Abstract
Idiosyncratic drug-induced liver injury (IDILI) continues to be a significant human health problem. IDILI is characterized as occurring in a minority of individuals exposed to a drug, yet it accounts for as much as 17% of all cases of acute liver failure. Despite these concerns, the mechanisms underlying IDILI remain unknown. Trovafloxacin (TVX), which causes IDILI in humans, also causes hepatocellular death in vitro when combined with tumor necrosis factor-alpha (TNF) treatment. However, the molecular mechanisms involved in this toxicity are not fully characterized. The purpose of this study was to identify mechanisms by which TVX and TNF interact to cause hepatocellular death, with a focus on a human hepatocyte cell line. TVX and TNF interacted to cause cytotoxicity in HepG2 cells at drug concentrations similar to those in people undergoing TVX therapy. TVX/TNF treatment caused apoptosis and DNA damage in HepG2 cells that depended on caspase activation. Prolonged activation of JNK occurred in TVX/TNF-induced cytotoxicity, and treatment with the JNK selective inhibitor SP600125 attenuated cytotoxicity. TVX/TNF cotreatment also caused cytotoxicity in isolated primary murine hepatocytes that was dependent on caspase activation. These results increase understanding of molecular signaling pathways involved in hepatocellular death caused by a drug with idiosyncratic liability in the presence of TNF.
Key Words: idiosyncratic drug-induced liver injury, hepatotoxicity, caspase, JNK, trovafloxacin.
Drug-induced liver injury is the leading cause of acute liver failure in the United States (Ostapowicz et al., 2002) as well as a common adverse effect preventing regulatory approval for new drugs. In addition, it is the primary cause of postmarketing actions resulting in warnings or withdrawal of drugs from the marketplace (Watkins, 2005). Idiosyncratic drug-induced liver injury (IDILI) usually occurs in a small minority of individuals exposed to a drug, yet these reactions account for 13%–17% of all acute liver failure cases (Hussaini and Farrington, 2007). Although current preclinical and clinical safety testing prevents many hepatotoxic drug candidates from entering the market, the ability to detect drugs capable of causing IDILI is still inadequate (Watkins, 2005).
Despite the prevalence of IDILI and the problems that it causes, mechanisms underlying the hepatotoxicity remain unknown. One hypothesis is that a modest inflammatory stress can render the liver sensitive to an otherwise nontoxic dose of a drug (Ganey et al., 2004). It has been demonstrated that administration of lipopolysaccharide (LPS) to generate a modest inflammatory stress precipitates liver injury in animals coexposed to drugs with IDILI liability. This phenomenon has been demonstrated in animal models using chlorpromazine, diclofenac, halothane, doxorubicin, amiodarone, trovafloxacin (TVX), and sulindac (Buchweitz et al., 2002; Deng et al., 2006; Dugan et al., 2010; Hassan et al., 2008; Lu et al., 2012; Shaw et al., 2007; Zou et al., 2009).
TVX is a fluoroquinolone antibiotic. Between February of 1998 and May of 1999, approximately 2.5 million prescriptions were written for TVX, and 140 adverse hepatic events were reported (Dembry et al., 1999), an incidence rate of 5.6 per 100 000 prescriptions written. These included 14 cases of acute liver failure, 6 of which were fatal (Nightingale, 1999). TVX is not hepatotoxic in mice but becomes so when combined with a modest inflammatory stress from LPS (Shaw et al., 2007). Histological examination of TVX/LPS-induced liver injury identified both apoptotic as well as oncotic necrosis, and the pathogenesis of the TVX-LPS-induced liver injury in mice depended on the proinflammatory cytokine tumor necrosis factor-α (TNF). Interference with TNF activity by treatment with etanercept or genetic knockout of TNF receptors (TNFRs) protected mice from TVX-LPS-induced liver injury, signifying the importance of TNF signaling in the generation of hepatotoxicity (Shaw et al., 2007, 2009c). Though TNF is able to induce apoptosis in many cell types, hepatocytes are largely resistant to its killing effects in the absence of a sensitizing event or factor (Leist et al., 1994). Ligation of TNFR1 on hepatocytes can activate several apoptotic factors, including caspase enzymes and JNK (Ding and Yin, 2004).
Exposure of cultured hepatocytes of human, rat, and murine lineage to cytokines in the presence of TVX and other drugs with IDILI potential caused cytotoxicity (Cosgrove et al., 2009; Shaw et al., 2009a). However, the intracellular signaling events in these models have not been elucidated fully. Accordingly, the aim of this study was to identify key signaling events in TVX/TNF-induced cytotoxicity. The hepatoblastoma HepG2 cell line was chosen for these studies because this cell is currently used in preclinical testing and is of human lineage. TVX also causes a number of similar changes in gene expression in both HepG2 cells and primary human hepatocytes (Liguori et al., 2008). Identification of critical signaling events could lead to development of more effective preclinical screening protocols to identify drugs with idiosyncratic liability.
MATERIALS AND METHODS
Materials.
Unless otherwise noted, all materials were purchased from Sigma-Aldrich (St Louis, Missouri). TVX was synthesized by Cayman Chemical (Ann Arbor, Michigan). Recombinant truncated form of murine TNF, recombinant human TNF, z-VAD-FMK, z-IETD-FMK, z-LEHD-FMK, z-DEVD-FMK, and caspase fluorometric assay kits were purchased from R&D Systems (Minneapolis, Minnesota). Antibiotic-antimycotic (ABAM), 0.25% trypsin-EDTA, PBS, L-glutamine, Dulbecco’s Modified Eagle’s Medium (DMEM), and Williams’ Medium E were purchased from Life Technologies (Carlsbad, California). SP600125 and lactate dehydrogenase (LDH) were purchased from Calbiochem (San Diego, California). Cell Staining Buffer, Annexin V Binding Buffer, Alexa Fluor 647 conjugated Annexin V (AnnV), and propidium iodide (PI) were all purchased from Biolegend (San Diego, California). Alanine aminotransferase (ALT) reagent was purchased from ThermoScientific (Pittsburgh, Pennsylvania).
Animals.
Mice received humane care, and the Michigan State University Committee on Animal Use and Care approved all procedures. Nine-week-old male C57Bl/6J mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Prior to use, mice were allowed 1 week to acclimate in a 12-h light/dark cycle. They had continual access to bottled spring water and were fed a standard chow (8640 Teklad 22/5 Rodent Diet, Harlan Laboratories, Madison, Wisconsin) ad libitum.
Cell culture.
HepG2 human hepatoblastoma cells (American Type Culture Collection, Manassas, Virginia) were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% ABAM in 25-cm2 tissue culture flasks. Primary murine hepatocytes were isolated as described by Klaunig et al. (1981) and modified by Bajt et al. (2004). Briefly, hepatocytes were isolated using a 2-step collagenase perfusion method. Isolated hepatocytes with a viability of 85% or greater, as determined by trypan blue exclusion, were plated and supplemented with Williams’ Medium E containing 10% FBS, 1% ABAM, 2mM L-glutamine, and 100nM insulin and allowed 3h to adhere before treatment. Both cell types were cultured at 37°C in a humidified atmosphere composed of 95% air and 5% CO2. Confluent HepG2 cell cultures were detached from the flask with 0.25% trypsin-EDTA and allowed to adhere in culture plates for at least 7h before treatment.
HepG2 cytotoxicity assessment.
