Summary:
ER activity in the colon prevents tumorigenesis by suppressing inflammation, regulating cytokine, Wnt and Ihh availability and providing maintenance of the ISC microenvironment through modulation of crosstalk in the ECM.
Abstract
Adenomatous polyposis coli (APC)-regulated Wnt and transforming growth factor-β (TGFβ) signaling cooperate in the intestine to maintain normal enterocyte functions. Human clinical trials showed that estrogen [17β-estradiol (E2)], the ligand of nuclear receptors estrogen receptor α (ERα) and ERβ, inhibited colorectal cancer (CRC) in women. Consistent with this finding, we reported that E2, ERα and ERβ suppressed intestinal tumorigenesis in the C57BL/6J-Min/+ (Min/+) mouse, a CRC model. Here, we extended our results with further comparisons of colon and small intestine from intact female Apc +/+ (WT), Min/+ and ER-deficient Min/+ mice. In the colon of ER-deficient Min/+ mice, ER loss reduced TGFβ signaling in crypt base cells as evidenced by minimal expression of the effectors Smad 2, 3 and 4 in these strains. We also found reduced expression of Indian hedgehog (Ihh), bone morphogenetic protein 4 and hepatocyte nuclear factor 3β or FoxA2, factors needed for paracrine signaling between enterocytes and mesenchyme. In proximal colon, ER loss produced a >10-fold increased incidence of crypt fission, a marker for wound healing and tumor promotion. These data, combined with our previous work detailing the specific roles of E2, ERα and ERβ in the colon, suggest that ER activity helps to maintain the intestinal stem cell (ISC) microenvironment by modulating epithelial–stromal crosstalk in ways that regulate cytokine, Wnt and Ihh availability in the extracellular matrix (ECM).
Introduction
Colorectal cancer (CRC) arises from a mutant intestinal stem cell (ISC) whose behavior is no longer regulated by its microenvironment (1,2). ISCs reside at the base of crypts in a niche specified by stromal–epithelial interactions that maintain and regulate their stem-like properties. Each crypt contains multiple monoclonal ISCs that can replicate by either asymmetrical or symmetrical cell division (3). Normal tissue renewal proceeds by asymmetrical replication, a cell division that produces a differentiation-committed stem cell daughter. Under conditions of crypt expansion, ISC division is symmetrical, thereby duplicating the stem cell. The physical result of symmetrical ISC duplication is crypt fission, which provides the means for intestinal tissue growth during development and peaks in mice during puberty (4,5). In the normal adult, intestinal crypt fission occurs at a slow rate; however, this process is induced at sites of chronic inflammation and tumorigenesis (6).
Adenomatous polyposis coli (APC)- and transforming growth factor-β (TGFβ)-dependent pathways regulate homeostasis in the intestinal mucosa and inhibit CRC development (7,8). APC negatively regulates Wnt signaling, a network that is essential for crypt morphology and ISC survival (7,9,10). Wnt ligand-mediated activation of frizzled-LRP5/6 surface receptors promotes β-catenin stabilization, nuclear localization and activation of Tcf/Lef transcription factors by β-catenin-Tcf4. Multiple gene targets of β-catenin-Tcf4 stimulate enterocyte growth. Canonical Wnt ligands are available only in the proliferative compartment at the base of intestinal crypts. In the majority of sporadic and familial CRC, genetic or epigenetic loss of the APC gene results in excess β-catenin expression that drives abnor-mal β-catenin-Tcf4 gene expression. C57BL/6J-Min/+ (Min/+) mice carry a germline mutation in one Apc allele. These animals develop numerous small intestinal adenomas that demonstrate unregulated Wnt signaling due to somatic loss of the wild-type allele.
TGFβ- and Wnt-signaling pathways cooperate in critical ways to regulate enterocyte proliferation, differentiation and migration. TGFβ cytokine family members are secreted by stromal cells and enterocytes and engage in different autocrine and paracrine signaling along the crypt-villus axis. The TGFβ ligand family activates type I and II receptors leading to phosphorylation of Smad2 and Smad3. A related ligand family including bone morphogenetic proteins (BMPs) activates similar receptors leading to phosphorylation of additional Smads (11). These phosphorylated proteins then form heteromeric complexes with Smad4 and regulate gene expression. Mutations that inactivate Smad4 are tumor-initiating events (12,13), even in the absence of APC loss.
Carefully controlled studies, including a large randomized trial, showed that hormone replacement therapy prevented CRC (14,15). The steroid hormone, 17β-estradiol (E2) binds to the nuclear receptors, estrogen receptor α (ERα) and ERβ, which in turn recruit coregulators and modulate transcription. These receptors are expressed in intestinal epithelial cells, immune effector CD4+ and CD8+ T cells, B cells, monocytes and macrophages (16). Epigenetic silencing of the human ESR1 gene encoding ERα is commonly observed in adenomas and CRC, and ESR1 promoter hypermethylation was found in studies of patients with CRC predisposing inflammatory bowel disease (17,18). Together, these findings suggest that sex hormone availability maintains ER expression and function in ways that inhibit inflammatory signals that promote CRC.
We reported that E2 deficiency in ER +/+Min/+ females increased tumor incidence and that ERα+/−Min/+, ERβ+/−Min/+ and ERβ− /−Min/+ mice developed more tumors in the proximal colon when compared with ER +/+Min/+ littermates (19,20). The incidence of dysplastic aberrant crypt foci (ACF) demonstrating APC functional loss in the colon of ER-deficient Min/+ strains was similar to that of Min/+ controls. Therefore, we concluded that ER loss stimulated proximal colon tumor progression without affecting Apc-dependent tumor initiation. Here, we extended our previous results by further studying intestinal tissues from these same animals to determine the relationship between ER function and the crypt base microenvironment.
