Abstract
Although Apc mutation is widely considered an initiating event in colorectal cancer, little is known about the earliest stages of tumorigenesis following sporadic Apc loss. Therefore, we have utilized a novel mouse model that facilitates the sporadic inactivation of Apc via frameshift reversion of Cre in single, isolated cells and subsequently tracks the fates of Apc-deficient intestinal cells. Our results suggest that consistent with Apc being a ‘gatekeeper’, loss of Apc early in life during intestinal growth leads to adenomas or increased crypt fission, manifested by fields of mutant but otherwise normal-appearing crypts. In contrast, Apc loss occurring later in life has minimal consequences, with mutant crypts being less prone to either increased crypt fission or adenoma formation. Using the stem cell-specific Lgr5-CreER mouse, we generated different sized fields of Apc-deficient crypts via independent recombination events and found that field size correlates with progression to adenoma. To evaluate this early stage prior to adenoma formation as a therapeutic target, we examined the chemopreventive effects of sulindac on Apc-deficient occult crypt fission. We found that sulindac treatment started early in life inhibits the morphologically occult spread of Apc-deficient crypts and thus reduces adenoma numbers. Taken together these results suggest that: (i) earlier Apc loss promotes increased crypt fission, (ii) a field of Apc-deficient crypts, which can form via occult crypt fission or independent neighboring events, is an important intermediate between loss of Apc and adenoma formation and (iii) normal-appearing Apc-deficient crypts are potential unappreciated targets for cancer screening and chemoprevention.
Introduction
The prevailing dogma of solid tumor progression is that cancer results from the stepwise accumulation of multiple mutations beginning within a single cell (1). In colorectal cancer, the adenomatous polyposis coli protein is frequently mutated in the earliest adenomas (1) and is the most commonly mutated gene in colorectal cancer (2). However, little is known about the earliest stages of tumor initiation following Apc loss, in particular when loss occurs in isolated stem cells in an otherwise wild-type intestine. Mutation in a single stem cell alone may be insufficient to confer a tumorigenic phenotype and the local environment may play a modifying role by creating a field effect (3,4). The resulting numerical increase in mutant progeny will both raise the odds of further alteration and form a field of adjacent mutant crypts that could enhance tumorigenesis (5). Field effects have been reported for genes such as p53 in esophageal cancer (6), ulcerative colitis-associated neoplasia (7) and Cdh3 in colorectal cancer (8). Here, we report for the first time evidence for field effects and occult crypt fission involving a strong tumor initiator, specifically the Apc gene, and how sulindac can inhibit the Apc-deficient crypt fission advantage, thereby reducing adenoma number.
A prevalent view is that Apc loss in intestinal crypt stem cells is sufficient for adenoma formation (9,10). However, our previous studies in which we examined the effects of Apc loss in single crypts surrounded by tissue expressing wild-type levels of Apc demonstrated a form of phenotypic plasticity. Namely, following Apc loss, adenoma formation could ensue, but the majority of Apc-deficient intestinal crypts retained a normal phenotype with increased clonal expansion (11). In both cases (adenoma formation or field formation), Apc loss functions as a gatekeeper mutation with net increases in Apc-mutant cells (12). Interestingly, a significant fraction of the morphologically normal Apc-deficient crypts exhibited a growth advantage resulting in clonal expansion and a field of mutant crypts, thus raising the possibility that crypt fission leading to an occult, horizontal spread of mutations is an important intermediate during tumorigenesis.
Here, we first show that the timing of Apc loss can affect field formation by strongly influencing the crypt fission advantage of Apc-deficient crypts, with later Apc loss resulting in decreased mutant field size and adenoma formation. Next, using the intestinal stem cell-specific Cre, Lgr5-CreER system (13,14), we generate different sized fields of Apc-deficient crypts and polyclonal adenomas, further substantiating that an expanded Apc-deficient field is important for adenoma formation (5,15,16). Finally, we demonstrate that the non-steroidal anti-inflammatory drug (NSAID) sulindac can act as a chemopreventive by inhibiting the Apc-deficient occult crypt fission that appears to precede subsequent adenoma formation. Based on these findings, we suggest that: (i) isolated Apc-deficient crypts are not highly prone to tumorigenesis, whereas a field of Apc-deficient crypts formed by either occult crypt fission or independent neighboring events creates a local environment that is highly conducive to adenoma formation and (ii) minimizing Apc-deficient occult crypt fission is a previously unappreciated mechanism of chemoprevention.
Materials and methods
Mice
Pms2 cre; Apc CKO/CKO; R26R mice were generated by interbreeding Pms2 cre/+; R26R mice with Apc CKO (17) mice. The R26R (Rosa26 Reporter mice with lox-stop-lox LacZ, YFP or Confetti) allele was used (18,19). Mice were housed in a specific pathogen-free High-efficiency particulate air filtered room and were fed a diet of Purina PicoLab Rodent Diet 20. The R26R and Lgr5-CreER (13) mouse strains were obtained from the Jackson Laboratory (Bar Harbor, ME). Tamoxifen (Sigma) was prepared in sunflower seed oil (Sigma) at a concentration of 10mg/ml or 1mg/ml. Two hundred microliters of the tamoxifen solution was intraperitoneally injected once into mice at the specified age. All experiments were approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University. Genotyping was performed as described previously (11) and as described in the supplementary methods, available at Carcinogenesis Online. Lgr5-CreER; Apc 580S/580S; R26R-Confetti mice were generated and treated as described previously (14). Intestinal organoids were cultured as described previously (20).
Sulindac treatment
Sulindac was administered at different time points through the drinking water and continued until time of sacrifice. The sulindac solution comprised 180mg/l of sulindac and 4mM sodium phosphate dibasic in distilled water (pH ~7.4).
Scoring of β-gal+ foci in wholemount intestine
β-gal staining was performed and scored as described previously (11). Nearby β-gal+ foci were considered independent if not arising from the same crypt and surrounded by non-staining crypts. Adenomas, which involve multiple villi, and microadenomas involving a single villus were scored in wholemount and cross sections. We concentrated on the proximal small intestine for β-gal+ foci counts because of inefficient recombination of the Cre reporter in the distal small intestine, as reported previously (21,22). The rate of cre reversion was determined by counting the number of colonies with β-gal+ cells versus those without, as described previously (23).
Statistics
Data were analyzed with StatistiXL for Windows in Microsoft Excel. Student–Newman–Keul post hoc test was used after analysis of variance. Fisher exact test was performed using a 2 × 2 table.
