based on the increased sensitivity of MRI in women at high risk due to an inherited predisposition or strong family history of BC, and specifically stated there was insufficient evidence to recommend for or against MRI screening in women with LCIS [5]. Although the lifetime risk for an individual with LCIS may exceed 20 % (depending on age at diagnosis), the biology of the breast cancers that develop in women with LCIS differs from those in women at risk on the basis of BRCA mutations, and the optimal screening strategy for women with LCIS remains uncertain. Here we report our longitudinal experience with breast MRI in a large population of women with LCIS screened at a single institution.
Methods
From a prospectively maintained database of 1064 women with LCIS having BC screening at Memorial Sloan-Kettering Cancer Center (MSKCC) between 1980 and 2009, we selected women diagnosed with LCIS after April 1999 when MRI screening for high-risk patients was introduced. All patients presenting with LCIS on core biopsy underwent surgical excision. Patients with a known BRCA mutation, history of, or concurrent diagnosis of ductal carcinoma in situ (DCIS) or invasive cancer, and those who chose bilateral risk-reducing mastectomy, were excluded. Medical records were abstracted after institutional review board approval.
All patients were offered annual or biannual CBE and annual screening mammography. Routine screening ultrasound was not performed. Frequency of CBE, use of MRI screening, and timing of MRI (i.e., at the time of annual mammography or the 6-month interval) were at physician and patient discretion. For study purposes, an MRI was considered a screening MRI only if done in the absence of concurrent malignancy. MRIs performed for extent of disease after cancer diagnosis were excluded. Breast MRI was performed with a 1.5 or 3.0T commercially available system (Sigma, GE Medical Systems, Milwaukee, WI) using a dedicated surface breast coil. All breast MRIs were interpreted by dedicated breast imagers.
The presence of additional high-risk lesions (atypical ductal or lobular hyperplasia) at the initial core biopsy or on subsequent biopsies was determined from pathology reports, as were subsequent cancer diagnoses. Mammographic density by the American College of Radiology Breast Imaging-Reporting and Data System (BI-RADS) classifications (1: fatty; 2: scattered fibroglandular density; 3: heterogeneous/moderately dense; 4: extremely dense) was taken from the clinical record when reported by an MSKCC radiologist. Cases where BI-RADS breast density was not reported by an MSKCC radiologist were considered missing data. Chemoprevention use was defined as at least 6 months of documented therapy.
Comparisons were made between patients undergoing conventional screening (CBE and mammography) and those undergoing conventional screening plus MRI. Chi square, Fisher’s exact, and the 2-sample t tests were used to test differences in variables between groups. Kaplan–Meier methodology was used to estimate cancer detection rates in the cohort as a whole. Time was measured from date of LCIS diagnosis to date of first-cancer diagnosis or date of last follow-up. As this is a retrospective analysis of a prospective database, the time to first MRI screen, the time interval between MRI screens, and the number of MRIs per patient, was not uniform; yet they reflect real-life screening experiences. We defined MRI screening in 3 ways: patients who started MRI screening within the first year of LCIS diagnosis; within the first 2 years of LCIS diagnosis; and within the first 3 years of LCIS diagnosis for landmark analyses. This reflects our assumption that in clinical practice, MRI screening would begin soon after an LCIS diagnosis. In each analysis, we sought to compare patients who had their first screening MRI prior to the landmark time to those who had their first screening MRI following the landmark time or did not have MRI screening at all. Patients taking chemoprevention were excluded and Landmark analyses were adjusted for family history and age, and in separate analyses, stratified by age at LCIS diagnosis (≤45 years; 46–60 years; ≥60 years). We also conducted sensitivity analyses to ensure our results were not strongly dependent on the assumptions made [i.e., we examined the number of MRIs received (0, 1, 2, or more) in all landmark analyses]. Additionally, for women who developed cancer, we examined the time from first MRI to cancer incidence. In all these analyses, our interpretation of the results did not change. Descriptive analyses of all cancer characteristics are presented as medians and frequencies. Comparisons by MRI receipt were done using Fisher’s exact and Wilcoxon rank-sum tests. All analyses were conducted in SAS v.9 and R2.15.1. P values < 0.05 were considered significant.
