Abstract
Background
To examine whether short-term, ie, five daily sessions, vigorous dynamic cycling exercise and heat exposure could achieve heat acclimation in trained athletes and the effect of heat acclimation on cutaneous blood flow in the active and nonactive limb.
Methods
Fourteen male badminton and table tennis athletes (age = 19.6 ± 1.2 years) were randomized into a heat acclimation (EXP, n = 7) or nonheat acclimation (CON, n = 7) group. For 5 consecutive days, the EXP group was trained using an upright leg cycle ergometer in a hot environment (38.4°C ± 0.4°C), while the CON group trained in a thermoneutral environment (24.1°C ± 0.3°C). For both groups, the training intensity and duration increased from a work rate of 10% below ventilatory threshold (VT) and 25 minutes per session on day 1, to 10% above VT and 45 minutes per session on day 5. Subjects performed two incremental leg cycle exercise tests to exhaustion at baseline and post-training in both hot and thermoneutral conditions. Study outcome measurements include: maximum oxygen uptake (VO2max); exercise heart rate (HR); O2 pulse; exercise time to exhaustion (tmax); skin blood flow in the upper arm (SkBFa) and quadriceps (SkBFq); and mean skin (Tsk).
Results
The significant heat-acclimated outcome measurements obtained during high-intensity leg cycling exercise in the high ambient environment are: (1) 56%–100% reduction in cutaneous blood flow to the active limbs during leg cycling exercise; (2) 28% drop in cutaneous blood flow in nonactive limbs at peak work rate; (3) 5%–10% reduction in heart rate (HR); (4) 10% increase in maximal O2 pulse; and (5) 6.6% increase in tmax.
Conclusion
Heat acclimation can be achieved with five sessions of high-intensity cycling exercise in the heat in trained athletes, and redistribution of cutaneous blood flow in the skin and exercising muscle, and enhanced cardiovascular adaptations provide the heat-acclimated athletes with the capability to increase their endurance time in the hot environment.
Keywords: VO2max, oxygen pulse, skin blood flow, cardiovascular adaptation, badminton and table tennis players, short-term heat acclimation
Introduction
It has been shown that heat acclimation enhances human ability to perform physical work in a hot ambient environment.1–3 Without heat acclimation, aerobic capacity and performance are impaired in high ambient temperatures (ie, earlier onset of fatigue and heat-related exhaustion).2,4,5 One mechanism after heat acclimation is to increase skin blood flow and the volume of sweat for dissipating heat to regulate body temperature during vigorous exercise in the heat.6–9 It has been reported that when a healthy person attains the “complete” effects of heat acclimation, a series of physiological adaptations or adjustments may occur during exercise in a hot environment: maintained body temperature by increased convective heat loss via distributing blood flow to the cutaneous tissue and by sustained sweating rates;2,7 increased plasma volume by maintaining cardiac-filling pressure and thus restoring arterial pressure and cardiac output;1,10 reduced cardiovascular stress by lowering heart rate and enhancing exercise capacity and endurance;2,3,11,12 induced compensatory reflexes by limiting blood volume contained in the splanchnic vascular bed and enhanced maximal cutaneous volume;2,10 reduced electrolyte loss (ie, sodium chloride) in the sweat;11,13 and increased heat-shock proteins’ synthesis in response to cellular stress.14 All of these enhance exercise performance11,12 and prevent the negative effect of heat-related illnesses, such as exertional heat stroke, heat exhaustion, and heat cramps.15
There is a scarcity of studies investigating short-term (ie, ≤5 days of physical exercise in high ambient environments) heat acclimation in well-trained competitive athletes. These athletes, when preparing to compete in hot environments, often have to rely on research information on heat acclimation obtained from moderately trained or untrained populations using long-term (ie, 12–14 days of physical exercise in high ambient environments) heat acclimation protocol.3,4,12 For competitive athletes, disruption of quality training time close to competitions with long-term heat acclimation could interfere with their training schedule, resulting in a negative effect on subsequent sport performance. Furthermore, competitive athletes also have to deal with environmental concerns when traveling from a mild climate region to a hot climate region (eg, countries of tropical climate) for competition, with the possibility of physical detraining (and jet lag), due to travel time and distance to the site of competition. It is critical to know the extent to which trained athletes may adapt to short-term heat acclimation to prevent negative thermoregulatory effects on physical performance. Thus, for the competitive athletes, the use of a short-term heat acclimation protocol of five daily sessions would be more economical and efficient than long-term protocols. The precise mechanisms responsible for the impaired performance in the high thermal environment in trained athletes are not yet fully understood.
During exercise in a high-thermal environment, cutaneous circulation undergoes periodic changes by increased local blood flow to meet the metabolic demands of muscular activity and to dissipate body heat.6,7 The mechanism of peripheral vascular reflex responses during exercise in the heat maintains adequate cardiac output and blood pressure during periods of decreased cardiac-filling pressure, suggesting that during exercise in the heat, blood flow in the nonactive region of the tissue is lowered.10 The effect of exercise in the heat on the distribution of SkBF in the active and nonactive limbs of highly trained athletes has not been fully investigated. Thus, it is unclear as to whether skin blood flow to the active region of the working tissue after heat acclimation decreases, remains unchanged, or increases during exhaustive leg cycle exercise in high ambient temperature in trained athletes. It would be of great interest for trained athletes to examine the extent of heat acclimation that could occur with respect to cardiovascular adaptations and body heat dissipation via SkBF in the active and nonactive limbs. Therefore, the purpose of this study was to determine the effect of five daily sessions of leg cycling exercise with an incremental work rate in high ambient temperature on physiological responses, as well as SkBF distribution in the active and inactive limb in trained athletes.
