Abstract
Background
Symptomatic hypocalcemia after thyroidectomy is a barrier to same day surgery, and the cause of ER visits. A standard protocol of calcium and vitamin D supplementation, dependent on intact parathyroid hormone (iPTH) levels, can address this issue. How effective is it? When does it fail?
Methods
We performed a retrospective review of the prospective Thyroid Database from January 2006 to December 2010. 620 patients underwent completion (CT) or total thyroidectomy (TT), and followed our post-operative protocol of calcium carbonate administration for iPTH levels ≥10pg/ml and calcium carbonate and 0.25μg calcitriol BID for iPTH <10pg/ml. Calcium and iPTH values, pathology and medication, were compared to evaluate protocol efficacy. A p value <0.05 was considered statistically significant.
Results
Using the protocol, sixty-one (10.2%) patients were chemically hypocalcemic but never developed symptoms and twenty-four (3.9%) patients developed breakthrough symptomatic hypocalcemia. The symptomatic (SX) and asymptomatic (ASX) groups were similar with regard to gender, cancer diagnosis, and pre-operative calcium and iPTH. The symptomatic group was significantly younger (39.6 ± 2.8 vs. 49 ± 0.6 years, p=0.01), with lower post-operative iPTH levels. 33% (n=8) of SX patients had an iPTH ≤5 pg/ml vs. only 6% (n=37) of ASX patients. While the majority of patients with a PTH <5 pg/ml were asymptomatic, 62.5% (n=5) of SX patients with iPTH levels ≤5 pg/ml, required an increased in calcitriol dose to achieve both biochemical correction and symptom relief.
Conclusion
Prophylactic calcium and vitamin D supplementation based on post-operative iPTH levels can minimize symptomatic hypocalcemia after thyroidectomy. An iPTH ≤ 5pg/ml may warrant higher initial doses of calcitriol in order to prevent symptoms.
Keywords: intact parathyroid hormone, symptomatic, hypocalcemia, protocol
Introduction
Hypocalcemia is the most common complication of thyroid surgery. Occurring most commonly after total and completion thyroidectomy, the incidence ranges from 1.6 to 50% [1, 2]. This incidence is dependent on the definition of hypocalcemia, what symptoms are included, permanent versus transient occurrence, and the type of resection. Even rarer, is the occurrence of permanent hypoparathyroidism, ranging from 0–3%, according to the experience of most surgeons [1, 3]. Regardless of the underlying cause, the symptoms can be debilitating, hence the need to minimize its occurrence. Studies have demonstrated the efficacy of supplemental calcium and/or vitamin D, in minimizing the occurrence of hypocalcemia and subsequent emergency room visits and hospital readmission [4–6]. Others have debated the efficacy and cost-effectiveness of standardized protocols, which provide supplementation to all patients. There are differences in the protocols used, including the utilization of ionized calcium versus serum calcium the timing of post-operative laboratory values and the dosage of vitamin D to administer. [6–15]. There are also questions of whether prophylactic vitamin D supplementation further suppresses parathyroid function, and hence recovery.
Here, we evaluate the efficacy of our post-operative parathormone (iPTH) based protocol, and attempt to identify any prognostic clinical factors, which would result in failure of the protocol.
Methods
We performed a retrospective review of our prospective Thyroid database from January 2006 to December 2011, and identified all patients who underwent a thyroid resection. Included in the study are all patients who underwent a total (TT) or completion (CT) thyroidectomy, either alone or in conjunction with another procedure. Both benign and malignant pathologies were included. We evaluated differences between patient demographics, including age, gender, pre-operative diagnosis, and clinical parameters, including pre-operative and post-operative calcium, intact parathormone (iPTH), operative procedure(s), pathologic diagnosis, hypocalcemia symptoms, and medications and their respective dosages. Patients were stratified into two groups, symptomatic (SX) and asymptomatic (ASX), for comparison. In an effort to identify those patients with significant symptomatic hypocalcemia while following the protocol, at least one of the following criteria had to be met: 1) two or more episodes of peri-oral or extremity paresthesias, muscles aches or cramps at any time in the post-operative period 2) symptoms presenting on or persistent after post-operative day 3; 3) emergency room visitation; or 4) hospital readmission for hypocalcemia management.