Results
Between November 1980 and December 2009, 1,222 patients with a diagnosis of LCIS were evaluated. After excluding those with concurrent (n = 120) or prior BC (n = 38), those undergoing bilateral prophylactic mastectomy (n = 55), and those diagnosed with LCIS before April 1999 (n = 233), the remaining 776 patients formed our study cohort. Of these, 455 (59 %) patients underwent conventional screening plus MRI; the remaining 321 (41 %) underwent conventional screening alone. Supplementary File 1 shows the number of LCIS patients entering surveillance per year and proportion of patients undergoing MRI screening. Median follow-up of the 678 patients who did not develop cancer was 58 months (range 0–151 months).
Patients undergoing MRI screening were younger (median 49 vs. 53 years; p < 0.0001), more likely to be premenopausal (65 vs. 46 %; p < 0.0001), and more likely to have at least one first-degree relative with BC (30 vs. 20 %; p = 0.009) (Table 1). Overall, 725 patients (93 %) had BI-RADS breast density assigned by an MSKCC radiologist. The majority of patients had moderately dense breasts [BI-RADS breast density 3; n = 444 (61 %)]. Concurrent atypical ductal hyperplasia (ADH) or atypical lobular hyperplasia (ALH) was present at LCIS diagnosis in 237 (31 %) and 138 (18 %) patients, respectively. BI-RADS breast density or the presence of atypical hyperplasia did not differ between screening groups. Patients undergoing MRI were more likely to use chemoprevention (21 vs. 9 %, p < 0.0001) and more likely to undergo at least 1 benign breast biopsy during surveillance (36 vs. 13 %, p < 0.0001) (Table 1).
Table 1.
Characteristics of 776 patients entering the LCIS screening population between April 1999 and December 2009, stratified by use of MRI
| Characteristics | Conventional screening (n = 321) | MRI screening (n = 455) | p value | |
|---|---|---|---|---|
| Age at LCIS diagnosis | ≤45 years | 49 (15 %) | 118 (26 %) | <0.0001 |
| 46–60 years | 183 (57 %) | 299 (66 %) | ||
| ≥60 years | 89 (28 %) | 38 (8 %) | ||
| Median age, years (range) | 53 (35–83) | 49 (27–77) | <0.0001 | |
| Menopausal status | Premenopausal | 147 (46 %) | 298 (65 %) | <0.0001 |
| Postmenopausal | 170 (53 %) | 153 (34 %) | ||
| Missing | 4 (1 %) | 4 (1 %) | ||
| ≥1 FDR with a history of breast cancer | No | 252 (79 %) | 310 (68 %) | 0.001 |
| Yes | 64 (20 %) | 137 (30 %) | ||
| Missing | 5 (2 %) | 8 (2 %) | ||
| ≥2 SDR with a history of breast cancer | No | 275 (86 %) | 385 (85 %) | 0.722 |
| Yes | 41 (13 %) | 62 (14 %) | ||
| Missing | 5 (2 %) | 8 (2 %) | ||
| LCIS laterality | Unilateral | 313 (98 %) | 435 (96 %) | 0.162 |
| Bilaterala | 8 (2 %) | 20 (4 %) | ||
| Concurrent ADH | No | 216 (67 %) | 323 (71 %) | 0.271 |
| Yes | 105 (33 %) | 132 (29 %) | ||
| Concurrent ALH | No | 265 (83 %) | 373 (82 %) | 0.902 |
| Yes | 56 (17 %) | 82 (18 %) | ||
| BI-RADS breast densityb | 1 | 9 (3 %) | 7 (2 %) | 0.101 |
| 2 | 59 (18 %) | 73 (16 %) | ||
| 3 | 178 (55 %) | 266 (58 %) | ||
| 4 | 43 (13 %) | 90 (20 %) | ||
| Not assigned | 32 (10 %) | 19 (4 %) | ||
| Chemopreventionc | No | 293 (91 %) | 361 (79 %) | <0.0001 |
| Yes | 28 (9 %) | 94 (21 %) | ||
| ≥1 biopsyd | No | 280 (87 %) | 293 (64 %) | <0.0001 |
| Yes | 41 (13 %) | 162 (36 %) |
BI-RADS American College of Radiology Breast Imaging Reporting and Data System, LCIS lobular carcinoma in situ, FDR first-degree relative, SDR second-degree relative, ADH atypical ductal hyperplasia, ALH atypical lobular hyperplasia
Bilateral LCIS defined as bilateral breast biopsies demonstrating LCIS within 6 months of initial diagnosis
Comparing BI-RADS breast density 1 + 2 versus 3 + 4, p = 0.090; BI-RADS breast density 1 + 2 + 3 versus 4, p = 0.050
Defined as any chemoprevention use >6 months
Does not include biopsy at diagnosis of LCIS or biopsy at diagnosis of cancer
Significant p-values are in bold
Median time from LCIS diagnosis to first MRI was 9 months (range 0.3–137.0 months), 289/455 (64 %) patients had an MRI within the first year following LCIS diagnosis, and 390/455 (86 %) patients had an MRI within the first 2 years following LCIS diagnosis. The median number of MRIs per patient was 3 (range 1–13). The number of MRI screens and interval between screens are shown in Table 2. On average, women received an MRI 0.84 times per follow-up year (interquartile range 0.5–1.1). Among the 232 benign biopsies performed during surveillance in the MRI screening cohort, 115 (50 %) were generated by MRI findings—the others were generated by conventional imaging or CBE findings. Patients undergoing conventional imaging had 47 benign biopsies; 41 were generated by imaging findings.