HepG2 cells were plated at 4×104 cells per well in white-walled 96-well tissue culture plates. For treatment, TVX and levofloxacin (LVX) were reconstituted to stock solutions of 200mM and 500mM, respectively, in dimethyl sulfoxide (DMSO), which resulted in a maximal final concentration of 0.01% DMSO in treated wells. Vehicle controls for TVX or LVX are represented as “Veh” throughout. LVX was used as a negative comparator drug for TVX in this model because it is in the same pharmacological class as TVX but has far less propensity to cause IDILI in humans or liver injury in mice cotreated with LPS (Shaw et al., 2007). TNF was reconstituted to a stock solution of 100 μg/ml in PBS. Cytotoxicity was measured using the CytoTox-Glo Cytotoxicity Assay from Promega (Madison, Wisconsin). After initial concentration-response studies, a combination treatment of 20μM TVX and 4ng/ml TNF was chosen for subsequent experiments. For the caspase inhibitor studies, the peptide-based caspase inhibitors were reconstituted in DMSO, and cells were exposed to 40μM of each inhibitor or 0.2% DMSO vehicle. For studies using the JNK inhibitor SP600125, cytotoxicity was assessed by trypan blue exclusion. This was done because SP600125 interfered with the CytoTox-Glo Cytotoxicity Assay.
Cytotoxicity time course study.
LDH activity was measured as described (Vanderlinde, 1985). Briefly, HepG2 cells were plated at 2.5×105 cells per well in 12-well tissue culture plates. Cells were treated, and 40 μl of culture medium was collected every 4h for 24h starting at the time of treatment and kept at 4°C until LDH activity was measured. LDH enzyme (Calbiochem) was used to create a standard curve of enzyme activity, and LDH activity was measured in the collected supernatants at each time spectrophotometrically.
Flow cytometry.
Cells were plated at 5×105 cells per well in 12-well tissue culture plates. After 24h of exposure, cells and supernatant were collected in 12×75mm round bottomed tubes (BD Biosciences, San Jose, California) on ice. Cells were pelleted by centrifugation at 4°C for 5min at 70 × g. Culture medium was aspirated before cells were washed with cold Cell Staining Buffer. After the cells were pelleted again and Cell Staining Buffer was removed, they were resuspended in 200 μl Annexin V Binding Buffer with 10 μl each of AnnV and PI. They were incubated for 15min at room temperature before an additional 300 μl Annexin V Binding Buffer was added to each sample. Cells were then analyzed using a BD FACS Canto II flow cytometer. All data were analyzed using Kaluza software (Beckman Coulter, Brea, California). Gating parameters were determined using Veh/PBS-treated cells as a negative control. Unstained cells were run to account for auto fluorescence.
Terminal deoxynucleotidyl transferase dUTP nick end labeling assay.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) labeling was conducted using the In Situ Cell Death Detection Kit from Roche Diagnostics Corporation (Indianapolis, Indiana). Cells were plated in 8-chamber culture slides (BD Falcon) at 9×104 cells per chamber. After 24h of treatment, the cells were processed following the manufacturer’s instructions. An antifade mounting medium containing 4′,6-diamidino-2-phenylindole was applied (Vector Laboratories, Burlingame, California). Slides were imaged using an Olympus IX71 inverted fluorescence microscope and appropriate filters. Images were taken with an Olympus F-View II digital monochrome camera and were processed using Image J software.
Caspase activity assay.
Caspase activity was determined using fluorometric caspase activity assay kits (R&D Systems). Briefly, cells were plated at 1.2×106 cells per well in 6-well tissue culture plates. Cells were treated for 8, 16, or 24h before collection. They were lysed, and lysates were centrifuged. Fifty microliters of supernatant from each sample was added to black-walled 96-well plates, along with reaction buffer and fluorogenic substrate and incubated at 37°C for 1h. After incubation, the plate was read on a fluorescent microplate reader with filters for excitation at 400nm and emission at 505nm.
Protein isolation.
HepG2 cells were plated at 1.2×106 cells per well in 6-well tissue culture plates. They were treated for 1 or 8h before being washed with ice-cold PBS and treated with radioimmunoprecipitation assay (RIPA) buffer containing HALT protease and phosphatase inhibitors (ThermoScientific). Cells were scraped, collected in tubes, and kept on ice. After incubating in RIPA buffer for 10min, each sample was sonicated with one 5-s pulse. Lysates were centrifuged at 20 000 × g for 20min, and supernatants containing whole cell extract were collected. Protein concentration was determined using the bicinchoninic acid assay (ThermoScientific).
Western blot analysis.
For phospho-JNK (p-JNK) and Lamin B1 (Lamin) detection, 15 μg of protein were loaded and separated on NuPAGE 12% Bis-Tris gels (Life Technologies) by electrophoresis. Proteins were transferred onto PVDF membranes (Millipore, Billerica, Massachusetts), which were then blocked for 1h with 5% bovine serum albumin (BSA) in Tris-buffered saline containing 0.1% Tween 20 (TBST). Membranes were then probed with p-JNK or Lamin primary antibodies (Cell Signaling Technology, Beverly, Massachusetts). Antibodies were diluted in 5% BSA in TBST to 1:1000 for p-JNK and 1:10 000 for Lamin. Membranes were incubated with primary antibodies at 4°C for at least 18h. PVDF membranes were then washed with TBST and probed with goat anti-rabbit horseradish peroxidase (HRP)–conjugated secondary antibody (Sana Cruz Biotechnology, Santa Cruz, California). Secondary antibodies were diluted in 5% BSA in TBST to 1:2500 for p-JNK and 1:10 000 for Lamin. HRP was visualized using Clarity Western ECL Substrate (Bio-Rad, Hercules, California) and developed on HyBlot CL Film (Denville Scientific, Metuchen, New Jersey). Densitometry was performed on the developed films using Image J software.
Primary murine hepatocyte studies.
After isolation, hepatocytes were plated at 1.25×105 cells per well in 24-well, collagen-coated tissue culture plates. After attachment, the cells were washed twice with warm PBS and treated with serum-free Williams’ Medium E containing 2mM glutamine and 1% ABAM. At 24h, medium and cell lysates were collected and analyzed for ALT activity as described previously (Luyendyk et al., 2005).
Statistical analysis.
Results are expressed as mean ± SEM. Percentile data were subjected to arcsine transformation. Analysis of data was performed using 1-way or 2-way ANOVA followed by pairwise multiple comparisons using the Holm Sidak or Tukey’s method when appropriate. Nonparametric data were analyzed using Kruskal-Wallis test followed by pairwise multiple comparisons using Tukey’s or Dunn’s method when appropriate. The criterion for statistical significance was p < .05.