Materials and methods
Materials
Min/+ and Apc +/+ (WT) mice were purchased from The Jackson Laboratory; ERα+/−Min/+, ERβ+/−Min/+ and ERβ− /−Min/+ were bred as described previously (20). Previous study showed that tumor numbers in ER-deficient Min/+ mice were not affected by gender, and ERβ+/−Min/+ and ERβ− /−Min/+ had the same tumor phenotypes. Also, none of the viable pups were of ERα− /− Apc Min/+ genotype (20). Antibodies used appear in a Supplementary Table 1, available at Carcinogenesis Online (20). Paraffin blocks containing colon specimens from patients with familial adenomatous polyposis (FAP) were obtained from Brigham and Women’s Hospital Department of Pathology following Institutional Review Board approval.
Immunohistochemical analysis
Serial sections of intestine, fixed in 10% neutral-buffered formalin and embedded in paraffin, were deparaffinized and rehydrated in a graded xylene–ethanol series. Experiments used WT, Min/+, ERα+/−Min/+, ERβ+/−Min/+ and ERβ− /−Min/+ sections of proximal colon that were all preserved at the same time. All experiments were repeated with tissues from four different mice of each genotype. Antigen retrieval was performed in citrate buffer, pH 6 in a Decloaking Chamber ProTM (Biocare Medical); and slides were cooled to room temperature before reacting with a peroxidase blocking reagent. Primary antibody reactions were performed in a phosphate-buffered saline solution containing 1% of an appropriate serum and biotin block at 4°C overnight. Sections were incubated with biotinylated secondary antibodies followed by reaction with Vectastain using ABC reagents. Color development utilized 3,3′-diaminobenzidine. Representative images were acquired using an Olympus BX40 microscope.
Determination of the relative percent of branching crypts, the relative number of HFN3β-positive nuclei or Paneth cells per crypt and statistical analyses
By microscopy at ×20 magnification, the number of branched and total crypts in well-oriented sections were counted in the non-tumor mucosa of ER +/+Min/+, ERα+/−Min/+ and ERβ− /−Min/+ proximal colon sections. The branched crypts counted were similar in appearance to those shown in Figure 1A. The percent of branched crypts was calculated as the total number of these structures in each specimen divided by the total number of crypts per high power field. The number of tissue sections analyzed for Min/+, ERα+/−Min/+ and ERβ− /− mice were 15, 10 and 15, and when pooled contained a total of 1347, 1368, 1462 crypts per genotype, respectively. For Paneth cell counting, sectioned small bowel was immunostained for lysozyme, and the number of mature Paneth cells (those with dark-stained and well-formed granules) was counted for each crypt. Fifty crypts were analyzed per genotype. Finally, colons of each strain of study mice were analyzed at ×20 magnification to determine the mean percentage of enterocyte nuclei that immunostained strongly for hepatocyte nuclear factor 3β (HNF-3β). For WT, Min/+ and ERβ− /−Min/+, 60 different well-oriented crypts were analyzed; for ERα+/−Min/+, 48 crypts were analyzed. In all instances, counting was evaluated by an individual blinded to the genotype of specimens. One way analysis of variance was used to test the overall differences among all groups. Closed testing procedure for subgroup comparison was used to control familywise error rate when the overall test was statistically significant at α = 0.05 level. All the statistical tests were two sided and were performed using SAS 9.3 (Cary, NC).
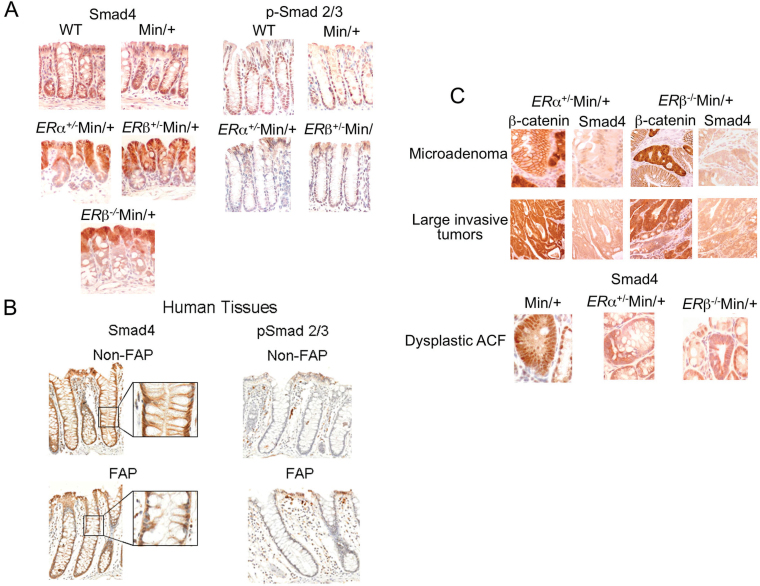
Fig. 1.