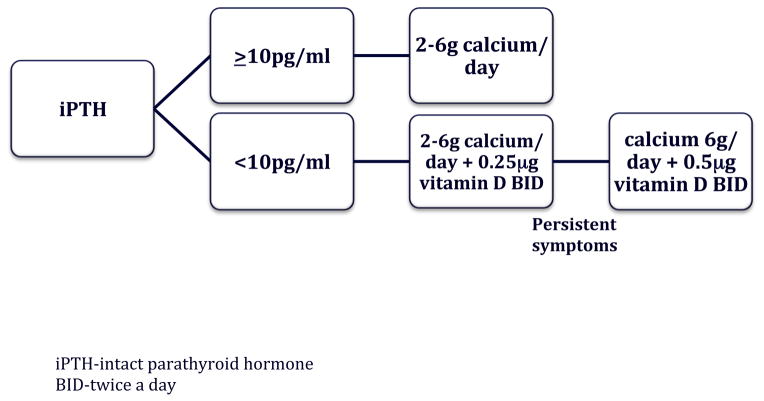
Post-operative iPTH protocol: Calcium and iPTH levels at 1–4 hours post operatively were evaluated (Elecsys; Roche Pharmaceuticals, Mannheim, Germany). The detection limit of the iPTH assay at our institution is down to <2 pg/ml, with a reference range of 14–72 pg/ml. Patients with an iPTH<10pg/ml were given 2–6g of oral calcium carbonate daily, scheduled and as needed for symptom relief, with 25g calcitriol twice daily. Patients with an iPTH ≥10 pg/ml were prescribed only 2–6 g daily of oral calcium carbonate scheduled, and for symptom relief. Verbal explanation of the post-operative protocol was discussed during the pre-operative clinic visit and at the time of discharge. The initial calcium dosage was based on patient symptoms and the risk of hypocalcemia (i.e. surgery for Graves’ disease). The patients are given instructions to start with 2 grams of calcium, at the onset of symptoms. If the symptoms persist or do not show signs of improvement, they are instructed to take an additional 1 gram of calcium every 30 minutes, until symptom resolution. All symptomatic patients were told to increase their scheduled calcium intake to 2 gm three times a day, if they were not already taking that much. Patients were provided with written post-operative instructions, followed up by a midlevel practitioner, and had the first post-operative visit within two weeks of surgery.
SPSS statistical software (SPSS Inc., Chicago, IL) was used to analyze the data. Student’s t test was used to determine the statistical significance between SX and ASX groups.
Results
From January 2006 to December 2011, 620 patients underwent either a total or completion thyroidectomy (figure 1). 596 patients were classified into the asymptomatic (ASX) group. In addition to patients who never experienced symptoms of hypocalcemia, this group includes patients with biochemical hypocalcemia without symptoms, those who experienced very mild hypocalcemia symptoms (had ≤ 2 episodes of symptoms within two days of surgery), and those patients who did not follow the protocol and had symptom resolution upon taking the previously recommended supplemental calcium. Twenty-four patients were considered symptomatic (SX) (see definition in methods).
Figure 1.
