Abstract
Recent efforts to update cumulative risk assessment procedures to incorporate nonchemical stressors ranging from physical to psychosocial reflect increased interest in consideration of the totality of variables affecting human health and the growing desire to develop community-based risk assessment methods. A key roadblock is the uncertainty as to how nonchemical stressors behave in relationship to chemical stressors. Physical stressors offer a reasonable starting place for measuring the effects of nonchemical stressors and their modulation of chemical effects (and vice versa), as they clearly differ from chemical stressors; and “doses” of many physical stressors are more easily quantifiable than those of psychosocial stressors. There is a commonly held belief that virtually nothing is known about the impact of nonchemical stressors on chemically mediated toxicity or the joint impact of coexposure to chemical and nonchemical stressors. Although this is generally true, there are several instances where a substantial body of evidence exists. A workshop titled “Cumulative Risk: Toxicity and Interactions of Physical and Chemical Stressors” held at the 2013 Society of Toxicology Annual Meeting provided a forum for discussion of research addressing the toxicity of physical stressors and what is known about their interactions with chemical stressors, both in terms of exposure and effects. Physical stressors including sunlight, heat, radiation, infectious disease, and noise were discussed in reference to identifying pathways of interaction with chemical stressors, data gaps, and suggestions for future incorporation into cumulative risk assessments.
Key Words: cumulative risk, nonchemical stressors, joint action, radiation, infectious disease, sunlight, temperature, noise.
Although the need for assessing cumulative risk from chemical and nonchemical stressors has long been acknowledged, cumulative risk assessment approaches have mostly been applied narrowly to groups of closely related chemicals (Sarigiannis and Hansen, 2012; Sexton, 2012). A key roadblock to advancement is uncertainty as to how nonchemical stressors behave in relationship to chemical stressors. A simplifying assumption is that nonchemical stressors act in the same manner as chemicals and can therefore be incorporated into current risk assessment paradigms. However, evidence is required to support this assumption.
Nonchemical stressors encompass a diverse set of variables including physical (eg, noise, temperature, and disease) and psychosocial stressors. Physical stressors offer a reasonable starting place for measuring the effects of nonchemical stressors and their interactions with chemical effects, as they clearly differ from chemical stressors and often have widespread and quantifiable exposures. A 2013 Society of Toxicology workshop session titled “Cumulative Risk: Toxicity and Interactions of Physical and Chemical Stressors” reviewed 5 examples of physical stressors: Sunlight, heat, radiation, infectious disease, and noise. Topics included the effects of these stressors on chemical exposure, known interactions between physical and chemical stressors, important knowledge gaps, and consideration of incorporating physical stressors into cumulative risk assessments.
SUNLIGHT ENHANCEMENT OF THE TOXICITY OF AIR POLLUTANT MIXTURES
There is clear evidence that sunlight modifies both exposure to chemicals and the toxicity of chemicals present in the air. Atmospheric chemistry alters the concentration and composition of air pollutant mixtures of primary emissions as they age (Jeffries, 1995; Liu et al., 1999). Sunlight and temperature can significantly drive photochemical reactions of air pollutants, producing ozone (from hydrocarbons and nitrogen oxides), nitrogen dioxide (from oxidation of nitrogen oxide), carbon monoxide (from oxidation of hydrocarbons), and many well-known toxic compounds such as formaldehyde, acetaldehyde, acrolein, and other carbonyl- and nitrate-containing products (from oxidation of hydrocarbons and nitrogen oxides) while reducing the concentrations of the primary emitted pollutants (Finlayson-Pitts and Pitts, 1999). These reactions contribute also to the formation of secondary organic aerosols (SOA) and modify the composition of existing aerosols or particulate matter (PM). Not only do sunlight and temperature affect atmospheric reactivity speed, temperature, and relative humidity but also determine which semivolatile secondary products are likely to partition onto existing particles and contribute to SOA yield (Zhang et al., 2011).
Even within the criteria pollutants, atmospheric transformation processes affect their relative composition and resulting cumulative health effects. There is great interest in how atmospheric transformations affect air pollution mixtures and PM composition and the resulting variations in toxicological risk from exposure to modified PM and associated copollutants. For example, these issues are part of the ongoing effort at the USEPA to develop policy and control strategies as part of the multipollutant program (http://www.gpo.gov/fdsys/pkg/FR-2011-01-27/html/2011-1773.htm; http://www.gpo.gov/fdsys/pkg/FR-2012-05-04/html/2012-10805.htm).
Smog chambers can be used to prepare repeatable, controlled mixtures of simple to increasing complexity for a range of primary pollutants and to study photochemical atmospheric transformation with natural sunlight or simulated sunlight as well as a range of temperatures and humidity. They allow for the study of sunlight and temperature effects without interference from meteorology and variable new emissions that occur in the atmosphere. Smog chambers can be interfaced with direct exposure to in vitro or in vivo models for toxicity studies including direct air-to-tissue (air-liquid interface) inhalation exposures (Lichtveld et al., 2012). This method does not require precollection and recovery of toxic agents using filters and liquids, which is a significant advantage over other methods as these steps can alter and reduce toxicological responses (Lichtveld et al., 2012). Photochemical experiments have been conducted in smog chambers with industrial compounds and nitrogen oxide mixtures and complex mixtures of motor vehicle exhaust in urban atmospheres, often demonstrating enhanced toxicity as measured by markers of inflammation and other biological endpoints such as cytotoxicity (Doyle et al., 2007). Additional experiments have been conducted with mixtures of the observed secondary products to confirm the causality of the observed effects.