Smad4 protein was downregulated in colon dysplastic ACF, microadenomas and invasive tumors of ER-deficient Min/+ mice. Representative serial sections of colon tissues from female ERα+/−Min/+, ERβ+/−Min/+, ERβ− /−Min/+, ERαβ+/+Min/+ (Min/+) and WT mice were used for Smad4 immunohistochemistry that showed different staining patterns at the base of crypts (A, left). Nuclear-localized Smad4 was apparent in enterocytes of normal adjacent crypts (A, top left). The absence of Smad4 expression was apparent in ×40 magnification images of ERα+/−Min/+ and ERβ− /−Min/+ colon (A, middle/bottom left). Smad4 and p-Smad2/3 immunostaining of FAP colon at different magnifications is also shown (B, left). The presence of nuclear p-Smad2/3 staining is shown in WT and Min/+ colon but not in ERα+/−Min/+, ERβ− /−Min/+ colon (A, right; B, right). The low abundance of nuclear Smad4 is shown in microadenomas, invasive tumors and dysplastic ACF, identified in serial-sectioned specimens by β-catenin immunohistochemistry in colon of ER-deficient Min/+ mice (C). Nuclear staining of Smad4 is visible in adjacent normal crypts.
Results
ER deficiency in Min/+ mice altered the nuclear localization in crypt base cells of Smad4, the common TGFβ- and BMP-signaling regulator
Unlike the small intestine, where Min/+ mice develop numerous adenomas, dysplastic ACF in the colon typically remain unicryptal and adenoma growth is curtailed (20). Because loss of signaling by TGFβ favors inflammation and colon tumor formation in mice, we speculated that tumor-suppressive effects of this signaling pathway inhibit de novo crypt expansion in the normal adult colon (21–23). First, we examined the location and relative expression of the transcriptional regulator, Smad4. In agreement with others, nuclear expression of Smad4 was prevalent in enterocytes at the base of crypts in the colon of WT and Min/+ mice (Figure 1A, top left) (22). A strikingly different staining pattern was consistently observed in ER-deficient Min/+ colon, with a relocation of the nuclear Smad4 signaling from the crypts to the luminal surface of the colon and minimal expression of Smad4 in crypt base enterocytes and nearby stromal cells (Figure 1A, middle/bottom left). In the small intestine, normal nuclear Smad4 expression in crypt base cells was seen in ER-deficient Min/+ mice (data not shown) (20). To investigate the relevance of this change in the location of Smad4 expression to human CRC, we performed immunohistochemistry for Smad4 using a colon specimen from a patient with FAP. A similar downregulation of Smad4 expression and nuclear localization from the middle to the base of crypts was noted (Figure 1B, left). To confirm peri- cryptal downregulation of TGFβ signaling, we examined expression of phosphorylated Smad2 and Smad3 (p-Smad2/3), downstream targets of TGFβ receptor activation. These proteins were expressed in the nucleus of colon crypt base cells of controls but not in the ER-deficient Min/+ mice (Figure 1A, right). Available methods for p-Smad2/3 immunohistochemistry of human tissues did not show this change (Figure 1B, right).
Previously, we showed that ER loss in Min/+ mice produced invasive carcinomas of the proximal colon. Here, we found that, as colon tumors grew by crypt fission from microadenomas to large invasive tumors, loss of Smad4 expression occurred in the ER-deficient Min/+ strains (Figure 1C, top/middle). Min/+ mice without ER loss very rarely develop colon adenomas but occasionally demonstrate dysplastic ACF. A comparison of dysplastic ACF between Min/+ mice with and without ER loss shows that nuclear Smad4 expression is lost in dysplastic ACF from ER-deficient Min/+ mice but preserved in those from Min/+ mice with intact ER (Figure 1C, bottom). Collectively, these results suggest that ER loss in Min/+ colon and tumors affected TGFβ signaling.
ER deficiency in Min/+ mice altered the colonic crypt microenvironment
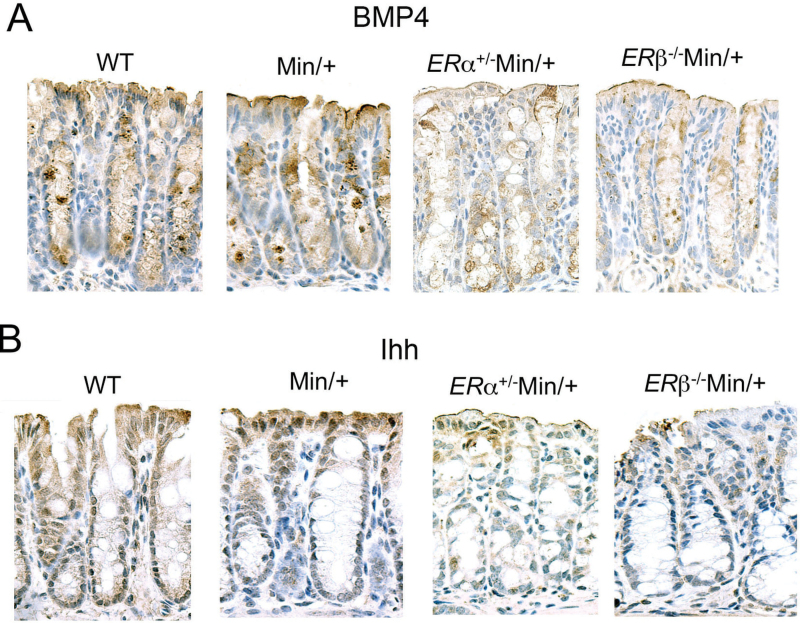
BMP4 is a member of the TGFβ ligand superfamily. Paracrine secretion of BMP4 by pericryptal myofibroblasts curtails stem cell duplication and limits Wnt signaling in crypt ISCs and progenitors (24,25). Bmp4 is also a β-catenin-Tcf4 target gene that is expressed by differentiated colonocytes near the mucosal surface (26). When we examined BMP4 expression, we found reduced protein levels in the colonic crypts of ERα+/−Min/+ and ERβ− /−Min/+ mice (Figure 2A). To modulate Wnt signaling, epithelial and adjacent mesenchymal cells communicate throughout the length of crypts via autocrine and paracrine BMPs and hedgehog pathways. Similar to BMP4, Indian hedgehog (Ihh) is expressed by enterocytes at the luminal end of crypts (27). Our staining conditions reproduced the increased expression pattern of Ihh in cells at the top of crypts in WT and Min/+ colon (Figure 2B, left) as reported (27). However, like BMP4, Ihh expression was reduced in colon of the ER-deficient Min/+ strains (Figure 2B, right).
