Abstract
Since p14 ARF and human papillomavirus (HPV) 16 E6/E7 oncoproteins are important regulators participating in the p53/Rb pathways, genetic variations of p14 ARF may modify tumor HPV16 status and survival of HPV16-positive squamous cell carcinoma of the oropharynx (SCCOP) patients. We determined tumor HPV16 status and expression of p14/p53 and genotyped p14 ARF-rs3731217 and -rs3088440 polymorphisms in 552 incident SCCOP patients. We found that patients having variant genotypes for each p14 ARF polymorphism were approximately two or three times as likely to have HPV16-positive tumors compared with patients with corresponding common homozygous genotype, and such an association was particularly pronounced in patients with variant genotypes of both polymorphisms. After definitive chemoradiotherapy, patients having p14 ARF rs3731217 TG/GG variant genotypes had significantly better overall, disease-specific and disease-free survival than those having TT genotype, respectively. Multivariable analysis found that patients with p14 ARF-rs3731217 TT genotype had an ~7-, 11- and 3-fold increased risk for death overall, death due to SCCOP and recurrence than those with TG/GG variant genotypes, respectively. Furthermore, such significantly prognostic effect was also found when survival analysis was limited to HPV16-positive patients. Additionally, potentially functional relevance of the two variants was characterized to explore the genotype–phenotype correlation. Our findings indicate p14 ARF variants may predict tumor HPV16-positive SCCOP patients and survival.
Introduction
Although tobacco and alcohol exposures remain significant risk factors for squamous cell carcinoma of the oropharynx (SCCOP), human papillomavirus (HPV) infection has accounted for a growing proportion of cases, particularly among the middle-aged population (1–3). Since tumor HPV-positive SCCOP patients have been shown to have a better prognosis than tumor HPV-negative SCCOP patients, it appears that HPV status is highly relevant to SCCOP prognosis (4). Thus, identification of individual SCCOP patient’s tumor HPV status is of utmost clinical relevance. Although there have been many attempts to develop biomarkers for progression and prognosis of SCCOP as well as for response to anticancer treatment, some results remain controversial because of confounding by HPV status and an admixture of cancer sites (5). Although HPV-positive SCCOP patients had improved survival and lower recurrence rates, the significant heterogeneity in outcomes among HPV-positive SCCOP patients remains a clinical problem: most do exceptionally well, but smokers with HPV-positive SCCOP have worse outcomes, in some studies similar to those of patients with HPV-negative SCCOP. To date, SCCOP have few prognostic markers targetable for improving prevention and treatment strategies. Since HPV status is highly relevant to SCCOP prognosis and there is significant heterogeneity in outcomes of HPV-positive SCCOP patients, new biomarkers that determine HPV status and further stratify HPV-positive SCCOP patients will help avoid both overtreatment and undertreatment of SCCOP, resulting in better survival and quality of life for patients with this disease.
Epidemiological studies have shown that infection of high-risk HPV plays an etiological role in SCCOP development (6–11), while only a small proportion of individuals who have contracted HPV16 infection will develop SCCOP, suggesting that genetic variants in key genes of the host may cause an interindividual variation in susceptibility to HPV16 infection and related SCCOP. p14 ARF plays an important role in the p53/Rb pathways to regulate the cell cycle and apoptosis (12–16). While the oncogenic potential of HPV16 has been recognized in negatively affecting cell cycle regulation, DNA repair and apoptosis by abrogating key functions of tumor suppressor genes, such as p53, Rb and p14 (17). Thus, p14 ARF may maintain genomic stability by mediating cellular activities, which could have a significant biological impact on tumor HPV status and prognosis of HPV-associated cancers (18). Few studies have been reported for p14 ARF germline genetic variants on tumor HPV status and prognosis of HPV-positive SCCOP. Identification of such biomarkers, such as p14 ARF variants, could help clinicians define an individualized genetic profile of HPV16-positive SCCOP, and lead to future individualized prevention and treatment. Therefore, in this study, we aimed to evaluate whether p14 ARF genetic variants modify tumor HPV16 status and survival among HPV-positive SCCOP patients as well as their potentially functional relevance in a cohort of 552 incident SCCOP patients. We included the two common p14 ARF-rs3731217 and rs3088440 tagging single-nucleotide polymorphisms with a minor allele frequency >5% and the linkage disequilibrium measure r 2 threshold at 0.8 for final analysis in this study.
Materials and methods
Study patients
Study participants, a homogeneous group of patients with an incident SCCOP, were recruited consecutively from an ongoing molecular epidemiology study of squamous cell carcinoma of the head and neck at The University of Texas MD Anderson Cancer Center after the approval of the institutional review board and informed consent from the patients. A total of 552 tumor specimens with genomic DNA samples from the blood between December 1996 and July 2011 were available from the parent study. Details for subject recruitment have been described previously (19). Medical record review for follow-up status of all patients was performed under direct supervision of the senior author and staff head and neck surgeon. Primary tumor subsite, clinical stage, treatment, recurrence-free survival, disease-specific survival (DSS) and overall survival (OS) were reviewed from medical records as assessed between the initial and final patient contact recorded and medical comorbidites. All patients included in survival analyses were treated with definitive chemoradiation for curative intent at our institution.