Breakdown of thyroid resection patients and hypocalcemia from January 2006 to December 2010
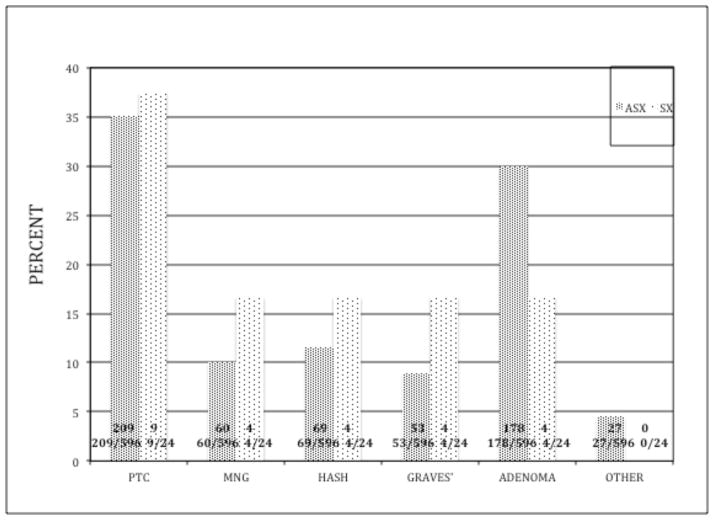
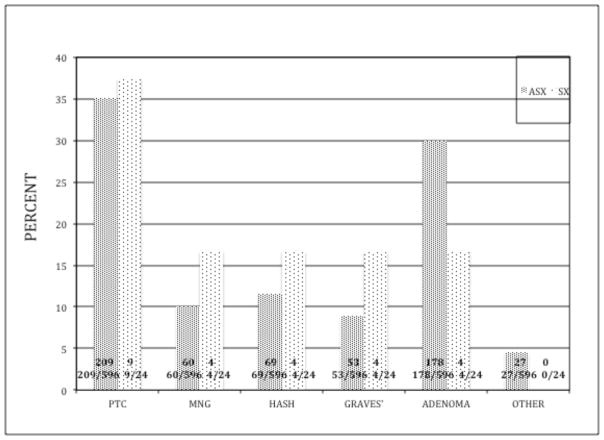
Both groups presented with similar pre-operative diagnoses and pathologic results (figure 2). The SX group had a higher incidence of both Hashimoto’s thyroiditis (16.7% versus 11.5%) and Graves’ disease (12.4% versus 8.8 %); however this was not statistically significant, even when combining all causes of autoimmune thyroiditis. Women represented the majority of patients in both groups. The SX group was significantly younger (39.6 ± 2.8 versus 49 ± 0.6 years, p=0.01) and had smaller thyroid glands (21.4 ± 3.2 versus 39.6 ± 1.8 grams, p<0.001). The size of the dominant nodule was similar in the two groups (table 1). Parathyroid autotransplantations were performed in 4 (17%) patients in the symptomatic group and thirty-one patients in the asymptomatic group (5%) (p=0.07). None of the 24 SX patients had a parathyroid gland identified in their pathologic specimen.
Figure 2.
iPTH based protocol
Table 1.
Comparison of clinical parameters for symptomatic and asymptomatic patients
| ASYMPTOMATIC | SYMPTOMATIC | P value | |
|---|---|---|---|
| Gender (%F) | 116M; 480F (81%) | 3M; 21F (90%) | NS |
| Age (years) | 49 ± 0.6 | 39.6 ± 2.8 | 0.01 |
| Nodule size (cm) | 2.9 ± 0.5 | 2.5 ± 0.4 | NS |
| Thyroid weight (gm) | 39.6 ± 1.8 | 21.4 ± 3.2 | <0.001 |
| Pre-op calcium (mg/dl) | 9.7 ± 0.4 | 9.3 ± 0.1 | NS |
| Pre-op iPTH (pg/ml) | 71 ± 14 | 44 ± 2 | NS |
| Post-op calcium (mg/dl) | 9.9 ± 0.0 | 8.6 ± 0.1 | NS |
| Post-op iPTH (pg/ml | 31 ± 1 | 16 ± 4 | 0.03 |
| POD 1 calcium (mg/dl) | 8.5 ± 0.0 | 8 ± 0.2 | 0.02 |
Pre-and post-operative calcium levels were similar in the ASX group (9.7 ± 0.4 and 9.9 ± 0.03 mg/dl); whereas the symptomatic group had an average of a 7.5% decline in serum calcium post-operatively (9.3 ± 0.1 and 8.6 ± 0.1). Both post-operative calcium (9.9 ± 0.03 versus 8.6 ± 0.1, p=0.06) and iPTH (31 ± 1 versus 16 ± 4, p=0.03) levels were higher in the ASX group (table 1). Sixty-one (10.2%) ASX patients were chemically hypocalcemic in the post-operative period. For patients who underwent same day surgery, laboratory values were obtained one hour post-operatively; whereas, those patients admitted for a 23-hr short stay, had laboratories values obtained both four hours post-operatively and the morning of post-operative day 1 (POD1), prior to discharge. For those patients admitted for a 23-hr short stay, the SX patients in this group continued to have a decrement in their serum calcium levels (9.2 ± 0.9 versus 8 ± 0.2 mg/dl, p<0.001), compared to their 4hr post-operative values. Though still significant, this depreciation was less significant in the short stay ASX group (8.8 ± 0.03 versus 8.5 ± 0.3, mg/dl, p<0.001).