Table 2.
Time in months between MRI assessments for 455 patients in the MRI screening group who developed cancer and patients who were cancer-free at last follow-up
| N with MRI | Median time between | IQR | ||
|---|---|---|---|---|
| Patients who developed cancer | LCIS to 1st MRI | 57 | 9.28 | 8.72 |
| 1st to 2nd | 31 | 7.54 | 5.44 | |
| 2nd to 3rd | 24 | 10.90 | 5.98 | |
| 3rd to 4th | 11 | 11.48 | 6.20 | |
| 4th to 5th | 8 | 10.41 | 4.69 | |
| 5th to 6th | 4 | 11.00 | 4.41 | |
| 6th to 7th | 2 | * | * | |
| 7th to 8th | 1 | * | * | |
| 8th to 9th | 1 | * | * | |
| Patients who were cancer-free at last follow-up (median follow-up time: 59 months, with an IQR of 59) | LCIS to 1st MRI | 398 | 8.80 | 10.75 |
| 1st to 2nd | 329 | 9.48 | 6.69 | |
| 2nd to 3rd | 266 | 11.92 | 6.39 | |
| 3rd to 4th | 212 | 12.08 | 2.82 | |
| 4th to 5th | 157 | 12.16 | 1.34 | |
| 5th to 6th | 119 | 12.16 | 1.31 | |
| 6th to 7th | 88 | 12.16 | 2.11 | |
| 7th to 8th | 53 | 12.16 | 3.54 | |
| 8th to 9th | 28 | 12.20 | 1.82 |
IQR interquartile range, LCIS lobular carcinoma in situ
NA due to small sample size
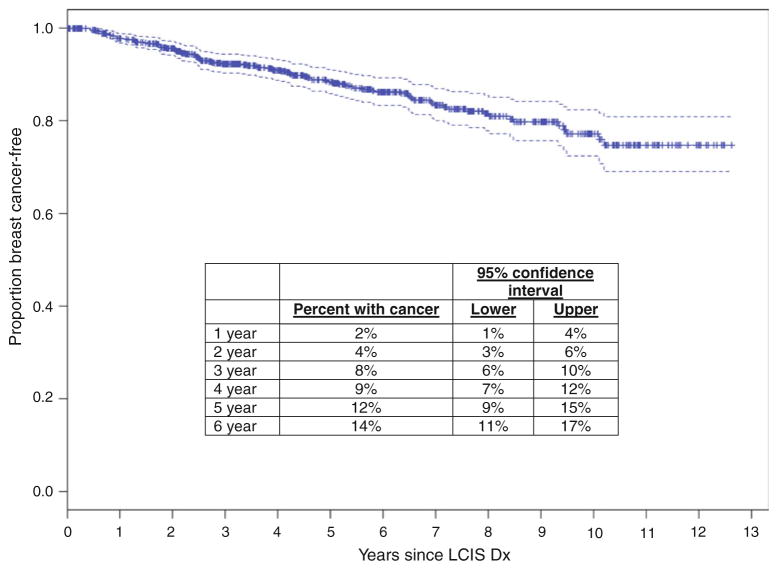
After a median follow-up of 58-months, 98/776 (13 %) patients have been diagnosed with 110 malignancies (12 bilateral cases). Cancers detected in the contralateral breast, at the time of CPM (n = 5) or during extent of disease workup (n = 1), were excluded, as our aim was to compare screening methods, leaving 104 cancers (6 bilateral). The crude screen-detected cancer rate in both groups was 13 %; 61 cancers (57 patients) in the MRI group and 43 cancers (41 patients) in the conventional screening group. Among the 6 bilateral cases, 3 were diagnosed synchronously and 3 were diagnosed metachronously at a median of 23 months (range 11–74 months) after the initial cancer diagnosis. The primary analysis includes the 101 first-cancer diagnoses in 98 patients. The actuarial rates of cancer development, from date of LCIS diagnosis, and yearly estimates, are shown in Fig. 1.