RESULTS
Concentration Response and Time Course of TVX/TNF-Induced Cytotoxicity
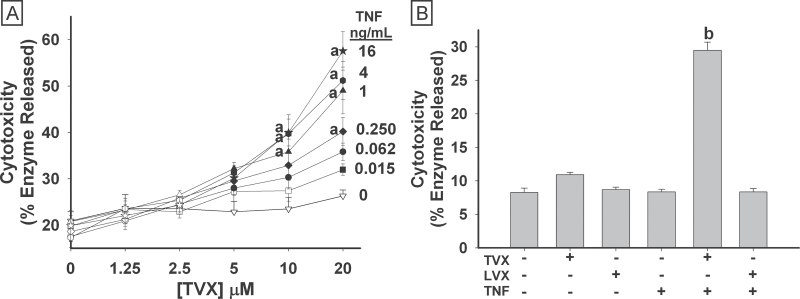
HepG2 cells were treated with TVX (1.25–20μM), TNF (0.015–16ng/ml), or their vehicles simultaneously for 24h before cytotoxicity was measured. TVX alone (0ng/ml TNF) did not cause significant cytotoxicity at any concentration. Similarly, TNF alone (0μM TVX) was not cytotoxic. In TVX/TNF-treated cells, synergistic cytotoxicity was observed (Fig. 1A). Cytotoxic interactions occurred with concentra-tions as small as 5μM TVX and 62.5 pg/ml TNF. LVX, another fluoroquinolone antibiotic with a much lower propensity for causing IDILI in humans, was used as a negative comparator drug for TVX in this model. Clinically, LVX is prescribed at a dose that is 2.5-fold greater than the dose of TVX to achieve a similar therapeutic effect (Lubasch et al., 2000). Accordingly, HepG2 cells were treated with a larger concentration of LVX (50μM). LVX treatment alone or in combination with TNF did not cause cytotoxicity in HepG2 cells (Fig. 1B).
Fig. 1.
Concentration response for TVX/TNF-induced cytotoxicity. A, HepG2 cells were treated simultaneously with Veh or TVX (1.25–20μM) and with PBS or TNF (0.015–16ng/ml). Cytotoxicity was measured 24h after treatment. Data represent the mean ± SEM of 4 separate experiments performed in duplicate. B, HepG2 cells were treated with either vehicle, 20μM TVX, or 50μM LVX in the presence of 4ng/ml TNF or PBS. Cytotoxicity was measured 24h after treatment. Data represent the mean ± SEM of 3 separate experiments performed in quadruplicate. Closed symbols (panel A) are significantly different from 0μM TVX at the same concentration of TNF. a, Significantly different from 0ng/ml TNF at the same concentration of TVX. b, Significantly different from all other treatment groups. Abbreviations: LVX, levofloxacin; TNF, tumor necrosis factor-alpha; TVX, trovafloxacin.
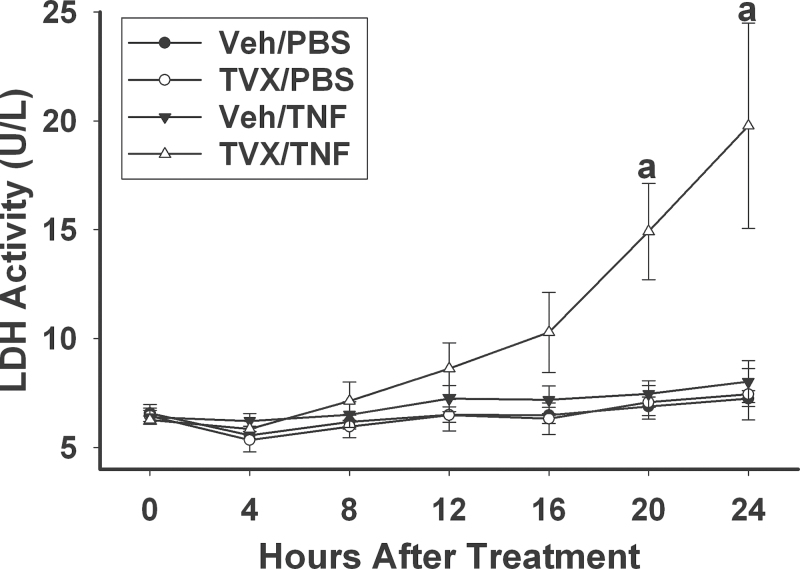
The activity of LDH released into medium was not increased in HepG2 cells treated with Veh/PBS, TVX/PBS, or Veh/TNF at any time up to 24h (Fig. 2). TVX/TNF cytotoxicity appeared to begin at 8h and became statistically significant by 20h.
Fig. 2.
Time course of TVX/TNF-induced cell death. HepG2 cells were treated with 20μM TVX or Veh and with 4ng/ml TNF or PBS. Cell culture supernatant was collected every 4h after treatment, and LDH activity was measured as described in Materials and Methods section. a, Significantly different from other treatment groups at same time point. Data represent the mean ± SEM of 3 separate experiments performed in triplicate. Abbreviations: LDH, lactate dehydrogenase; TNF, tumor necrosis factor-alpha; TVX, trovafloxacin.
Flow Cytometric Analysis of Cell Viability
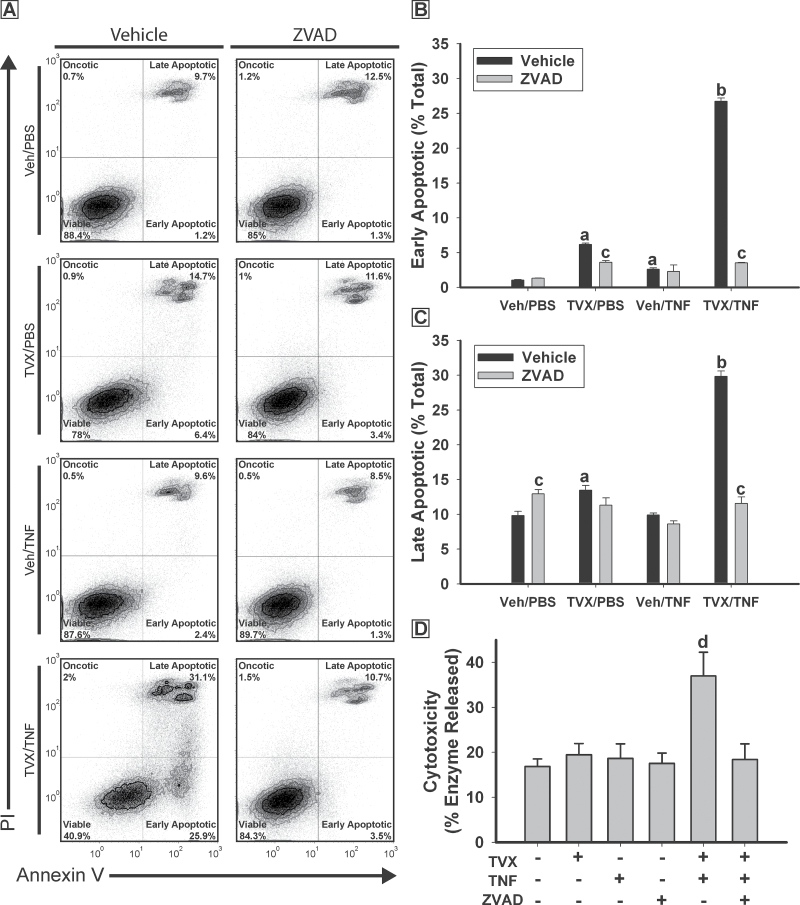
To determine if the cytotoxicity induced by TVX/TNF cotreatment was oncotic or apoptotic, cells were stained with cell-impermeable PI to identify compromised plasma membranes characterized by oncosis and with Annexin V that binds to exposed phosphatidylserine residues on the outer leaflet of the plasma membrane during apoptosis (Vermes et al., 1995). Cells were analyzed using flow cytometry after 24h of exposure (Fig. 3). TVX/PBS and Veh/TNF treatments each caused a small increase in the percentage of early apoptotic (AnnV+/PI−) cells compared with Veh/PBS-treated group (Fig. 3B). TVX/TNF treatment caused a much greater increase in the percentage of early apoptotic cells. Cotreatment with ZVAD resulted in a decrease in the percentage of early apoptotic cells treated with TVX/PBS or TVX/TNF. Treatment of cells with TVX/PBS resulted in an increase in the percentage of late apoptotic (AnnV+/PI+) cells (Fig. 3C), and TVX/TNF treatment caused a more pronounced increase. Treatment with ZVAD decreased the percentage of late apoptotic cells in the TVX/TNF-treated group. Cytotoxicity, measured by enzyme release, was also increased in cells treated with TVX/TNF, an effect that was prevented in the presence of ZVAD (Fig. 3D). A small percentage (less than 2%) of cells appeared to have undergone oncotic necrosis (AnnV−/PI+) and this percentage was similar in all treatment groups (data not shown).