An interpretation of the mechanism and mode of action of air pollution can be improved by consideration of modifying and varying pollutant sources, component composition and concentrations, sunlight exposure conditions, and including additional toxicological analyses. For example, the same initial primary pollutant mixture designed to represent the average volatile organic compounds (VOCs) observed in 40U.S. cities will have different ozone concentrations, copollutants, and toxicity after 1 day of “aging” depending on the cloud cover and resulting sunlight intensity and temperature (ie, more sunlight and higher temperatures transform more primary pollutants into toxic secondary products) (Sexton et al., 2004). This type of study has been used to develop air quality chemical models to predict ozone concentration (Sexton et al., 1988). As toxic secondary products are identified using biological models and endpoints of interest, those secondary products with the greatest toxicity can be included in similar air quality simulation models for multipollutant risk assessments.
Measures of cytotoxicity and inflammation have been used to investigate the effects of sunlight and temperature on atmospheric transformations of air pollutants (Lichtveld et al., 2012; Sexton et al., 2004). The choice of biological models and endpoints is almost unlimited. Novel genomic analyses of cells exposed to an urban-like mixture showed transcriptional changes on a subset of genes, increasing the number of genes with altered expressions from 19 for the unirradiated mixture to 709 after a 1-day sunlight irradiation (Rager et al., 2011). This example illustrates how potential health outcomes from exposures to complex mixtures generated by sunlight-induced atmospheric chemistry can be explained and potentially quantified, which is especially promising if such in-field toxicology is teamed with future epidemiology studies.
Both sunlight and temperature can affect the toxicity of air pollutants by transforming pollutants to other pollutants, altering exposure profiles and potentially enhancing toxicity. Fortunately, both physical stressors can be quantified (Jeffries et al., 1989), and air quality simulation models can estimate the exposure concentrations and distribution in an area (Vizuete et al., 2010). The model results can be used to estimate total exposure if the population distribution is known; health impact assessments can be calculated, including excess deaths and morbidity (Li et al., 2010). For ambient exposures, researchers and risk assessors should consider studies that include mixtures resulting from natural atmospheric photochemical reactivity driven by sunlight and temperature, the resulting transformation of air components, and the enhancement of toxic effects of air pollutants and their products. Not considering such effects could result in misinterpreting the mode of action and underestimating the potential risk of exposure to air pollution mixtures or its sources.
EXACERBATION OF TOXICITY OF AIR POLLUTANTS AND PESTICIDES BY THERMAL STRESS
Global warming necessitates consideration of heat stress and its incorporation into cumulative risk assessments (Peng et al., 2011). Increased body temperatures have the potential to alter absorption, clearance, and toxicity of chemicals in humans. The following discussion addresses the use of rodent models in assessment of potential interactions between heat and chemical stressors. The internal body or core temperature of mammals and birds is maintained relatively constant over a wide range of ambient temperatures through the activation of autonomic and behavioral motor systems. Motor responses, termed thermoeffectors, maintain a balance between the organism’s heat production and heat loss to the environment. A stable core temperature with a circadian modulation of approximately 1°C is maintained over most of the organisms’ life span and is altered under conditions of work and exercise, exposure to relatively hot or cold environments, or during fever. Environmental toxicants also affect core temperature through modulation of central nervous system (CNS) regulatory mechanisms and/or direct modulation of thermoeffector systems (ie, skin blood flow, sweating, and skeletal muscle thermogenesis). Because a toxicant-induced change in body temperature can indirectly influence the activity of essentially all physiological systems, it is important to understand how toxicants affect the thermoregulatory system.
Acute Hypothermic Responses
Mice and rats exposed acutely to most chemical toxicants generally exhibit an abrupt reduction in core temperature that persists for many hours (Gordon, 2005). Organophosphate (OP) pesticides, heavy metals, solvents, and air pollutants such as ozone will lead to marked hypothermia in mice and rats—a response directly dependent on the prevailing ambient temperature. Most studies are performed at standard room temperature of 22°C, which is 6°C–9°C below the thermoneutral zone of rats and mice, respectively (Gordon, 1993). Within the thermoneutral zone, temperature regulation is achieved by modulation of skin blood flow while metabolic rate is maintained at basal levels. At temperatures below thermoneutrality, metabolic rate must be elevated to maintain a balance between heat production and heat loss. Because the depth of the hypothermic response worsened with cooler temperatures, it was assumed to be a forced thermoregulatory response meaning that the toxicant led to dysfunction of thermoeffectors for heat production and possible heat loss. However, mice and rats that are allowed to behaviorally thermoregulate show a marked preference for cooler ambient temperatures during the hypothermic phase (Gordon, 2005; Watanabe and Suzuki, 1986). Hence, the toxicants appear to activate CNS thermoregulatory centers leading to a regulated reduction in core temperature.
Hypothermia generally ameliorates the toxic effects of drugs and environmental chemicals (Gordon, 2005). A regulated hypothermic response following exposure to a toxicant could thus be viewed as an adaptive response that minimizes toxicity. Doull (1972) noted that “temperature is directly correlated with the magnitude and inversely correlated with the duration of a drug response in biological systems.” In other words, hypothermia causes the drug or chemical to remain in the system longer because its metabolism and clearance are slowed; however, its toxicity to biological systems is also reduced, improving likelihood of survival. When heat stressed (ie, exposed at temperatures above the thermoneutral zone), the hypothermic response is blocked and, in most cases, the toxicity of the chemical is exacerbated.
Delayed Febrile Response
The acute hypothermic responses observed in mice and rats generally recover within 24h. The hypothermic response to toxic agents such as ethanol (Gallaher and Egner, 1987), OP insecticides (Gordon et al., 1997, 2012; Gordon and Mack, 2003), and ozone (Gordon, unpublished) is followed by an elevation in daytime core temperature but close to normal nighttime temperature (note that mice and rats are nocturnal). This day-night pattern is typical in rodents subjected to fevers such as seen following administration of pyrogens (Gordon et al., 2012). The overall responses point to a delayed fever by OP toxicants regulated by the CNS thermoregulatory centers. The acute hypothermic responses observed in mice and rats are not observed in humans and other larger species. In fact, a fever is the most common thermoregulatory response in humans exposed to toxicants such as OP insecticides (Gordon, 1994, 2005). Fever is a common clinical observation in patients exposed to OP insecticides, persisting for many days after exposure. Although the rise in core temperature during the fever is not considered dangerous, it nonetheless suggests a unique mechanism of action of some toxicants on the thermoregulatory system.
