Abstract
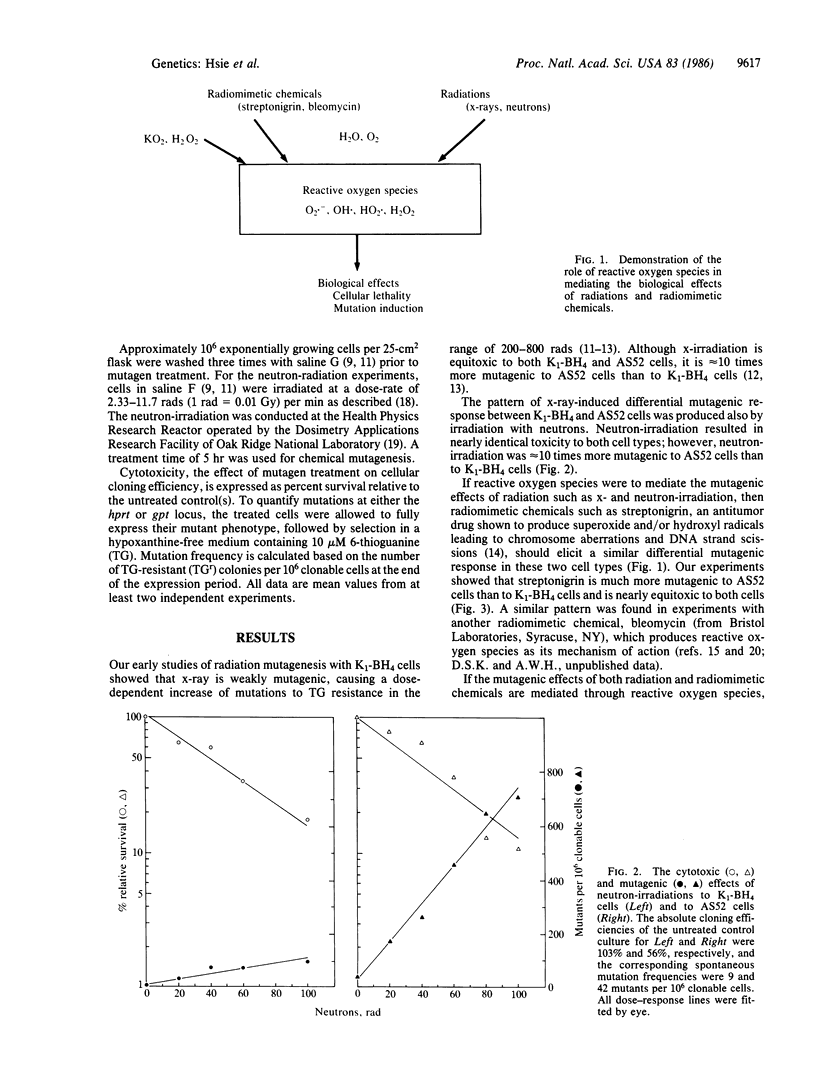
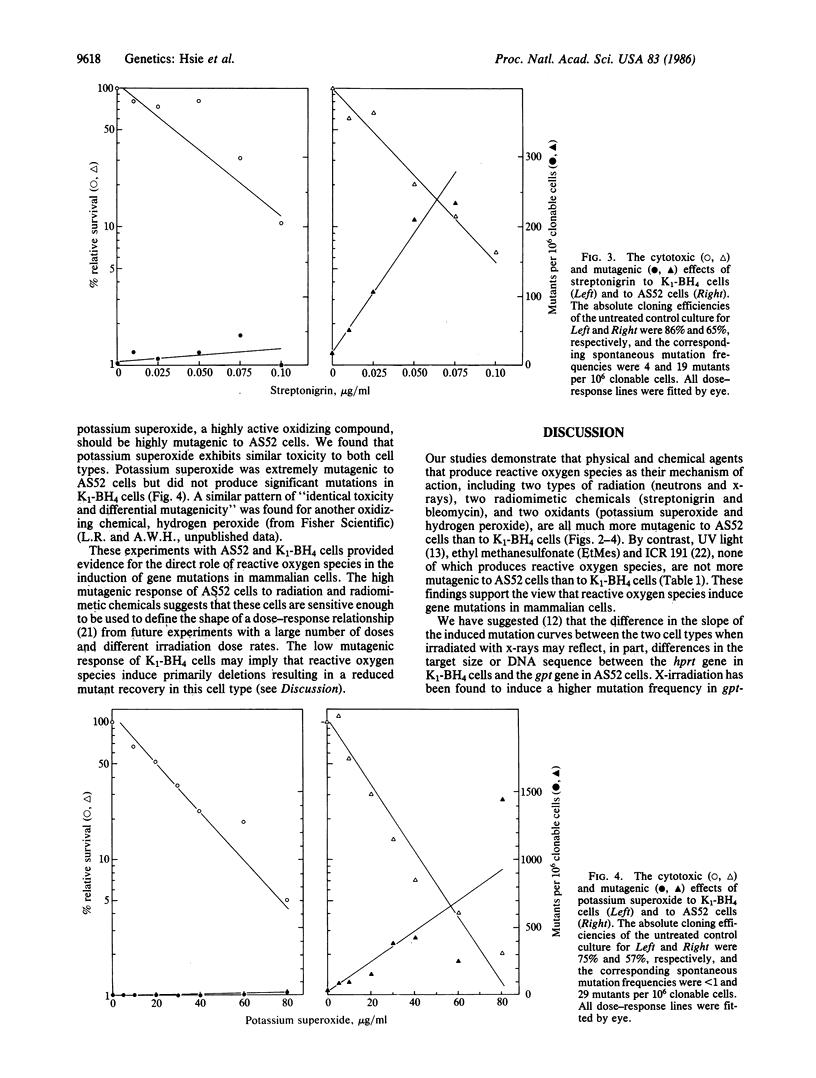
We have studied the mutagenicity (by selecting for mutants resistant to 6-thioguanine) and cytotoxicity (by determining cellular cloning efficiency) of physical and chemical agents in Chinese hamster ovary (CHO) cells, clone CHO-K1-BH4 (K1-BH4), and its radiation-hypersensitive transformant, AS52. AS52 cells contain a single functional copy of a bacterial gene, the xanthine/guanine phosphoribosyltransferase (gpt) gene instead of its mammalian equivalent, the hypoxanthine/guanine phosphoribosyltransferase (hprt) gene. We found that x-ray and neutron irradiations are equally toxic to both cell types; however, these physical agents are approximately equal to 10 times more mutagenic to AS52 cells than to K1-BH4 cells. Our earlier studies using Southern blot analysis showed that x-irradiation produces mostly or exclusively deletion mutations in both cell types. If reactive oxygen species mediate the mutagenic effects of radiations and chemicals, then radiomimetic compounds such as streptonigrin and bleomycin, which exert their biological effects via reactive oxygen species, and oxidizing compounds such as potassium superoxide and hydrogen peroxide should elicit a similar differential mutagenic response in both cell types. On the other hand, agents such as ethyl methanesulfonate, ICR 191, and UV light, which do not produce reactive oxygen species, should not elicit differential mutagenicity. Our results fulfill such predictions. The apparent hypermutability of AS52 cells probably results from a higher recovery of multilocus deletion mutants in AS52 cells than in K1-BH4 cells, rather than a higher yield of induced mutants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Albertini R. J., O'Neill J. P., Nicklas J. A., Heintz N. H., Kelleher P. C. Alterations of the hprt gene in human in vivo-derived 6-thioguanine-resistant T lymphocytes. Nature. 1985 Jul 25;316(6026):369–371. doi: 10.1038/316369a0. [DOI] [PubMed] [Google Scholar]