Fig. 3.
TVX/TNF treatment causes caspase-dependent apoptosis. HepG2 cells were treated with 20μM TVX or Veh and with 4ng/ml TNF or PBS, as well as 40μM ZVAD or its vehicle control. After 24h, viability was assessed using flow cytometry and the Cytotox-Glo Cytotoxicity Assay. A, Representative quadrant plots for each treatment. B, Percentage of the total cells gated undergoing early apoptosis. C, Percentage of the total cells gated undergoing late apoptosis. a, Significantly different from Veh/PBS-treated group without ZVAD. b, Significantly different from Veh/PBS-, TVX/PBS-, and Veh/TNF-treated groups without ZVAD. c, Significantly different from same treatment without ZVAD. Data represent the mean ± SEM of 3 separate experiments. One hundred thousand cells were sampled from each experiment. Oncotic necrosis (AnnV−, PI+) represents less than 2% of the total cells gated in any experiment. D, Cytotoxicity results assessed by Cytotox-Glo. d, Significantly different from all other treatment groups. Data represent the mean ± SEM of 4 separate experiments in quadruplicate. Abbreviations: AnnV, Annexin V; PI, propidium iodide; TNF, tumor necrosis factor-alpha; TVX, trovafloxacin.
TUNEL Staining
DNA strand breakage was examined using the TUNEL assay. Compared with Veh/PBS-treated cells, treatment with TVX/PBS or Veh/TNF did not increase TUNEL staining (Figs. 4A and 4B). TVX/TNF treatment increased TUNEL staining compared with all other treatment groups.
Fig. 4.
TVX/TNF-induced DNA damage. HepG2 cells were treated with 20μM TVX or Veh and with 4ng/ml TNF or PBS. Twenty-four hours after treatment cells were subjected to TUNEL labeling as described in Materials and Methods section and imaged using fluorescence microscopy. A, Representative images from each treatment. Gray areas represent DAPI signal. Black areas represent colocalized FITC and DAPI signal indicating TUNEL-positive signal located in nuclei. B, Quantification of the area of TUNEL signal colocalizing with DAPI in response to treatment. a, Significantly different from all other treatment groups. Data represent the mean ± SEM of 3 separate experiments and 3–4 images from each treatment group. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate; TNF, tumor necrosis factor-alpha; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; TVX, trovafloxacin.
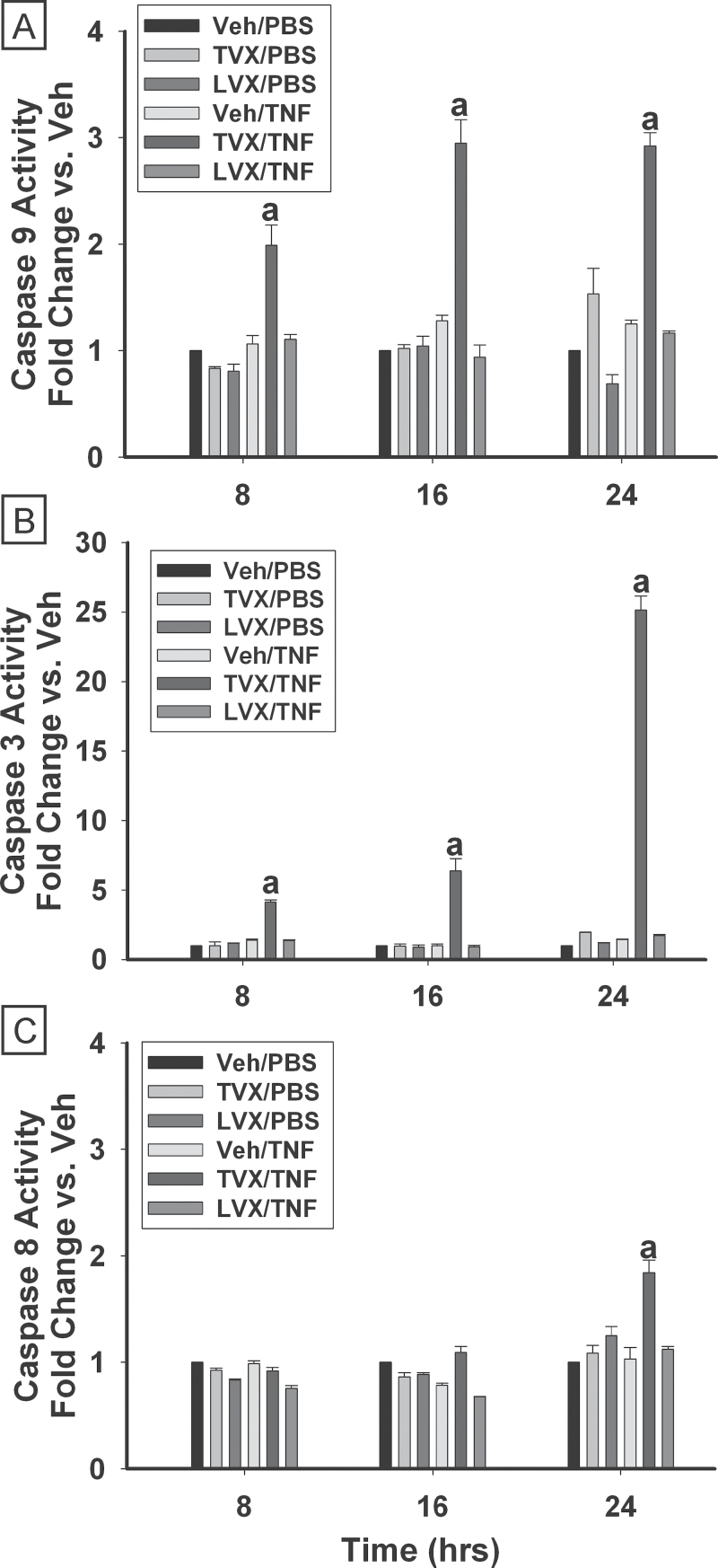
Caspase Activity Time Course and Inhibition
The activities of caspases 8, 9, and 3 were measured 8, 16, and 24h after treatment. Treatment with TVX/PBS or Veh/TNF did not cause significant changes in the activities of any of the caspases at any time examined (Fig. 5). In cells treated with TVX/TNF, the activity of caspase 9 was increased 2-fold 8h after treatment (Fig. 5A) and 3-fold by 16h after treatment. Caspase 9 activity remained increased 24h after TVX/TNF treatment. Caspase 3 activity was increased 4-fold compared with Veh/PBS-treated cells 8h after treatment with TVX/TNF and increased with time, reaching 25-fold after 24h (Fig. 5B). TVX/TNF treatment caused a significant increase (1.8-fold) in activity of caspase 8 at 24h (Fig. 5C). At no time did LVX/PBS or LVX/TNF increase activity of any of the caspases.