Translating From Animal to Human
The core temperature of mice, rats, and humans differs by just 1°C–2°C, whereas their rate of whole-body heat production differs by 5- to 10-fold. As body size increases, the surface area:volume ratio decreases, meaning that the rate of heat loss normalized to body weight increases with a reduction in body size (Gordon, 2005). Small endotherms such as mice and rats are considered to be metabolic specialists—relying on a continuous level of metabolic heat production to maintain a constant core temperature. Any small decrement in metabolism will be reflected quickly in a reduction in core temperature. Large mammals such as humans rely more on vasomotor control of heat loss through the skin to thermoregulate. Due to their large thermal inertia, metabolic rate and core temperature of relatively large mammals are generally more stable when challenged with heat or cold stress or when exposed to toxicants.
The toxic response of heat-stressed humans may be exacerbated but for reasons other than what was described for rodents. Heat stress, especially when combined with a work load, results in sweating, peripheral vasodilation, and increased respiration, which are all factors that may exacerbate toxicity. Ventilatory intake of airborne toxicants is predicted to increase and cutaneous absorption of OP’s is enhanced under heat stress, raising the biological dose of the toxicant.
The hypothermic responses manifested in small rodents can be used as an adaptive, protective response to minimize toxicity. Such a hypothermic response is not possible in large mammals. Thus, in the extrapolation of toxicological responses from small experimental test subjects to humans, one must be cognizant of size-dependent hypothermic responses and the impact of ambient temperature.
MODULATION OF X-RAY-MEDIATED TOXICITY BY CHEMICAL EXPOSURE
X-irradiation (x-ray) is known to elicit DNA damage and therefore has the potential to interact with genotoxic chemicals. In assessing the interactions of physical and chemical stressors, targeted molecular studies can inform risk assessment by testing joint action hypotheses based on knowledge of toxicological pathways. 2,5-Hexanedione (HD) and x-ray are 2 testicular toxicants that have distinct cellular targets with differing modes of action. HD is a chemical stressor that disrupts microtubule function within Sertoli cells, whereas x-ray is a physical stressor that results in double strand breaks in the DNA of germ cells (Boekelheide, 2005; Hasegawa et al., 1997). Despite their differing modes of action, exposure to either stressor ultimately results in increased germ cell apoptosis owing to the supportive role that the Sertoli cells have within the testis (Boekelheide, 2005).
High-density microarrays and a detailed bioinformatics analytical approach were used to demonstrate that an initial chemical exposure to HD altered the rat testis to ameliorate the response to a subsequent exposure to x-ray. Adult male rats were exposed to HD (0.33% or 1%) in the drinking water for 18 days followed by x-ray exposure (2Gy or 5Gy). Multiple exposure patterns were used in a 3×3 factorial design that resulted in a total of 9 treatment groups (Campion et al., 2010a). Testis samples were collected after 3h, and gene array analysis was performed. A novel bioinformatic approach was used to summarize the effect of HD across all treatment groups, with the focus on HD modification of x-ray-induced gene alterations (Campion et al., 2010a). Enrichment analysis was used to identify biological pathways where HD modification of gene expression was the greatest. HD exerted a significant influence on genes involved in cell cycle and DNA replication, recombination, and repair. HD also had an antagonistic effect on x-ray-induced alterations of several proapoptotic genes (Fas, BBC3, AEN) while enhancing antiapoptotic genes (IκBα) (Campion et al., 2010a).
In addition to the complexity of the 2 toxicants and their specific cellular targets, germ cell apoptosis that is induced by x-ray exposure is stage specific. The testis not only has several interacting cell types but is highly synchronized in the cell type associations that make up spermatogenesis. The process of rat spermatogenesis has been mapped into 14 different “stages,” with each stage containing unique combinations of cells that can be particularly susceptible to different toxicants (Leblond and Clermont, 1952). To further investigate the specific cell populations and stages in which these critical toxicant-associated gene alterations occur, laser capture microdissection (LCM) samples were collected from the basal compartment of the seminiferous epithelium, enriching for those germ cells most susceptible to x-ray-induced apoptosis (Campion et al., 2010b). Quantitative RT-PCR of the LCM samples confirmed the suppression of apoptosis-inducing genes by HD coexposure, with Fas emerging as an important regulator in the attenuation of x-ray-induced apoptosis following an HD priming exposure (Campion et al., 2010b).
The ability to study LCM-captured cell- and stage-specific populations avoids the diluting effect of other cell types and stages within the testis that are not susceptible to the toxicants. This method can be applied broadly to study the entire apoptosis pathway, rather than just a few selected genes within that pathway. Recently, we have developed a streamlined approach to LCM that allows for the collection of specific RNA from toxicant-sensitive cell populations that can be amplified and examined using apoptosis-specific PCR arrays.
The coexposure attenuation of germ cell apoptosis is the result of an adaptive response to the chemical exposure, causing altered paracrine signaling of the supportive cells in the seminiferous epithelium. These results suggest that toxicity pathway responses determine the outcome of coexposures, whether chemical or physical in nature, and that complex paracrine interactions between cells modulate the extent of injury. In this specific example, a risk assessment based on HD alone would provide a conservative estimate of risk from coexposure to HD and x-ray. The degree to which this example can be extrapolated to other stressors that share similar mechanisms of testicular toxicity requires further study.