Methods and materials
Subjects
Fourteen male athletes were recruited from active members of the national all-star table tennis and the national all-star badminton teams from Taiwan. The athletes recruited for this study were six badminton and eight table tennis players. (See exclusion criteria below.) The athletes were full-time college students at the National Taiwan Sport University in Taoyuan, Taiwan, not heat acclimated, and had been residing at the same location (altitude of 250 meters above sea level) for 3–4 years. During the study period, the months of November through March, the average monthly ambient temperature and relative humidity were: 19.9°C and 68% (November); 16.5°C and 67% (December); 15.3°C and 79% (January); 14.4°C and 77% (February); and 17.5°C and 82% (March), respectively. Summer month ambient temperature and relative humidity averaged 28°C–30°C and 80%–88%, respectively. The age, height, body mass, body surface area, and maximum oxygen uptake (VO2max) of the subjects were (mean ± standard deviation [SD]): 19.6 ± 1.2 years; 171.6 ± 4.7 cm; 64.7 ± 5.0 kg; 1.78 ± 0.08 m2; and 53.0 ± 5.3 mL/kg−1 per minute−1 (with a cycle ergometer test), respectively. Before the subjects’ randomization, the participants were fully informed of potential risks and anticipated discomforts associated with the experimental procedure and allowed to have two familiarization sessions – one for the cycle ergometer test protocol and one for the training protocol. All participants gave their written, informed consent to participate as study subjects. This research protocol was conducted according to the principles of the Declaration of Helsinki on human research and approved by the National Taiwan Sport University Ethics Committee.
Study design
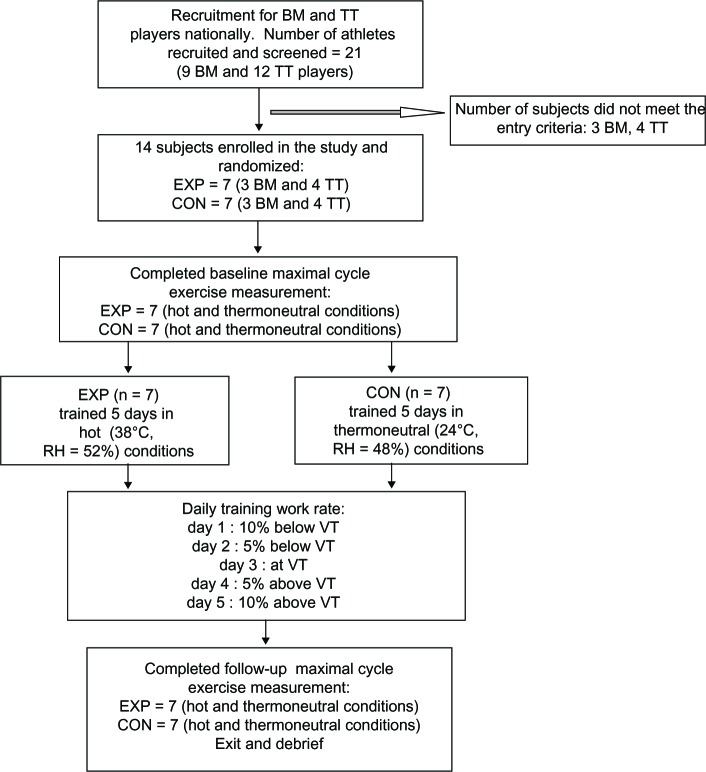
All subjects underwent pre-participation screening using a Health and Exercise History Questionnaire and a blood chemistry profile test.15 Subjects were excluded from the study if they: (1) had a cardiovascular, liver, kidney, metabolic, or pulmonary disease; (2) were recovering from musculoskeletal injuries or respiratory tract infections that could affect the outcomes of maximal leg cycle exercise test (GXT) and aerobic training in the heat; (3) were heat acclimatized during physical training within the past 6 months; (4) had been diagnosed with Raynaud’s disease, a vasospastic disease of small arteries and arterioles affecting circulation to the digits of the hands; (5) currently used any tobacco product; (6) were heavy caffeine users, ie, >500 mg per day; or (7) used vasodilator drugs. We excluded subjects with Raynaud’s disease because intermittent occlusions of circulation to the small arteries and arterioles affect arterial blood flow to the skin region and thus compromise skin blood measurements. The subjects were randomly assigned to an experimental (EXP, n = 7; three badminton players and four table tennis players) or control (CON, n = 7; three badminton players and four table tennis players) group. After randomization, the participants followed the experimental procedure outlined in Figure 1. Two separate laboratory visits were required for baseline study outcome measurements in thermoneutral and hot conditions, separated by at least a 24-hour rest. The outcome measurements were performed using a leg cycle ergometer (Model 818E, Monark Exercise AB, Vansbro, Sweden) and an incremental leg cycle protocol with 3-minute increment per stage until reaching volitional fatigue or exhaustion. The Monark cycle ergometer was calibrated daily before the measurement. The GXTs were conducted at the same time of the day on each visit (ie, ±30 minutes), and the test order was randomly allocated and counterbalanced. Twenty-four hours after the baseline measurements, the EXP began to train in a hot environment, mean ± SD room temperature = 38.4°C ± 0.4°C, and relative humidity (RH) = 52.0% ± 4.6%, while the CON began to train in a thermoneutral environment with mean ± SD room temperature = 24.1°C ± 0.3°C and RH = 51.5% ± 4.5%, both groups on upright cycle ergometers. Both EXP and CON were trained using the following cycling exercise protocol from day 1 to day 5. On day 1, the training duration and work rate were 25 minutes per day and at 10% below the individual’s ventilatory threshold (VT) obtained from the GXT in the thermoneutral condition. The subsequent training work rates for days 2, 3, 4, and 5 were increased to 5% below the VT, equal to the VT, 5% above the VT, and 10% above the VT, respectively (Figure 1). The subsequent training duration was increased by 5 minutes each day, until reaching 45 minutes per day on day 5. At the conclusion of the training period, within 24 hours, subjects repeated the same baseline measurements. All subjects completed the 5-day training sessions without any incident of heat-related illnesses or muscle soreness. The thermal-controlled room used for exercise training and testing was equipped with a thermal-controlled system and 122 square meters wide with an 2.44 meter ceiling. Temperature in the testing room was manually controlled by an investigator using a wall-mounted thermostat outside the testing room. One investigator (TIC) accompanied the subject in the room at all times to perform the measurements. The hot thermal room condition was significantly higher than the thermoneutral condition.