Fig. 5.
Time course of caspase activation. HepG2 cells were treated with 20μM TVX, 50μM LVX, or their vehicle control in the presence of 4ng/ml TNF or PBS. Activities of caspase 9 (A), caspase 3 (B), and caspase 8 (C) are represented relative to activities measured in Veh/PBS-treated groups. Caspase activities were measured 8, 16, and 24h after treatment as described in Materials and Methods section. a, Significantly different from all other treatment groups within the same time. Data represent the mean ± SEM of 3 separate experiments run in triplicate. Abbreviations: LVX, levofloxacin; TNF, tumor necrosis factor-alpha; TVX, trovafloxacin.
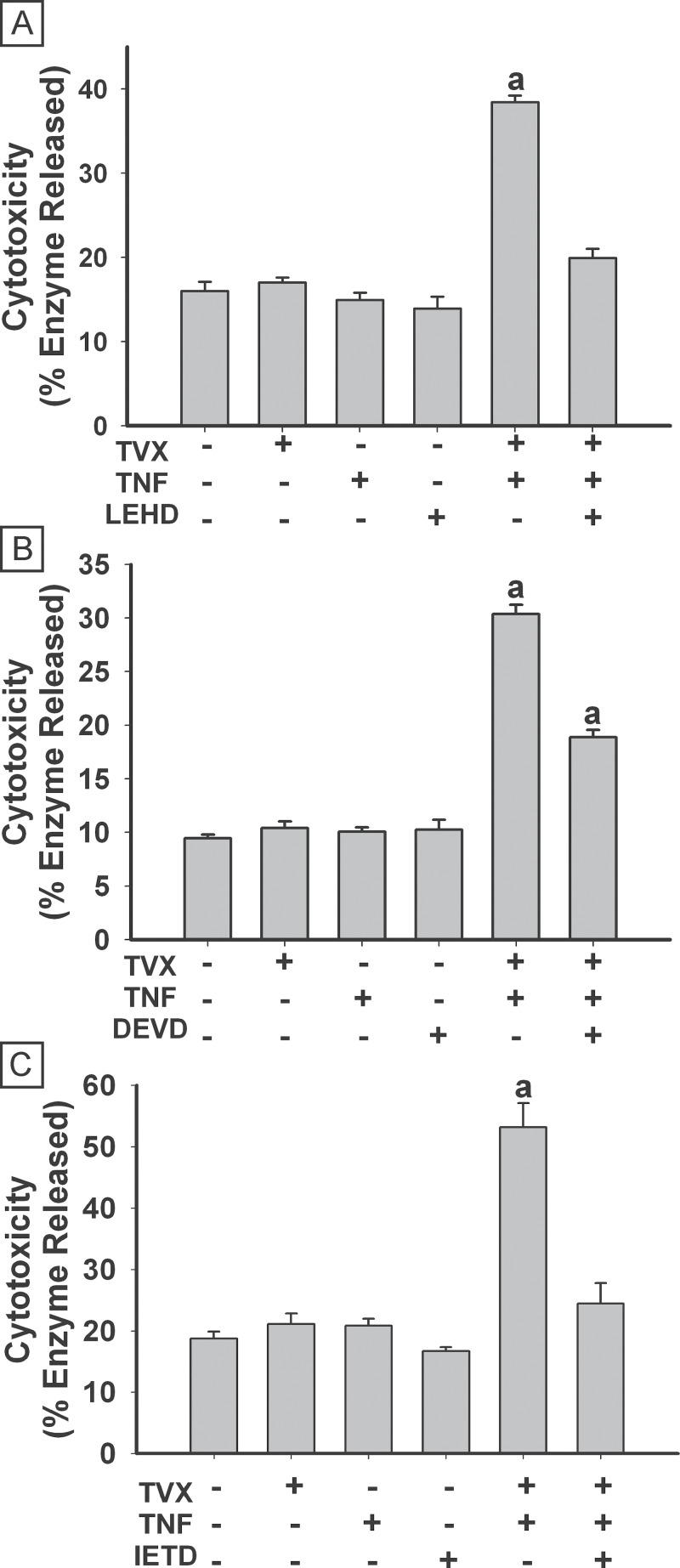
The effects of selective caspase inhibitors on TVX/TNF-induced cytotoxicity were evaluated (Fig. 6). In all studies, TVX/TNF treatment significantly increased cytotoxicity. Treatment with the caspase inhibitors themselves did not cause any cytotoxicity. Treatment with either LEHD (caspase 9 inhibitor) or IETD (caspase 8 inhibitor) abolished TVX/TNF-induced cytotoxicity (Figs. 6A and 6C), whereas DEVD (caspase 3/7 inhibitor) attenuated TVX/TNF-induced cytotoxicity (Fig. 6B).
Fig. 6.
Caspase inhibition protects against TVX/TNF-induced cytotoxicity. HepG2 cells were treated simultaneously with 20μM TVX or vehicle and with 4ng/ml TNF or PBS. At the same time some cells also received 40μM of z-LEHD-fmk to inhibit caspase 9 (A), z-DEVD-fmk to inhibit caspase 3 (B), or z-IETD-fmk to inhibit caspase 8 (C). Cytotoxicity was measured 24h after treatment. a, Significantly different from all other treatment groups. In panel (B), bars labeled (a) are also different from one another. Data represent the mean ± SEM of 4–5 separate experiments. Abbreviations: TNF, tumor necrosis factor-alpha; TVX, trovafloxacin.
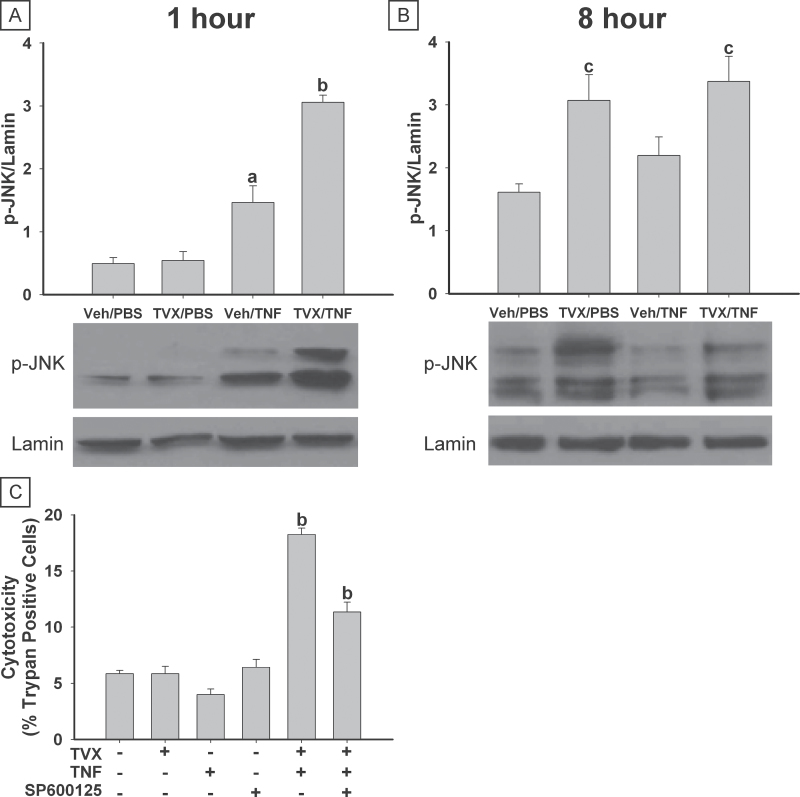
JNK Activation and SP600125 Treatment
TVX/PBS treatment did not affect JNK phosphorylation after 1h (Fig. 7A). Treatment with Veh/TNF led to JNK activation after 1h. A greater increase in this early JNK activation was observed in TVX/TNF-treated cells. After 8h of treatment, Veh/PBS and Veh/TNF had similar levels of phosphorylated JNK (Fig. 7B). Both TVX/PBS and TVX/TNF treatments were associated with increased JNK phosphorylation at this time. Total JNK expression was not affected by any treatment (data not shown).