EXPOSURE TO TOXICANTS INCREASES SUSCEPTIBILITY TO MICROBIAL DISEASE AND VICE VERSA
Mechanisms underlying interactions between toxicants and infectious disease include the following: (1) chemical-mediated immune suppression resulting in increased infectious disease; (2) alteration of chemical toxicity by immune mediators; (3) chemically enhanced inflammation and immune pathology associated with infection; and (4) enhancement of chemical-induced lesions by infection. These mechanisms, although imperfectly understood, provide a useful framework to further explore the interactions between infectious disease and toxic chemicals with the ultimate goal of improving our understanding of cumulative risk.
A number of chemicals suppress a variety of immune responses in laboratory rodents, and there is some indication that many chemicals affect immune functions in humans (Selgrade, 2010). One well-documented example is decreased alveolar macrophage function following exposure to several air pollutants accompanied by enhanced risk of certain bacterial infections (Selgrade and Gilmour, 2006). Data on ozone indicate that cells from both species respond almost identically as measured by macrophage phagocytic capability. These data suggest that the effects of ozone exposure on murine alveolar macrophage function are predictive of effects on human alveolar macrophage function, and effects of in vitro exposure of macrophages to ozone are predictive of effects that result from in vivo exposure. For purposes of risk assessment, the immune system is considered the target. The uncertainty factor for interspecies variability can be eliminated by applying inhalation dosimetry methods (EPA, 2012) to deal with toxicokinetics; a toxicodynamic factor is not needed because sensitivity is comparable across species. Other examples include developmental exposures to arsenic, polychlorinated biphenyls, and cigarette smoke (Selgrade, 2007), which demonstrate at least a qualitative relationship between immune suppression in rodents and increased risk of disease. In the case of arsenic and PCBs, a quantitative relationship exists between exposure to the chemical and immune suppression in humans. It is difficult to predict how immune suppression will impact the incidence or severity of infection in a human population (ie, risk). Immune competence in a population may be represented as a bell-shaped curve including sensitive individuals (eg, young, old, or immune-compromised people) and robust individuals (eg, healthy workers). Under that bell-shaped curve, the portion of the population at risk of infection depends not only on the level of immune competence, but also increases with the dose and virulence of infectious agents encountered. The bell-shape curve would be expected to shift in a population exposed to immunosuppressive agents, putting a larger portion of population at risk.
Infections can also affect host defenses against toxicants by interfering with metabolic enzymes and transporters. There are numerous examples demonstrating increased chemical and drug toxicity during infections and other diseases that involve inflammation (Morgan et al., 2008). In one of the earliest examples, an influenza epidemic resulted in decreased clearance of theophylline in children taking the drug for asthma. Murine cytomegalovirus infection increased the toxicity of parathion (Selgrade et al., 1984), increased sodium pentobarbital–induced sleeping time, and decreased cytochrome P450 levels in liver microsomes in mice (Catignani et al., 1989). Infection and inflammatory diseases have been shown, in multiple tissues, to downregulate ATP Binding-Cassette (ABC) drug transporters involved in cellular efflux of drugs/xenobiotics (Morgan et al., 2008). Currently, risk assessment deals with such effects by invoking a 10-fold intraspecies uncertainty factor to account for conditions that affect individual susceptibility in a population. However, the pathways involved in the effects of infection/inflammation on chemical toxicity are at least partially understood. Thus, as we begin to use toxicity pathways to characterize complex mixtures, infection and inflammation may simply become part of that complex mixture.
A third possible interaction between infections and toxic chemicals involves exacerbation of infection-induced inflammation and pathology as a result of chemical exposure. This is best illustrated by effects of ozone (Selgrade et al., 1988), ultraviolet radiation (Ryan et al., 2002), TCDD (Head and Lawrence, 2009), and acrolein (Ong et al., 2012) on influenza infection. In all of these exposure conditions, higher mortality rates have been reported in the absence of increased virus titers in the lung or viral dissemination. Thus, although all of these chemicals have immunosuppressive properties, enhanced mortality does not appear to be due to reduced viral clearance as a result of immune suppression. In fact, morbidity and mortality occur very early in infection, too soon for adaptive immunity to be involved, and surviving mice develop protective immunity that prevents subsequent reinfection. Deaths appear to be due to increased inflammatory responses (Head and Lawrence, 2009). Similarities exist between receptors and subsequently triggered signaling pathways for pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), which trigger innate inflammatory responses (Kono and Rock, 2008), the first line of defense against infection and also important responses to tissue injury and repair. A systems approach that examines the integration of these pathways is needed to better describe this phenomenon.
Finally, infection may enhance chemical-induced lesions (eg, p53 mutations, inflammation, and cell proliferation). Such mechanisms might explain the interaction between hepatitis B virus infection and aflatoxin in induction of liver cancer (Kensler et al., 2011). An increased relative risk of hepatocellular carcinoma of 3.4, 7, and 59 was associated with aflatoxin exposure only, hepatitis B only, and both, respectively. The results strongly suggest an interaction between aflatoxin and hepatitis B in the etiology of hepatocellular carcinoma. It is suggested that hepatitis B infection and the resulting chronic inflammation may promote DNA lesions leading to P53 mutations and cell proliferation, contributing to chronic hepatitis and/or cirrhosis and ultimately carcinoma. Similar interactions may exist between other types of liver infections and liver toxicants, which could be elucidated through a systems biology approach. In the meantime, there is sufficient evidence to justify public health interventions to limit exposure to aflatoxin and vaccinate against hepatitis B.