Fig. 2.
BMP4 expression was reduced in ER-deficient Min/+ mice. Representative immunohistochemistry of sectioned proximal colons from female WT and Min/+ mice compared with ERα+/−Min/+ and ERβ−/−Min/+ mice for BMP4 (A). Original magnification was ×40. Note, there is artificial uptake of antibody reagents with mucins in the goblet cells. Representative immunohistochemistry of sectioned proximal colons from study mice for Ihh (B). Original magnification was ×40.
ER deficiency in Min/+ mice reduced nuclear HNF3β signaling in non-tumor mucosa
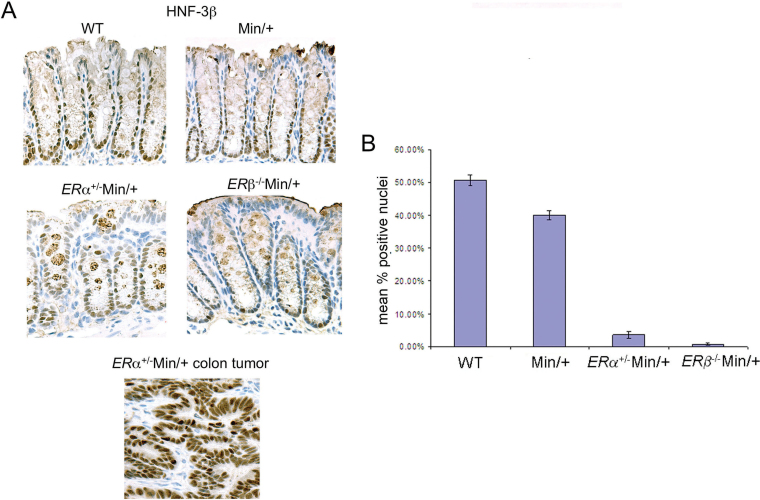
HNF3β is an adhesion-dependent transcriptional activator required for gut development that cooperates with hedgehog signaling in enterocytes at the crypt base (28). Appropriately, immunohistochemistry for HNF3β in non-tumor colon mucosa of WT and Min/+ mice showed strong positive nuclear staining of crypt base cells, but in the ER-deficient Min/+ animals, staining for this protein was reduced (Figure 3A, top/middle). Counting the number of strongly stained nuclei in these mice showed that ER loss decreased the number of HNF3β+ cells per crypt, relative to ER +/+Min/+ mice (Figure 3A, middle). In contrast, strong nuclear HNF3β expression was present in ER-deficient colonic tumors (Figure 3A, bottom).
Fig. 3.
Nuclear HNF3β expression was diminished in crypt base cells of ER-deficient Min/+ mice but not in tumors. Representative immunohistochemistry of sectioned proximal colons from female WT and Min/+ mice compared with ERα+/−Min/+ and ERβ− /−Min/+ mice for HNF3β at ×40 magnification and a graph of the relative number of strongly stained nuclei (A). HNF3β immunohistochemistry performed in parallel showed intense nuclear staining in a large colon tumor (A, bottom). Compared with WT mice, ERα+/−Min/+, ERβ− /−Min/+ and ER +/+Min/+ all had significantly less HNF3β+ cells per crypt (47, 50 and 11% reduction, respectively; P < 0.0001) (B). In addition, mice with either ERα+/−Min/+ or ERβ− /−Min/+ had significantly decreased numbers of HNF3β+ cells per crypt than ER +/+Min/+ mice (36 and 39% reduction, respectively; P < 0.0001). There was no difference in the number of HNF3β+ cells per crypt between ERβ−/−Min/+ and ERα+/−Min/+ mice (3% reduction, P = 0.10).