In evaluating protocol efficacy, 3.9% of patients were symptomatic, despite the use of the protocol. For the eight patients with an iPTH >10pg/ml, all had symptom relief without an increase in vitamin D supplementation, and the symptoms persisted for less than three months. Fifteen (62.5%) of the symptomatic patients had a post-operative iPTH <10pg/ml. Eight (33%) SX patients had a post-operative iPTH ≤ 5pg/ml and due to the severity of their symptoms, 62.5% required an increase in vitamin D supplementation for relief, as compared to only 2 (28.5%) of the SX patients with a post-operative iPTH >5pg/ml (table 2).
Table 2.
Distribution of asymptomatic and symptomatic patients based on post-operative iPTH values, with percentage of iPTH group total. Number of SX patients who required an increased in calcitriol dosage for symptom relief.
| iPTH | ASYMPTOMATIC | SYMPTOMATIC | Increased calcitriol requirement |
|---|---|---|---|
| <2pg/ml | 4 (57.1) | 3 (42.9) | 2(28.5) |
| 2–5 pg/ml | 24 (82.8) | 5 (17.2) | 3 (60) |
| 6–9 pg/ml | 36 (83.7) | 7 (16.3) | 2 (4.7) |
| 10–15 pg/ml | 54 (95.6) | 3 (6.4) | 0 |
| 16–20 g/ml | 60 (98.8) | 1 (1.2) | 0 |
| ≥21 pg/ml | 418(98.2) | 5( 1.2) | 0 |
Discussion
Traditionally an inpatient procedure, due to concerns for bleeding, hematoma, airway compromise and symptoms related to hypocalcemia, there has been a movement towards outpatient and short stay thyroid surgery. In order to have a successful outpatient thyroid program, it is essential that you have a low bleeding rate, and do a good job of predicting who might have post-operative hypocalcemia, and hence, treat it pre-emptively. Several studies have demonstrated the efficacy of prophylactic calcium and vitamin D administration, structured around the iPTH and/or calcium levels [16, 10, 4, 5, 17 18, 11]. As a result, the Australian Endocrine Surgeons Society instituted recommendations for same day discharge with the presence of a 4 hour post-operative iPTH level within the normal range [19, 20]. The use of post-operative parathyroid hormone (iPTH) levels as a guide to determine which patients will experience hypocalcemia, has not been universally adopted, as the studies are conflicting. Several have demonstrated its success, using levels obtained within the first twenty-four hours [19, 21, 22]. Whereas others have noted its inaccuracy when used alone, reporting an incidence of 13.4% hypocalcemia, with 2.1% of those patients being symptomatic [23].
We obtained pre-operative intact PTH and calcium levels in all of our patients undergoing total or completion thyroidectomy, even if the risk to the parathyroid glands was small. Our reasoning is to recognize concurrent primary hyperparathyroidism. We have found a high incidence of concurrent hyperparathyroidism in our patients who undergo thyroid surgery [24]. In order to guide our intra-operative decision making, should an enlarged parathyroid gland be identified, we have found it helpful to check intact PTH pre-operatively in all patients.
Utilizing either the one or four hour iPTH level as a guide, 3.9% of our patients presented with breakthrough symptoms. These results are comparative to prospective studies performed by Roh et al, who had 7% symptomatic patients in their protocol with multiple lab draws on post-operative day (POD) 1–7 and administering 1g every 8hr calcium with 0.5μg calcitriol every 12hr [4]. Wiseman et al reported similar results, with significantly fewer patients requiring readmission and intravenous calcium, and more patients being able to be discharged on POD # 1, with the administration of up to 1.5g of calcium carbonate every 6hr with 0.25μg calcitriol every 8hr, for those patients with PTH <10 and calcitriol 0.25μg every 12hr for PTH 10–15 pg/ml, using an algorithm based on 1hr iPTH levels [8]. Youngwirth et al noted the economic benefits of such a protocol as well, noting fewer emergency room visits with the administration of calcium to all patients, and 0.25μg calcitriol every 12 hr to those patients with an iPTH < 10pg/ml [5].