ENHANCEMENT OF NOISE-INDUCED HEALTH EFFECTS BY CHEMICALS
Noise can enhance the toxicity of chemicals both indirectly through a stress hormone pathway and through direct joint action on ear physiology. Hearing loss and respiratory and cardiovascular disease are some of the health outcomes associated with common coexposure scenarios involving noise and chemicals. The noise stress hypothesis is well understood (Babisch et al., 2013). Acute and chronic noise experiments have consistently shown changes in the production of stress hormones. Multiple factors and the incidence of ischemic heart disease were examined in 3950 middle-aged men (Babisch et al., 2003). The participants, who were highly annoyed by noise but free of any chronic disease at the beginning of the follow-up intervention, had significant odds ratios between 1.7 and 3.0 for heart disease. Another emerging area for studies on noise interactions are respiratory tract diseases. Generally urban environments with high noise pollution also have high air pollution. During sleep, noise signals that are associated with danger (ie, truck noise) have the potential to trigger stress reactions even if the noise level is “low.” Studies on the effects of road traffic pollution have often attributed the effects of combined air pollution and noise pollution solely to one or the other pollutant, without recognizing the relative contribution of each or the interaction of both. However, evidence has shown that in areas of high noise pollution there is an increased incidence of asthma and bronchitis, suggesting an interaction between noise and air pollution–induced effects (Ising et al., 2003, 2004). Similarly, skin diseases have been studied in association with traffic air and noise pollution (Ising et al., 2003).
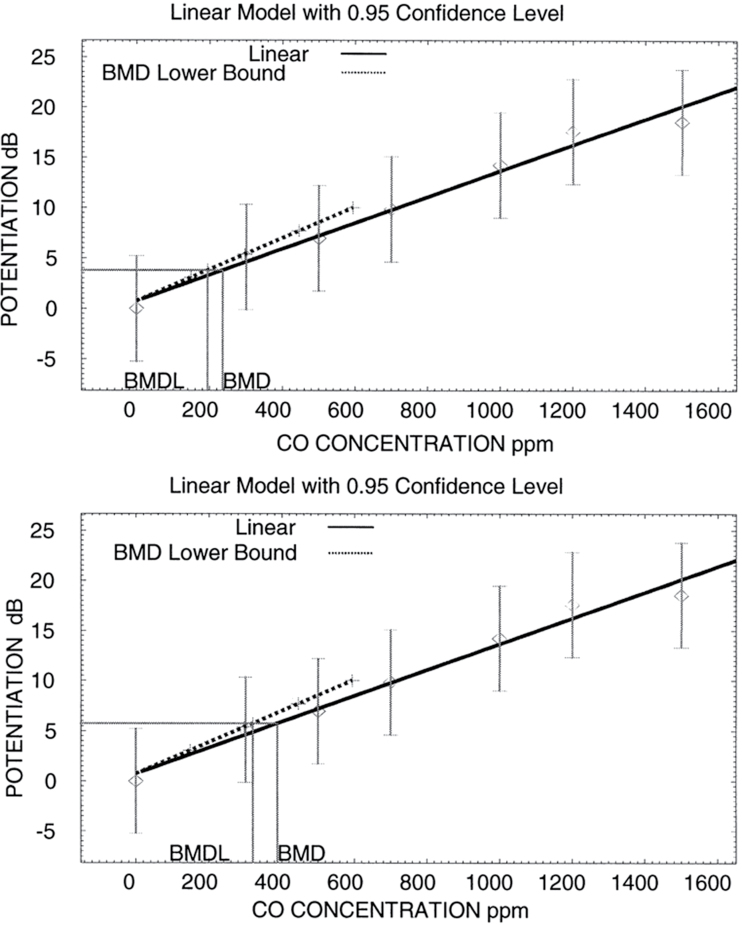
Work is the setting where noise exposures can be highest and most consistent. Noise exposure at work can interact with other factors causing both auditory and nonauditory effects. Chemicals identified as ototoxicants are heavy metals, pesticides, solvents, and asphyxiants, substances with diverse chemical structures, which suggest a number of targets for injury within the auditory system and an array of possible underlying mechanisms (Fechter, 1995; Johnson and Morata, 2010). Metabolic processes involving oxidative stress have been shown to contribute to noise-induced and chemical-induced hearing loss. The generation of reactive oxygen species or free radicals has been associated with cellular injury in different organ systems. Free radicals produce cell damage by macromolecule binding and lipid peroxidation. Free radicals are considered a basic mechanism of toxicity and thought to be part of the mechanism underlying acquired hearing losses. If solvent exposures occur in sufficiently high concentrations, hearing may be affected despite the lack of occupational exposure to noise. In addition, solvents can enhance the effects of noise on hearing, beyond what would be predicted from their individual effects (Johnson and Morata, 2010). Other studies have indicated that carbon monoxide (CO) exposure can potentiate noise-induced hearing loss (Rao and Fechter, 2000). Fechter et al. (2000) did a series of calculations using a benchmark-dose approach for risk assessment analysis (USEPA BMDS version 1.3). Exposures of 195–320 ppm CO would be the lower bound of the benchmark dose that would yield a 10% increase in noise-induced hearing loss or a 5-dB elevation in thresholds (Fig. 1). These levels are less than 1 order of magnitude, which is larger than the CO permissible exposure level in work settings. Moreover, under intermittent noise exposure with long quiet periods, CO exposure can produce unexpectedly large, permanent threshold shifts (Chen and Fechter, 1999; Rao and Fechter, 2000). Surprisingly, the mildest noise duty cycle produced maximal hearing loss when CO was also present. Finally, studies have also demonstrated that as the number of stress factors increase, the lowest-observable-adverse-effects level for hearing loss decreases.
FIG. 1.
Benchmark-dose analysis documenting a linear relationship between carbon monoxide concentration and potentiation of noise-induced hearing loss. The upper panel shows the predicted benchmark dose that would yield an elevation in auditory threshold equivalent to 10% of the effect produced by noise alone and its lower bound. The predicted benchmark concentration is 236 ppm CO, and the lower bound for the benchmark concentration is 195 ppm. The lower panel shows the predicted benchmark dose that would yield a 5-dB elevation in auditory threshold beyond that produced by noise alone and its lower bound. The predicted benchmark concentration is 388 ppm CO, and the lower bound for the benchmark concentration is 320 ppm. Reproduced from Fechter et al., 2000.