Fig. 7.
TVX treatment enhances JNK activation. HepG2 cells were treated simultaneously with 20μM TVX or vehicle and with 4ng/ml TNF or PBS for 1 or 8h. Phosphorylation of JNK was determined by Western blot analysis. Representative blots are shown. Densitometry was performed on phospho-JNK (p-JNK) and Lamin bands, and the ratio of p-JNK to Lamin is represented. A, p-JNK 1h after treatment. B, p-JNK 8h after treatment. C, Cells were also treated with 10μM SP600125. Cytotoxicity was assessed 24h after treatment by trypan blue exclusion: A minimum of 300 cells was counted for each treatment group. a, Significantly different from Veh/PBS-treated group. b, Significantly different from all other treatment groups. c, Significantly different from respective group in the absence of TVX. Data represent the mean ± SEM of 4 separate experiments. Abbreviations: TNF, tumor necrosis factor-alpha; TVX, trovafloxacin.
HepG2 cells were treated with SP600125, a selective inhibitor of JNK (Bennett et al., 2001), and cytotoxicity was assessed. Treatment with TVX, TNF, or SP600125 alone did not increase cytotoxicity (Fig. 7C). Cytotoxicity was increased by treatment with TVX/TNF, and addition of SP600125 significantly reduced this effect.
Studies Using Isolated Primary Murine Hepatocytes
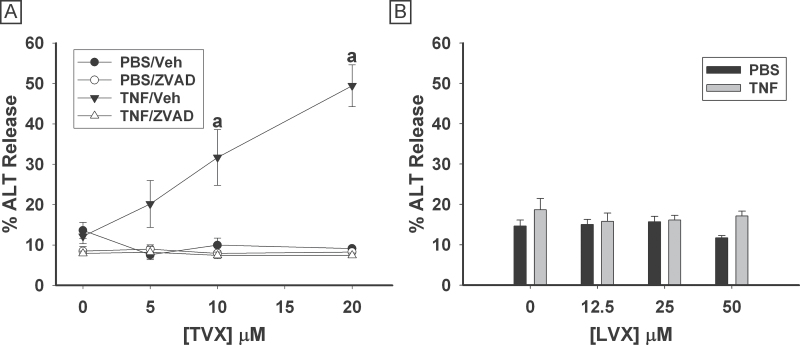
Primary murine hepatocytes were treated simultaneously with TVX (0–20μM) and/or TNF (10ng/ml). ALT activity released into the medium was measured 24h after treatment. Neither TNF nor TVX alone at any concentration tested caused an increase in ALT release (Fig. 8A). Treatment with 10 or 20μM TVX in combination with TNF caused a significant increase in release of ALT compared with all other treatment groups. Treatment of cells with ZVAD decreased the TVX/TNF-induced ALT release. Treatment of primary murine hepatocytes with concentrations of LVX up to 50μM caused no significant increase in release of ALT, regardless of the inclusion of TNF (Fig. 8B).
Fig. 8.
TVX/TNF-induced cytotoxicity of murine hepatocytes is caspase-dependent. A, Primary murine hepatocytes were treated with vehicle or TVX (5–20μM) and with 10ng/ml TNF or PBS, as well as 10μM ZVAD or its vehicle. ALT release was measured 24h after treatment. B, Cells were treated with LVX or Veh at various concentrations and with 10ng/ml TNF or PBS. a, Significantly different from all other treatment groups at same TVX concentration. Data represent the mean ± SEM of hepatocytes isolated from 4 mice run in duplicate. Abbreviations: LVX, levofloxacin; TNF, tumor necrosis factor-alpha; TVX, trovafloxacin.
DISCUSSION
For the initial experiments, the range of TVX concentrations was based on the plasma concentrations of TVX observed in patients treated with the drug, which ranged from 2 to 10μM (Melnik et al., 1998; Teng et al., 1996; Vincent et al., 1997). These concentrations should reflect those to which the human liver might be exposed during TVX therapy. Similarly, the concentrations of TNF (0–16ng/ml) were chosen to mimic what is experienced physiologically in humans during inflammatory stress (Copeland et al., 2005; Taudorf et al., 2007), as well as what was observed in the mouse model of TVX/LPS-induced liver injury (Shaw et al., 2007). Treatment with either TVX or TNF alone at any of the concentrations tested did not cause cytotoxicity in HepG2 cells. Also, treatment with TVX at concentrations smaller than 5μM in combination with TNF at any concentration did not cause statistically significant cytotoxicity (Fig. 1A). However, a combination of TVX at 5 or 10μM interacted with TNF at concentrations as small as 62.5 pg/ml to cause cytotoxicity. Previous studies have demonstrated a cytotoxic interaction between TVX and TNF on HepG2 cells, but those studies used a combined treatment of 450μM TVX and 100ng/ml TNF (Cosgrove et al., 2009). A treatment combination of 20μM TVX and 4ng/ml TNF was chosen for additional study because it (1) caused a robust and reproducible increase in cytotoxicity and (2) represents concentrations of TVX and TNF that are similar to those occurring in vivo. The cytotoxic interaction of TVX and TNF was not limited to the HepG2 transformed human cell line; TVX also interacted with TNF to kill primary murine hepatocytes in a concentration-dependent manner. Unlike TVX, LVX did not interact with TNF to cause cytotoxicity in either cell type; thus, the cytotoxic interaction of these 2 drugs with TNF matched their propensity to cause IDILI in humans. The cell death that occurred from TVX/TNF in HepG2 cells appeared to start about 8–12h after treatment and became significant at 20h.
The livers of mice treated with TVX/LPS displayed histological signs of both apoptotic and oncotic necrosis, and this injury depended on TNF (Shaw et al., 2009b). In a previous study, effector caspase activation occurred in liver after treatment of mice with TVX/TNF and in hepatocytes treated with TVX/TNF in vitro, suggesting that cells died at least partially by apoptosis (Shaw et al., 2009a). Flow cytometric analysis corroborated that observation (Fig. 3). TVX/TNF-treated cells with compromised plasma membranes registered as positive for PI uptake largely costained with Annexin V, suggesting an apoptotic cell death. TVX/TNF treatment also caused an increase in the fraction of cells in the early apoptotic (AnnV+/PI−) population. Interestingly, TVX treatment alone caused a small but significant increase in both the early and late apoptotic population. These findings suggest that TVX stressed cells in a way that did not result in overt cell death after 24h of treatment.