Results
Pms2cre system for fate mapping of mutant intestinal stem cells
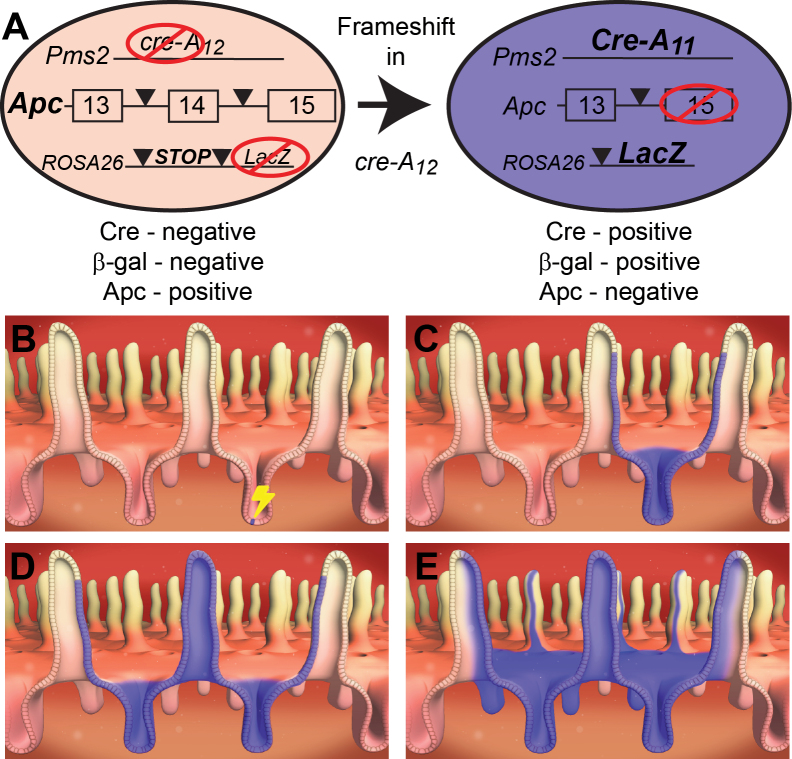
To better understand the fates of normal and mutant intestinal cells, we developed the Pms2 cre mouse system that features an out-of-frame cre allele to stochastically recombine floxed target genes and the marker gene, β-gal (Figure 1A) (21). This system enables not only the monitoring of tumor formation following stochastic genetic manipulations but also facilitates the tracking of normal or mutant stem cell fates. Replication slippage into frame is a function of cell division (DNA replication), and therefore Cre activation can occur any time after conception. Whereas Cre reversion will occur most often in any dividing cell, we stress that only the products of reversion events that take place in either intestinal stem cells (regardless of their position within the crypt) or long-lived progenitors will persist. Finally, we are able to alter the overall timing of Cre reversion by modulating the mismatch repair status, such that there is either a high frequency [Pms2 cre/cre, mismatch repair (MMR)-deficient background] or low frequency (Pms2 cre/+, MMR-proficient background) of Cre reversion.
Fig. 1.
Progression, following Cre reversion, of a β-gal+ stem cell to a field of β-gal+ crypts. (A) Diagram summarizing the Pms2 cre mouse system combined with a conditional Apc allele. An out-of-frame cre gene is located at the Pms2 locus. Upon frameshift mutation within the mononucleotide repeat (A12), Cre protein becomes active and recombines the conditional target allele Apc and the reporter allele LacZ. (B) Cre reversion occurs stochastically at low frequency, resulting in a single Apc-deficient β-gal+ cell within a crypt. Only Cre activation in a stem cell will have the chance to spread throughout the crypt. (C) The end product of crypt succession by a single β-gal+ stem cell, namely, a monoclonal, β-gal+ crypt. (D) The end product of crypt fission of the monoclonal, β-gal+ crypt producing a pair of neighboring, Apc-deficient β-gal+ crypts. (E) The consequence of continued crypt fission resulting in a field of Apc-deficient β-gal+ crypts.
Because Cre reversion rates are low (~1 in 700 cell divisions in Pms2 cre/cre mouse embryonic fibroblasts), Cre activation overwhelmingly occurs in isolated cells. In Pms2 cre mice carrying a lox-stop-lox β-gal allele (R26R), clonal patches composed of different numbers of β-gal+ crypts/villi are observed. Each β-gal+ focus should represent a single Cre reversion and is classified by size as either small (1–3 villi), medium (4–9 villi) or large (10+ villi per focus). The different sized foci reflect the normal process of crypt fission that takes place during growth and development of the intestine (Figure 1B–E and Supplementary Figure 1A –D, available at Carcinogenesis Online). We directly compared Pms2 cre/cre intestines with Pms2 cre/+ intestines, in which Cre reversion occurs at a ~50-fold lower rate than in Pms2 cre/cre mice (Figure 2A and B). Because clonal expansion via crypt fission is in part a function of the timing of Cre reversion, we predicted that fewer and smaller β-gal+ patches should be observed in Pms2 cre/+ mice. Consistent with this prediction, the lower reversion rate of Pms2 cre/+; R26R mice results in a slower accumulation of reversion events with age (Supplementary Figure 2, available at Carcinogenesis Online). As a control, we examined the intestines of 6 Pms2 +/+; R26R mice and saw no β-gal+ foci, as expected for mice without a Cre gene. Therefore, the pattern of β-gal+ crypts in either Pms2 cre/cre; R26R or Pms2 cre/+; R26R mice provides a baseline of how individual stem cells normally form clonal patches throughout life. Depending on the constellation of the Cre-target alleles, we can measure not only whether mutant intestinal stem cells progress to tumors but also whether mutant cells have undergone altered crypt succession, indicated by the number of β-gal+ foci, and/or altered crypt fission, indicated by the size of β-gal+ foci.
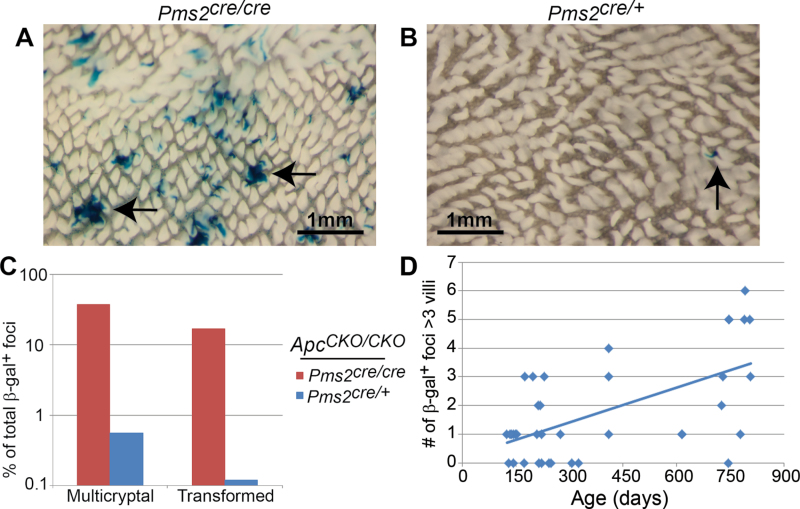
Fig. 2.
β-gal+ foci number, crypt fission and adenoma formation in Pms2 cre/cre and Pms2 cre/+ intestines. Wholemount images of the proximal small intestine from a Pms2 cre/cre (A) and Pms2 cre/+ (B) mouse. Arrows show multicryptal β-gal+ foci in (A) and a single β-gal+ focus in (B). Note the difference in both number and size of β-gal+ foci between Pms2 cre/cre and Pms2 cre/+ intestines. (C) Pms2 cre/cre; Apc CKO/CKO intestines have a higher percentage of normal-appearing multicryptal β-gal+ foci and transformed β-gal+ foci compared with Pms2 cre/+; Apc CKO/CKO intestines. The percentage of transformed β-gal+ foci was determined by scoring frozen sections counterstained with nuclear fast red. (D) The number of β-gal+ foci containing more than three villi results in a linear regression of y = 0.004x + 0.2, which is a statistically significant increase with age (P < 0.001). The number of β-gal+ foci containing more than three villi was determined via wholemount.