Evans et al. (2013) conducted a cumulative risk assessment (CRA) case study characterizing combined exposures to noise and VOCs and examined ways to estimate small-scale, population-specific exposures by extrapolating from large-scale, population-based surveys and integrating data analyzed at different analytical scales (eg, area vs. individual). A quantile regression model of sociodemographic and personal predictors from the 1999–2000U.S. National Health and Nutrition Examination Survey VOC dataset was used along with block group-level sociodemographic and personal variables to estimate VOC exposures. Hazard indices (HIs) for potential hearing impairment due to joint noise and VOC exposures were calculated. The HIs for hearing impairment ranged from 0.8 (10th total VOCs percentile and 45–60 dB) to 1.7 (90th total VOCs percentile and 71–75 dB). Limitations of the exposure and health effects data included issues combining heterogeneous data and a lack of established threshold levels for combined low-level exposures; yet, this case study illustrates that screening-level CRAs, including nonchemical stressors, can be accomplished with publicly available data and existing methods.
Recognizing the risk of occupational chemical exposure on hearing, comprehensive evaluations of ototoxic substances and of the hazards of combined workplace exposure to noise and ototoxic chemical substances have recently been published (EU-OSHA, 2009; Johnson and Morata, 2010). These documents include information on noise/chemical interactions, focused on the qualitative properties of chemicals inducing ototoxic effects, highlight policies from specific countries or multinational agencies, and the possible impact of the 2007 European Commission regulations for the Registration, Evaluation, Authorization and Restriction of Chemical substances (REACH).
CONCLUSIONS
It is clear that a diverse array of physical stressors can modify chemical exposure and effects and should be included in cumulative risk assessments. Currently, the vast majority of data describing the toxicity of physical stressors and interactions between physical and chemical stressors is qualitative and not quantitative. More work is needed to understand the dose-response relationships of physical stressors and to quantify the magnitude of interactions between physical and chemical stressors. An example of such work is NoMiracle, a European Union project that developed new tools to evaluate chemicals in combination with biological or physical factors (Løkke, 2010). Despite the significant knowledge gaps that exist, the approaches described above offer promise for incorporating nonchemical stressors into future cumulative risk assessments.
Key recommendations for cumulative risk assessment emerging from this review of the toxicity and interactions of physical and chemical stressors include the following:
Multiple physical stressors including sunlight, noise, radiation, temperature, and infectious disease can elicit toxicity and/or modify the toxicity of chemical stressors and should be considered in cumulative risk assessments.
Linking chemical and biological analysis of environmental samples with the physical factors affecting the site of collection (eg, low/high pH or temperature of water samples, bright sunlight vs. extensive cloud cover for air samples, ambient noise levels, the infectious microbial load of the soil/water/air) is recommended for assessing physical and chemical interactions across exposure and effect.
A systems-based approach that evaluates disruption of pathways can be used to predict potential interactions between physical and chemical stressors.
Modeling and simulation approaches can be used with existing monitoring data in cumulative risk assessments to incorporate some physical stressors.
In many cases, the direction of the stressor interaction is known. Therefore, estimates (derived using the available data) of the magnitude of the physical stressor-mediated modification of chemical toxicity could be added as modifiers to the cumulative risk assessment process. Additionally, these would provide testable hypotheses for future laboratory and epidemiology studies.
Finally, more risk assessment case studies are required to evaluate the relative contribution of physical stressors to overall cumulative risk calculation.
FUNDING
Superfund Research Program grant (P42ES013660 to K.B.); Training grant (NC; T32ES07272); the Intramural Research Program of the National Institute of Health, National Institute of Environmental Health Sciences.
DISCLOSURES
K.B. has funding from NIEHS, USEPA, and the American Chemistry Council. He is an occasional expert consultant for chemical and pharmaceutical companies and owns stock in CytoSolv, an early stage biotechnology company developing a wound healing therapeutic.
REFERENCES
- Babisch W., Ising H., Gallacher J. E. (2003). Health status as a potential effect modifier of the relation between noise annoyance and incidence of ischaemic heart disease. Occup. Environ. Med. 60, 739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babisch W., Pershagen G., Selander J., Houthuijs D., Breugelmans O., Cadum E., Vigna-Taglianti F., Katsouyanni K., Haralabidis A. S., Dimakopoulou K., et al. (2013). Noise annoyance–a modifier of the association between noise level and cardiovascular health? Sci. Total Environ. 452-453, 50–57 [DOI] [PubMed] [Google Scholar]
- Boekelheide K. (2005). Mechanisms of toxic damage to spermatogenesis. J. Natl. Cancer Inst. Monogr. 34, 6–8 [DOI] [PubMed] [Google Scholar]