HPV16 detection and p14 ARF genotyping
Paraffin-embedded tissues of 309 patients were used to extract DNA for tumor HPV16 detection using the PCR and in situ hybridization methods described in our previous studies (19,20). We also included additional 243 SCCOP patients whose tumor HPV status was clinically available for HPV16 by in-situ hybridization and p16 immunohistochemisrty. More recently, the tumor HPV data were now part of the patient’s clinical record as the pathology laboratory at MD Anderson classified all SCCOP specimens as a standard clinical practice. p14 ARF polymorphisms were genotyped with isolated genomic DNA from a leukocyte cell pellet of the blood samples, and details for genotyping were also described previously (21). For the quality control purpose, ~10% of the samples for tumor HPV16 status and p14 genotyping were repeated, and the results of the rerun samples were 100% concordant.
Immunohistochemical staining
A total of 57 tissue specimens from another subset of 57 incident SCCOP patients, who were more recently recruited, were used for the staining. After the formalin-fixed tissue specimens were deparaffinized, rehydrating and blocking endogenous peroxidase activity antigen was retrieved by microwaving slides in 10mM citrate buffer, pH 6.0, for 15min on high plus 5min on 50% power. Slides were treated with blocking reagent (Biocare, Concord, CA) and incubated with antibodies specific for p14 and p53 (Cell signaling Technologies, Danvers, MA) for 2 h. After washing, slides were incubated with secondary anti-rabbit horseradish peroxidase (Dako, Carpinteria, CA) for 30min and stained using 3,3'-diaminobenzidine reagent (Dako). Stained slides were then examined microscopically and scored for average positive intensity (API) or percentage of positive nuclei.
Statistical analysis
Statistical Analysis System software (Version 9.3; SAS Institute, Cary, NC) was used for all of the statistical analyses. All of the tests were two sided, and statistical significance was determined by P < 0.05. We first evaluated the differences in the distributions of selected variables and p14 ARF genotype frequencies between HPV16-positive and HPV16-negative cases by using the χ2 test, and t-test was also used to compare the expression levels of p14/p53 between HPV16-positive and HPV16-negative SCCOP patients and between different genotyping groups. We estimated the association between p14 ARF genotypes and tumor HPV16 positivity among SCCOP by computing the odds ratios (ORs) and 95% confidence intervals (CIs) using both univariate and multivariable logistic regression analyses. For survival analysis, only patients treated with definitive chemoradiotherapy at our institution for curative intent (n = 285) were included. SCCOP patients were typically followed and monitored through their treatment and posttreatment courses with regularly scheduled clinical and radiographic examinations. OS was defined as the time from first appointment to death from any cause or date of last follow-up. Participants who were alive at the end of the study period or lost to follow-up were considered censored. DSS was defined as the time from first appointment to death from disease or date of last follow-up. Participants who were alive at the end of the study period or lost to follow-up were considered censored. Disease-free survival (DFS) was defined as the time from date of end of treatment to date of last follow-up or date of clinically detectable recurrent cancer (local, regional and distant). Participants who were recurrence-free or lost to follow-up were considered censored. Log-rank test was carried out for analyses between various variables and survival endpoints, and Cox’s proportional hazards regression analysis was used to calculate hazard ratio (HR) and 95% CI. The Cox model included adjustment for potential confounding effect.
Results
Patient characteristics
Of the 552 patients with SCCOP enrolled in this study, a total of 74 patients died. Thirty-five patients died of their primary malignancy (e.g. cervical node metastasis, local recurrence). The second most frequent cause of death was second primary cancer (n = 19) including 9 head and neck cancers, 4 lung cancers, 3 esophageal cancers, 1 bladder cancer and others, followed in frequency by death from causes unrelated to cancer (n = 20). Table I presents the distribution of demographic variables, risk factors and clinical characteristics of the study patients. There was no statistically significant difference in age (P = 0.209), ethnicity (P = 0.141), alcohol drinking (P = 0.583), comorbidity (P = 0.387), disease staging (P = 0.741) and treatment (P = 0.100) between HPV16-negative and HPV16-positive SCCOP patients. However, a significant or borderline significant difference was observed for sex or smoking status (P = 0.001 for sex and P = 0.047 for smoking).
Table I.