ER deficiency in Min/+ mice stimulated intestinal crypt expansion
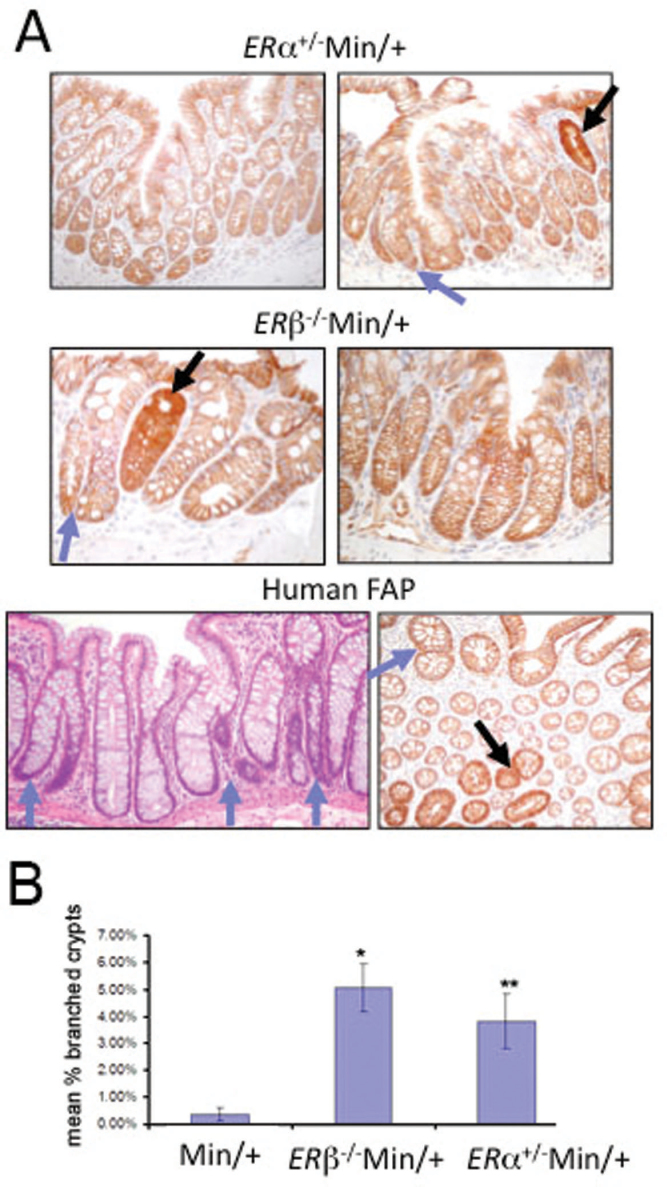
In the colon of both humans with FAP and Min/+ mice, APC-deficient dysplastic ACF are the earliest adenoma precursors. These dysplastic lesions grow into visible tumors by symmetrical stem cell replication and crypt branching and are identifiable from normal crypts by strongly increased expression of β-catenin (29). Previously, we performed β-catenin immunohistochemistry on colon sections to determine the incidence of dysplastic ACF in 3- to 4-month-old mice, and found increased frequency of these lesions in ER-deficient Min/+ compared with ER +/+Min/+ strains (20). In female, age-matched ER +/+Min/+ mice, branching in non-tumor colon mucosa was rarely observed, and these mice had a low incidence of colon tumors (20). As shown in Figure 4A, dysplastic ACF (black arrows) were commonly observed in both ERα+/−Min/+ and ERβ− /−Min/+ mice in regions of colon that also showed increased branching of non-dysplastic crypts (blue arrows). Similar branching can be found in human colon specimens from patients with FAP (data not shown). The incidence of non-dysplastic branched crypts was counted in non-tumor colon of Min/+ mice relative to those of ERα+/−Min/+ and ERβ− /−Min/+ mice and was ~5-fold higher in the ER-deficient Min/+ strains (Figure 4B). Crypt branching is produced by symmetrical stem cell division, an event which does not occur in an adult animal under normal conditions. Because of this, our data suggest that ER loss alters stem cell behavior in the proximal colon of ER-deficient mice.
Fig. 4.
ER deficiency stimulated intestinal crypt expansion in adult Min/+ mice. Representative immunohistochemistry for β-catenin revealed crypt fission in the colon of ER-deficient Min/+ mice (A, top). Dysplastic ACF are indicated by increased β-catenin expression (black arrows); crypt fission in non-dysplastic tissue are illustrated (blue arrows). Hematoxylin and eosin staining (A, bottom left) shows crypt fission in non-tumor colon from a patient with FAP. Primary branching appears within three crypts (blue arrows, A, bottom right). In tissue sectioned obliquely with respect to the crypt axis, β-catenin immunostaining (bottom right) shows crypt fission in colon of another FAP patient. The mean percent of branched crypts in Min/+ versus ER-deficient Min/+ mouse colons were counted using four mice of each genotype (B). Error bars represent standard error of the mean. Comparing with Min/+ mice, ERα+/−Min/+ and ERβ− /−Min/+ mice had significantly more non-dysplastic branched crypts (0.8, 5 and 3.8% for Min/+, ERα+/−Min/+ and ERβ− /−Min/+ mice, respectively; P = 0.0002 for Min/+ versus ERα+/−Min/+ and P = 0.002 for Min/+ versus ERβ− /−Min/+). It indicated ER-deficient Min/+ strains had about a 5-fold increase in the incidence of non-dysplastic branched crypts.