- Campion S. N., Houseman E. A., Sandrof M. A., Hensley J. B., Sui Y., Gaido K. W., Wu Z., Boekelheide K. (2010a). Suppression of radiation-induced testicular germ cell apoptosis by 2,5-hexanedione pretreatment. II. Gene array analysis reveals adaptive changes in cell cycle and cell death pathways. Toxicol. Sci. 117, 457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion S. N., Houseman E. A., Sandrof M. A., Hensley J. B., Sui Y., Gaido K. W., Wu Z., Boekelheide K. (2010b). Suppression of radiation-induced testicular germ cell apoptosis by 2,5-hexanedione pretreatment. III. Candidate gene analysis identifies a role for fas in the attenuation of x-ray-induced apoptosis. Toxicol. Sci. 117, 466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catignani J. C., Ménache M. G., Selgrade M. K. (1989). Increased susceptibility to pentobarbital following mouse cytomegalovirus infection: Relative roles of viral-induced interferon and viral infection of the liver. J. Biochem. Toxicol. 4, 221–229 [DOI] [PubMed] [Google Scholar]
- Chen G. D., Fechter L. D. (1999). Potentiation of octave-band noise induced auditory impairment by carbon monoxide. Hear. Res. 132, 149–159 [DOI] [PubMed] [Google Scholar]
- Doull J. (1972). The Effect of Physical Environmental Factors on Drug Response (Hayes W. J., Ed.). Academic Press, New York, NY [Google Scholar]
- Doyle M., Sexton K. G., Jeffries H., Jaspers I. (2007). Atmospheric photochemical transformations enhance 1,3-butadiene-induced inflammatory responses in human epithelial cells: The role of ozone and other photochemical degradation products. Chem. Biol. Interact. 166, 163–169 [DOI] [PubMed] [Google Scholar]
- EPA (2012). Status Report: Evidence Based Advances in Inhalation Dosimetry for Gases With Effects in the Lower Respiratory Tract and in the Body. U.S. EPA, Washington, DC [Google Scholar]
- EU-OSHA, European Agency for Safety and Health at Work (2009). Combined Exposure to Noise and Ototoxic Substances. Office for Official Publications of the European Communities, Luxembourg, Germany: Available at: https://osha.europa.eu/en/publications/literature_reviews/combined-exposure-to-noise-and-ototoxic-substances [Google Scholar]
- Evans A. M., Rice G., Wright J. M., Teuschler L. K. (2013). Exploratory cumulative risk assessment (CRA) approaches using secondary data. Hum. Ecol. Risk Assess. 10.1080/10807039.2013.764771 [Google Scholar]
- Fechter L. D. (1995). Combined effects of noise and chemicals. Occup. Med. 10, 609–621 [PubMed] [Google Scholar]
- Fechter L. D., Chen G. D., Rao D., Larabee J. (2000). Predicting exposure conditions that facilitate the potentiation of noise-induced hearing loss by carbon monoxide. Toxicol. Sci. 58, 315–323 [DOI] [PubMed] [Google Scholar]
- Finlayson-Pitts B. J., Pitts J. N., Jr, eds. (1999). Chemistry of the Upper and Lower Atmosphere, Theory, Experiments and Applications, pp. 969 Academic Press, New York, NY [Google Scholar]
- Gallaher E. J., Egner D. A. (1987). Rebound hyperthermia follows ethanol-induced hypothermia in rats. Psychopharmacology (Berl). 91, 34–39 [DOI] [PubMed] [Google Scholar]
- Gordon C. J. (1993). Temperature Regulation in Laboratory Rodents. Cambridge University Press, New York, NY [Google Scholar]
- Gordon C. J. (1994). Thermoregulation in laboratory mammals and humans exposed to anticholinesterase agents. Neurotoxicol. Teratol. 16, 427–453 [DOI] [PubMed] [Google Scholar]
- Gordon C. J. (2005). Toxicology and Temperature: An Integrative, Comparative, and Environmental Approach. CRC Press, Boca Raton, FL [Google Scholar]
- Gordon C. J., Grantham T. A., Yang Y. (1997). Hypothermia and delayed fever in the male and female rat exposed to chlorpyrifos. Toxicology 118, 149–158 [DOI] [PubMed] [Google Scholar]
- Gordon C. J., Katz L., Leon L. R. (2012). Mechanisms of hypothermia, delayed hyperthermia, and fever following CNS injury. Am. J. Neuroprot. Neurogen. 4, 1–16 [Google Scholar]
- Gordon C. J., Mack C. M. (2003). Influence of gender on thermoregulation and cholinesterase inhibition in the long-evans rat exposed to diazinon. J. Toxicol. Environ. Health. A 66, 291–304 [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Wilson G., Russell L. D., Meistrich M. L. (1997). Radiation-induced cell death in the mouse testis: Relationship to apoptosis. Radiat. Res. 147, 457–467 [PubMed] [Google Scholar]
- Head J. L., Lawrence B. P. (2009). The aryl hydrocarbon receptor is a modulator of anti-viral immunity. Biochem. Pharmacol. 77, 642–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising H., Lange-Asschenfeldt H., Lieber G. F., Weinhold H., Eilts M. (2003). Effects of long-term exposure to street traffic exhaust on the development of skin and respiratory tract diseases in children. Schriftenr. Ver. Wasser Boden Lufthyg. 112, 81–99 [PubMed] [Google Scholar]
- Ising H., Lange-Asschenfeldt H., Moriske H. J., Born J., Eilts M. (2004). Low frequency noise and stress: Bronchitis and cortisol in children exposed chronically to traffic noise and exhaust fumes. Noise Health 6, 21–28 [PubMed] [Google Scholar]
- Jeffries H. E. (1995). Photochemical air pollution: Chapter 9. In Composition, Chemistry and Climate of the Atmosphere (Singh H. B., Ed.), pp. 308–348 Van Nostand-Reinhold, New York, NY [Google Scholar]
- Jeffries H. E., Sexton K. G., Arnold J. R., Kale T. L. (1989). Validation testing of new mechanisms with outdoor chamber data. Volume 3: Calculation of Photochemical Reaction Photolysis Rates in the UNC Outdoor Chamber. Report No. EPA/600/3-89/010C. U.S. Environmental Protection Agency, Research Triangle Park, NC [Google Scholar]
- Johnson A. C., Morata T. C. (2010). Occupational Exposure to Chemicals and Hearing Impairment. (The Nordic Expert Group), pp. 177 University of Gothenburg, Gothenburg, Sweden: Available at: http://hdl.handle.net/2077/23240 [Google Scholar]