Figure 1.
Experimental protocol from subject recruitment, five-daily exercise training, to completion of follow-up measurements.
Note: Number of dropouts = zero.
Abbreviations: BM, badminton; TT, table tennis; EXP, experimental; CON, control; VT, ventilator threshold; RH, relative humidity.
Outcome measurements
Determination of VO2max and time to reach exhaustion
The subjects’ VO2max was measured on a cycle ergometer in a thermoneutral room with a mean ± SD, 24.1°C ± 0.3°C, and RH of 51.5% ± 4.5% and in a hot room with 38.4°C ± 0.4°C and RH of 52.0% ± 4.6%. The GXT protocol consisted of a 5-minute warm-up at a work rate of 30 watts (W), and 3 minutes of stretching exercises. During the VO2max measurement, the initial cycling work rate was 60 W for 3 minutes; thereafter, the work rate was increased by 30 W every 3 minutes until reaching volitional fatigue. VO2max was determined when the subject met at least two of the following criteria: (1) failed to maintain the prescribed pedaling frequency of 60 revolutions per minute for more than 5 seconds with repeated verbal encouragement (from researcher TIC); (2) a rating of perceived exertion (RPE) ≥19 (Borg 6–20 scale); (3) respiratory exchange ratio (RER) >1.10; or (4) exercise heart rate >95% of the predicted maximal heart rate. Expired gases were collected and recorded breath-by-breath, using an automated metabolic cart system (Sensor Medics System, Model 2900, Yorba Linda, CA, USA), which was calibrated 2 hours before all testing. All subjects reached peak work rates between 210 W and 240 W before stopping the test. During the VO2max measurement, exercise time to exhaustion (tmax) was determined by the same researcher (TIC). The time-to-exhaustion criteria was recorded in seconds and determined when the subject stopped pedaling the cycle ergometer, despite repeated verbal encouragement given by the same researcher. In addition, one of the following criteria must be attained: (1) a rating of RPE ≥19 (Borg 6–20 scale); or (2) an exercise heart rate >95% of the age-predicted maximal heart rate. Note that during the tmax measurement, all the subjects met the above end-point criteria. In addition to the VO2max and tmax, study outcome measurements also included: maximal minute ventilation (VE); oxygen consumption (VO2); rate of elimination of carbon dioxide (VCO2); VT; heart rate (HR); maximal oxygen-pulse; skin blood flow in the quadriceps, (SkBFq); skin blood flow of the upper arm (SkBFa); mean Tsk; and total body sweat loss. HR was recorded during all training sessions using a Polar heart rate monitor (Model #1901201, Polar Electro, Kempele, Finland). The equipment used for the outcome measurements was calibrated daily, 2 hours before testing or data collection. Over the 5-day period, average training HR between the EXP in the heat (163 ± 5.2 beats per minute [BPM]−1) and the CON in the thermoneutral condition (164 ± 3.0 BPM−1) were not significantly different. The exercise intensity ranged from 75% on day 1% to 89% of maximum heart rate on day 5. Core temperature was not obtained, due to more than half of the subjects experiencing esophageal discomfort or gastroesophageal reflux while swallowing the esophageal thermostat probe during cycling exercise in the hot environment. Twenty-four hours prior to the GXT test, the athletes were instructed to adequately hydrate, abstain from any physical training, drinking alcohol, caffeine or tea, and no food intake 2 hours before testing or training. Each subject was provided with ten 450 mL bottles of water daily and instructed to drink three bottles of fluid 2 hours before the GXT or exercise training and at least three to four bottles of fluid following the GXT test or exercise training. Subjects maintained their regular diet by consuming cafeteria-prepared standard meals for breakfast, lunch, and dinner at the campus dining hall. During the study period, the standard meals prepared by the cafeteria consisted of approximately 3500 kilocalories per day with 55%–60% calories from carbohydrate, 15%–20% calories from protein, and 20%–25% calories from fat.
Determination of VT level
During the GXT in the thermoneutral and hot conditions, VT was determined according to Wasserman et al,16 using the criteria of systematic increase in ventilatory equivalents for oxygen (VE/VO2) with no concomitant increase of VE/VCO2. The same investigator (TIC) performed determination of the point at which these two values crossed each other from the measured parameters.
