Between-group differences and on-road driving errors that predicted pass or fail on-road outcomes are described in a comparison of 101 drivers with PD and 138 healthy control drivers.
MeSH TERMS: automobile driving, forecasting, Parkinson disease, task performance and analysis, safety
Abstract
Age-related medical conditions such as Parkinson’s disease (PD) compromise driver fitness. Results from studies are unclear on the specific driving errors that underlie passing or failing an on-road assessment. In this study, we determined the between-group differences and quantified the on-road driving errors that predicted pass or fail on-road outcomes in 101 drivers with PD (mean age = 69.38 ± 7.43) and 138 healthy control (HC) drivers (mean age = 71.76 ± 5.08). Participants with PD had minor differences in demographics and driving habits and history but made more and different driving errors than HC participants. Drivers with PD failed the on-road test to a greater extent than HC drivers (41% vs. 9%), χ2(1) = 35.54, HC N = 138, PD N = 99, p < .001. The driving errors predicting on-road pass or fail outcomes (95% confidence interval, Nagelkerke R2 =.771) were made in visual scanning, signaling, vehicle positioning, speeding (mainly underspeeding, t(61) = 7.004, p < .001, and total errors. Although it is difficult to predict on-road outcomes, this study provides a foundation for doing so.
Age-related medical conditions such as Parkinson’s disease (PD) compromise driver fitness. The current and growing body of literature suggests that drivers with PD, when compared with healthy control (HC) drivers, make more driving errors and have impaired on-road driving outcomes (Classen et al., 2009, 2011; Cordell, Lee, Granger, Vieira, & Lee, 2008; Uc et al., 2006a, 2006b, 2007; Uc, Rizzo, Johnson, et al., 2009; Wood, Worringham, Kerr, Mallon, & Silburn, 2005). However, studies to date have not reported on the specific driving errors that are predictive of on-road outcomes in a large sample of drivers with PD compared with HC drivers.
On-Road Driving Error Studies
In five on-road studies, researchers examined the types of errors made during on-road performance in participants with mild to moderate PD compared with HC participants (Cordell et al., 2008; Scally et al., 2011; Stolwyk, Triggs, Charlton, Iansek, & Bradshaw, 2005; Uc et al., 2006b, 2007). Uc et al. (2006b) examined 79 participants with PD (mean [M] age = 66.0 ± 8.6) and 151 HC participants (M age not reported) and found that the PD group identified fewer landmarks and traffic signs and committed more at-fault safety errors than the HC group. In a follow-up study of similar design (N = 77), Uc et al. (2007) reported that drivers in the PD group took longer to perform a route-following task than HC drivers, made more incorrect turns, got lost more often, and committed more at-fault safety errors.
Stolwyk et al. (2005) examined the impact of impaired internal cueing on driving performance in 18 participants with PD (M age = 67.6 ± 6.5) and 18 HC drivers (67.1 ± 6.5). They found that the PD group had difficulties using internal cues around traffic signals and curves and failed to adjust speed without receiving external cues.
Scally et al. (2011) compared 19 participants with mild to moderate PD (M age = 68.7 ± 6.7) with 19 HC participants (68.1 ± 7.2) and found that during no-cue conditions, the drivers with PD braked later and traveled greater distances between deceleration and braking points. However, no difference was observed in braking distance or deceleration in response to cues.
Finally, Cordell et al. (2008) examined the driving performance of 53 participants with PD (M age = 69.3 ± 8.3) and 129 HC drivers (72.9 ± 7.1). They found that the PD group performed worse at T junctions, when using rear and side mirrors, and when maintaining speed.
On-Road Performance Outcomes Studies
In five studies, researchers examined on-road outcomes (safe vs. unsafe, pass vs. fail an on-road assessment) in drivers with mild to moderate PD as a single group (Radford, Lincoln, & Lennox, 2004; Singh, Pentland, Hunter, & Provan, 2007) or compared with HC drivers (Classen et al., 2011; Grace et al., 2005; Heikkilä, Turkka, Korpelainen, Kallanranta, & Summala, 1998).
Singh et al. (2007) examined 154 drivers with PD (M age = 67.6) and identified various clinical variables associated with unsafe driving but ascertained that 66% of the drivers with PD were safe to drive. Radford et al. (2004) examined the driving performance of 51 drivers with PD (M age = 64.4 ± 9.1) in a one-group prospective study. The driving instructor found that 43 of 51 drivers with PD (84.3%) were considered safe to drive. Grace et al. (2005) studied 21 drivers with PD (M age = 68.1 ± 8.5) and 21 HC drivers (M age = 69.0 ± 10.4) and found that although drivers with PD made more driving errors than HC drivers, 67% of those drivers were considered “safe” and 33% only “marginally safe” to drive. Heikkilä et al. (1998) examined 20 drivers with PD (M age = 59.0 ± 11.0) and 20 HC drivers (M age = 55.0 ± 6.0). Drivers in the PD group performed worse than HC drivers on visual memory, choice reaction time, and information-processing speed and drove worse than those in the HC group, although most were deemed safe to drive. Classen et al. (2011) conducted a comprehensive driving evaluation on 41 drivers with PD (M age = 73.1 ± 6.0) and 41 HC drivers (M age = 73.0 ± 5.2). The PD group failed the road test almost 5 times more often than the HC group (56.1% vs. 12.2%), χ2(1) = 17.6, p < .001.