In contrast to early loss, Apc loss later in life is not conducive to either increased crypt fission or adenoma formation
We previously reported that Pms2 cre/cre; Apc CKO/CKO mice exhibited an increase in the size of β-gal+ foci compared with Pms2 cre/cre; Apc +/+ mice, with only a minority (<20%) of Apc loss events resulting in either a microadenoma or adenoma. In fact, >80% of β-gal+; Apc − /− foci retained a normal phenotype, including normal levels and cellular distribution of β-catenin (11). While our studies were concentrated on the proximal small intestine, we observed a similar increase in the proportion of larger Apc-deficient foci in the medial and distal small intestines of Pms2 cre/cre mice (Supplementary Figure 3, available at Carcinogenesis Online). We also cultured crypts from Pms2 cre/cre; Apc CKO/CKO intestine and found that recombined β-gal+ cells can contribute to normal-appearing portions of organoids or cysts typical of an adenoma (20,24) (Supplementary Figure 4, available at Carcinogenesis Online). Furthermore, we found that somatic loss of a single Apc allele in Pms2 cre/cre; Apc CKO/+ mice did not increase the number of larger β-gal+ foci when compared with Pms2 cre/cre; Apc +/+ mice, showing that only complete loss of Apc function results in increased field size (11) (Supplementary Figure 5, available at Carcinogenesis Online). We also compared the number of β-gal+ foci with the fraction of β-gal+ foci that occupied more than one crypt (multicryptal) and found that >75% of the multicryptal β-gal+ foci cannot be explained by independent, neighboring events (Supplementary Table 1, available at Carcinogenesis Online). Overall, these results illustrate morphologic phenotypic plasticity of Apc-deficient crypts because early Apc loss increases clonal expansion (crypt fission) without necessarily resulting in visible adenoma formation.
In contrast to results with Pms2 cre/cre mice, we found that conditional alteration of Apc in Pms2 cre/+ mice results in significantly less subsequent crypt clonal expansion and adenoma formation (Figure 2C). Less than 1% of the β-gal+ foci in the Pms2 cre/+; Apc CKO/CKO mice occupied multiple crypts, starkly different from the 24% of multicryptal patches observed in sections of intestine from Pms2 cre/cre; Apc CKO/CKO mice (Figure 2C) (11). In fact, the Pms2 cre/+; Apc CKO/CKO mice did not show any significant change in the number or size distribution of β-gal+ patches compared with Pms2 cre/+; Apc +/+ controls (Supplementary Table 2, available at Carcinogenesis Online). Interestingly, we observed a significant increase in the number of multicryptal β-gal+ foci with age (Figure 2D), suggesting that a low level of crypt fission persists in the adult intestine. Tumorigenesis was also much less evident in the Pms2 cre/+ mice compared with Pms2 cre/cre mice. Even at ~2 years of age, Pms2 cre/+; Apc CKO/CKO mice developed an average of only one intestinal adenoma. Tumorigenesis was rare in Pms2 cre/+; Apc CKO/CKO β-gal+ foci, as less than 1% of β-gal+ foci were scored as microadenomas or adenomas in sections (1/176) or in wholemount (10/8387). In contrast, in Pms2 cre/cre; Apc CKO/CKO mice, 17% (22/129) of β-gal+ foci were scored as either microadenomas or adenomas in sections (P < 0.001) (Figure 2C). To test the importance of later Apc loss in a background of Apc-heterozygosity, we generated Pms2 cre/+; Apc Δ/CKO or Pms2 cre/+; Apc 1638N/CKO mice. We found that later loss of Apc, even in a background of Apc-heterozygosity, did not alter the size distribution of β-gal+ foci compared with Pms2 cre/+ mice (Supplementary Table 2, available at Carcinogenesis Online) or increase the number of tumors compared with Apc Δ/+ or Apc 1638N/+ mice, respectively (Supplementary Figure 6, available at Carcinogenesis Online), suggesting that a field of Apc deficiency is important, even in the setting of an inherited Apc mutation. Taken together, these results suggest that promotion of adenoma formation following Apc loss is much more probable earlier in life during intestinal growth because Apc-deficient crypts have a greater chance of increased occult crypt fission.
Both ApcCKO target alleles are efficiently recombined in β-gal+ cells from Pms2cre/+ mice
We showed previously that both Apc CKO alleles were efficiently recombined in β-gal+ normal and tumor tissue from Pms2 cre/cre mice (11). Nevertheless, the relative lack of increased crypt fission and adenoma formation in Pms2 cre/+ mice could simply reflect inefficient Cre-mediated recombination of both Apc target alleles. To further test the efficiency of Cre recombination at Apc, we used either fluorescence-activated cell sorting of recombined (YFP+) cells or laser capture microdissection samples coupled with PCR to genotype individual recombined (β-gal+) foci.
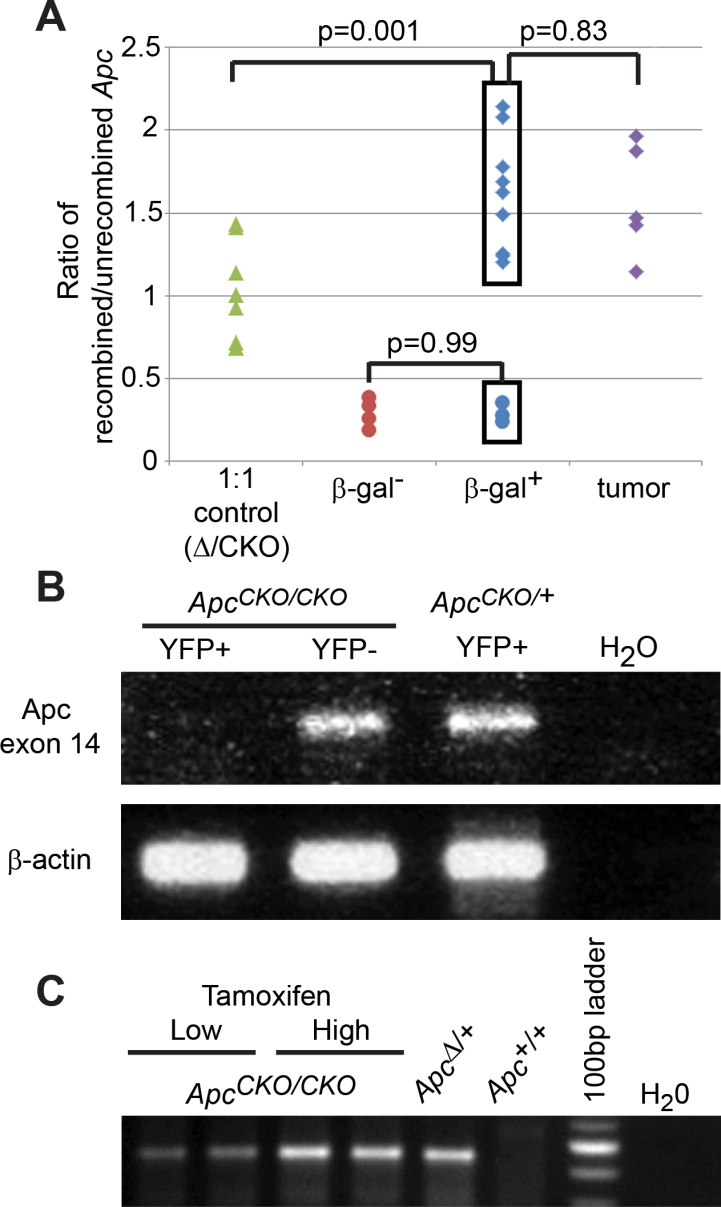
For the laser capture microdissection-PCR, we calculated the ratio of the recombined Apc allele (Apc Δ) to the unrecombined Apc allele (Apc CKO) and compared the ratios to control samples, β-gal−; Apc CKO/CKO or Apc Δ/CKO. In Pms2 cre/+; Apc CKO/CKO mice, 75% (9/12) of normal-appearing β-gal+ foci showed a similar ratio of Apc Δ:Apc CKO as tumor tissue (Apc Δ/Δ, P = 0.83), but an increased ratio when compared with either β-gal− patches (P < 0.001) or control, heterozygous (Apc Δ/CKO) tissue (P = 0.001) (Figure 3A). The increased Apc Δ:Apc CKO ratio in normal-appearing β-gal+ foci compared with control Apc Δ/CKO samples strongly supports efficient recombination of both Apc alleles in a majority of β-gal+ foci.