Our protocol is efficacious for multiple reasons. First, it is easy to administer and follow. Second, it is inexpensive. We initially prescribe one half of the calcitriol as other studies [4, 8, 13]. Only 83 (13.4%) of our patients were prescribed calcitriol, based on the protocol. Taking into account only the cost of the prescription, 87.6% of our patients saved a total of $23,134 per month, by not routinely being prescribed calcitriol [25]. Some studies favor routine supplementation, as the optimal algorithm is unknown. Cost-analysis studies suggest that either prophylactic treatment with vitamin D or metabolites and calcium, or calcium alone or routine supplementation, is cost effective [25, 26]. Even with routine vitamin D administration with calcium, patients pay on average one half the cost of emergency room visitation and evaluation, and evaluation and treatment during a one-day hospital readmission is more than three times the cost [25]. Tartaglia et al demonstrated the effectiveness of prophylactic calcium therapy; however due to their inability to perform iPTH testing, all patients received vitamin D [16]. Considering the determination of the development of hypocalcemia based on post-operative iPTH levels is not an exact science, we believe giving lower doses of calcitriol only to those patients with an iPTH < 10 pg/ml, captures the majority of patients who would have been symptomatic, as well as minimizes the risks hypocalcemia and the costs of routine supplementation. Noting that the critical days for hypocalcemia occur with the nadir of calcium and the recovery time of the parathyroid glands, on POD # 2 and # 3, the amount of calcitriol given is pertinent [4, 27]. Calcitriol induces a fourfold increase in oral calcium uptake, as well as suppresses parathyroid function [28]. Hence, higher initial doses may result in a higher incidence of hypercalcemia and prolong parathyroid gland recovery; thus starting with a lower dosage, and administering higher dosages on an individual basis, may be sound.
Another issue is the timing of the post-operative laboratory studies. Several studies have suggested that four hours or later while others suggest 1 hour or earlier [5, 7, 8, 11, 15, 17]. Our protocol is effective, using both one and four hour post-operative laboratory values. With only 3.9% symptomatic patients and 0.1% readmission rate, this time point, allows for monitoring and observation for the more dire complications of bleeding, hematoma, and airway compromise. Our results coincide with others, demonstrating safety and efficacy in both total and completion thyroidectomy patients [4, 29, 30].
There are a few limitations to the study. First, it is a retrospective non-random review, which may result in selection bias. Secondly, our definition of symptomatic hypocalcemia, focused on symptoms, which are inherently subjective. Taking this into consideration, in an effort to minimize the bias, we implemented a stricter definition. This allowed us to exclude patients who were non-compliant with the protocol, only reported one episode of paresthesias lasting less than 5 minutes, or did not maintain telephone and office visit follow-up. This was done in an effort to truly scrutinize the protocol when it was adhered to. Our protocol relies on patient reporting hence this may not reflect the true symptoms of hypocalcemia because of reporting error. The stricter definition aided in our ability to better assess the efficacy of the protocol, and to assess where changes may need to be made. The variability in post-operative calcium administration was also a concern, considering the wide range (2–6g daily) that patients take. Despite this range, patients took the initial prescribed dose, and then additional doses with the occurrence of symptoms, until resolution, as per the protocol. The majority of patients were initially prescribed calcitriol 0.25μg twice daily; however, there was some variability between individual surgeons, with respect to the threshold to increase the dose. The strengths of the study were our inclusion of completion thyroidectomies and exclusion of lobectomies to more accurately include the population at risk. It has been well documented that completion thyroidectomies have higher complication rates, as compared to other thyroid procedures, due to the presence of adhesions and a more tenuous surgical field [27, 29]. In addition, we included patients who had a parathyroid gland re-implanted or included in the pathology specimen