Fig. 1.
Kaplan–Meier curve depicting rate of cancer detection [ductal carcinoma in situ (DCIS) or invasive cancer] and yearly estimates among 776 women in the lobular carcinoma in situ (LCIS) screening population, April 1999–December 2009
Table 3 shows a comparison of clinicopathologic characteristics of the 104 screen-detected cancers by MRI group. We observed a higher proportion of screen-detected DCIS in the MRI group (41 vs. 26 %), but this was not statistically significant (p = 0.10). Among the invasive cancers, tumor size for MRI screen-detected cancers appeared smaller (median 0.5 vs. 0.95 cm), but also was not significant (p = 0.09). Similarly, there were no differences in the proportion of patients with positive nodes or in the breakdown by IHC subtype based on MRI receipt (p = 0.74 and 0.52, respectively).
Table 3.
Characteristics of 104 screen-detected cancers diagnosed in patients with LCIS by screening group
| Screen-detected tumors | All (n = 104) | MRI screening group (n = 61) | Conventional screening group (n = 43) | p value |
|---|---|---|---|---|
| Histology | 0.10** | |||
| DCIS | 36 (35 %) | 25 (41 %) | 11 (26 %) | |
| IDC | 30 (28 %) | 14 (21 %) | 16 (37 %) | |
| ILC | 29 (28 %) | 17 (28 %) | 12 (28 %) | |
| IDC with lobular features | 6 (6 %) | 4 (7 %) | 2 (5 %) | |
| Special types | 3 (3 %) | 1 (2 %) | 2 (5 %) | |
| Median tumor size* | 0.80 cm (0.1–3.5) | 0.5 cm (0.1–3.5) | 0.95 cm (0.1–2.7) | 0.09 |
| Node status | 0.74 | |||
| Positive | 14 (22 %) | 7 (21 %) | 7 (24 %) | |
| Negative | 49 (78 %) | 27 (79 %) | 22 (76 %) | |
| Not available | 5 | 2 | 3 | |
| IHC profile | 0.53 | |||
| ER+ HER2− | 55 (89 %) | 28 (88 %) | 27 (90 %) | |
| ER+ HER2+ | 4 (6 %) | 3 (9 %) | 1 (3 %) | |
| ER− HER2+ | 3 (5 %) | 1 (3 %) | 2 (7 %) | |
| Not available | 4 | 2 |
DCIS ductal carcinoma in situ, IDC infiltrating ductal carcinoma, ILC infiltrating lobular carcinoma, ER estrogen receptor, PR progesterone receptor
For 63/68 invasive cancers with complete size information
DCIS versus invasive histology
Diagnostic imaging and biopsy procedure reports were available for 99/104 (95 %) cases. Of these, detailed review demonstrated only 29/58 (50 %) screen-detected cancers in the MRI group were initially detected as an abnormality on MRI; 24 were detected by conventional imaging and 5 by CBE (Table 3). However, for this analysis, all 58 cancers were included in the MRI group. Among the 24 patients in this group whose cancers were detected by conventional imaging, 19/24 (79 %) had a negative MRI within the previous 12 months, and 16/24 (67 %) had a negative MRI within 6 months. MRI-detected cancers accounted for 3 of the 6 bilateral cases—1 diagnosed synchronously, the other 2 on subsequent MRI screens at 12 and 23 months following the first-cancer diagnosis. Among patients not having MRI screening, 33/41 (80 %) cancers were detected on conventional imaging, and 8 (20 %) were detected by CBE. In Table 4, a comparison of MRI-detected cancers versus conventional imaging-detected cancers, as defined by detection method, irrespective of screening group, demonstrates very small differences in the proportion of patients with DCIS versus invasive histology (p = 0.69), and small differences in median tumor size (p = 0.36), and node status (p = 0.69) among those with invasive disease. Characteristics of patients who had cancer detected by clinical exam are also provided.