ER deficiency results in decreased Paneth cell numbers
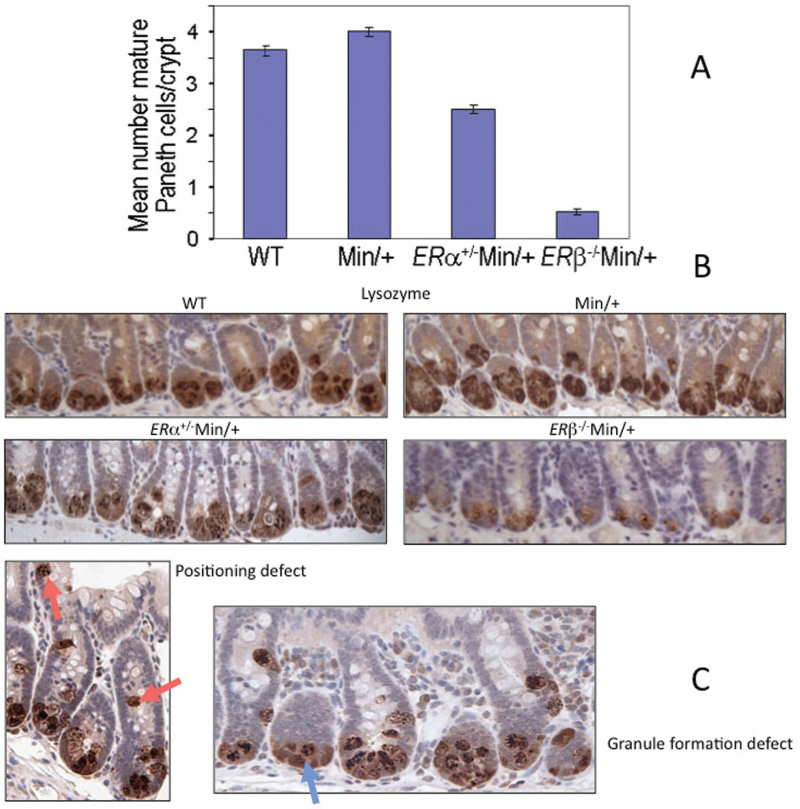
Studies to this point showed differences in the colon but not in the small intestine of ERα+/−Min/+ and ERβ−/−Min/+ mice compared with tissues from ER +/+Min/+ animals. Previously, we found that ER loss altered colonic differentiation, as evidenced by markedly reduced numbers of mature goblet cells in ERα+/−Min/+, ERβ+/−Min/+ and ERβ− /−Min/+ mice (20). To study differentiation in the small intestine, we determined the relative number of mature Paneth cells, which are specialized secretory enterocytes that form postnatally in the small intestine and localize to the base of crypts interspersed among ISCs. Maturation of these cells depends on β-catenin-mediated Wnt signaling (30). The relative number of Paneth cells is relevant to the incidence of crypt fission in the intestine because these cells provide essential niche support for ISCs (31,32). We found that ER deficiency in Min/+ mice reduced the mean number of Paneth cells in ileum relative to Min/+ and WT animals, yielding Paneth cell numbers per crypt that were decreased by 38% in ERα+/−Min/+ and 88% in ERβ− /−Min/+ mice (Figure 5A). Immunohistochemistry for Paneth cell marker lysozyme revealed reduced granule formation, suggesting decreased cell differentiation, in specimens from both ERα+/−Min/+ and ERβ− /−Min/+ mice but not in controls (Figure 5B). In ERα+/−Min/+ mice only, Paneth cell location was not restricted to the crypt base (Figure 5C, left). Given that crypt fission reflects stem cell duplication and that Paneth cells in crypts are derived from ISCs, these results suggest that ER loss affects both ISC duplication and differentiation.
Fig. 5.
ER-deficient Min/+ mice have reduced numbers of ISC-supporting Paneth cells in the small intestine compared with ERα/β+/+Min/+ and WT mice. Immunohistochemistry for lysozyme was used to identify Paneth cells, and the mean number per crypt for sectioned WT, Min/+, ERα+/−Min/+ and ERβ− /−Min/+ small intestine was counted using four mice of each genotype (A). Error bars represent standard error of the mean. All other comparisons between genotypes were significant (P < 0.0001). There was a trend toward more mature Paneth cells per crypt in Min/+ compared with WT, but it was not significant (P = 0.1817). Representative lysozyme immunohistochemistry shows Paneth cells localized to the base of crypts in sectioned small bowel of study mice (B). Examples of positioning (left, red arrows) and maturation (right, blue arrow) defects in ERα+/−Min/+ mice (C). The latter, but not the positioning defect, was also observed in ERβ− /−Min/+ mice.
Discussion
Previously, we reported that ERα+/−Min/+, ERβ+/−Min/+ and ERβ− /−Min/+ mice developed increased numbers of proximal colon tumors and accelerated tumor progression when compared with intact ER +/+ female Min/+ mice (20). Here, we used tissues obtained from these experiments to show that ER functions regulate elements of crypt homeostasis in the setting of Apc deficiency. The presence of branched structures in the base of colonic crypts indicated symmetrical stem cell duplication in ER-deficient animals, an event that is normally prevented by properly coordinated signals in the microenvironment of the stem cell niche. This additional characterization of the tumor-prone proximal colon of ER-deficient Min/+ mice showed a shift of location of Smad4 signaling from the crypt base to the luminal surface (Figure 1), as well as reduced expression of the Wnt-signaling target protein, BMP4, and associated stromal factors Ihh and HNF3β (Figures 2 and 3). These changes are expected to alter regulation of the stem cell niche, and evidence of this is provided by the branching of crypts and propensity for tumor formation in the proximal colon.
TGFβ signaling in crypt base enterocytes recruits stromal cells, and in its classical role induces expression of integrin adhesion receptors and extracellular matrix (ECM) constituents. Loss of either β1 integrin or proper ECM sequestering of latent TGFβ causes intestinal tumor formation (33,34). Hedgehog proteins, including Ihh, are secreted from epithelial cells downstream of HNF3β activity and activate transcription regulator Gli1 in nearby myofibroblasts. This leads to expression of Foxf1, another transcription factor exclusively expressed in mesenchyme. Foxf1 and Foxf2 control gut development by limiting Wnt signaling and promoting ECM production (34). Foxf1 activation also induces expression of BMP4, which we showed was also reduced in ER-deficient Min/+ mice (Figure 2A and B). Wnts, hedgehog-signaling ligands and TGFβ cytokines are sequestered in the ECM and must be presented in specific ways for cognate receptor activation. Taken together, our data suggest that ER loss in the setting of Apc deficiency altered the restrictive signaling microenvironment of the crypt stem cell niche.