Distribution of selected variables in SCCOP patients by tumor HPV16 status
| Variable | HPV16-positive patients (n = 439) | HPV16-negative patients (n = 113) | P valuea | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Age | |||||
| ≤50 years | 136 | 31.0 | 42 | 37.2 | 0.209 |
| >50 years | 303 | 69.0 | 71 | 62.8 | |
| Sex | |||||
| Male | 384 | 87.5 | 85 | 75.2 | 0.001 |
| Female | 55 | 12.5 | 28 | 24.8 | |
| Ethnicity | |||||
| Non-Hispanic white | 404 | 92.0 | 99 | 87.6 | 0.141 |
| Others | 35 | 8.0 | 14 | 12.4 | |
| Tobacco smoking | |||||
| Ever | 246 | 56.0 | 75 | 66.4 | 0.047 |
| Never | 193 | 44.0 | 38 | 33.6 | |
| Alcohol drinking | |||||
| Ever | 331 | 75.4 | 88 | 77.9 | 0.583 |
| Never | 108 | 24.6 | 25 | 22.1 | |
| Comorbidity | |||||
| None to mild | 407 | 92.7 | 102 | 90.3 | 0.387 |
| Moderate to severe | 32 | 7.3 | 11 | 9.7 | |
| Staging | |||||
| I–II | 31 | 7.1 | 9 | 8.0 | 0.741 |
| III–IV | 408 | 92.9 | 104 | 92.0 | |
| Treatment | |||||
| XRTb | 119 | 27.1 | 35 | 31.0 | 0.100 |
| XRT + chemo. and/or surgery | 280 | 63.8 | 61 | 54.0 | |
| Othersc | 40 | 9.1 | 17 | 15.0 | |
aTwo-sided χ2 test.
bXRT, radiotherapy; chemo, chemotherapy.
cPalliative treatment and/or treatment at outside institution.
Association between p14 ARF genotypes and HPV16-positive SCCOP
Table II summarizes the associations between tumor HPV16 status and genotypes of p14 ARF-rs3088440 and -rs3731217 polymorphisms. Genotype distributions for both p14 ARF-rs3731217 and -rs3088440 were significantly different between HPV16-positive and HPV16-negative patients (P = 0.001 for p14 ARF-rs3731217 and P = 0.002 for p14 ARF-3088440). Multivariable analyses showed that for each polymorphism, patients with variant genotypes were approximately two or three times more likely to have HPV16-positive tumors than those with wild-type homozygous genotypes (OR, 2.1; 95% CI, 1.3–3.6 for p14 ARF-rs3731217 and OR, 2.4; 95% CI, 1.4–3.8 for p14 ARF-3088440). Furthermore, we found patients who carried two variant genotypes were approximately eight times more likely to be tumor HPV16-positive than those with wild-type homozygous genotype of both polymorphisms (OR, 8.0; 95% CI, 2.8–22.3) (Table II) and patients possessing either variant allele (p14 ARF G or p14 ARF A) were approximately two times more likely to have a HPV16-positive tumor than those without any variant genotypes (OR, 1.9; 95% CI, 1.2–2.9) (data not shown).
Table II.
Association of p14 ARF genotypes with tumor HPV16 status in SCCOP patients
| p14 ARF genotypes | HPV16-positive patients (n = 439) | HPV16-negative patients (n = 113) | P | Adjusted OR (95% CI)a | ||
|---|---|---|---|---|---|---|
| Number | % | Number | % | |||
| p14 ARF-rs3731217 | ||||||
| TT (Ref.b) | 269 | 61.3 | 88 | 77.9 | 0.001 | 1.0 |
| TG+GG | 170 | 38.7 | 25 | 22.1 | 2.1 (1.3–3.6) | |
| p14 ARF-rs3088440 | ||||||
| GG | 276 | 62.9 | 89 | 78.8 | 0.002 | 1.0 |
| GA+AA | 163 | 37.1 | 24 | 21.2 | 2.4 (1.4–3.9) | |
| Combined variant genotypes | ||||||
| 0 (Ref.b,c) | 196 | 44.6 | 68 | 60.2 | <0.001 | 1.0 |
| 1c | 153 | 34.9 | 41 | 36.3 | 1.3 (0.8–2.1) | |
| 2c | 90 | 20.5 | 4 | 3.5 | 8.0 (2.8–22.3) | |
| Trend | <0.0001 | |||||
P values for χ2 test for genotype distribution.
aAdjusted for age, sex, ethnicity, smoking and alcohol status in a logistic regression model.
bRef. = reference group.
c0: p14 ARF-rs3731217 TT and p14 ARF-rs3088440 GG genotypes; 1: p14 ARF-rs3731217 TT and p14 ARF-rs3088440 GA+AA or p14 ARF-rs3731217 TG+GG and p14 ARF-rs3088440 GG genotypes and 2: p14 ARF-rs3731217 TG+GG and p14 ARF-rs3088440 GA+AA genotypes.