Fig. 3.
Efficient recombination of Apc CKO in Pms2 cre and Lgr5-CreER intestines. (A) Laser capture microdissection-PCR was used on isolated β-gal+ foci to detect the recombined Apc allele (Apc Δ) and unrecombined Apc allele (Apc CKO). DNA from Pms2 +/+; Apc Δ/CKO mice, which contains equal amounts of Apc CKO and Apc Δ (1:1 control), was given an average ratio of 1 and used to normalize all experimental samples. Therefore, a ratio >1 shows a population of cells with more of the Apc Δ alleles, while a ratio of <1 shows a population of cells with more of the Apc CKO alleles. Tumors (purple diamonds) and 75% of the normal-appearing β-gal+ foci (blue diamonds) had a similar ratio of Apc Δ to Apc CKO, while the same β-gal+ foci had an increased ratio compared with either the β-gal− foci (red circles) or control, heterozygous (Apc Δ/CKO) samples (green triangles). (B) Fluorescence-activated cell sorting was used to isolate 1000 YFP+ and YFP− cells from crypts of either Pms2 cre/cre; Apc CKO/CKO or Pms2 cre/cre; Apc CKO/+ intestine. RNA was isolated from the different populations and then analyzed for the presence of the floxed Apc exon (exon 14). The floxed exon was lost in YFP+; Apc CKO/CKO cells, but not in YFP−; Apc CKO/CKO or YFP+; Apc CKO/+ cells. β-actin was used as a control. (C) PCR was used to detect the recombined Apc allele in intestinal sections from Lgr5-CreER; Apc CKO/CKO mice injected with either the high or low dose of tamoxifen. Apc was recombined in both treatments. More importantly, the 3.6-fold increase in recombined Apc DNA with high-dose treatment was in agreement with the observed 4-fold increase in β-gal+ crypts (see Figure 4A).
We also generated Pms2 cre/cre; ROSA-lox-stop-lox-Yfp mice for fluorescence-activated cell sorting of YFP+ and YFP− cells from Apc CKO/+ or Apc CKO/CKO mice. RNA isolated from each population was assayed for recombination, which results in deletion of Apc exon 14. We found that RNA from Apc CKO/CKO; YFP+ cells lacked signal for exon 14, while both Apc CKO/CKO; YFP− and Apc CKO/+; YFP+ cells contained Apc exon 14 RNA (Figure 3B). These results demonstrated efficient Cre-mediated recombinational deletion of exon 14 of both Apc alleles in YFP+ cells.
Apc-deficient field size potentiates tumor initiation independently of mismatch repair status
Potentially, the greater propensity for adenoma formation in Pms2 cre/cre; Apc CKO/CKO mice compared with Pms2 cre/+; Apc CKO/CKO mice could be due to the higher level of background mutation in the MMR-deficient Pms2 cre/cre mice. Indeed, mice heterozygous for the Apc min mutation do in fact develop more adenomas in a MMR-deficient, Pms2 null background (25). To compare the tumorigenic potential of isolated versus fields of Apc-deficient crypts independently of MMR status, we used the Lgr5-CreER mouse model (13) in combination with the conditional Apc CKO allele. Using this mouse model, we varied the frequency and field size of Apc-deficient crypts by altering the dose of tamoxifen given at similar times. The use of this system eliminates any effects of MMR status, removes possible differences based on timing of Apc loss and generates fields of Apc-deficient crypts from independent, neighboring events.
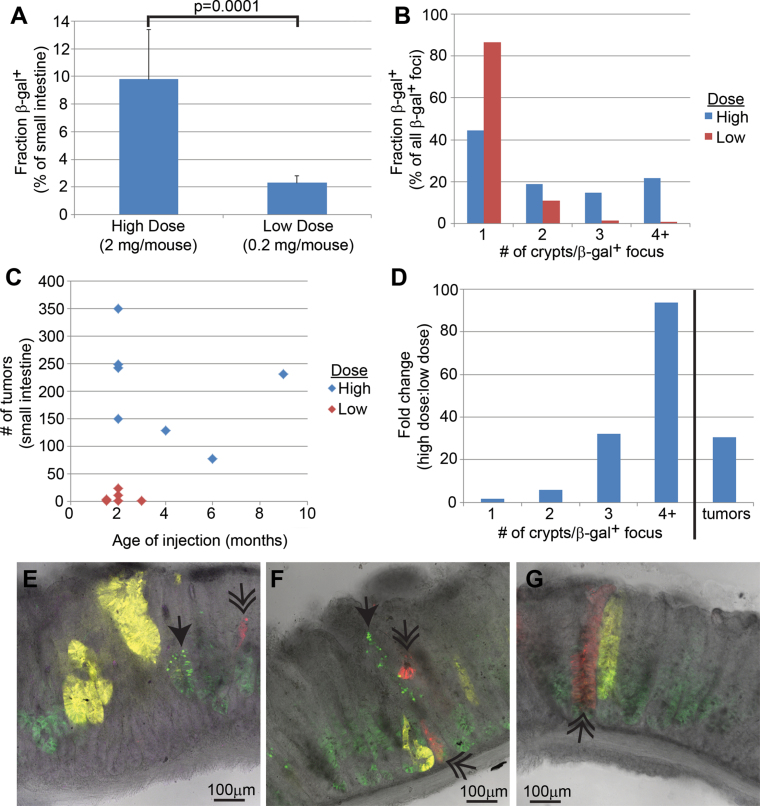
We administered tamoxifen via intraperitoneal injection into mice at two doses, 2mg/mouse (high) and 0.2mg/mouse (low). The higher dose, similar to that used by other investigators (9), resulted in 9.8% β-gal+ crypts, whereas the lower dose resulted in 2.3% β-gal+ crypts (P = 0.0001) (Figure 4A). Furthermore, with the higher dose, 56% (41/74) of β-gal+ foci were multicryptal, of which 21% (16/74) comprised at least four neighboring β-gal+ crypts. In contrast, the lower dose produced only 13% (17/127) multicryptal β-gal+ foci (P < 0.001), with only 0.8% (1/127) comprising at least four neighboring β-gal+ crypts (P < 0.001) (Figure 4B). These results show that varying the tamoxifen dose alters both the frequency and field size of recombined crypts.
Fig. 4.
Characterization of recombination in Lgr5-CreER mice. (A) A 4-fold difference between the high (n = 4) and low (n = 8) dose of tamoxifen in the percentage of tissue with marker gene recombination. (B) The increased β-gal+ focus size in the intestine following high-dose tamoxifen as measured by the number of neighboring β-gal+ crypts. (C) The increased number of tumors in Lgr5-CreER; Apc CKO/CKO intestine following high-dose tamoxifen. (D) The higher dose of tamoxifen gives a higher ratio of larger β-gal+ fields. As discussed in the text, we estimate that at least three neighboring crypts are required for efficient adenoma initiation. (E– G) Images of adenomas from an Lgr5-CreER; Apc 580S/580S; R26R-Confetti mouse injected with a single, high dose of tamoxifen. Notice the multiple different fluorescent proteins found in single adenomas [nuclear green fluorescent protein (single arrowhead), red fluorescent protein (double arrowhead) and yellow fluorescent protein]. Cytoplasmic green fluorescent protein is from the Lgr5-targeted transgene.