Gene knockout studies clearly show that Wnt signaling is essential for ISC viability (4,5,9), and activities that alter the level of Wnt signaling modulate the behavior of resident stem cells (2,6,10). Proteins produced from truncated Apc alleles fail to regulate β-catenin protein levels properly in stem/progenitor cells, altering Wnt signaling in ways that inhibit differentiation (35). ERα and corepressor NcoR1 are expressed in the stem cell compartment of crypts where they may influence Wnt signaling and stem cell maintenance in a setting of Apc deficiency (36). This view is in agreement with work showing that a hypomorphic ERα allele increased tumor incidence in Min/+ mice (37). In the small intestine, the relative number of Paneth cells is a surrogate for Wnt signaling, since β-catenin-Tcf4 activity is required for their differentiation (30). For instance, the β-catenin-Tcf4 target gene, Sox9, is needed for Paneth cell maturation (38). Interestingly, liganded ERα repressed Sox9 gene transcription in mammary epithelial cells (39). If E2-ERα normally inhibits Sox9 in ISC daughter cells, this could explain why ERβ− /−Min/+ mice had the fewest Paneth cells (Figure 5B), because in these circumstances ERα activity would be unopposed. Paneth cells provide crucial support functions for ISCs in small bowel crypts but are not present in the colon, suggesting that colonic pericryptal stroma plays a more stringent role in regulating the ISC microenvironment. Concerning the Paneth cell mislocalization we observed in ERα-deficient Min/+ mice (Figure 5C), winged helix transcription factor Foxl1, which is expressed in pericryptal mesenchyme, regulates Wnt signaling in crypt base cells, and our phenotype resembles that of Foxl1 null mice (40). Consistent with the role of mesenchyme-inhibiting tumorigenesis in the context of the Apc Min allele, colon tumor multiplicity was also increased in Foxl1 − /−Min/+ mice (41). In humans, Paneth cell deficiency is associated with chronic intestinal inflammation in ileal Crohn’s disease and causes barrier function defects (31). Taken together, these findings are consistent with the hypothesis that ER loss affects ISC survival within its microenvironment, for both the murine colon and small intestine.
ERα and ERβ begin to be expressed in the gut and elsewhere during the hormonal surge of prepuberty, a period when the rate of crypt fission is normally accelerated (6). Intestinal tumors in patients with classical FAP and the Min/+ mouse also arise at this time. Crypt expansion indicates effects at the level of the stem cell population that necessarily involve epithelial–mesenchymal signaling crosstalk and that directly or indirectly change the crypt cycle rate. These considerations and our results implicate ERα and ERβ functions in negatively regulating the crypt cycle in the normal adult colon. Present data do not permit application of safe and effective chemoprevention to target ER activity as E2 replacement after menopause stimulates mammary and uterine epithelial cell growth contributing to the cancer burden in these tissues, and the role of selective estrogen response modifiers in CRC development is unclear. Nevertheless, our work and results from prevention clinical trials argue for continued study of ER modulation to achieve CRC prevention.
Supplementary material
Supplementary Table 1 can be found at http://carcin.oxfordjournals.org/
Funding
National Cancer Institute (K05-CA131504 to M.M.B.); National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases (2T32DK007754-12 to R.M.H.).
Supplementary Material
Acknowledgements
We wish to thank Dr Mark Redston for his help with histological interpretations and Ms Hira Rizvi for her technical support.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- ACF
aberrant crypt foci
- APC/Apc
adenomatous polyposis coli
- BMP
bone morphogenetic protein
- CRC
colorectal cancer
- E2
17β-estradiol
- ECM
extracellular matrix
- ERα/β
estrogen receptor α/β
- FAP
familial adenomatous polyposis
- HNF3β
hepatocyte nuclear factor 3β
- Ihh
Indian hedgehog
- ISC
intestinal stem cell
- Min/+
C57BL/6J-Min/+
- TGFβ
transforming growth factor-β
- WT
C57BL/6J-Apc+/+.
References
- 1. Barker N., et al. (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature, 457, 608–611 [DOI] [PubMed] [Google Scholar]
- 2. Medema J.P., et al. (2011). Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature, 474, 318–326 [DOI] [PubMed] [Google Scholar]
- 3. Zeki S.S., et al. (2011). Stem cells and their implications for colorectal cancer. Nat. Rev. Gastroenterol. Hepatol., 8, 90–100 [DOI] [PubMed] [Google Scholar]
- 4. Cheng H., et al. (1985). Whole population cell kinetics and postnatal development of the mouse intestinal epithelium. Anat. Rec., 211, 420–426 [DOI] [PubMed] [Google Scholar]
- 5. Dehmer J.J., et al. (2011). Expansion of intestinal epithelial stem cells during murine development. PLoS ONE, 6, e27070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bjerknes M., et al. (1997). APC mutation and the crypt cycle in murine and human intestine. Am. J. Pathol., 150, 833–839 [PMC free article] [PubMed] [Google Scholar]
- 7. Aoki K., et al. (2007). Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J. Cell. Sci., 120(Pt 19), 3327–3335 [DOI] [PubMed] [Google Scholar]
- 8. Engles S.J., et al. (1999). Transforming growth factor beta1 suppresses nonmetastatic colon cancer at an early stage of tumorigenesis. Cancer Res., 59, 3379–3386 [PubMed] [Google Scholar]
- 9. Korinek V., et al. (1998). Depletion of epithelial stem cell compartment in the small intestine of mice lacking Tcf-4. Nature Genet., 19, 379–383 [DOI] [PubMed] [Google Scholar]