Table 4.
Comparison of screen-detected cancers by method of detection only
| MRI | Conventional imaging | Clinical exam | p value* | |
|---|---|---|---|---|
| Total number of cancers detected** | 29/104 (28 %) | 57/104 (55 %) | 13/104 (13 %) | |
| DCIS | 13/29 (45 %) | 23/57 (40 %) | 0 | 0.69*** |
| IDC | 5/29 (17 %) | 19/57 (33 %) | 4/13 (31 %) | |
| ILC | 10/29 (34 %) | 10/57 (18 %) | 6/13 (46 %) | |
| IDC with lobular features | 1/29 (3 %) | 3/57 (5 %) | 2/13 (15 %) | |
| Special subtypes | 0 | 2/57 (4 %) | 1/13 (8 %) | |
| Median tumor size**** (range) | 0.80 cm (0.1–2.3) | 0.5 cm (0.1–1.7) | 1.25 cm (0.5–3.5) | 0.36 |
| Nodal status | 0.68 | |||
| Positive | 3 (19 %) | 5 (15 %) | 6 (46 %) | |
| Negative | 13 (81 %) | 28 (85 %) | 7 (54 %) | |
| Not available | 0 | 1 | 0 | |
| IHC profile | 0.59 | |||
| ER +/HER2− | 14 (93 %) | 28 (85 %) | 12 (92 %) | |
| ER +/HER2+ | 1 (7 %) | 3 (9 %) | 0 | |
| ER −/HER2+ | 0 | 2 (6 %) | 1 (8 %) | |
| Not available | 1 | 1 | 0 |
DCIS ductal carcinoma in situ, IDC infiltrating ductal carcinoma, ILC infiltrating lobular carcinoma, ER estrogen receptor
p value for conventional imaging versus MRI
Initial method of detection could not be confirmed for 5/104 cases
p value for invasive versus DCIS for conventional imaging versus MRI
For 61/63 invasive cancers with detailed information
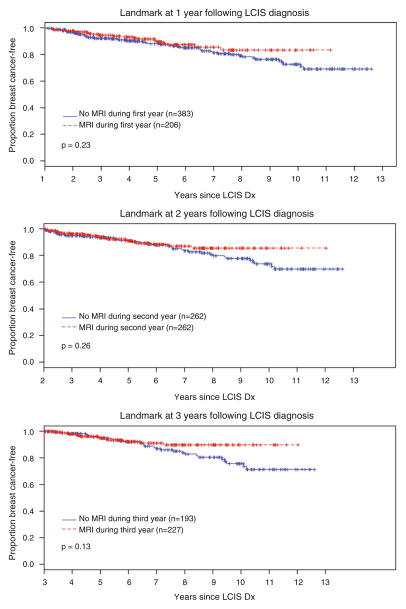
As described, we performed landmark analyses at 3 different time points and by age at LCIS diagnosis. After excluding patients taking chemoprevention and those with <1 year of follow-up, we compared patients who had an MRI within the first year of LCIS diagnosis to those not having an MRI within the first year or never having an MRI, and found no difference in cancer detection rates for these 2 groups (Fig. 2a, p = 0.23; note that Time 0 is pushed forward 1 year on the x-axis). We performed the same analysis for patients who had an MRI within the first 2 years of LCIS diagnosis (Fig. 2b; note that Time 0 is pushed forward 2 years on the x-axis) and for those having an MRI within the first 3 years of LCIS diagnosis (Fig. 2c). Landmark analyses at these time points also failed to demonstrate an increased rate of cancer detection among women having MRI (p = 0.26 and 0.13, respectively). Similarly, landmark analyses at 1 year by age group (≤45 years; 46–60 years; ≥60 years) also failed to demonstrate an increased cancer detection rate among women having MRI (p = 0.67, 0.41, and 0.43, by age group, respectively).
Fig. 2.