Next, we injected Lgr5-CreER; Apc CKO/CKO mice with two different doses of tamoxifen. We used PCR to show that the conditional Apc allele was efficiently recombined in the intestine of mice injected with either the low or high dose of tamoxifen (Figure 3C). Whereas mice receiving the higher dose developed an average of 204 adenomas in the entire small intestine, we found that the lower dose tamoxifen resulted in an average of only seven tumors (P = 0.0002) (Figure 4C). Therefore, the lower tamoxifen dose resulted in a dramatic ~30-fold lower incidence of tumors while reducing the number of β-gal+ crypts by only 4-fold and in turn increasing the proportion of isolated β-gal+ crypts. Furthermore, by comparing the fold difference in β-gal+ foci and tumors between the low and high dose, we estimate that three or more neighboring Apc-deficient crypts are needed for tumorigenesis (Figure 4D). Although the increased background mutation in the MMR-deficient Pms2 cre/cre; Apc CKO/CKO mice could have enhanced adenoma formation, results with MMR-proficient Lgr5-CreER; Apc CKO/CKO mice provide further evidence that the field size of Apc-deficient crypts is an important determinant for adenoma initiation.
We predicted that large fields of mutant Apc-deficient crypts characteristically generated ‘de novo’ by high-dose tamoxifen administration should predispose to polyclonal adenomas because such fields should include numerous independent Apc loss events. Therefore, we analyzed adenomas from Lgr5-CreER; Apc 580S/580S; R26R-Confetti mice 28 days after a single injection of high-dose tamoxifen (5 mg) (14). In these ‘Confetti’ mice, adenomas arising from a single mutant cell should express only a single color, whereas adenomas arising from multiple independent mutant cells should display multiple color markers. As predicted, we found that a majority of adenomas (11/14) exhibited more than one fluorescent marker, illustrating that adenomas were mostly polyclonal, forming from multiple neighboring mutant crypts (Figure 4E–G).
Early administration of sulindac reduces the increased crypt fission and adenoma formation associated with early Apc loss
Because our present and previous (11) studies revealed the importance of mutant crypt clonal expansion for fostering adenoma initiation, we determined the effects of sulindac (26) on this early stage of tumorigenesis. Sulindac is an NSAID that reduces adenoma numbers in humans and mice (26–28), with earlier exposure having an improved response (29). We administered sulindac to Pms2 cre/cre; Apc CKO/CKO mice (i) prior to conception—before any reversion events have occurred, (ii) at weaning—after early reversion events have occurred but before obvious adenoma formation or (iii) in the adult at 2 months of age—after most reversion events have occurred and adenomas have begun to form.
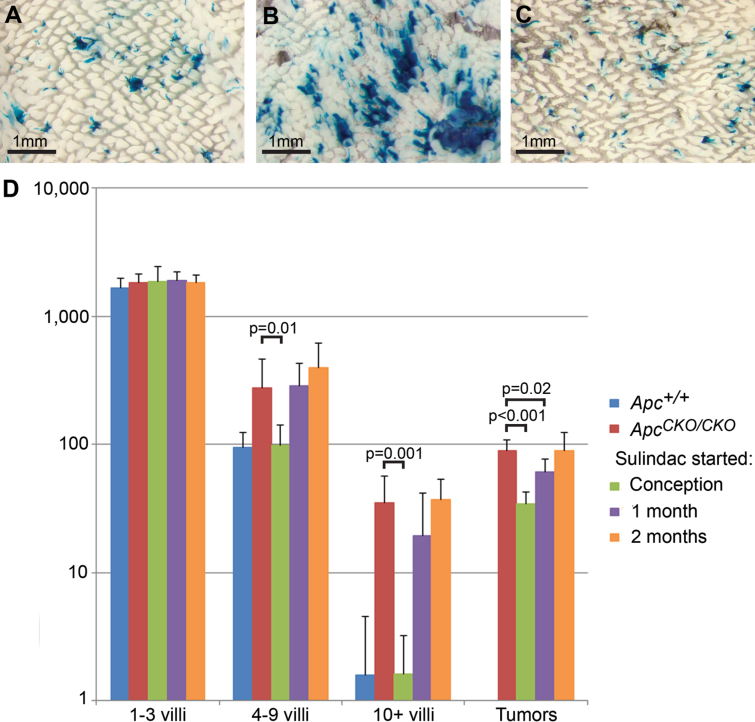
When sulindac was administered prior to conception via the mothers’ drinking water and continued throughout life, the total number of β-gal+ foci remained unchanged compared with untreated mice (P = 0.95), suggesting that sulindac did not noticeably eliminate Apc-mutant cells through apoptosis or alter the rate of Cre reversion. However, we did observe a reduction in the number of medium and large β-gal+ foci in Pms2 cre/cre; Apc CKO/CKO mice (P = 0.01 and P = 0.001) to levels similar to Pms2 cre/cre; Apc +/+ control mice, consistent with sulindac suppressing the increased crypt fission associated with early Apc loss (Figure 5D). Furthermore, we observed in tissue sections that the percentage of multicryptal β-gal+ patches returned to levels seen in the untreated mice when sulindac treatment was started at conception (Supplementary Figure 7A, available at Carcinogenesis Online). To determine whether sulindac generally affected the rate of crypt fission or had a targeted effect on Apc-deficient crypt fission, we scored the number of β-gal-negative crypts undergoing fission in the Pms2 cre/cre; Apc CKO/CKO intestine. We found that sulindac had no detectable effect on the fission rate of Apc +/+ crypts (Supplementary Figure 7B, available at Carcinogenesis Online). Similar to our findings for Apc +/+ crypts, we found that treatment of Pms2 cre/cre; Apc CKO/+ mice with sulindac had no effect on the size or number of β-gal+, Apc +/− foci (Supplementary Figure 5, available at Carcinogenesis Online). Taken together, our results strongly suggest that crypt fission inhibition by sulindac was specific to Apc-deficient crypts. In addition to the suppressive effect on Apc-deficient crypt fission, sulindac treatment initiated prior to conception resulted in a 3-fold decrease in adenomas compared with untreated Pms2 cre/cre; Apc CKO/CKO mice (P < 0.001) (Figure 5D). The inhibition by sulindac of both increased crypt fission and adenoma formation suggests a direct relationship and reinforces the idea that a field of Apc-deficient crypts is conducive to adenoma formation.
Fig. 5.
Effects of sulindac treatment on crypt fission and adenoma formation in Pms2 cre/cre mice. Pms2 cre/cre; Apc +/+ (A), Pms2 cre/cre; Apc CKO/CKO (B) and Pms2 cre/cre; Apc CKO/CKO (C) mice administered sulindac continuously from conception. Note the striking increase in β-gal+ focus size when comparing Pms2 cre/cre; Apc +/+ (A) and Pms2 cre/cre; Apc CKO/CKO mice (B) versus the obvious similarity between the β-gal+ focus sizes in the sulindac-treated intestine (C) and the control intestine (A). (D) β-gal+ foci and tumor numbers in Pms2 cre/cre control mice (n = 7) and Pms2 cre/cre; Apc CKO/CKO mice untreated (n = 6) or treated with sulindac started at conception (n = 8), 1 month (n = 6) or 2 months (n = 3). Sulindac treatment started prior to conception, via the mother’s drinking water, resulted in a significant decrease in medium (4–9 villi) and large (10+ villi) β-gal+ foci and tumor number compared with untreated Apc CKO/CKO mice. Sulindac started at weaning (1 month) resulted in a significant decrease in tumor number, but not β-gal+ focus size. Sulindac started in adult mice at 2 months had no significant effect on β-gal+ focus size or tumor number. Total β-gal+ foci numbers and tumor numbers were determined in wholemount.