Skin blood flow measurement
SkBFa and SkBFq during the GXT in both thermal conditions was measured concurrently using two calibrated laser Doppler flowmeters (LDF) (Vasamedics Inc, model BPM2; St, Paul, MN, USA) and two hardtip pencil probes (model BPM2, Vasamedics Inc). The subjects’ skin sites were chosen and marked with a small circle, using a waterproof permanent black marker for the attachment of the LDF probe head.17 To obtain the resting LDF value, the subject entered the thermal-controlled room for 10 minutes before undertaking three resting LDF measurements every 20 seconds, from which the averaged value was taken and used for data analysis. These same skin sites were used for subsequent LDF study during the GXT measurements. The following LDF recorder settings were used: speed, 100 mm per minute; sensitivity, X20; and averaging time, 10 seconds.17,18 During the GXT, the highest and lowest blood perfusion values were recorded for 10 seconds. These five 10-second SkBF values were averaged and reported as a relative perfusion unit (% RU).19 The SkBF values were used as indexes for estimating continuous SkBF of the region being measured. The LDF can be applied to any region of the skin with a measuring depth limited to cutaneous tissue and was not affected by the underlying muscle blood flow.20 The LDF instrument used for these outcome measurements was calibrated daily, at least 2 hours before testing or data collection.
Skin temperature measurement
During the GXT in both thermal conditions, Tsk was measured at the same time as SkBF in an upright position. The following skin sites – chest (Tch); thigh (Tth); forearm (Tfa); and medial calf (Tca) – were recorded using a Tele-Thermometer (YSI Tele-Thermometer model 34, YSI Inc, Yellow Springs, OH, USA) and four attachable surface temperature probes (YSI model 409B).17 Whole body mean Tsk was estimated on the basis of regional area and thermal sensitivity of each site as follows:21
| (1) |
Estimation of total body sweat loss
Total body sweat loss was estimated using the pre- and post-body weight difference divided by the individual’s body surface area, and expressed as kg per meter−2 per hour.−1 Before each weighing, subjects were asked to void their bladder completely. Weighing of the subject was performed with subject wearing only thin athletic shorts, no shoes, and no socks, using an automated electronic weight-height scale (model NK-3000, Nakamura Medical Industry, Tokyo, Japan) measured to the nearest 0.05 kg. After termination of the GXT, subjects towel dried completely, changed into freshly dried athletic shorts, and then their postexercise body weight was measured. Note that during the GXT and all the training sessions, subjects were allowed to drink water ad libitum (ie, not required). The weight of fluid they drank was accounted for by weighing the water prior to ingestion and was subtracted from body weight obtained. From this procedure, whole body sweat loss was calculated.
Statistical procedures
All statistical analyses were performed using the Statistical Product and Service Solutions statistical software package (SPSS version 20, IBM Corporation, Armonk, NY, USA). A two-way (group × time point) analysis of variance (ANOVA), with repeated measures on the time factor and two-way ANOVA (group × work rate), with repeated measures on the work factor, was used to determine significant main effects for the study outcomes. When there was a significant main effect, the Tukey’s test was used to locate the source of the difference. A probability of P< 0.05 was taken to indicate statistical significance. Based on the sample size calculation for the SkBF study outcomes, with a statistical power of 0.80 (β = 0.2); α = 0.05; and effect size = 1.75 and 1.5; respectively, for leg SkBF and arm SkBF, a sample size of seven would provide sufficient statistical power for this study.
Results
At baseline, physical and physiological characteristics of the subjects were not significantly different between the EXP and CON for age (19.9 ± 1.9 years versus 19.4 ± 0.5 years); height (172.5 ± 4.7 cm versus 170.6 ± 4.8 cm); weight (65.9 ± 6.1 kg versus 63.4 ± 3.8 kg); body surface area (1.8 ± 0.09 m2 versus 1.76 ± 0.07 m2); and resting heart rate (72.4 ± 6.2 BPM−1 versus 68.6 ± 7.2 BPM−1). During the post-test measurements, the EXP showed a 6.6% or 1.1 minute (P < 0.05) gain in endurance time in the hot environment, while the CON exhibited no improvement (ie, 0.4 minutes) in this outcome measurement (Table 1). When the leg cycling exercise was performed in the thermoneutral condition, both groups showed no improvement in endurance time between pre- and post-test. The VO2max measured at baseline was not significantly different between the EXP and CON, in the thermoneutral (53.8 ± 5.2 versus 52.4.0 ± 4.7 mL per kg−1 per minute−1) and the hot conditions (52.3 ± 8.2 versus 49.2 ± 3.3 mL per kg−1 per minute−1). At the post-test measurements, both groups show minimal improvement in VO2max, and mean Tsk relative to the pre-test values in the hot and thermoneutral conditions (Table 1). The subject’s VT in terms of VO2 levels obtained at post-test was also unchanged relative to the pre-test values (data not shown).
Table 1.
Mean ± SD of measured study outcomes between the experimental and control groups
| Thermoneutral, 24°C
|
P-valuea | Hot, 38°C
|
P-valuea | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-test
|
Post-test
|
Pre-test
|
Post-test
|
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Time to exhaustion, minutes | ||||||||||
| EXP | 17.3 | 1.8 | 18.1 | 2.6 | NS | 16.6 | 2.2 | 17.7 | 2.5 | <0.05 |
| CON | 15.9 | 1.8 | 16.1 | 1.8 | NS | 15.2 | 1.9 | 15.6 | 2.4 | NS |
| VO2max, mL · kg−1 · minutes−1 | ||||||||||
| EXP | 53.8 | 5.2 | 53.0 | 6.5 | NS | 52.3 | 8.2 | 53.4 | 6.7 | NS |
| CON | 52.4 | 4.7 | 52.4 | 4.1 | NS | 49.2 | 3.3 | 50.6 | 5.2 | NS |
| Heart rate maximal, beats · minutes−1 | ||||||||||
| EXP | 196.1 | 9.2 | 186.9 | 6.7 | <0.05 | 197 | 10.1 | 186.9 | 6.7 | <0.05 |
| CON | 194.7 | 10.2 | 186.9 | 5.4 | <0.05 | 195 | 12.2 | 190.1 | 5.3 | NS |
| O2-pulse maximal, mL · beat−1 | ||||||||||
| EXP | 18.8 | 2.9 | 20.0 | 3.9 | <0.05 | 18.3 | 3.7 | 20.2 | 3.8 | <0.05 |
| CON | 17.4 | 2.2 | 18.2 | 3.9 | NS | 16.2 | 2.0 | 17.0 | 2.4 | NS |
| Total sweat loss, kg · m2 · hour−1 | ||||||||||
| EXP | 0.64b | 0.36 | 0.47b | 0.16 | NS | 1.26 | 0.47 | 1.41 | 0.55 | NS |
| CON | 0.60b | 0.30 | 0.46 | 0.30 | NS | 1.14 | 0.30 | 0.65 | 0.30 | <0.05 |
| Peak Tsk, °C | ||||||||||
| EXP | 32.4 | 0.60 | 32.2 | 0.20 | NS | 35.9 | 0.80 | 35.7 | 0.50 | NS |
| CON | 32.2 | 0.50 | 32 | 0.60 | NS | 36.2 | 0.80 | 35.1 | 0.30 | NS |
Notes:
Pre- and post-test comparison
P < 0.05 compared to hot conditions.