- 10. Fevr T., et al. (2007). Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell. Biol., 27, 7551–7559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massagué J. (2008). TGFbeta in cancer. Cell, 134, 215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Labbé E., et al. (2007). Transcriptional cooperation between the transforming growth factor-beta and Wnt pathways in mammary and intestinal tumorigenesis. Cancer Res., 67, 75–84 [DOI] [PubMed] [Google Scholar]
- 13. Zhang M., et al. (2007). Polarity of response to transforming growth factor-beta1 in proximal tubular epithelial cells is regulated by beta-catenin. J. Biol. Chem., 282, 28639–28647 [DOI] [PubMed] [Google Scholar]
- 14. Rossouw J.E., et al. ; Writing Group for the Women’s Health Initiative Investigators (2002). Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA, 288, 321–333 [DOI] [PubMed] [Google Scholar]
- 15. Chlebowski R.T., et al. ; Women’s Health Initiative Investigators (2004). Estrogen plus progestin and colorectal cancer in postmenopausal women. N. Engl. J. Med., 350, 991–1004 [DOI] [PubMed] [Google Scholar]
- 16. Phiel K.L., et al. (2005). Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol. Lett., 97, 107–113 [DOI] [PubMed] [Google Scholar]
- 17. Issa J.P., et al. (1994). Methylation of the oestrogen receptor CpG island link ageing and neoplasia in human colon. Nature Genet., 7, 536–540 [DOI] [PubMed] [Google Scholar]
- 18. Tominaga K., et al. (2005). Prediction of colorectal neoplasia by quantitative methylation analysis of estrogen receptor gene in nonneoplastic epithelium from patients with ulcerative colitis. Clin. Cancer Res., 11(24 Pt 1), 8880–8885 [DOI] [PubMed] [Google Scholar]
- 19. Weyant M.J., et al. (2001). Reciprocal expression of ERalpha and ERbeta is associated with estrogen-mediated modulation of intestinal tumorigenesis. Cancer Res., 61, 2547–2551 [PubMed] [Google Scholar]
- 20. Cho N.L., et al. (2007). Estrogen receptors alpha and beta are inhibitory modifiers of Apc-dependent tumorigenesis in the proximal colon of Min/+ mice. Cancer Res., 67, 2366–2372 [DOI] [PubMed] [Google Scholar]
- 21. Shull M.M., et al. (1992). Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature, 359, 693–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takaku K., et al. (1998). Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell, 92, 645–656 [DOI] [PubMed] [Google Scholar]
- 23. Sodir N.M., et al. (2006). Smad3 deficiency promotes tumorigenesis in the distal colon of ApcMin/+ mice. Cancer Res., 66, 8430–8438 [DOI] [PubMed] [Google Scholar]
- 24. Mifflin R.C., et al. (2011). Intestinal myofibroblasts: targets for stem cell therapy. Am. J. Physiol. Gastrointest. Liver Physiol., 300, G684–G696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He X.C., et al. (2004). BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat. Genet., 36, 1117–1121 [DOI] [PubMed] [Google Scholar]
- 26. van de Wetering M., et al. (2002). The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell, 111, 241–250 [DOI] [PubMed] [Google Scholar]
- 27. van den Brink G.R., et al. (2004). Indian hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat. Genet., 36, 277–282 [DOI] [PubMed] [Google Scholar]
- 28. Zaret K. (1999). Developmental competence of the gut endoderm: genetic potentiation by GATA and HNF3/fork head proteins. Dev. Biol., 209, 1–10 [DOI] [PubMed] [Google Scholar]
- 29. Yamada Y., et al. (2005). Opposing effects of DNA hypomethylation on intestinal and liver carcinogenesis. Proc. Natl Acad. Sci. USA, 102, 13580–13585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Es J.H., et al. (2005). Wnt signaling induces maturation of Paneth cells in intestinal crypts. Nature Cell Biol., 7, 381–386 [DOI] [PubMed] [Google Scholar]
- 31. Wehkamp J., et al. (2010). Paneth’s disease. J. Crohns. Colitis, 4, 523–531 [DOI] [PubMed] [Google Scholar]
- 32. Sato T., et al. (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature, 469, 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoshinaga K., et al. (2008). Perturbation of transforming growth factor (TGF)-beta1 association with latent TGF-beta binding protein yields inflammation and tumors. Proc. Natl Acad. Sci. USA, 105, 18758–18763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ormestad M., et al. (2006). Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development, 133, 833–843 [DOI] [PubMed] [Google Scholar]
- 35. Brabletz S., et al. (2009). Gastrointestinal stem cells in development and cancer. J. Pathol., 217, 307–317 [DOI] [PubMed] [Google Scholar]
- 36. Giannakis M., et al. (2006). Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J. Biol. Chem., 281, 11292–11300 [DOI] [PubMed] [Google Scholar]
- 37. Cleveland A.C., et al. (2009). Disruption of estrogen receptor signaling enhances intestinal neoplasia in ApcMin/+ mice. Carcinogenesis, 30, 1581–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bastide P., et al. (2007). Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J. Cell Biol., 178, 635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carroll J.S., et al. (2006). Genome-wide analysis of estrogen receptor binding sites. Nat. Genet., 38, 1289–1297 [DOI] [PubMed] [Google Scholar]
- 40. Takano-Maruyama M., et al. (2006). Foxl1-deficient mice exhibit aberrant epithelial cell positioning resulting from dysregulated EphB/EphrinB expression in the small intestine. Am. J. Physiol. Gastrointest. Liver Physiol., 291, G163–G170 [DOI] [PubMed] [Google Scholar]
- 41. Perreault N., et al. (2005). Foxl1 is a mesenchymal Modifier of Min in carcinogenesis of stomach and colon. Genes Dev., 19, 311–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.