Apoptosis is associated with DNA damage, which can be detected using the TUNEL method (Heatwole, 1999). In this study, significant DNA damage was observed only in cells treated with TVX/TNF. Though not statistically significant, there was a trend toward an increase in DNA damage caused by TVX treatment alone. This suggests that TVX might have caused some modest genomic stress by itself. Taken together, these results demonstrate that TVX/TNF treatment causes HepG2 cell apoptosis.
Hepatocellular apoptosis is largely regulated by activation of caspase enzymes and can be either extrinsic or intrinsic (Malhi and Gores, 2008). Treatment with the pancaspase inhibitor ZVAD resulted in fewer early apoptotic cells after both TVX and TVX/TNF treatment, and it also decreased late apoptotic cells as well as cytotoxicity after TVX/TNF treatment. Similarly, the cytotoxic interaction of TVX and TNF to kill primary murine hepatocytes was completely abrogated when caspases were inhibited with ZVAD. These results suggest that caspase activation was critically involved in the TVX/TNF-induced apoptosis. Further investigation revealed that caspases 9 and 3 were significantly activated starting 8h after TVX/TNF treatment, a time corresponding to the onset of cell death. Caspase 8 was also significantly activated, but not until 24h after treatment and to a lesser extent than caspases 9 and 3. The latency in caspase 8 activation suggests that it might not play a role in the initiation of TVX/TNF-induced cell death. Taken together, these results suggest that TVX/TNF treatment initiated an intrinsic pathway to apoptosis.
To demonstrate the role that these activated caspases play in TVX/TNF-induced apoptosis, selective inhibitors of individual capases were used. Treatment with IETD or LEHD, inhibitors of caspases 8 and 9, respectively, resulted in complete protection. The effectiveness of IETD was surprising, given that caspase 8 activation was detected only after the onset of cell death (Fig. 5C). However, recent findings indicate that caspase inhibitors are less selective for individual caspases than previously reported (Pereira and Song, 2008). Treatment with DEVD to inhibit caspases 3 and 7 attenuated the cytotoxicity caused by TVX/TNF, but did not fully prevent it. Attempts to inhibit cytotoxicity further by increasing the concentration of DEVD resulted in DEVD-induced cytotoxicity. It is possible that the degree of caspase 3 activation, which was much greater than the activation of initiator caspases 8 or 9, was too extensive to be fully inhibited by DEVD. Nevertheless, these findings identify caspase activation as being critical to TVX/TNF-induced apoptosis, and the latency in caspase 8 activation suggests that apoptosis was intrinsically mediated.
Another signaling pathway involved in hepatocellular apoptosis involves JNK (Ding and Yin, 2004). JNK can be activated in the liver by many cellular stresses, including cytokines such as TNF (Seki et al., 2012). If cellular stress is short lived, JNK activation is typically transient and does not result in cell death. Prolonged activation of JNK is associated with cell death signaling in hepatocytes and can occur during oxidative or genotoxic stress (Kobayashi and Tsukamoto, 2001; Seok et al., 2008; Win et al., 2011; Wullaert et al., 2006). TVX by itself did not cause early (1h) JNK activation, but the early activation of JNK by TNF was enhanced by TVX coexposure. At a later time when the degree of activated JNK was similar in TNF-treated cells and vehicle controls (8h), TVX caused more JNK activation by itself, independent of TNF. Thus, because of the early enhancement of JNK activation by TVX, cells exposed to both TVX and TNF experienced JNK activation that was prolonged. Treatment of cells with SP600125 to inhibit JNK attenuated TVX/TNF-induced cytotoxicity. These results indicate that prolonged JNK activation is important in promoting cytotoxicity from TVX/TNF coexposure.
In summary, TVX and TNF interact to cause hepatocellular toxicity in a concentration-dependent manner in both HepG2 human hepatoblastoma cells and cultured primary murine hepatocytes at drug concentrations that are near what is measured in humans undergoing TVX therapy. Cotreated hepatocytes undergo an apoptotic mode of cell death that requires active caspases. LVX is not associated with causing IDILI in humans, and treatment of hepatocytes with LVX, in the presence or absence of TNF, does not cause hepatocellular toxicity. TVX alone significantly increases the percentage of early apoptotic cells, and in the presence of TNF, it prolongs JNK activation that is required for cytotoxicity. Apoptotic cells were identified in vivo in the mouse model of TVX/LPS- or TVX/TNF-induced liver injury. However, oncotic necrosis was also observed (Shaw et al., 2007, 2009a). Identifying the signaling mechanisms involved in hepatocellular toxicity due to TVX/TNF could help the understanding of IDILI pathogenesis and the development of more effective preclinical screening tools to prevent drugs with idiosyncratic liability from reaching the market.
FUNDING
National Institutes of Health (RO1DK061315).
ACKNOWLEDGMENTS
We thank Ryan Albee for technical assistance. The authors have no conflicts of interest to disclose.
REFERENCES
- Bajt M. L., Knight T. R., Lemasters J. J., Jaeschke H. (2004). Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: Protection by N-acetyl cysteine. Toxicol. Sci. 80, 343–349 [DOI] [PubMed] [Google Scholar]
- Bennett B. L., Sasaki D. T., Murray B. W., O’Leary E. C., Sakata S. T., Xu W., Leisten J. C., Motiwala A., Pierce S., Satoh Y., et al. (2001). SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. U.S.A. 98, 13681–13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchweitz J. P., Ganey P. E., Bursian S. J., Roth R. A. (2002). Underlying endotoxemia augments toxic responses to chlorpromazine: Is there a relationship to drug idiosyncrasy? J. Pharmacol. Exp. Ther. 300, 460–467 [DOI] [PubMed] [Google Scholar]
- Copeland S., Warren H. S., Lowry S. F., Calvano S. E., Remick D. (2005). Acute inflammatory response to endotoxin in mice and humans. Clin. Diagn. Lab. Immunol. 12, 60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove B. D., King B. M., Hasan M. A., Alexopoulos L. G., Farazi P. A., Hendriks B. S., Griffith L. G., Sorger P. K., Tidor B., Xu J. J., et al. (2009). Synergistic drug-cytokine induction of hepatocellular death as an in vitro approach for the study of inflammation-associated idiosyncratic drug hepatotoxicity. Toxicol. Appl. Pharmacol. 237, 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembry L. M., Farrington J. M., Andriole V. T. (1999). Fluoroquinolone antibiotics: Adverse effects and safety profiles. Infect. Dis. Clin. Pract. 8, 421–428 [Google Scholar]
- Deng X., Stachlewitz R. F., Liguori M. J., Blomme E. A., Waring J. F., Luyendyk J. P., Maddox J. F., Ganey P. E., Roth R. A. (2006). Modest inflammation enhances diclofenac hepatotoxicity in rats: Role of neutrophils and bacterial translocation. J. Pharmacol. Exp. Ther. 319, 1191–1199 [DOI] [PubMed] [Google Scholar]