- Kensler T. W., Roebuck B. D., Wogan G. N., Groopman J. D. (2011). Aflatoxin: A 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 120(Suppl. 1), S28–S48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H., Rock K. L. (2008). How dying cells alert the immune system to danger. Nat. Rev. Immunol. 8, 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond C. P., Clermont Y. (1952). Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. N.Y. Acad. Sci. 55, 548–573 [DOI] [PubMed] [Google Scholar]
- Li Y., Gibson J. M., Jat P., Puggioni G., Hasan M., West J. J., Vizuete W., Sexton K., Serre M. (2010). Burden of disease attributed to anthropogenic air pollution in the United Arab Emirates: Estimates based on observed air quality data. Sci. Total Environ. 408, 5784–5793 [DOI] [PubMed] [Google Scholar]
- Lichtveld K. M., Ebersviller S. M., Sexton K. G., Vizuete W., Jaspers I., Jeffries H. E. (2012). In vitro exposures in diesel exhaust atmospheres: Resuspension of PM from filters versus direct deposition of PM from air. Environ. Sci. Technol. 46, 9062–9070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Jeffries H. E., Sexton K. G. (1999). Hydroxyl radical and ozone initiated photochemical reactions of 1,3-butadiene. Atmos. Environ. 33, 3005–3022 [Google Scholar]
- Løkke H. (2010). Novel methods for integrated risk assessment of cumulative stressors–results from the NoMiracle project. Sci. Total Environ. 408, 3719–3724 [DOI] [PubMed] [Google Scholar]
- Morgan E. T., Goralski K. B., Piquette-Miller M., Renton K. W., Robertson G. R., Chaluvadi M. R., Charles K. A., Clarke S. J., Kacevska M., Liddle C., et al. (2008). Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab. Dispos. 36, 205–216 [DOI] [PubMed] [Google Scholar]
- Ong F. H., Henry P. J., Burcham P. C. (2012). Prior exposure to acrolein accelerates pulmonary inflammation in influenza A-infected mice. Toxicol. Lett. 212, 241–251 [DOI] [PubMed] [Google Scholar]
- Peng R. D., Bobb J. F., Tebaldi C., McDaniel L., Bell M. L., Dominici F. (2011). Toward a quantitative estimate of future heat wave mortality under global climate change. Environ. Health Perspect. 119, 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager J. E., Lichtveld K., Ebersviller S., Smeester L., Jaspers I., Sexton K. G., Fry R. C. (2011). A toxicogenomic comparison of primary and photochemically altered air pollutant mixtures. Environ. Health Perspect. 119, 1583–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D. B., Fechter L. D. (2000). Increased noise severity limits potentiation of noise induced hearing loss by carbon monoxide. Hear. Res. 150, 206–214 [DOI] [PubMed] [Google Scholar]
- Ryan L. K., Copeland L. R., Daniels M. J., Costa E. R., Selgrade M. J. (2002). Proinflammatory and Th1 cytokine alterations following ultraviolet radiation enhancement of disease due to influenza infection in mice. Toxicol. Sci. 67, 88–97 [DOI] [PubMed] [Google Scholar]
- Sarigiannis D. A., Hansen U. (2012). Considering the cumulative risk of mixtures of chemicals—A challenge for policy makers. Environ. Health 11(Suppl. 1), S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selgrade M. J. K. (2010). Immune system. In A Textbook of Modern Toxicology (Hodgson E, 4th Ed.), pp. 387–404 J. Wiley & Sons, New York, NY [Google Scholar]
- Selgrade M. K. (2007). Immunotoxicity: The risk is real. Toxicol. Sci. 100, 328–332 [DOI] [PubMed] [Google Scholar]
- Selgrade M. K., Daniels M. J., Illing J. W., Ralston A. L., Grady M. A., Charlet E., Graham J. A. (1984). Increased susceptibility to parathion poisoning following murine cytomegalovirus infection. Toxicol. Appl. Pharmacol. 76, 356–364 [DOI] [PubMed] [Google Scholar]
- Selgrade M. K., Gilmour M. I. (2006). Immunotoxicology of inhaled compounds—Assessing risks of local immune suppression and hypersensitivity. J. Toxicol. Environ. Health A 69, 827–844 [Google Scholar]
- Selgrade M. K., Illing J. W., Starnes D. M., Stead A. G., Ménache M. G., Stevens M. A. (1988). Evaluation of effects of ozone exposure on influenza infection in mice using several indicators of susceptibility. Fundam. Appl. Toxicol. 11, 169–180 [DOI] [PubMed] [Google Scholar]
- Sexton K. (2012). Cumulative risk assessment: An overview of methodological approaches for evaluating combined health effects from exposure to multiple environmental stressors. Int. J. Environ. Res. Public Health 9, 370–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton K. G., Arnold J. R., Jeffries H. E., Kale T. L., Kamens R. M. (1988). Validation Data For Photochemical Mechanisms, Experimental Results (Dodge M., Ed.), pp. 73 U.S. Environmental Protection Agency, Atmospheric Sciences Research Laboratory, Research Triangle Park, NC [Google Scholar]
- Sexton K. G., Jeffries H. E., Jang M., Kamens R. M., Doyle M., Voicu I., Jaspers I. (2004). Photochemical products in urban mixtures enhance inflammatory responses in lung cells. Inhal. Toxicol. 16(Suppl. 1), 107–114 [DOI] [PubMed] [Google Scholar]
- Vizuete W., Biton L., Jeffries H. E., Couzo E. (2010). Evaluation of relative response factor methodology for demonstrating attainment of ozone in Houston, Texas. J. Air Waste Manag. Assoc. 60, 838–848 [DOI] [PubMed] [Google Scholar]
- Watanabe C., Suzuki T. (1986). Sodium selenite-induced hypothermia in mice: Indirect evidence for a neural effect. Toxicol. Appl. Pharmacol. 86, 372–379 [DOI] [PubMed] [Google Scholar]
- Zhang H. F., Rattanavaraha W., Zhou Y., Bapat J., Rosen E. P., Sexton K. G., Kamens R. M. (2011). A new gas-phase condensed mechanism of isoprene-NOx photooxidation. Atmos. Environ. 45, 4507–4521 [Google Scholar]