Abbreviations: Pre-test, baseline measurement; Post-test, follow-up measurement; EXP, experimental group; CON, control group; NS, not statistically significant; Tsk, mean skin temperature; SD, standard deviation; VO2max, maximum volume of oxygen.
Figure 2A and B depict leg SkBF of the EXP and CON from the hot and thermoneutral conditions, respectively. During incremental leg cycling exercise in hot conditions, the post-heat acclimation of the leg SkBF of the EXP was significantly lower than the pre-heat acclimation leg SkBF: 71% at 120 W; 56% at 180 W; and 100% at 240 W (all P < 0.05) (Figure 2A). However, when the leg cycling exercise was performed in the thermoneutral condition, post-heat acclimation of the leg SkBF of the EXP were significantly higher than pre-heat acclimation: 65% at 60 W; 63% at 120 W; 80% at 180 W; and 73% at 240 W (all P < 0.05) (Figure 2B). For the EXP, the rise in leg SkBF during incremental leg cycling exercise, measured at post-heat acclimation in the thermoneutral condition, was not significantly higher than the rise in leg SkBF measured in hot conditions, except at the 180 W work rate (70%, P < 0.05) (Figure 2A and B). None of the above changes in the leg SkBF during incremental leg cycling exercise was observed in the CON.
Figure 2.
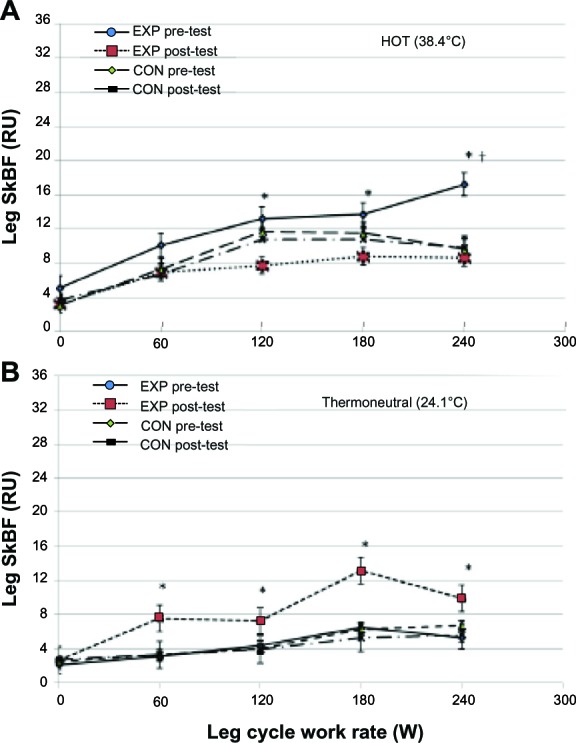
Leg skin blood flow in the hot (A) and thermoneutral (B) conditions of the EXP and CON group.
Notes: *Significant between pre-test and post-test of EXP, P < 0.05. †Significant compared to other groups at all work rates, P < 0.05. Values are means ± SD as determined by repeated measures ANOVA (group × work rate).
Abbreviations: RU, relative perfusion unit; SkBF, skin blood flow; W, watt; EXP, experimental; CON, control; SD, standard deviation; ANOVA, analysis of variance.
Figure 3 depicts arm SkBF of the EXP and CON. When leg exercise was performed in hot conditions, after heat acclimation, the EXP exhibited steady increase in arm SkBF during incremental leg exercise. The increase in arm SkBF at 180 W was significant (P < 0.05), compared to all other work rates (Figure 3A). This finding in the arm SkBF was not observed when leg cycling exercise was performed in the thermoneutral condition (Figure 3B). Again, the above changes were not observed in the CON, except when the cycling work rate reached 180 W arm SkBF dropped 28% (P > 0.05) (Figure 3A). We performed a regression analysis of SkBF during leg cycling exercise to exhaustion for the EXP and CON and observed that the arm SkBF showed a linear increase during leg exercise at pre-test (correlation coefficient [r] = 0.996, P < 0.001) and post-test (r = 0.996, P < 0.001) in both thermal conditions.
Figure 3.