Landmark analyses at 1, 2, and 3 years after lobular carcinoma in situ (LCIS) diagnosis. a Rate of cancer detection among patients having an MRI within the first year following LCIS diagnosis (red line, median follow-up 44.67 months; range 3.57–121.93 months) versus those not having an MRI during the first year or never having an MRI (blue line, median follow-up, 49.87 months; range 0.16–139 months. b Rate of cancer detection among patients having an MRI within the first 2 years following LCIS diagnosis (red line, median follow-up, 36.59 months; range 0.07–120.39 months) versus those not having an MRI during the first 2 years or never having an MRI (blue line, median follow-up 49.08 months; range 0.07–127.4 months). c Rate of cancer detection among patients having an MRI during the first 3 years following LCIS diagnosis (red line, median follow-up 31.8 months; range 0.07–108.39 months) versus those not having an MRI during the first 3 years or never having an MRI (blue line, median follow-up 49.8 months; range 0.16–115.44 months). For each analyses, patients who were lost to follow-up or developed cancer before the landmark time and those taking chemoprevention were excluded
Discussion
The observation that an LCIS diagnosis confers an increased risk of BC of approximately 1 % per year was initially made in the 1970s [1, 3]. Our experience with a large population of women with LCIS undergoing BC screening confirms that LCIS remains a significant risk factor and underscores the need for evidence-based clinical management guidelines. Breast MRI has proven to be a valuable adjunct to conventional imaging in patients at increased risk due to strong family history and/or genetic predisposition [6–8], leading to the assumption that the increased sensitivity of MRI should translate into clinical benefit for other high-risk groups; however, data directly addressing this question in women with LCIS are limited [9–11]. Here we have demonstrated in a large, well-annotated screening population of women with LCIS that adjunctive MRI screening in the first 3 years following a diagnosis of LCIS does not translate into an increased cancer detection rate or earlier stage at diagnosis.
This study expands and updates an earlier report by Port et al. in 2007 that included 378 women who participated in our program from 1999 to 2005. This study included 252 women with LCIS, 135 (54 %) of whom were participating in MRI screening [10]. There were 6 mammographically occult cancers detected in 5 LCIS patients by MRI screening, an occult cancer detection rate of 4 % (5/135 patients)—similar to the crude cancer detection rate in patients with LCIS who did not undergo MRI screening (5/117, 4 %). Our current study, which now includes 776 patients with LCIS (59 % undergoing MRI screening), continues to demonstrate no difference in the crude cancer detection rate among women having conventional screening or conventional screening plus MRI, nor do we find an association with MRI screening and earlier stage at diagnosis. The 101 first-cancer diagnoses on which this observation is now based significantly strengthens the validity of our earlier conclusion that routine MRI screening does not translate into a clinical benefit for women with LCIS.
Similar to the earlier report, this series demonstrates that physicians and/or patients are more likely to pursue MRI screening in patients who are younger (p < 0.0001), pre-menopausal (p < 0.0001), and have stronger family histories of BC (p = 0.0009), although somewhat surprisingly, not in those with higher breast density. These characteristics resemble those of women at increased risk due to a genetic predisposition, where MRI screening is known to be beneficial [12, 13]. Yet, unlike young women with an inherited mutation who have a propensity to develop high-grade, triple-negative breast cancers, which frequently occur in the interval between mammographic screens, this longitudinal dataset clearly demonstrates that >90 % of women with LCIS will develop estrogen receptor-positive breast cancers that are likely to be detected at a small size, irrespective of the screening strategy used. This finding is consistent with previous reports from us [14] and others [15] regarding the phenotype of cancers developing after an LCIS diagnosis.
The higher frequency of infiltrating lobular carcinoma (ILC) that develops in women with LCIS as compared to the general population [15] has led to the popular belief that MRI should be beneficial in women with LCIS because of its reported increased sensitivity for detecting ILC [16]. In this large, modern cohort of women with LCIS followed prospectively, the subsequent invasive cancers that developed were equally divided between those of ductal and lobular phenotype—an observation first made decades ago by Haagensen and Rosen [1, 3]. Of the 26 lobular cancers diagnosed, 10 were diagnosed by MRI imaging, 10 by conventional imaging, and 6 by CBE, emphasizing the importance of CBE in this high-risk population. Another frequent misconception is the propensity of lobular cancers to be bilateral—of the 12 LCIS patients diagnosed with bilateral BC, none were bilateral lobular cancers. SEER data also show that an initial lobular cancer diagnosis does not increase the risk of a metachronous contralateral cancer compared to patients with ductal disease [17].