- Carver J. H., Adair G. M., Wandres D. L. Mutagenicity testing in mammalian cells. II. Validation of multiple drug-resistance markers having practical application for screening potential mutagens. Mutat Res. 1980 Sep;72(2):207–230. doi: 10.1016/0027-5107(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Clive D., McCuen R., Spector J. F., Piper C., Mavournin K. H. Specific gene mutations in L5178Y cells in culture. Mutat Res. 1983 Jun;115(2):225–251. doi: 10.1016/0165-1110(83)90005-2. [DOI] [PubMed] [Google Scholar]
- Cunningham M. L., Lokesh B. R. Superoxide anion generated by potassium superoxide is cytotoxic and mutagenic to chinese hamster ovary cells. Mutat Res. 1983 Sep;121(3-4):299–304. doi: 10.1016/0165-7992(83)90218-x. [DOI] [PubMed] [Google Scholar]
- Deaven L. L., Petersen D. F. The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma. 1973;41(2):129–144. doi: 10.1007/BF00319690. [DOI] [PubMed] [Google Scholar]
- Dormandy T. L. An approach to free radicals. Lancet. 1983 Oct 29;2(8357):1010–1014. doi: 10.1016/s0140-6736(83)90989-3. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Brimer P. A., Mitchell T. J., Gosslee D. G. The dose-response relationship for ethyl methanesulfonate-induced mutations at the hypoxanthine-guanine phosphoribosyl transferase locus in Chinese hamster ovary cells. Somatic Cell Genet. 1975 Jul;1(3):247–261. doi: 10.1007/BF01538449. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Schenley R. L. Utilization of Chinese hamster cells in vitro and in vivo in genetic toxicology: a multiphasic approach. Environ Mutagen. 1983;5(5):733–744. doi: 10.1002/em.2860050511. [DOI] [PubMed] [Google Scholar]
- Kappus H., Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981 Dec 15;37(12):1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Levin D. E., Hollstein M., Christman M. F., Schwiers E. A., Ames B. N. A new Salmonella tester strain (TA102) with A X T base pairs at the site of mutation detects oxidative mutagens. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7445–7449. doi: 10.1073/pnas.79.23.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H. J. ARTIFICIAL TRANSMUTATION OF THE GENE. Science. 1927 Jul 22;66(1699):84–87. doi: 10.1126/science.66.1699.84. [DOI] [PubMed] [Google Scholar]
- O'Neill J. P., Couch D. B., Machanoff R., San Sebastian J. R., Brimer P. A., Hsie A. W. A quantitative assay of mutation induction at the hypoxanthine-guanine phosphoribosyl transferase locus in Chinese hamster ovary cells (CHO/HGPRT system): utilization with a variety of mutagenic agents. Mutat Res. 1977 Oct;45(1):103–109. doi: 10.1016/0027-5107(77)90048-3. [DOI] [PubMed] [Google Scholar]