Association of p14 ARF genetic variants with survival and risk of deaths/recurrence
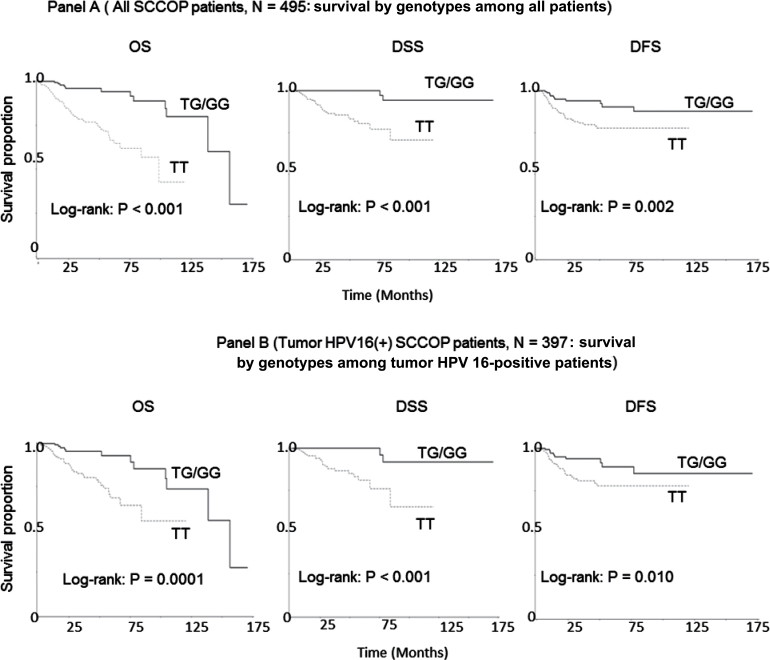
Figure 1A shows the univariate Kaplan–Meier analyses of survival with respect to the death from all causes (OS), death from SCCOP (DSS) and disease recurrence (DFS). Among all 552 SCCOP patients, 57 patients did not receive treatment for curative intent at our institution and were excluded for survival analysis, resulting in 495 cases with chemoradiotherapy and follow-up time for final survival analysis. At a median follow-up time of 24.0 months (mean, 34.7 months; range, 0.2–171 months), 74 deaths from any causes occurred, of which 35 died from SCCOP, and 59 patients had experienced disease recurrence. Survival analyses demonstrated that the differences in OS, DSS and DFS of SCCOP between carriers of p14 ARF-rs3731217 wild-type homozygous TT and variant TG/GG genotypes were statistically significant (log-rank tests: P < 0.001; P < 0.001 and P = 0.002, respectively). SCCOP patients with p14 ARF-rs3731217 TG/GG variant genotypes had better OS, DSS and DFS than the patients with the TT genotype, whereas such differences in survival were not significant for p14 ARF-3088440 polymorphism (log-rank tests: P = 0.982 for OS; P = 0.362 for DSS and P = 0.919 for DFS, respectively).
Fig. 1.
Survival by the genotypes of p14 ARF polymorphisms in SCCOP patients.
The results of multivariable Cox proportional hazards regression analysis regarding the association between p14 ARF polymorphisms and risk of death overall (death from all cause), death from SCCOP and disease recurrence among SCCOP patients are shown in Table III. Estimates of association were adjusted for potential confounders including age, sex, ethnicity, smoking and alcohol status, treatment, T and N stages, comorbidity and tumor HPV status. Compared with patients having p14 ARF-rs3731217 TG/GG variant genotypes, patients with the TT genotype had significantly increased risk of death overall, death from SCCOP and disease recurrence (HR, 6.7; 95% CI, 2.6–17.1 for overall death; HR, 11.2; 95% CI, 2.5–50.6 for death from SCCOP and HR, 2.6; 95% CI, 1.2–5.8 for recurrence). The increased risk for death overall, death from SCCOP and recurrence was also observed for patients with the p14 ARF-3088440 GG genotype compared with GA/AA variant genotypes, but the associations were not statistically significant (Table III). When we further stratified the patients with radiotherapy only and chemoradiotherapy, we found that the significant effect of p14 ARF-rs3731217 on OS only (log-rank test: P = 0.005) was observed among patients with radiotherapy only, whereas such significant modifying effects of p14 ARF-rs3731217 on OS, DSS and DFS were found among patients with chemoradiotherapy (log-rank tests: P < 0.001 for OS, P < 0.001 for DSS and P = 0.007 for DFS, respectively). Moreover, all these significant effects remained after adjustment with potential confounders. However, no significant effect on survival was found for p14 ARF-3088440 polymorphism (log-rank test: all P values >0.05).
Table III.
Multivariable survival analysis by p14 ARF genotypes in 495 SCCOP patients
| p14 ARF genotypes | Total (495) | Events | Survival | ||||
|---|---|---|---|---|---|---|---|
| Death (all causes) | Death (owing to disease) | Recurrence | OS | DSS | DFS | ||
| aHR*, 95% CI | aHR, 95% CI | aHR, 95% CI | |||||
| p14 ARF-rs3731217 | |||||||
| TG+GG | 179 | 12 | 2 | 12 | 1.0 | 1.0 | 1.0 |
| TT | 316 | 62 | 33 | 47 | 6.7 (2.6–17.1) | 11.2 (2.5–50.6) | 2.6 (1.2–5.8) |
| p14 ARF-rs3088440 | |||||||
| GA+AA | 163 | 22 | 8 | 19 | 1.0 | 1.0 | 1.0 |
| GG | 332 | 52 | 27 | 40 | 1.2 (0.6–2.2) | 2.1 (0.8–5.4) | 1.8 (0.8–3.7) |
*Adjusted for age, sex, ethnicity, smoking, alcohol, T stage, N stage, treatment, comorbidity and tumor
HPV16 status in Cox’s models.