Two recent radiology studies demonstrate that MRI identifies otherwise occult BC in women with LCIS [9, 11], as it does in other high-risk cohorts [18]. Friedlander et al. [9] reported that MRI-detected occult cancer in 5/133 (3.8 %) patients with LCIS, and Sung et al. [11] reported that MRI identified occult cancer in 10/220 patients (4.5 %); however, as these were both single-arm studies, this should not be interpreted as evidence that MRI results in either short-term or long-term clinical benefit for patients with LCIS. Our study, although not a prospective randomized trial, is, to our knowledge, the largest reported cohort of LCIS patients undergoing screening at a single institution and, as such, provides increased clarity to the risks and benefits of MRI screening in this population. Accounting for other BC risk factors, follow-up duration, MRI frequency, and the time dependency of BC development, these data demonstrate that routine MRI screening does not result in increased cancer detection rates (short-term) or earlier stage at diagnosis. Unsurprisingly, women in the MRI-screened group were significantly more likely to undergo ≥1 benign biopsies during the surveillance period (36 vs. 13 %, p < 0.0001); a problem that translates to increased patient anxiety and increased healthcare costs [6, 19, 20], and highlights the need to define specific subsets of high-risk patients who truly benefit from this type of breast imaging.
Our study clearly demonstrates that a diagnosis of LCIS remains a significant risk factor for BC, with a cumulative risk of 14 % at 6 years of follow-up in this cohort, and estimated cancer rates at 10 years exceeding 20 %, the lifetime risk threshold currently considered to warrant MRI screening. It is important to remember, however, that these guidelines were developed based on rates of cancer detection among women at increased risk due to strong family history and/or genetic predisposition, and do not account for the biological features of the cancers that are most likely to develop in other high-risk screening groups. This factor should be considered when making screening recommendations; emerging data in BRCA mutation carriers, taking into account the differences in the natural history of BRCA1- versus BRCA2-associated breast cancers, also support this contention [21]. It should also be noted that the landmark analysis curves for the MRI and no-MRI groups in this analysis do not separate until approximately 10 years, and that at this separation, patients undergoing mammographic screening are more likely to be diagnosed with BC, suggesting that any clinical benefit to MRI screening should be seen in the earlier years—a finding not confirmed by this analysis. Further, there is no evidence suggesting that “late” cancers that develop in women with LCIS are more likely to be mammographically occult than the cancers that develop early. As such, the clinical benefit of long-term MRI screening in women with LCIS remains unproven and must be weighed against the likelihood that long-term MRI screening will continue to result in increased numbers of benign biopsies performed during surveillance.
Supplementary Material
Acknowledgments
This study was supported by funding from the Cary Grossman Breast Cancer Research Fellowship, and funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Footnotes
Presented in part in poster format at the Society of Surgical Oncology Annual Meeting, San Antonio, TX, March 2011.
Electronic supplementary material The online version of this article (doi:10.1007/s10549-013-2725-5) contains supplementary material, which is available to authorized users.
Conflict of interest The authors report no potential conflict of interest.
Contributor Information
Tari A. King, Email: kingt@mskcc.org, Breast Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 300 E. 66th St, New York, NY 10065, USA
Shirin Muhsen, Email: muhsens@mskcc.org, Breast Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 300 E. 66th St, New York, NY 10065, USA.
Sujata Patil, Email: patils@mskcc.org, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY 10065, USA.
Starr Koslow, Email: koslows@mskcc.org, Breast Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 300 E. 66th St, New York, NY 10065, USA.
Sabine Oskar, Email: oskars@mskcc.org, Breast Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 300 E. 66th St, New York, NY 10065, USA.
Anna Park, Email: parka@mskcc.org, Breast Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 300 E. 66th St, New York, NY 10065, USA.
Mary Morrogh, Email: morroghm@mskcc.org, Breast Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 300 E. 66th St, New York, NY 10065, USA.
Rita A. Sakr, Email: sakrr@mskcc.org, Breast Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 300 E. 66th St, New York, NY 10065, USA
Monica Morrow, Email: morrowm@mskcc.org, Breast Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 300 E. 66th St, New York, NY 10065, USA.