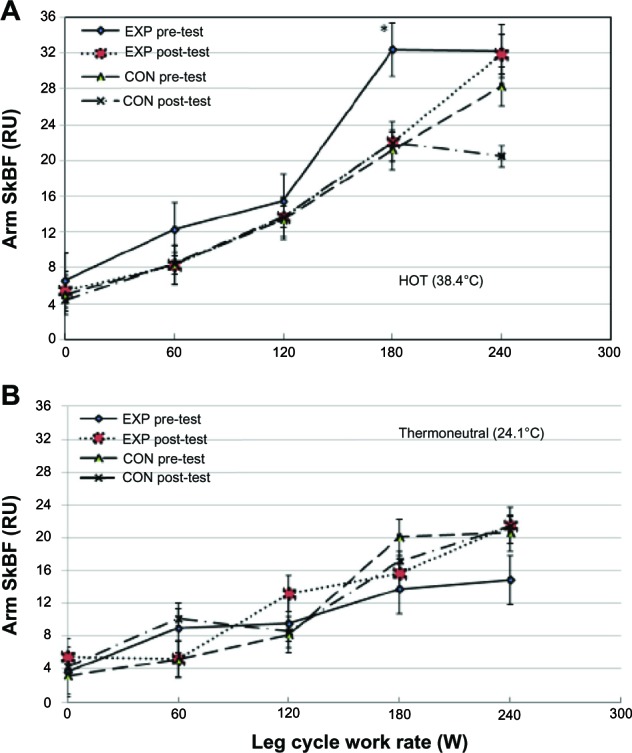
Arm skin blood flow in the hot (A) and thermoneutral (B) conditions of the EXP and CON group.
Notes: *Significant compared to resting, 60 W and 120 W, P < 0.05. Values are means ± SD as determined by repeated-measures ANOVA (group × work rate).
Abbreviations: RU, relative perfusion unit; SkBF, skin blood flow; EXP, experimental; CON, control; W, watt; SD, standard deviation; ANOVA, analysis of variance.
Table 1 depicts study outcome measurements for time to exhaustion: VO2max; maximal heart rate; maximal O2 pulse; total sweat loss; and peak Tsk. After heat acclimation, in the hot environment, the EXP showed lowered HR at rest (−2%, P > 0.05) and during the leg cycling exercise at 60 W (−2.5%, P > 0.05), 120 W (−10%, P < 0.05), 180 W (−7.7%, P < 0.05), and 240 W (−5.1%, P < 0.05). In the thermoneutral condition, the reduction in exercise HR was also observed in the EXP and CON. For O2 pulse measurement, after heat acclimation, the EXP showed significant increases during incremental leg cycling exercise (13.3%–15.7%, P < 0.05) in the thermoneutral condition, and only modest increases (1.2%–10%, P < 0.05) in the hot environment. However, at maximal cycling work rate in the hot environment, the EXP showed a 10.4% (P < 0.05) increase in peak O2 pulse (all P < 0.05) (Table 1). The observed changes in peak O2 pulse were not observed in the CON at post-test, relative to pre-test in both thermal conditions (Table 1). No change in mean skin temperature (Tsk °C) in the EXP and the CON were observed at post-test in both thermal conditions (Table 1). Also, no change in sweat loss in the EXP was observed at post-test in both thermal conditions, relative to the pre-test values. Note that the sweat loss of the CON at post-test was lowered (P < 0.05) in hot conditions, but not in the thermoneutral condition (Table 1).
Discussion
After five daily sessions of upright leg cycling exercise in the heat, the EXP showed a 6.6% or 1.1 minute (P < 0.05) gain in endurance time in the hot environment, while the CON exhibited no improvement in this outcome measurement (Table 1). Our results agreed with that of Hales et al22 and Lorenzo et al,2 who reported increased endurance time after heat acclimation in trained athletes in hot conditions. On HR response during incremental exercise in the hot environment, our results agreed with that of Garrett et al4 and Nielsen et al.8 In light of the VO2max and HR response during incremental leg exercise obtained for the EXP and CON in both thermal conditions, it is reasonable to suggest that the training protocol used in the present study was effective for inducing heat acclimation and training effect, ie, enhancing cardiovascular adaptations.
For the EXP group, pre-heat acclimation leg SkBF response to incremental cycling works was significantly higher than post-heat acclimation in the hot condition (Figure 2A). The mechanism(s) responsible for this phenomenon is unclear. These observations may be interpreted as that before heat acclimation, leg SkBF in the hot environment was largely directed to the skin region, due to cutaneous vasodilator drive in response to exercise and heat stress.10 However, after a 5-day heat acclimation process, the observed reduction in leg SkBF at 120 W, 180 W, and 240 W work rate (all P < 0.05; Figure 2A) in the hot condition may be explained as follows. Before heat acclimation during dynamic exercise in the heat, blood flow in the exercising muscle increases to meet the increased metabolic demand and that is accompanied by cutaneous vasoconstriction and substantial vasodilation in the active muscle until reaching exhaustion.6,7 In this case, we hypothesize that after heat acclimation, cutaneous blood flow during dynamic leg exercise was lowered so that blood flow in the active muscle can be maintained in the high thermal environment and thus increased the time to exhaustion.
In the thermally neutral environment, after heat acclimation, at all levels of exercise work rates, leg skin blood flow increases steadily, due to cutaneous vasodilation until work rate reached 180 W and then leg SkBF dropped (Figure 2B). This assumption is based on: (1) during leg exercise in the hot environment, internal body temperature also increases; and (2) a constant cardiac output and cardiac-filling pressure.7 The above observations were not detected in the CON.