Effect of p14 ARF genetic variants on prognosis among HPV16-positive SCCOP patients
When the survival analyses were restricted to tumor HPV16-positive SCCOP patients, we observed similar findings in risk of death and recurrence. Among all 397 tumor HPV16-positive SCCOP patients, 51 deaths from any causes occurred, of which 25 died due to SCCOP, and 45 patients had experienced a disease relapse. As shown in Figure 1B, the differences in OS, DSS and DFS of SCCOP between carriers of p14 ARF-rs3731217 wild-type homozygous TT and variant TG/GG genotypes were statistically significant (log-rank tests: P < 0.001; P < 0.001 and P = 0.010, respectively). The SCCOP patients with p14 ARF-rs3731217 TG/GG variant genotypes had significantly better OS, DSS and DFS than the patients with the TT genotype, whereas such differences in survival were not statistically significant for the p14 ARF-3088440 polymorphism (log-rank tests: P = 0.786; P = 0.710 and P = 0.788, respectively). Multivariable analysis showed that patients with p14 ARF-rs3731217 TT genotype had significantly increased risk of death overall, death from SCCOP and disease recurrence compared with patients with the TG/GG variant genotypes (HR, 5.4; 95% CI, 2.0–14.6 for overall death; HR, 10.1; 95% CI, 2.1–48.7 for death from SCCOP and HR, 2.5; 95% CI, 1.0–5.9 for recurrence) (Table IV). The increased risk for overall death, death from SCCOP and recurrence did not reach statistically significant levels for the p14 ARF-3088440 polymorphism (Table IV).
Table IV.
Multivariable survival analysis by p14 ARF genotypes in 397 tumor HPV16(+) SCCOP patients
| p14 ARF genotypes | Total (397) | Events | Survival | ||||
|---|---|---|---|---|---|---|---|
| Death (all causes) | Death (owing to disease) | Recurrence | OS | DSS | DFS | ||
| aHR*, 95% CI | aHR, 95% CI | aHR, 95% CI | |||||
| p14 ARF-rs3731217 | |||||||
| TG+GG | 157 | 12 | 2 | 11 | 1.0 | 1.0 | 1.0 |
| TT | 240 | 39 | 23 | 34 | 5.4 (2.0–14.6) | 10.1 (2.1–48.7) | 2.5 (1.0–5.9) |
| p14 ARF-rs3088440 | |||||||
| GA+AA | 144 | 17 | 7 | 17 | 1.0 | 1.0 | 1.0 |
| GG | 253 | 34 | 18 | 28 | 1.1 (0.6–2.3) | 1.6 (0.6–4.5) | 1.6 (0.7–3.6) |
*Adjusted for age, sex, ethnicity, smoking, alcohol, T stage, N stage, treatment, comorbidity and tumor
HPV16 status in Cox’s models
Genotype–phenotype correlation of p14 ARF polymorphisms
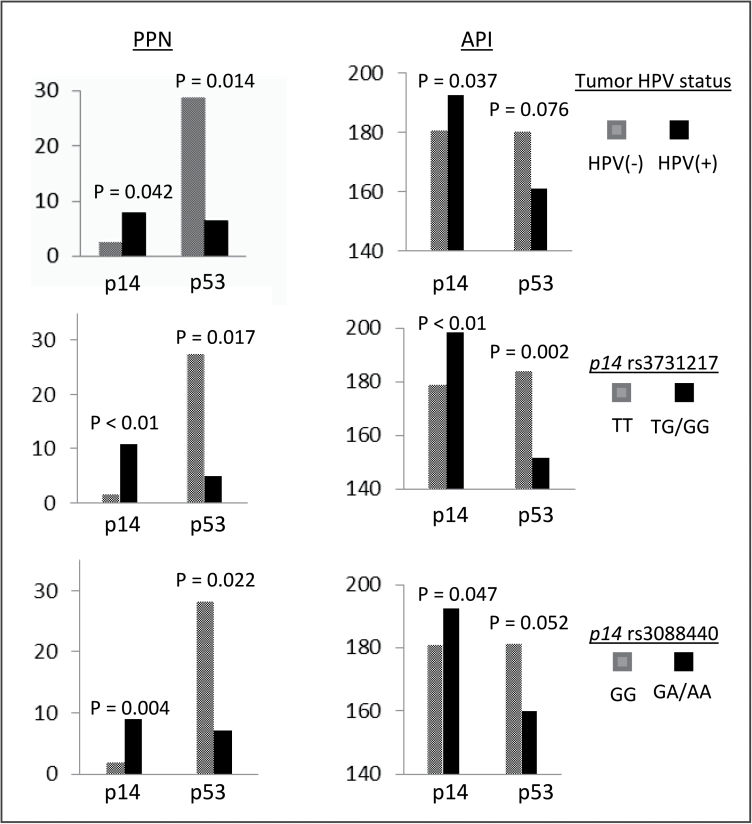
To further characterize the potentially functional relevance of the two polymorphisms in p14 ARF promoter and 3′-untranslated region, we determined p14 and p53 expression levels in tumor tissue specimens among another subset of 57 SCCOP patients by immunohistochemical staining and conducted a correlation analysis between tumor HPV16 status and genotypes of the two polymorphisms and the expression of p14 and p53. Among 57 SCCOP tissue specimens, we found that 33 were tumor HPV16 positive and 24 were tumor HPV16 negative. For p14 ARF-rs3731217 polymorphism, 37 were TT genotype and 20 were TG or GG genotypes. For p14 ARF-3088440 polymorphism, there were 33 patients with GG genotype and 24 with GA or AA genotypes. As shown in Figure 2, the expression of p14 (either p14 percentage of positive nuclei or API) was significantly higher in tumor HPV16-positive patients than the HPV16-negative cases, whereas the expression of p53 (p53 percentage of positive nuclei) was significantly lower in tumor HPV16-positive patients than the HPV16-negative cases, and such a difference was a borderline significant for the p53 API (P = 0.076). The similar significance was also found for both p14 ARF polymorphisms, with a lower expression of p14 or higher expression of p53 in patients with homozygous genotype than the cases with variant genotypes of both p14 ARF polymorphisms except a borderline significance for p14 ARF-3088440 polymorphism with relation to expression of p53 API (P = 0.052). In this study, due to the small sample size and few events of outcome among HPV-negative SCCOP patients, we did not perform such similar survival analysis for this subgroup of patients.
Fig. 2.
Comparison of p14 and p53 expression levels [percentage of positive nuclei (PPN) or API] in tumor tissue specimens among 57 SCCOP patients with different tumor HPV16 status and genotypes of p14 ARF-rs3731217 (promoter) and rs3088440 (3′-untranslated region) polymorphisms.