- Sims C. S., Patterson G. R., Jr Reference dosimetry for the health physics research reactor. Health Phys. 1983 Nov;45(5):1003–1005. [PubMed] [Google Scholar]
- Stankowski L. F., Jr, Hsie A. W. Quantitative and molecular analyses of radiation-induced mutation in AS52 cells. Radiat Res. 1986 Jan;105(1):37–48. [PubMed] [Google Scholar]
- Stankowski L. F., Jr, Tindall K. R., Hsie A. W. Quantitative and molecular analyses of ethyl methanesulfonate- and ICR 191-induced mutation in AS52 cells. Mutat Res. 1986 Apr;160(2):133–147. doi: 10.1016/0027-5107(86)90037-0. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Flude E., Garner C. M., Campbell J. B., Edwards M. J. Effects of the DNA strand-cleaving antitumor agent, streptonigrin, on ataxia telangiectasia cells. Cancer Res. 1983 Jun;43(6):2700–2703. [PubMed] [Google Scholar]
- Thacker J., Debenham P. G., Stretch A., Webb M. B. The use of a cloned bacterial gene to study mutation in mammalian cells. Mutat Res. 1983 Sep;111(1):9–23. doi: 10.1016/0027-5107(83)90003-9. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Stankowski L. F., Jr, Machanoff R., Hsie A. W. Analyses of mutation in pSV2gpt-transformed CHO cells. Mutat Res. 1986 Apr;160(2):121–131. doi: 10.1016/0027-5107(86)90036-9. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Stankowski L. F., Jr, Machanoff R., Hsie A. W. Detection of deletion mutations in pSV2gpt-transformed cells. Mol Cell Biol. 1984 Jul;4(7):1411–1415. doi: 10.1128/mcb.4.7.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. R., Morley A. A., Haliandros M., Kutlaca R., Sanderson B. J. In vivo somatic mutations in human lymphocytes frequently result from major gene alterations. Nature. 1985 May 23;315(6017):343–345. doi: 10.1038/315343a0. [DOI] [PubMed] [Google Scholar]
- Umezawa H. Chemistry and mechanism of action of bleomycin. Fed Proc. 1974 Nov;33(11):2296–2302. [PubMed] [Google Scholar]
- WATSON J. D., CRICK F. H. Genetical implications of the structure of deoxyribonucleic acid. Nature. 1953 May 30;171(4361):964–967. doi: 10.1038/171964b0. [DOI] [PubMed] [Google Scholar]