Several others have reported the feasibility and safety of outpatient thyroid surgery [29, 31–34]. Our clinical protocol for the prophylactic administration of supplemental calcium and/or vitamin D, based on the one or four hour iPTH value is efficacious and supports an algorithmic approach. The protocol is easy to follow, and implementation is inexpensive. The costs are minimal, with each PTH assay being $7 per usage at our institution [5]. Both calcium and calcitriol can be purchased in a generic formulation, the majority of patients require less than one month of medication, and patients can undergo same day thyroidectomy or a 23-hour short stay, as opposed to being admitted as an inpatient, without a high risk of readmission [5]. Serial laboratory studies are not necessary, as an iPTH value < 10 pg/ml, adequately identifies those patients who require supplementation with both calcium and vitamin D. This minimizes the costs of phlebotomy and laboratory charges. An algorithm proposed by Wiseman et al, significantly decreased costs associated with reduced length of stay, lab draws, need for IV calcium, and hospital readmissions, reporting an average savings $1631 per patient [8]. Our protocol is comparative, with only 3.9% of patients being symptomatic, all patients being discharged on hospital day 0 or 1, and a 0.1% readmission rate.
At the crux of such a protocol, is patient education, as the protocol is not perfect. Patients must be educated and take an active role in their post-operative care. This effect can be seen in the younger patients with thyroiditis and those with post-operative iPTH ≤5 pg/ml. Even though there was no significant difference in the groups, a combination of these characteristics were more common in the symptomatic patients with an iPTH ≤ 5pg/ml. Patients in both cohorts were, on average, in the pre- and peri-menopausal age range. The younger age and lower gland weight of the SX group is likely a result of the higher incidence of Hashimoto’s thyroiditis and hypothyroidism, as the inflammation and vascularity associated with this disease has been shown to increase the risk of complications [35]. In order for the protocol to work best, patients must have access to medical professionals when questions arise. Our program is supported by a nurse practitioner who is able to answer patient questions and adjust calcium or calcitriol doses if necessary in order to prevent emergency room visits and/or readmissions. Our low incidence of symptomatic hypocalcemia with adherence to the protocol supports the notion that total and completion thyroidectomies can be performed safely on an outpatient basis, with the institution of an algorithm for prophylactic therapy.
Conclusion
A standardized iPTH protocol for the prevention of symptomatic hypocalcemia following total or completion thyroidectomy is highly efficacious. By using a PTH cutoff of <10 pg/mL to determine which patients should be treated with calcitriol we were able to minimize the number of patients requiring treatment and reduce our rate of symptomatic hypocalcemia to 3.9%. Since most of the patients that required an increase in their calcitriol dose despite the protocol had PTH levels <5 pg/mL, we have modified our protocol to increase the dose of calcitriol from 0.25 mcg to 0.5 mcg twice a day, for patients with PTH <5 pg/ml and we anticipate that this change will help lower our rate of symptomatic hypocalcemia even further..
Figure 3.
Pathologic diagnoses (given as percentage of total) for symptomatic and asymptomatic patients (absolute numbers within bar)
Acknowledgments
This research was supported by the National Institutes of Health/ National Cancer Institutes RO1CA12115-S1 Supplemental Grant.