Discussion
The current study demonstrates that p14 ARF genetic variants may be associated with tumor HPV16-positive SCCOP and survival of patients with SCCOP, particularly HPV16-positive SCCOP cases, and the correlation between both p14 ARF genotypes and tumor HPV status as well as p14/p53 expression might further indicate their potentially functional roles in HPV-associated SCCOP. Such genetic variants could affect tumor HPV status and survival of HPV16-positive SCCOP patients and may help identify individuals at high risk of deaths/recurrence and developing HPV16-positive SCCOP for potentially optimizing patient’s stratification for HPV16-targeted therapies.
Chronic HPV infection can cause SCCOP by encoding viral oncoproteins E6 and E7 that inhibit p53 and Rb tumor suppressors in human oropharyngeal mucosa. Recent investigation reported that p14 ARF may play critical roles in the development of these HPV-associated cancers. Mutation analysis further showed that p14 ARF mutations less likely occurred in tumors without p53 mutations (more common of HPV-positive SCCOP) than in those with p53 mutations (typically HPV negative), and this difference was statistically significant (18). These findings suggest an interaction between p14 ARF alterations and tumor HPV status as well as p53 mutations. In fact, the alterations of p14 ARF were found to be inversely correlated with HPV positivity in SCCOP (22). As somatic mutations of p14 ARF can cause changes in p53 activity, which might have a significant impact in therapy against HPV-related tumors (23–25), it is speculated that other genetic variations, such as p14 ARF polymorphisms, may also affect HPV-associated SCCOP and related outcomes in subgroups of patients.
p14 ARF expression is positively regulated by transcription factors, such as E2F-1 and E2F-2, thus preventing Rb proteasomal degradation (12,14–16), whereas HPV E7 inhibits Rb, resulting in the release of the active form of E2F-1, and consequently E7 has the ability to induce p14 ARF expression. In contrast, p53 negatively regulates p14 ARF expression through elimination of MDM2-mediated inhibition of the p53 activity (13). As E6 inhibits p53 by binding to E6-AP ubiquitin ligase, which results in ubiquitination and subsequent degradation of p53, E6 can upregulate p14 ARF expression. Therefore, inactivation of p53 and Rb might upregulate p14 ARF expression, and overexpression of p14 ARF could be induced by E6/E7 expression in HPV16-positive SCCOP. In this report, we have shown that tumor HPV16 status of SCCOP patients is associated with significantly increased expression of p14 and reduced expression of p53, and such results may further support abovementioned mechanisms involved in interaction between HPV and these tumor suppressors. In a study by Hafkamp et al. (26), HPV16-postive tumors were strongly correlated with p14ARF overexpression and downregulation of Rb in contrast to HPV16-negative tumors. Overexpression of p14 ARF was also strongly associated with HPV-positive cervical cancers, whereas low expression of p14 ARF was associated with HPV-negative cervical cancers (27). Furthermore, the overexpression of p14ARF protein levels in E6/E7-expressing cells was observed in an in vitro study (28). In contrast, our results from this study indicate that p14 ARF genetic variants were significantly associated with tumor HPV status and could serve as a marker for HPV-positive SCCOP tumors among patients with SCCOP. Therefore, in addition to HPV and/or p16 expression, other biomarkers, such as p14 ARF genetic variants, for identifying subgroups to further stratify SCCOP patients may ensure appropriate therapy and therefore better survival. For example, among the SCCOP with radiotherapy only, the variant genotypes of p14 ARF-rs3731217 only had better OS than the corresponding wild-type genotype, whereas among the SCCOP patients with chemoradiotherapy, the same variant genotypes had better OS, DSS and DFS than the wild-type genotype. However, such stratified effect by treatment on prognosis was not significantly different among the SCCOP patients with different genotypes of p14 ARF-3088440 polymorphism.