In stark contrast to ‘preconception’ administration, sulindac treatment initiated at weaning had no effect on the increased crypt fission associated with Apc loss, as evidenced by no significant reduction in the number of medium and large β-gal+ foci compared with untreated mice (P = 0.82 and P = 0.94) (Figure 5D). Presumably, the growth advantage of Apc − /− crypts had already manifested prior to weaning. However, we did observe a slight reduction in adenoma number (P = 0.02) (Figure 5D). Sulindac treatment initiated at 2 months of age failed to alter either the size distribution of normal-appearing Apc-deficient crypts (P = 0.99 and P = 0.39) or reduce adenoma number when compared with untreated mice (P = 0.93) (Figure 5D). These data show that similar to prior studies (29), sulindac is most effective in reducing adenoma formation when administered early. In addition, sulindac does not appear to act only by eliminating Apc-deficient cells via increased apoptosis. Rather, our results suggest that sulindac can also reduce adenoma number by inhibiting the accelerated crypt fission associated with Apc loss and is most effective in reducing the tumor burden when Apc-mutant field size is minimized. Taken together, the sulindac results combined with the Pms2 cre and Lgr5-CreER data above suggest that adenomas arise most efficiently from a field of Apc-mutant crypts, which can form from either occult crypt fission or independent, neighboring events.
Discussion
In the current study, we have exploited Cre/lox systems in the mouse to alter Apc in the intestine. From our results, we offer some novel conclusions concerning intestinal tumor initiation and prevention. Specifically: (i) Sporadic Apc loss in single isolated cells does not usually change morphologic phenotype, with most Apc − /− cells residing in normal-appearing crypts. (ii) Early Apc loss results in increased crypt fission that in turn enhances adenoma formation. (iii) The observed phenotypic plasticity of Apc-deficient crypts is regulated by field size, with larger field size fostering adenoma formation. (iv) Sulindac can suppress tumor formation by inhibiting the increased occult crypt fission of Apc-deficient cells.
Rather than ‘instantaneous’ transformation and a direct correlation between genotype and phenotype, our studies revealed considerable phenotypic plasticity with the majority of Apc-deficient single crypts retaining normal morphologic phenotypes. Although Apc is a driver mutation, we suggest that the local environment can influence the phenotype in Apc-deficient crypts. For example, the genome from a fully transformed melanoma cell can be completely ‘reprogrammed’ by nuclear transfer and contribute to the formation of a normal mouse (30). However, we do show that Apc-mutant crypts are functionally abnormal because they can exhibit accelerated crypt fission, as manifested by increased numbers of larger mutant patches. Moreover, these advantages are more highly expressed during early development when intestinal growth by crypt fission is ongoing and mutant crypts can more readily outcompete normal crypts (31). Once intestinal growth has slowed in the adult mouse, most phenotypically normal Apc-deficient cells have a reduced chance of displacing surrounding normal crypts because most mutant patches remain isolated. This observation is consistent with data from Apc Min/+ mice demonstrating that carcinogens administered early in life produce the highest numbers of adenomas (32). Our data add to this concept and illustrate how early mutations are inherently better able to spread due to the normal crypt fission that occurs during growth.
We have utilized the MMR status of Pms2 cre mice to alter the frequency and time frame of Cre reversion. Compromised MMR in Pms2 cre/cre mice will result in both increased Cre reversion and a higher mutation rate throughout the genome. However, while an increased mutation rate could complicate our observations, we offer several reasons for why our data are not hindered by Pms2 deficiency. First, we compare Pms2-deficient experimental mice harboring floxed target genes with Pms2-deficient control mice that lack targets. Second, we analyze hundreds of reversion events per mouse, thus the stochastic nature of any ‘background’ mutations makes modifying a consequential gene in a significant fraction of β-gal+ foci highly unlikely. Furthermore, Pms2-null mice are not prone to either intestinal adenomas or carcinomas (33). Although we cannot rule out some undefined synergistic effect between the Cre targeted mutations and Pms2 deficiency, we believe such an effect is highly unlikely to play a significant role in influencing the phenotypes of Cre-expressing cells in Pms2 cre/cre mice.
The idea that fields of mutant crypts can promote tumorigenesis was proposed previously to explain polyclonal adenomas often found in mice and humans heterozygous for Apc mutation (5,15,16). Furthermore, tumor polyclonality was explained by short-range interactions between neighboring mutant crypts (34). In contrast, other studies showed that intestinal adenomas could also be monoclonal and thus derived from single crypts (35). Our data using the Pms2 cre system indicated that a field of Apc-deficient crypts originated in a single crypt followed by occult crypt fission. In addition, our results using the inducible Lgr5-CreER system showed that multiple independent, neighboring events can also produce a field that is conducive to the formation of polyclonal adenomas. Our analysis suggests that a field of at least three crypts is necessary for tumorigenesis. Therefore, we propose that adenomas can initiate by occult crypt fission and/or independent, neighboring events. Taken together, our results suggest that Apc-mutant fields, regardless of how formed, are conducive to adenoma formation. While our data are focused on intestinal cancer and Apc mutation, field effects have also been reported for other genes/cancers, such as p53 in esophageal cancer (6), ulcerative colitis-associated neoplasia (7) and Cdh3 in colorectal cancer (8). Field effects can manifest in a variety of ways, such as transcriptional and proliferative changes in benign prostate from prostate cancer patients (36,37), metabolic changes in non-dysplastic tissue from esophageal cancer patients (38) and methylation defects in the colonic mucosa from colorectal cancer patients (39).
A phenotypically normal phase of mutant cells before tumorigenesis has important implications for human cancer progression. First, the lack of visible adenomas early in life in mice and humans with heterozygous germline Apc mutations is difficult to explain if adenoma formation requires a simple loss of the normal Apc allele in a single stem cell. Based on our findings, we propose that loss of the functional Apc allele regularly occurs but that phenotypic plasticity allows Apc-deficient mutant crypts arising early in life to accumulate and only subsequently evolve into adenomas during adolescence. For example, in familial adenomatous polyposis patients there are hundreds of visible monocryptal lesions that seem to progress through crypt fission (40). Our data suggest that an even earlier stage in adenoma initiation is a phenotypically normal, but Apc-deficient crypt, which can remain normal for an extended length of time until a field of Apc-deficient crypts arises. Second, the large number of passenger mutations in human colorectal cancers implies that many mutations accumulate in normal intestine (41). Indeed, it has been estimated that over half of all the mutations in a cancer accumulated in normal colon tissue (41–43). Phenotypic plasticity facilitates the accumulation of both driver and passenger mutations throughout life as opposed to a simple stepwise progression, in which driver mutations accumulate only in visible tumors.