References
- 1.Reeve T, Thompson NW. Complications of thyroid surgery: how to avoid them, how to manage them and observations on their possible effect on the whole patient. World J Surg. 2000;24:971–75. doi: 10.1007/s002680010160. [DOI] [PubMed] [Google Scholar]
- 2.Pattou F, Combemale F, Fabre S, et al. Hypocalcemia following thyroid surgery: incidence and prediction of outcome. World J Surg. 1998;22:718–24. doi: 10.1007/s002689900459. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs JK, Aland JW, Jr, Ballinger JF. Total thyroidectomy. A review of 213 patients. Ann Surg. 1983;197:542–49. doi: 10.1097/00000658-198305000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roh J-L, Park CI. Routine oral calcium and vitamin D supplements for prevention of hypocalcemia after total thyroidectomy. Am J Surg. 2006;192:675–58. doi: 10.1016/j.amjsurg.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Youngwirth L, Benavidez J, Sippel R, Chen H. Postoperative parathyroid hormone testing decreases symptomatic hypocalcemia and associated emergency room visits after total thyroidectomy. Surgery. 2010;148:841–46. doi: 10.1016/j.surg.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 6.Sanabria A, Dominguez LC, Vega V, Osorio C, Duarte D. Routine postoperative administration of vitamin D and calcium after total thyroidectomy: a meta-analysis. Int J Surg. 2011;9:46–51. doi: 10.1016/j.ijsu.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Kara M, Tellioglu G, Krand O, et al. Predictors of hypocalcemia occurring after a total/near total thyroidectomy. Surg Today. 2009;39:752–27. doi: 10.1007/s00595-009-3957-1. [DOI] [PubMed] [Google Scholar]
- 8.Wiseman JE, Mossanen M, Ituarte PHG, et al. An algorithm informed by the parathyroid hormone level reduces hypocalcemic complications of thyroidectomy. World J Surg. 2010;34:532–27. doi: 10.1007/s00268-009-0348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards ML, Bingener-Casey J, Pierce D, et al. Intraoperative parathyroid hormone assay: an accurate predictor of symptomatic hypocalcemia following thyroidectomy. Arch Surg. 2003;138:632–36. doi: 10.1001/archsurg.138.6.632. [DOI] [PubMed] [Google Scholar]
- 10.Gentileschi P, Gacek I, Manzell A, et al. Early (1 hour) post-operative parathyroid hormone (PTH) measurement predicts hypocalcaemia after thyroidectomy: a prospective case-control single-institution study. Chir Ital. 2008;60:519–28. [PubMed] [Google Scholar]
- 11.Lim JP, Irvine R, Bugis S, et al. Intact parathyroid hormone measurement 1 hour after thyroid surgery identifies individuals at high risk for the development of symptomatic hypocalcemia. Am J Surg. 2009;197:648–54. doi: 10.1016/j.amjsurg.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Moore FD., Jr Oral calcium supplements to enhance early hospital discharge after bilateral surgical treatment of the thyroid gland or exploration of the parathyroid glands. J Am Coll Surg. 1994;178:11–16. [PubMed] [Google Scholar]
- 13.Bellantone R, Lombardi CP, Raffaelli M, et al. Is routine supplementation therapy (calcium and vitamin D) useful after total thyroidectomy? Surgery. 2002;132:1109–12. doi: 10.1067/msy.2002.128617. [DOI] [PubMed] [Google Scholar]
- 14.Tredici P, Grosso E, Gibelli B, et al. Identification of patients at high risk for hypocalcemia after total thyroidectomy. Acta Otorhinolaryngol Ital. 2011;31:144–48. [PMC free article] [PubMed] [Google Scholar]
- 15.Pfleiderer AG, Ahmad N, Draper MR, et al. The timing of calcium measurements in helping to predict temporary and permanent hypocalcaemia in patients having completion and total thyroidectomies. Ann R Coll Surg Engl. 2009;91:140–46. doi: 10.1308/003588409X359349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartaglia F, Giuliani A, Sgueglia M, et al. Randomized study on oral administration of calcitriol to prevent symptomatic hypocalcemia after total thyroidectomy. Am J Surg. 2005;190:424–49. doi: 10.1016/j.amjsurg.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Grodski S, Lundgren C, Sidhu S, et al. Postoperative PTH measurement facilitates day 1 discharge after total thyroidectomy. Clin Endocrinol (Oxf) 2009;70:322–25. doi: 10.1111/j.1365-2265.2008.03317.x. [DOI] [PubMed] [Google Scholar]