Arm SkBF response during leg cycling exercise in the thermoneutral condition support Smolander’s findings9,23 that skin blood flow measured at the forearm increased linearly in physically active men. We observed a plateau in arm SkBF during leg exercise in the hot (Figure 3A) and thermoneutral condition (Figure 3B). In the hot environment, our results did not support that of Hales et al22 who observed a remarkable 80% decrease in SkBF in the nonactive arm during leg cycle exercise at intensity of 60% VO2max. Both EXP and CON groups showed a larger and steeper increase in arm SkBF in response to increased work rate in hot conditions than in the thermoneutral environments. However, in both thermal conditions, arm SkBF plateau or drop when the exercise work rate reached 180 W or higher (Figure 3A and B). The observed plateau in arm SkBF is due to reflex effects from the skin temperature that work at suppressing the cutaneous vasodilator drive to rising internal body temperature by raising the threshold for vasodilation.7,24 We did not measure body temperature in this study and could only assume that during incremental leg cycling exercise in the hot environment, internal temperature was elevated and thus raised the cutaneous vasodilation threshold. Another possible mechanism is that when arm SkBF approaches its peak value, it begins to redirect cutaneous blood flow away from nonexercising tissues (including blood volume in the visceral vascular bed) to meet the metabolic demands of the exercising skeletal muscle.7 To meet the high metabolic demands and optimize cardiac output during heavy exercise in the high ambient environment, the thermoregulatory drive that is exercise must place a constraint on cutaneous vasodilation.7 Stephens et al suggest that in the cutaneous blood flow, the control is from sympathetic vasoconstrictor nerves manifested through the release of norepinephrine and the vasoconstricting cotransmitter.25 Other researchers suggest that in the exercising skeletal muscles, increased blood flow may be achieved via the release of local vasodilating substances, such as increased hydrogen (H+) and carbon dioxide partial pressure (Pco2),6,8,10 and release of nitric oxide synthase,7 overcoming the effect of sympathetic vasoconstrictor drive in those exercising muscles. These observations, redistribution of SkBF from skin presumably to exercising muscle in the active limb, led us to believe that after heat acclimation, vasodilation in the exercising muscle provides the capability for the EXP to increase their endurance time in the hot environment.
After heat acclimation, the EXP group significantly increased O2 pulse during incremental leg cycle exercise in the hot and thermoneutral conditions (10.4% and 6.4%, respectively, both P < 0.05) (Table 1). Wasserman et al16 suggested that when the exercise work rate reached a critical intensity level, the O2 pulse increases primarily because of an increasing arterial-mixed venous O2 difference, suggesting that after heat acclimation, active tissue receives adequate arterial blood perfusion during exercise in the heat. One benefit of heat acclimation is the redistribution of blood flow away from the skin in the active muscles, or to increase blood perfusion to the working muscle by enhancing peripheral working muscle O2 extraction.5,6 This may be attributed to the increase in O2 pulse, lowering of the heart rate, and the endurance time of the EXP after heat acclimation.
Throughout the study, our subjects were encouraged to drink fluid during the incremental leg cycle exercise in the hot and thermoneutral conditions and were encouraged to drink three 450 mL bottles fluid following each training session and to continue drinking three to four 450 mL bottles fluid throughout the day. It is possible that these athletes may have induced mild degrees of dehydration because of ad libitum (ie, not required) water intake. If a severe dehydration had occurred, due to incomplete fluid replacement from previous training sessions, the exercise time to exhaustion would have been affected.1,5,26 This could be the case for the CON at post-test measurement because sweat loss of the CON was reduced by approximately 43% (P < 0.05) in the thermoneutral conditions and 23% (P > 0.05) in hot conditions. Thus, the subjects’ hydration status may have influenced the skin blood flow outcome measurements.
The present study was novel in that we focused on the training of the lower limbs to separate training effects on SkBF in the active and nonactive limbs, with and without heat exposure. This study was unique with regard to the short-term (five daily sessions) training protocol and the exercise intensities at or above the individual’s ventilation threshold with heat exposure and that can be generalized to all athletic groups. Together, the present study makes a contribution to the body of knowledge in the field of sports medicine, particularly given the dearth of studies investigating this population.
Summary and conclusion
The purpose of this study was to examine whether short-term, ie, five daily sessions of vigorous dynamic cycling exercise and heat exposure could achieve heat acclimation in trained athletes and the effect of cutaneous blood flow in the active region of the skeletal muscle during all-out leg cycle exercise in the heat-acclimated athletes. The significant heat-acclimated outcome measurements during incremental leg cycling exercise in high ambient environment are: (1) 56%–100% reduction in cutaneous blood flow to the active limbs during leg cycling exercise; (2) 28% drop in cutaneous blood flow in nonactive limb at peak work rate; (3) 5%–10% reduction in exercise heart rate; (4) 10% increase in maximal O2 pulse; and (5) 6.6% increase in endurance time to exhaustion. Based on these findings, we conclude that: (1) heat acclimation can be achieved with five sessions of dynamic cycling exercise in the heat in trained athletes; and (2) reduction of cutaneous blood flow in the exercising limb, lower exercise heart rate, and increase oxygen pulse during cycling exercise provided the heat-acclimated athletes the capability to increase their endurance time in the hot environment. Considering the importance of cutaneous circulation in body temperature regulation and blood flow distribution to the working muscle and skin, heat acclimation should be considered advantageous for trained, competitive athletes. Future research may consider using endurance athletes who compete outdoors for long-distance events with a VO2max level >65 mL per kg per minute and using laser Doppler images to estimate regional skin blood perfusion with heat acclimation.