Because our studies with the Pms2 cre mice revealed the importance of mutant crypt clonal expansion for fostering adenoma initiation, we sought to determine the effects of a chemopreventive on this early stage before tumor progression. Sulindac is an NSAID that reduces adenoma numbers in humans and mice (26–29). Previous data from mice have led investigators to postulate that sulindac acts via stimulating apoptosis of Apc-deficient stem cells (44). Such an apoptotic mechanism should result in a marked reduction in β-gal+; Apc-deficient foci in our system. However, we found that the numbers of normal-appearing β-gal+; Apc − /− foci were similar between treated and untreated mice. Interestingly, however, sulindac treatment resulted in smaller β-gal+; Apc − /− foci. Therefore, we suggest that sulindac reduces adenoma numbers in the mouse not only by increasing apoptosis within the adenoma, as shown convincingly by previous investigators (44), but also by limiting the increased crypt fission and subsequent mutant field formation associated with Apc loss. Sulindac may therefore block the gatekeeper function of Apc deficiency or the net increase in mutant cells. Elucidating the mechanism by which sulindac can inhibit increased crypt fission associated with Apc loss is worthy of further investigation.
In terms of human cancer prevention, low-dose NSAID (e.g. aspirin) use has been associated with significantly reduced colorectal cancer risk (45), most often explained by increased apoptosis of Apc-deficient cells (46–48). However, reductions in cancer risk require 5 or more years of aspirin use (49), and the chronicity of use rather than the dosage appears to be critical for prevention (50). These epidemiologic findings are difficult to explain if the only chemopreventive mechanism is by apoptotic elimination of mutant cells. An additional explanation, based on our findings with sulindac reported here, is that chronic low-dose NSAIDs limit the expansion of Apc-deficient cells, therefore reducing tumorigenesis by inhibiting the otherwise enhanced crypt fission of Apc-mutant cells (51–53). As reported for adult humans (8,54), we observed continued crypt fission in adult mice (Figure 2D), albeit at a slower rate than during development (31,55). Because a low level of crypt fission persists in the adult, the possibility remains that new sporadic Apc mutations can accumulate in isolated crypts and over years create small fields of mutant crypts. The reduction in tumors after chronic aspirin use in humans could reflect the inhibition of the enhanced crypt fission of Apc-deficient crypts observed with sulindac in this study. This inhibition would reduce, but not completely prevent, any new field expansions of Apc-deficient crypts. In addition, fields of Apc-deficient crypts that were already established would not be eliminated. Therefore, we believe that our data can help explain why low-dose aspirin does not immediately reduce cancer risks, does not completely eliminate the chance of getting a tumor and requires at least 5 years of chronic administration to reduce the chance of cancer by 30–40% (45,56).
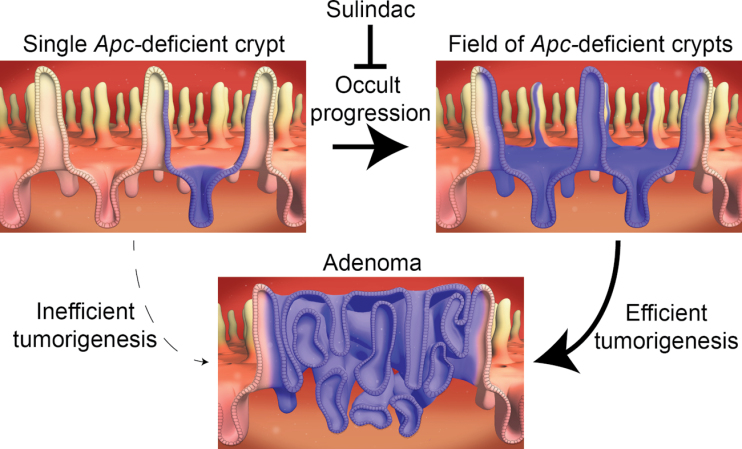
In summary, the ability to engineer and follow the fates of specific sporadic mutations in marked single cells has revealed both the phenotypic plasticity of Apc-mutant intestinal cells and the subsequent occult clonal expansion of mutant crypts via increased crypt fission. Such increased crypt clonal expansion by normal-appearing Apc-deficient crypts is consistent with the gatekeeper role of Apc but occurs without the microscopic or macroscopic manifestations of adenomas. Instead of a simple linear progression to cancer with genotype intrinsically associated with morphologic phenotype, our system reveals that the timing of mutation can influence morphologic phenotype, crypt fission and adenoma progression. Mutations can hitchhike and augment the crypt fission that occurs during normal growth and development. Most notably, the formation of a field of mutant crypts appears to be a more efficient tumor initiation pathway compared with the same mutations that remain isolated in single crypts. In addition, we find that limiting Apc-deficient occult crypt fission can serve as a previously unappreciated mechanism of chemoprevention (Figure 6).
Fig. 6.
Model for progression from an isolated Apc-deficient crypt to adenoma. First, an isolated stem cell loses Apc function during development of the intestine. Next, the Apc-mutant stem cell spreads by crypt succession to monoclonality. Possessing a selective advantage, the Apc-deficient crypt forms a field of mutant crypts via increased crypt fission. A field of Apc-deficient crypts is now primed to undergo tumorigenesis and produce an adenoma. Finally, sulindac blocks the crypt fission advantage of Apc-deficient crypts, thus preventing a field of mutant crypts from forming and hence tumorigenesis. Sulindac seems to have minimal effect on tumorigenesis after the field of Apc-deficient crypts has formed.
Supplementary material
Supplementary methods and Supplementary Tables 1 and 2 and Figures 1–7 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health grant (2R01GM032741-28 to R.M.L. and D.S.); American Cancer Society (PF-11-067-01-TBE to J.M.F.).
Supplementary Material
Acknowledgements
We thank Dr Raju Kucherlapati for Apc CKO mice. We also thank Sandy Dudley and Ashleigh Miller for technical assistance and Anthony Garay for assistance with figure design.
Conflict of Interest Statement: H.C. is an inventor of several patents involving the organoid culture system. The remaining authors disclose no conflicts.
Glossary
Abbreviations:
- MMR, mismatch repair; NSAID
non-steroidal anti-inflammatory drug.