References
- 1.Haagensen CD, Lane N, Lattes R, Bodian C. Lobular neoplasia (so-called lobular carcinoma in situ) of the breast. Cancer. 1978;42:737–769. doi: 10.1002/1097-0142(197808)42:2<737::aid-cncr2820420247>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 2.Page DL, Kidd TE, Jr, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol. 1991;22:1232–1239. doi: 10.1016/0046-8177(91)90105-x. [DOI] [PubMed] [Google Scholar]
- 3.Rosen PP, Kosloff C, Lieberman PH, Adair F, Braun DW., Jr Lobular carcinoma in situ of the breast: detailed analysis of 99 patients with average follow-up of 24 years. Am J Surg Pathol. 1978;2:225–251. doi: 10.1097/00000478-197809000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Chuba PJ, Hamre MR, Yap J, Severson RK, Lucas D, Shamsa F, Aref A. Bilateral risk for subsequent breast cancer after lobular carcinoma-in situ: analysis of surveillance, epidemiology, and end results data. J Clin Oncol. 2005;23:5534–5541. doi: 10.1200/JCO.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 5.Saslow D, Boetes C, Burke W, et al. American cancer society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 6.Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 7.Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R, Kuhn W, Schild HH. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23:8469–8476. doi: 10.1200/JCO.2004.00.4960. [DOI] [PubMed] [Google Scholar]
- 8.Lehman CD, Blume JD, Weatherall P, et al. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103:1898–1905. doi: 10.1002/cncr.20971. [DOI] [PubMed] [Google Scholar]
- 9.Friedlander LC, Roth SO, Gavenonis SC. Results of MR imaging screening for breast cancer in high-risk patients with lobular carcinoma in situ. Radiology. 2011;261:421–427. doi: 10.1148/radiol.11103516. [DOI] [PubMed] [Google Scholar]
- 10.Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann Surg Oncol. 2007;14:1051–1057. doi: 10.1245/s10434-006-9195-5. [DOI] [PubMed] [Google Scholar]
- 11.Sung JS, Malak SF, Bajaj P, Alis R, Dershaw DD, Morris EA. Screening breast MR imaging in women with a history of lobular carcinoma in situ. Radiology. 2011;261:414–420. doi: 10.1148/radiol.11110091. [DOI] [PubMed] [Google Scholar]
- 12.Kaas R, Muller SH, Hart AA, Rutgers EJ. Stage of breast cancers found during the surveillance of women with a familial or hereditary risk. Eur J Surg Oncol. 2008;34:501–507. doi: 10.1016/j.ejso.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Tilanus-Linthorst MM, Obdeijn IM, Hop WC, Causer PA, Leach MO, Warner E, Pointon L, Hill K, Klijn JG, Warren RM, Gilbert FJ. BRCA1 mutation and young age predict fast breast cancer growth in the Dutch, United Kingdom, and Canadian magnetic resonance imaging screening trials. Clin Cancer Res. 2007;13:7357–7362. doi: 10.1158/1078-0432.CCR-07-0689. [DOI] [PubMed] [Google Scholar]
- 14.King TA, Sakr RA, Muhsen S, Andrade VP, Giri D, Van Zee KJ, Morrow M. Is there a low-grade precursor pathway in breast cancer? Ann Surg Oncol. 2012;19:1115–1121. doi: 10.1245/s10434-011-2053-0. [DOI] [PubMed] [Google Scholar]
- 15.Fisher ER, Land SR, Fisher B, Mamounas E, Gilarski L, Wolmark N. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: twelve-year observations concerning lobular carcinoma in situ. Cancer. 2004;100:238–244. doi: 10.1002/cncr.11883. [DOI] [PubMed] [Google Scholar]
- 16.Mann RM, Hoogeveen YL, Blickman JG, Boetes C. MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast Cancer Res Treat. 2008;107:1–14. doi: 10.1007/s10549-007-9528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003;56:1038–1045. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 18.Lehman CD. Role of MRI in screening women at high risk for breast cancer. J Magn Reson Imaging. 2006;24:964–970. doi: 10.1002/jmri.20752. [DOI] [PubMed] [Google Scholar]
- 19.Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005;365:1769–1778. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 20.Morris EA, Liberman L, Ballon DJ, Robson M, Abramson AF, Heerdt A, Dershaw DD. MRI of occult breast carcinoma in a high-risk population. AJR Am J Roentgenol. 2003;181:619–626. doi: 10.2214/ajr.181.3.1810619. [DOI] [PubMed] [Google Scholar]
- 21.Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI Screening-MRISC, MA-RIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21:1458–1468. doi: 10.1158/1055-9965.EPI-11-1196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.