Although the precise mechanisms by which p14 ARF genetic variants play a role in the development of HPV16-associated SCCOP has not been clarified, these two p14 ARF genetic variants may result in different splice transcripts that may have different consequences of cellular activities, subsequently contributing to interindividual variations in susceptibility to tumor HPV status among HPV-associated cancers (29,30). So far, no studies on functional relevance of these two p14 ARF polymorphisms have been reported. Since expression of p14 ARF is negatively regulated by p53 through MDM2-mediated inhibition of the p53 activity and these two p14 ARF polymorphisms are within the functional regions of the gene’s promoter (p14 ARF-rs3731217) and 3′-untranslated region (p14 ARF-rs3088440), we speculated that these two p14 ARF variants may have potentially functional effect on expression levels of p14ARF and p53 by altering the efficiency of translational initiation, leading to interindividual differences in susceptibility to tumor HPV16 status among patients with SCCOP and treatment response. Indeed, in this study, we found that the variant genotypes of these two polymorphisms are significantly or borderline significantly correlated with increased expression of p14ARF but with decreased expression of p53 in 57 SCCOP specimens. Although the functional relevance of these two polymorphisms has not yet been elucidated, our results might partially suggest a functional correlation between the two polymorphisms and expression of p14ARFand p53, which may provide preliminary evidence of biological plausibility for the observed association in the current study. In addition, p14 ARF polymorphisms may be in linkage disequilibrium with other functional polymorphisms or adjacent susceptibility loci of the gene, thereby affecting p14 ARF gene expression and activity. This could lead to altered interaction of p14 with HPV16 or other cell cycle-related regulators, thus modulating tumor HPV status and prognosis of HPV-associated cancers. However, understanding of the exact mechanisms by which these two polymorphisms affect p14 and p53 expression and interaction with HPV warrant further in vitro and in vivo studies.
We have previously reported an association of these two polymorphisms with risk of second primary tumors among patients with index head and neck cancers (21). Other data on these two putative functional polymorphisms are lacking and our data here suggest that these two genetic variants may have functional significance as they appear to correlate with tumor HPV status and p14/p53 expression and affect survival among this cohort of SCCOP patients. These results imply that, if validated, germline variations should be taken into consideration for future treatment and prevention strategies of SCCOP, because such variations may contribute to the susceptibility to radio- and chemotherapy-induced cellular activities, such as apoptosis. It may ultimately be possible to alter the intensity of current treatments in HPV-positive SCCOP patients with known p14 ARF genotypes in order to improve functional and quality of life outcomes while maintaining high survival rates. In conclusion, our results demonstrated that these p14 ARF genetic variants were significantly associated with tumor HPV16-positive SCCOP as well as the survival of such patients. Further larger studies are required for validation of our findings and an exploration of the molecular mechanisms underlying the observed associations.
Funding
National Institutes of Health (R01 ES011740 to Q.W., CA128110-01A1 to E.M.S., CA135679 to G.L., CA133099 G.L.).
Acknowledgements
We thank Margaret Lung, Jenny Vo and Jessica Fiske for their assistance in recruiting the subjects and gathering the questionnaire information; Chong Zhao and Yingdong Li for laboratory assistance.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- API
average positive intensity
- CI
confidence interval
- DFS
disease-free survival
- DSS
disease-specific survival
- HPV
human papillomavirus
- HR
hazard ratio
- OR
odds ratio
- OS
overall survival
- SCCOP
squamous cell carcinoma of the oropharynx.