References
- 1. Vogelstein B., et al. (1985). Use of restriction fragment length polymorphisms to determine the clonal origin of human tumors. Science, 227, 642–645 [DOI] [PubMed] [Google Scholar]
- 2. Network T.C.G.A. (2012). Comprehensive molecular characterization of human colon and rectal cancer. Nature, 487, 330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garcia S.B., et al. (1999). Field cancerization, clonality, and epithelial stem cells: the spread of mutated clones in epithelial sheets. J. Pathol., 187, 61–81 [DOI] [PubMed] [Google Scholar]
- 4. Shen L., et al. (2005). MGMT promoter methylation and field defect in sporadic colorectal cancer. J. Natl Cancer Inst., 97, 1330–1338 [DOI] [PubMed] [Google Scholar]
- 5. Thliveris A.T., et al. (2005). Polyclonality of familial murine adenomas: analyses of mouse chimeras with low tumor multiplicity suggest short-range interactions. Proc. Natl Acad. Sci. USA, 102, 6960–6965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prevo L.J., et al. (1999). p53-mutant clones and field effects in Barrett’s esophagus. Cancer Res., 59, 4784–4787 [PubMed] [Google Scholar]
- 7. Leedham S.J., et al. (2009). Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology, 136, 542–550.e6 [DOI] [PubMed] [Google Scholar]
- 8. Milicic A., et al. (2008). Ectopic expression of P-cadherin correlates with promoter hypomethylation early in colorectal carcinogenesis and enhanced intestinal crypt fission in vivo . Cancer Res., 68, 7760–7768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barker N., et al. (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature, 457, 608–611 [DOI] [PubMed] [Google Scholar]
- 10. Clarke A.R. (2005). Studying the consequences of immediate loss of gene function in the intestine: APC. Biochem. Soc. Trans., 33(Pt 4), 665–666 [DOI] [PubMed] [Google Scholar]
- 11. Fischer J.M., et al. (2012). Different phenotypic consequences of simultaneous versus stepwise Apc loss. Oncogene, 31, 2028–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwong L.N., et al. (2009). APC and its modifiers in colon cancer. Adv. Exp. Med. Biol., 656, 85–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barker N., et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature, 449, 1003–1007 [DOI] [PubMed] [Google Scholar]
- 14. Schepers A.G., et al. (2012). Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science, 337, 730–735 [DOI] [PubMed] [Google Scholar]
- 15. Thliveris A.T., et al. (2011). Clonal structure of carcinogen-induced intestinal tumors in mice. Cancer Prev. Res. (Phila)., 4, 916–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Novelli M.R., et al. (1996). Polyclonal origin of colonic adenomas in an XO/XY patient with FAP. Science, 272, 1187–1190 [DOI] [PubMed] [Google Scholar]
- 17. Kuraguchi M., et al. (2006). Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet., 2, e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zambrowicz B.P., et al. (1997). Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc. Natl Acad. Sci. USA, 94, 3789–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Snippert H.J., et al. (2010). Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell, 143, 134–144 [DOI] [PubMed] [Google Scholar]
- 20. Sato T., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature, 459, 262–265 [DOI] [PubMed] [Google Scholar]
- 21. Miller A.J., et al. (2008). Tractable Cre-lox system for stochastic alteration of genes in mice. Nat. Methods, 5, 227–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sangiorgi E., et al. (2008). Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet., 40, 915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fischer J.M., et al. (2008). Visualizing loss of heterozygosity in living mouse cells and tissues. Mutat. Res., 645, 1–8 [DOI] [PubMed] [Google Scholar]
- 24. Sato T., et al. (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature, 469, 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baker S.M., et al. (1998). Enhanced intestinal adenomatous polyp formation in Pms2-/-;Min mice. Cancer Res., 58, 1087–1089 [PubMed] [Google Scholar]
- 26. Boolbol S.K., et al. (1996). Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res., 56, 2556–2560 [PubMed] [Google Scholar]
- 27. Waddell W.R., et al. (1983). Sulindac for polyposis of the colon. J. Surg. Oncol., 24, 83–87 [DOI] [PubMed] [Google Scholar]
- 28. Giardiello F.M., et al. (1993). Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N. Engl. J. Med., 328, 1313–1316 [DOI] [PubMed] [Google Scholar]
- 29. Beazer-Barclay Y., et al. (1996). Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis, 17, 1757–1760 [DOI] [PubMed] [Google Scholar]
- 30. Hochedlinger K., et al. (2004). Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev., 18, 1875–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheng H., et al. (1985). Whole population cell kinetics and postnatal development of the mouse intestinal epithelium. Anat. Rec., 211, 420–426 [DOI] [PubMed] [Google Scholar]
- 32. Shoemaker A.R., et al. (1995). N-ethyl-N-nitrosourea treatment of multiple intestinal neoplasia (Min) mice: age-related effects on the formation of intestinal adenomas, cystic crypts, and epidermoid cysts. Cancer Res., 55, 4479–4485 [PubMed] [Google Scholar]
- 33. Prolla T.A., et al. (1998). Tumour susceptibility and spontaneous mutation in mice deficient in Mlh1, Pms1 and Pms2 DNA mismatch repair. Nat. Genet., 18, 276–279 [DOI] [PubMed] [Google Scholar]
- 34. Halberg R.B., et al. (2007). Polyclonal tumors in the mammalian intestine: are interactions among multiple initiated clones necessary for tumor initiation, growth, and progression? Cell Cycle, 6, 44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fearon E.R., et al. (1987). Clonal analysis of human colorectal tumors. Science, 238, 193–197 [DOI] [PubMed] [Google Scholar]
- 36. Kosari F., et al. (2012). Shared gene expression alterations in prostate cancer and histologically benign prostate from patients with prostate cancer. Am. J. Pathol., 181, 34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ananthanarayanan V., et al. (2006). Alteration of proliferation and apoptotic markers in normal and premalignant tissue associated with prostate cancer. BMC Cancer, 6, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yakoub D., et al. (2010). Metabolic profiling detects field effects in nondysplastic tissue from esophageal cancer patients. Cancer Res., 70, 9129–9136 [DOI] [PubMed] [Google Scholar]
- 39. Svrcek M., et al. (2010). Methylation tolerance due to an O6-methylguanine DNA methyltransferase (MGMT) field defect in the colonic mucosa: an initiating step in the development of mismatch repair-deficient colorectal cancers. Gut, 59, 1516–1526 [DOI] [PubMed] [Google Scholar]
- 40. Preston S.L., et al. (2003). Bottom-up histogenesis of colorectal adenomas: origin in the monocryptal adenoma and initial expansion by crypt fission. Cancer Res., 63, 3819–3825 [PubMed] [Google Scholar]
- 41. Tomasetti C., et al. (2013). Half or more of the somatic mutations in cancers of self-renewing tissues originate prior to tumor initiation. Proc. Natl Acad. Sci. USA, 110, 1999–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Calabrese P., et al. (2004). Colorectal pretumor progression before and after loss of DNA mismatch repair. Am. J. Pathol., 164, 1447–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calabrese P., et al. (2004). Pretumor progression: clonal evolution of human stem cell populations. Am. J. Pathol., 164, 1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qiu W., et al. (2010). Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proc. Natl Acad. Sci. USA, 107, 20027–20032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rothwell P.M. (2013). Aspirin in prevention of sporadic colorectal cancer: current clinical evidence and overall balance of risks and benefits. Recent Results Cancer Res., 191, 121–142 [DOI] [PubMed] [Google Scholar]
- 46. Stark L.A., et al. (2007). Aspirin activates the NF-kappaB signalling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer. Carcinogenesis, 28, 968–976 [DOI] [PubMed] [Google Scholar]
- 47. Ashktorab H., et al. (2005). Apoptosis induced by aspirin and 5-fluorouracil in human colonic adenocarcinoma cells. Dig. Dis. Sci., 50, 1025–1032 [DOI] [PubMed] [Google Scholar]
- 48. Yoshikawa R., et al. (1995). Effect of aspirin on the induction of apoptosis in colorectal adenocarcinoma cell-lines. Oncol. Rep., 2, 361–364 [DOI] [PubMed] [Google Scholar]
- 49. Rothwell P.M., et al. (2012). Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet, 379, 1602–1612 [DOI] [PubMed] [Google Scholar]
- 50. Rothwell P.M., et al. (2010). Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet, 376, 1741–1750 [DOI] [PubMed] [Google Scholar]
- 51. Wasan H.S., et al. (1998). APC in the regulation of intestinal crypt fission. J. Pathol., 185, 246–255 [DOI] [PubMed] [Google Scholar]
- 52. Bjerknes M., et al. (1997). APC mutation and the crypt cycle in murine and human intestine. Am. J. Pathol., 150, 833–839 [PMC free article] [PubMed] [Google Scholar]
- 53. Bjerknes M. (1996). Expansion of mutant stem cell populations in the human colon. J. Theor. Biol., 178, 381–385 [DOI] [PubMed] [Google Scholar]
- 54. Totafurno J., et al. (1987). The crypt cycle. Crypt and villus production in the adult intestinal epithelium. Biophys. J., 52, 279–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cummins A.G., et al. (2008). Crypt fission peaks early during infancy and crypt hyperplasia broadly peaks during infancy and childhood in the small intestine of humans. J. Pediatr. Gastroenterol. Nutr., 47, 153–157 [DOI] [PubMed] [Google Scholar]
- 56. Rothwell P.M., et al. (2011). Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet, 377, 31–41 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.