- Ding W. X., Yin X. M. (2004). Dissection of the multiple mechanisms of TNF-alpha-induced apoptosis in liver injury. J. Cell. Mol. Med. 8, 445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan C. M., MacDonald A. E., Roth R. A., Ganey P. E. (2010). A mouse model of severe halothane hepatitis based on human risk factors. J. Pharmacol. Exp. Ther. 333, 364–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganey P. E., Luyendyk J. P., Maddox J. F., Roth R. A. (2004). Adverse hepatic drug reactions: Inflammatory episodes as consequence and contributor. Chem. Biol. Interact. 150, 35–51 [DOI] [PubMed] [Google Scholar]
- Hassan F., Morikawa A., Islam S., Tumurkhuu G., Dagvadorj J., Koide N., Naiki Y., Mori I., Yoshida T., Yokochi T. (2008). Lipopolysaccharide augments the in vivo lethal action of doxorubicin against mice via hepatic damage. Clin. Exp. Immunol. 151, 334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatwole V. M. (1999). TUNEL assay for apoptotic cells. Methods Mol. Biol. 115, 141–148 [DOI] [PubMed] [Google Scholar]
- Hussaini S. H., Farrington E. A. (2007). Idiosyncratic drug-induced liver injury: An overview. Expert Opin. Drug Saf. 6, 673–684 [DOI] [PubMed] [Google Scholar]
- Klaunig J. E., Goldblatt P. J., Hinton D. E., Lipsky M. M., Chacko J., Trump B. F. (1981). Mouse liver cell culture. I. Hepatocyte isolation. In Vitro 17, 913–925 [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Tsukamoto I. (2001). Prolonged Jun N-terminal kinase (JNK) activation and the upregulation of p53 and p21(WAF1/CIP1) preceded apoptosis in hepatocytes after partial hepatectomy and cisplatin. Biochim. Biophys. Acta 1537, 79–88 [DOI] [PubMed] [Google Scholar]
- Leist M., Gantner F., Bohlinger I., Germann P. G., Tiegs G., Wendel A. (1994). Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J. Immunol. 153, 1778–1788 [PubMed] [Google Scholar]
- Liguori M. J., Blomme E. A., Waring J. F. (2008). Trovafloxacin-induced gene expression changes in liver-derived in vitro systems: Comparison of primary human hepatocytes to HepG2 cells. Drug Metab. Dispos. 36, 223–233 [DOI] [PubMed] [Google Scholar]
- Lu J., Jones A. D., Harkema J. R., Roth R. A., Ganey P. E. (2012). Amiodarone exposure during modest inflammation induces idiosyncrasy-like liver injury in rats: Role of tumor necrosis factor-alpha. Toxicol. Sci. 125, 126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubasch A., Keller I., Borner K., Koeppe P., Lode H. (2000). Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob. Agents Chemother. 44, 2600–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyendyk J. P., Shaw P. J., Green C. D., Maddox J. F., Ganey P. E., Roth R. A. (2005). Coagulation-mediated hypoxia and neutrophil-dependent hepatic injury in rats given lipopolysaccharide and ranitidine. J. Pharmacol. Exp. Ther. 314, 1023–1031 [DOI] [PubMed] [Google Scholar]
- Malhi H., Gores G. J. (2008). Cellular and molecular mechanisms of liver injury. Gastroenterology 134, 1641–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnik G., Schwesinger W. H., Teng R., Dogolo L. C., Vincent J. (1998). Hepatobiliary elimination of trovafloxacin and metabolites following single oral doses in healthy volunteers. Eur. J. Clin. Microbiol. Infect. Dis. 17, 424–426 [DOI] [PubMed] [Google Scholar]
- Nightingale S. L. (1999). From the Food and Drug Administration. JAMA 282, 19. [DOI] [PubMed] [Google Scholar]
- Ostapowicz G., Fontana R. J., Schiødt F. V., Larson A., Davern T. J., Han S. H., McCashland T. M., Shakil A. O., Hay J. E., Hynan L., et al. (2002). Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 137, 947–954 [DOI] [PubMed] [Google Scholar]
- Pereira N. A., Song Z. (2008). Some commonly used caspase substrates and inhibitors lack the specificity required to monitor individual caspase activity. Biochem. Biophys. Res. Commun. 377, 873–877 [DOI] [PubMed] [Google Scholar]
- Seki E., Brenner D. A., Karin M. (2012). A liver full of JNK: Signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 143, 307–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok J. H., Park K. A., Byun H. S., Won M., Shin S., Choi B. L., Lee H., Kim Y. R., Hong J. H., Park J., et al. (2008). Long-term activation of c-Jun N-terminal kinase through receptor interacting protein is associated with DNA damage-induced cell death. Korean J. Physiol. Pharmacol. 12, 185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. J., Beggs K. M., Sparkenbaugh E. M., Dugan C. M., Ganey P. E., Roth R. A. (2009a). Trovafloxacin enhances TNF-induced inflammatory stress and cell death signaling and reduces TNF clearance in a murine model of idiosyncratic hepatotoxicity. Toxicol. Sci. 111, 288–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. J., Fullerton A. M., Scott M. A., Ganey P. E., Roth R. A. (2009b). The role of the hemostatic system in murine liver injury induced by coexposure to lipopolysaccharide and trovafloxacin, a drug with idiosyncratic liability. Toxicol. Appl. Pharmacol. 236, 293–300 [DOI] [PubMed] [Google Scholar]
- Shaw P. J., Ganey P. E., Roth R. A. (2009c). Tumor necrosis factor alpha is a proximal mediator of synergistic hepatotoxicity from trovafloxacin/lipopolysaccharide coexposure. J. Pharmacol. Exp. Ther. 328, 62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. J., Hopfensperger M. J., Ganey P. E., Roth R. A. (2007). Lipopolysaccharide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol. Sci. 100, 259–266 [DOI] [PubMed] [Google Scholar]
- Taudorf S., Krabbe K. S., Berg R. M., Pedersen B. K., Møller K. (2007). Human models of low-grade inflammation: Bolus versus continuous infusion of endotoxin. Clin. Vaccine Immunol. 14, 250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng R., Liston T. E., Harris S. C. (1996). Multiple-dose pharmacokinetics and safety of trovafloxacin in healthy volunteers. J. Antimicrob. Chemother. 37, 955–963 [DOI] [PubMed] [Google Scholar]
- Vanderlinde R. E. (1985). Measurement of total lactate dehydrogenase activity. Ann. Clin. Lab. Sci. 15, 13–31 [PubMed] [Google Scholar]
- Vermes I., Haanen C., Steffens-Nakken H., Reutelingsperger C. (1995). A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 184, 39–51 [DOI] [PubMed] [Google Scholar]
- Vincent J., Venitz J., Teng R., Baris B. A., Willavize S. A., Polzer R. J., Friedman H. L. (1997). Pharmacokinetics and safety of trovafloxacin in healthy male volunteers following administration of single intravenous doses of the prodrug, alatrofloxacin. J. Antimicrob. Chemother. 39(Suppl. B), 75–80 [DOI] [PubMed] [Google Scholar]
- Watkins P. B. (2005). Idiosyncratic liver injury: Challenges and approaches. Toxicol. Pathol. 33, 1–5 [DOI] [PubMed] [Google Scholar]
- Win S., Than T. A., Han D., Petrovic L. M., Kaplowitz N. (2011). c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J. Biol. Chem. 286, 35071–35078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullaert A., Heyninck K., Beyaert R. (2006). Mechanisms of crosstalk between TNF-induced NF-kappaB and JNK activation in hepatocytes. Biochem. Pharmacol. 72, 1090–1101 [DOI] [PubMed] [Google Scholar]
- Zou W., Devi S. S., Sparkenbaugh E., Younis H. S., Roth R. A., Ganey P. E. (2009). Hepatotoxic interaction of sulindac with lipopolysaccharide: Role of the hemostatic system. Toxicol. Sci. 108, 184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]