References
- 1. Parkin D.M., et al. (2005). Global cancer statistics, 2002. Cancer J. Clin., 55, 74–108 [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J., et al. (2004). GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. IARC Press, Lyon [Google Scholar]
- 3. Gillison M.L. (2007). Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck, 29, 779–792 [DOI] [PubMed] [Google Scholar]
- 4. Vokes E.E., et al. (1993) Head and neck cancer. N. Engl. J. Med., 328, 184–194 [DOI] [PubMed] [Google Scholar]
- 5. Matthias C., et al. (2006). Influential factors on tumor recurrence in head and neck cancer patients. Eur. Arch. Otorhinolaryngol., 263, 37–42 [DOI] [PubMed] [Google Scholar]
- 6. Shiboski C.H., et al. (2005). Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer, 103, 1843–1849 [DOI] [PubMed] [Google Scholar]
- 7. Li G., et al. (2006). The role of human papillomavirus in squamous carcinoma of the head and neck. Curr. Oncol. Rep., 8, 130–139 [DOI] [PubMed] [Google Scholar]
- 8. Herrero R., et al. ; IARC Multicenter Oral Cancer Study Group (2003) Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J. Natl Cancer Inst., 95, 1772–1783 [DOI] [PubMed] [Google Scholar]
- 9. Gillison M.L., et al. (2000). Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl Cancer Inst., 92, 709–720 [DOI] [PubMed] [Google Scholar]
- 10. Fakhry C., et al. (2006). Clinical implications of human papillomavirus in head and neck cancers. J. Clin. Oncol., 24, 2606–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. deVilliers E.M., et al. (2004). Classification of papillomaviruses. Virology, 324, 17–27 [DOI] [PubMed] [Google Scholar]
- 12. Leduc C., et al. (2006). p14ARF promotes RB accumulation through inhibition of its Tip60-dependent acetylation. Oncogene, 25, 4147–4154 [DOI] [PubMed] [Google Scholar]
- 13. Gjerset R.A., et al. (2006). Regulation of p14ARF through subnuclear compartmentalization. Cell Cycle, 5, 686–690 [DOI] [PubMed] [Google Scholar]
- 14. Eymin B., et al. (2006). p14ARF activates a Tip60-dependent and p53-independent ATM/ATR/CHK pathway in response to genotoxic stress. Mol. Cell. Biol., 26, 4339–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chin L., et al. (1998). The INK4a/ARF tumor suppressor: one gene–two products–two pathways. Trends Biochem. Sci., 23, 291–296 [DOI] [PubMed] [Google Scholar]
- 16. Bates S., et al. (1998). p14ARF links the tumour suppressors RB and p53. Nature, 395, 124–125 [DOI] [PubMed] [Google Scholar]
- 17. Termine N., et al. (2008). HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: a meta-analysis (1988-2007). Ann. Oncol., 19, 1681–1690 [DOI] [PubMed] [Google Scholar]
- 18. Weber A., et al. (2002) INK4a-ARF alterations and p53 mutations in primary and consecutive squamous cell carcinoma of the head and neck. Virchows Arch., 441, 133–142 [DOI] [PubMed] [Google Scholar]
- 19. Guan X., et al. (2010). Association of TGF-beta1 genetic variants with HPV16-positive oropharyngeal cancer. Clin. Cancer Res., 16, 1416–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ji X., et al. (2009). Association of p73 G4C14-to-A4T14 polymorphism with human papillomavirus type 16 status in squamous cell carcinoma of the head and neck in non-Hispanic whites. Cancer, 115, 1660–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y., et al. (2011). p14ARF genetic polymorphisms and susceptibility to second primary malignancy in patients with index squamous cell carcinoma of the head and neck. Cancer, 117, 1227–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghosh A., et al. (2009). SH3GL2 and CDKN2A/2B loci are independently altered in early dysplastic lesions of head and neck: correlation with HPV infection and tobacco habit. J. Pathol., 217, 408–419 [DOI] [PubMed] [Google Scholar]
- 23. Pan W., et al. (2003). P19ARF inhibits the functions of the HPV16 E7 oncoprotein. Oncogene, 22, 5496–5503 [DOI] [PubMed] [Google Scholar]
- 24. Oath S., et al. (2009). Alterations of p16 and p14ARF genes and their 9p21 locus in oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod., 107, 81–91 [DOI] [PubMed] [Google Scholar]
- 25. Bradley G., et al. (2001). Abnormalities of the ARF-p53 pathway in oral squamous cell carcinoma. Oncogene, 20, 654–658 [DOI] [PubMed] [Google Scholar]
- 26. Hafkamp H.C., et al. (2009). P21 Cip1/WAF1 expression is strongly associated with HPV-positive tonsillar carcinoma and a favorable prognosis. Mod. Pathol., 22, 686–698 [DOI] [PubMed] [Google Scholar]
- 27. Kanao H., et al. (2004). Correlation between p14(ARF)/p16(INK4A) expression and HPV infection in uterine cervical cancer. Cancer Lett., 213, 31–37 [DOI] [PubMed] [Google Scholar]
- 28. McCloskey R., et al. (2010). Human papillomavirus type 16 E6/E7 upregulation of nucleophosmin is important for proliferation and inhibition of differentiation. J. Virol., 84, 5131–5139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jurinke C., et al. (2005). A single nucleotide polymorphism based approach for the identification and characterization of gene expression modulation using MassARRAY. Mutat. Res., 573, 83–95 [DOI] [PubMed] [Google Scholar]
- 30. Haber D.A., et al. (1998). The promise of cancer genetics. Lancet, 351 (suppl. 2), SII1–SII8 [DOI] [PubMed] [Google Scholar]