- 18.Wu G, Pai SI, Agrawal N, et al. Profile of patients with completion thyroidectomy and assessment of their suitability for outpatient surgery. Otolaryngol Head neck Surg. 2007;136:556–59. doi: 10.1177/0194599811416749. [DOI] [PubMed] [Google Scholar]
- 19.Grodski S, Serpell J. Evidence for the role of perioperative PTH measurement after total thyroidectomy as a predictor of hypocalcemia. World J Surg. 2008;32:1367–73. doi: 10.1007/s00268-008-9545-5. [DOI] [PubMed] [Google Scholar]
- 20.Australian Endocrine Surgeons Guidelines AES06/01 Group. Postoperative parathyroid hormone measurement and early discharge after total thyroidectomy: analysis of Australian data and management recommendations. Aust N Z J Surg. 2007;77:199–202. doi: 10.1111/j.1445-2197.2007.04018.x. [DOI] [PubMed] [Google Scholar]
- 21.McCleod IK, Arciero C, Noordzij JP, et al. The use of rapid parathyroid hormone assay in predicting postoperative hypocalcemia after total or completion thyroidectomy. Thyroid. 2006;16:259. doi: 10.1089/thy.2006.16.259. [DOI] [PubMed] [Google Scholar]
- 22.Sywak MS, Palazzo FF, Yeh M, et al. Parathyroid hormone assay predicts hypocalcemia after total thyroidectomy. Aust N Z J Surg. 2007;77:667–70. doi: 10.1111/j.1445-2197.2007.04183.x. [DOI] [PubMed] [Google Scholar]
- 23.Lombardi CP, Raffaelli M, Princi P, et al. Early prediction of post thyroidectomy hypocalcemia by one single iPTH measurement. Surgery. 2004;136:1236–41. doi: 10.1016/j.surg.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 24.Murray SE, Sippel RS, Chen H. Incidence of concomitant hyperparathy-roidism in patients in patients with thyroid disease requiring surgery. J Surg Res. 2012;178:264–267. doi: 10.1016/j.jss.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanabri A, Dominguez LC, Vegas V, et al. Cost-effectiveness analysis regarding postoperative administration of vitamin-D and calcium after thyroidectomy to prevent hypocalcemia. Rev Salud Publica. 2011;13:804–813. doi: 10.1590/s0124-00642011000500009. [DOI] [PubMed] [Google Scholar]
- 26.Wang TS, Roman SA, Sosa JA. Postoperative calcium supplementation in patients undergoing thyroidectomy. Curr Opin Oncol. 2012;24:22–28. doi: 10.1097/CCO.0b013e32834c4980. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Chung MK, Son Y-I. Reliable early prediction for different types of post-thyroidectomy hypocalcemia. Clin Exper Otorhinol. 2011;4:95–100. doi: 10.3342/ceo.2011.4.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 29.Terris DJ, Moister B, Seybt MW, et al. Outpatient thyroid surgery is safe and desirable. Otolaryngol Head Neck Surg. 2007;136:556–59. doi: 10.1016/j.otohns.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Roh JL, Park CI. Prevention of postoperative hypocalcemia with routine oral calcium and vitamin D supplements in patients with differentiated papillary thyroid carcinoma undergoing total thyroidectomy plus central neck dissection. Cancer. 2009;115:251–58. doi: 10.1002/cncr.24027. [DOI] [PubMed] [Google Scholar]
- 31.Trottier DC, Barron P, Moonje V, et al. Outpaitent thyroid surgery: should patients be discharged on the day of their procedures? Can J Surg. 2009;52:182–86. [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder SK, Maid KS, Roberson CR, et al. Outpatient thyroidectomy is safe and reasonable: experience with more than 1,000 planned outpatient procedures. J Am Coll Surg. 2010;210:575–82. 582–84. doi: 10.1016/j.jamcollsurg.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 33.Chin CW, Loh KS, Tan KS. Ambulatory thyroid surgery: an audit of safety and outcomes. Singapore Med J. 2007;48:720–24. [PubMed] [Google Scholar]
- 34.Kulkarni RP, Ituarte PHG, Gunderson D, Yeh MW. Clinical pathways improve hospital resource utilization in endocrine surgery. J Am Coll Surg. 2011;212:35–41. doi: 10.1016/j.jamcollsurg.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Luo J, McManus C, Chen H, Sippel RS. Are there predictors of malignancy in patients with multinodular goiter? J Surg Res. 2012;174:207–210. doi: 10.1016/j.jss.2011.11.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]