Acknowledgments
The authors wish to thank the coaches for their support and the athletes for their time and effort in participating as study subjects. Many thanks go to the graduate students for their assistance in data collection and to Claire A Liang for manuscript preparation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cheuvront SN, Kenefick RW, Montain SJ, Sawka MN. Mechanisms of aerobic performance impairment with heat stress and dehydration. J Appl Physiol. 2010;109(6):1989–1995. doi: 10.1152/japplphysiol.00367.2010. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzo S, Halliwill JR, Sawka MN, Minson CT. Heat acclimation improves exercise performance. J Appl Physiol. 2010;109(4):1140–1147. doi: 10.1152/japplphysiol.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor NAS, Cotter JD. Heat adaptation: guidelines for the optimization of human performance. Int Sport Med J. 2006;7(2):1–37. [Google Scholar]
- 4.Garrett AT, Rehrer NJ, Patterson MJ. Induction and decay of short-term heat acclimation in moderately and highly trained athletes. Sports Med. 2011;41(9):757–771. doi: 10.2165/11587320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Tucker R, Rauch L, Harley YX, Noakes TD. Impaired exercise performance in the heat is associated with an anticipatory reduction in skeletal muscle recruitment. Pflugers Arch. 2004;448(4):422–430. doi: 10.1007/s00424-004-1267-4. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JM. Physical training and the control of skin blood flow. Med Sci Sports Exerc. 1998;30(3):382–386. doi: 10.1097/00005768-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JM. Exercise in a hot environment: the skin circulation. Scan J Med Sci Sports. 2010;20(Suppl 3):29–39. doi: 10.1111/j.1600-0838.2010.01206.x. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol. 1993;460:467–485. doi: 10.1113/jphysiol.1993.sp019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smolander J, Kolari P, Korhonen O, Ilmarinen R. Skin blood flow during incremental exercise in a thermoneutral and a hot dry environment. Eur J Appl Physiol Occup Physiol. 1987;56(3):273–280. doi: 10.1007/BF00690892. [DOI] [PubMed] [Google Scholar]
- 10.Tripathi A Mack GW, Nadel ER. Cutaneous vascular reflexes during exercise in the heat. Med Sci Sports Exerc. 1990;22(6):796–803. doi: 10.1249/00005768-199012000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Sawka MN, Young AJ. Physiological systems and their responses to conditions of heat and cold. In: Tipton CM, American College of Sports Medicine, editor. ACSM’s Advanced Exercise Physiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 535–563. [Google Scholar]
- 12.Horowitz M. Do cellular heat acclimation responses modulate central thermoregulatory activity? News Physiol Sci. 1998;13:218–225. doi: 10.1152/physiologyonline.1998.13.5.218. [DOI] [PubMed] [Google Scholar]
- 13.Wyndham C. The physiology of exercise under heat stress. Annu Rev Physiol. 1973;35:193–220. doi: 10.1146/annurev.ph.35.030173.001205. [DOI] [PubMed] [Google Scholar]
- 14.Locke M. The cellular stress response to exercise: role of stress proteins. Exerc Sport Sci Rev. 1997;25:105–136. [PubMed] [Google Scholar]
- 15.American College of Sports Medicine . Preparticipation health screening and risk stratification. In: Thompson WR, Gordon NF, Pescatello LS, editors. ACSM’s Guidelines for Exercise Testing and Prescription. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. pp. 18–39. [Google Scholar]
- 16.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2012. Measurements during Integrative Cardiopulmonary Exercise Testing; pp. 82–83. [Google Scholar]
- 17.Johnson JM, Taylor WF, Shepherd AP, Park MK. Laser-Doppler measurement of skin blood flow: comparison with plethysmography. J Appl Physiol. 1984;56(3):798–803. doi: 10.1152/jappl.1984.56.3.798. [DOI] [PubMed] [Google Scholar]
- 18.Liang MT, Su HF, Lee NY. Skin temperature and skin blood flow affect bioelectric impedance study of female fat-free mass. Med Sci Sports Exerc. 2000;32(1):221–227. doi: 10.1097/00005768-200001000-00033. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd AP, Oberg PA, editors. Laser-Doppler Blood Flowmetry (Developments in Cardiovascular Medicine) Hingham, MA: Kluwer Academic Publishers; 1990. [Google Scholar]
- 20.Saumet JL, Kellogg DL, Jr, Taylor WF, Johnson JM. Cutaneous laser-Doppler flowmetry: influence of underlying muscle blood flow. J Appl Physiol. 1988;65(1):478–481. doi: 10.1152/jappl.1988.65.1.478. [DOI] [PubMed] [Google Scholar]
- 21.Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol. 1964;19:531–533. doi: 10.1152/jappl.1964.19.3.531. [DOI] [PubMed] [Google Scholar]
- 22.Hales JRS, Nielsen B, Yanase M. Skin blood flow during severe heat stress: regional variations and failure to maintain maximal levels. In: Milton AS, editor. Temperature Regulation: Advances in Pharmacological Sciences. Switzerland: Birkhauser Verlag Basel; 1994. pp. 195–200. [Google Scholar]
- 23.Smolander J, Saalo J, Korhonen O. Effect of work load on cutaneous vascular response to exercise. J Appl Physiol. 1991;71(4):1614–1619. doi: 10.1152/jappl.1991.71.4.1614. [DOI] [PubMed] [Google Scholar]
- 24.Taylor WF, Johnson JM, O’Leary DS, Park MK. Modification of the cutaneous vascular responses to exercise by local skin temperature. J Appl Physiol. 1984;57(6):1878–1884. doi: 10.1152/jappl.1984.57.6.1878. [DOI] [PubMed] [Google Scholar]
- 25.Stephens DP, Aoki K, Kosiba WA, Johnson JM. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol Heart Circ Physiol. 2001;280(4):H1496–H1504. doi: 10.1152/ajpheart.2001.280.4.H1496. [DOI] [PubMed] [Google Scholar]
- 26.Watt MJ, Garnham AP, Febbraio MA, Hargreaves M. Effect of acute plasma volume expansion on thermoregulation and exercise performance in the heat. Med Sci Sports Exerc. 2000;32(5):958–962. doi: 10.1097/00005768-200005000-00013. [DOI] [PubMed] [Google Scholar]