Rationale and Significance
This extensive review portrays that most of the reported driving errors identified in PD appear to be mainly tactical and some operational in nature (Michon, 1985). Studies have generally not reported on the specific type and number of driving errors or how such errors explain driver performance outcomes. Studies of driver performance outcomes in PD clearly indicate that drivers with PD perform worse than HC drivers. The definition of outcomes varies, however: Some studies identify driving errors, others identify safety, and others assess fitness to drive through a comprehensive driving evaluation to make pass–fail determinations a primary outcome (Classen et al., 2011).
Purpose
The goal of this study was to determine the between-group differences in a large sample of drivers with PD compared with HC drivers. We were able to identify the on-road driving errors (type and number) that predicted pass–fail on-road driver performance outcomes. Understanding the specific on-road driving errors that underlie the outcome may create plausible opportunities for occupational therapy practitioners and driving rehabilitation specialists to tailor their evaluations and to identify targeted intervention strategies.
Method
Design
We used a prospective design with a convenience sample of drivers with PD and a cohort of HCs drivers. Participants with PD and the HC participants underwent the same testing protocol (with the exception of obtaining neurological data) during the same period. The institutional review board of the University of Florida approved this study. All participants provided written informed consent.
Participants
Participants with PD were recruited through the University of Florida’s Center for Movement Disorders and Neurorestoration (CMDNR), support groups, newspaper and other local advertisements, word-of-mouth referral, and Web site postings (e.g., American Parkinson Disease Association). All participants from the CMDNR (N = 80) met the United Kingdom Parkinson’s Disease Society Brain Bank Criteria for a diagnosis of PD and were evaluated by a neurologist trained in movement disorders (Hughes, Daniel, Blankson, & Lees, 1993). An additional 21 participants with PD not recruited through the CMDNR had a reported confirmed diagnosis of PD by their neurologist or movement disorder specialist. Participants were included if they were diagnosed with PD; were between ages 35 and 89; were currently driving with a valid driver’s license or had quit driving within the past 3 mo; had ≥10 yr of driving experience; met the Florida state statute requirement for visual acuity (i.e., 20/70)1; lived independently in the community; and were proficient in reading and speaking English. Participants were excluded if they had other neurological conditions (e.g., stroke, uncontrolled seizures, dementia); had active, untreated psychiatric disorders (e.g., psychosis) or physical conditions (e.g., missing limb) precluding full participation; or used psychotropic medications that impaired mental or physical functioning. Participants received $100 for study participation and completion.
HC participants were community-dwelling drivers recruited by flyer distribution in local community facilities, through local newspaper advertisements, and by word-of-mouth referrals in North Central Florida. Drivers were included if they were age 65–89; had a valid driver’s license; were driving 3 mo prior to or at the time of recruitment; met the Florida state statute requirement for visual acuity; had Mini-Mental State Examination scores of >24 (Folstein, Folstein, & McHugh, 1975); and were physically able to complete a clinical battery of tests and to participate in an on-road driving assessment. Drivers were excluded if they had received medical advice not to drive, had uncontrolled seizures in the past year, or used medications that impaired mental or physical functioning (self-report). Like the drivers with PD, HC participants received $100 for study participation and completion.
Procedure
A certified driver rehabilitation specialist (CDRS) conducted all aspects of the evaluation. Participants first completed general questionnaires on demographics and driving history and habits before undergoing a standardized clinical battery and an on-road test during the self-reported on medication state (participants with PD only).2 Participants drove a 45-min road course consisting of residential, suburban, and highway areas during the daytime and outside of peak traffic hours. During inclement weather (e.g., rain, heavy winds), the road test was deferred to a different day. We tested all participants in a dual-brake 2004 Buick Century with the CDRS sitting in the passenger seat to evaluate the driver. Beyond collecting the clinical and driving data on the 101 participants with PD, we also collected neurological data (e.g., confirmation of diagnoses, disease staging) through the data repository of the CMDNR. We included 138 HC drivers for a total sample of 239.
Measures
Questionnaires.
From the questionnaires, we collected information on demographics (e.g., age, gender, education, race) and medications. We also collected driving history and habits, including driving frequency, avoidance of driving situations, number of crashes and citations, and use of alternative transportation.
PD Staging and Severity.
Participants with PD were evaluated with the motor subscale of the Unified Parkinson’s Disease Rating Scale (UPDRS; Part 3) in the on medication state) by CMDNR neurologists prior to the driving evaluation (Ramaker, Marinus, Stiggelbout, & Van Hilten, 2002). Scores on the UPDRS motor subscale range from 0 to 108, with higher scores indicating greater disease severity.
The neurologists evaluated participants by using the modified Hoehn and Yahr, a numerical ranking that indicates the stage of PD (only on medication reported) as follows: Stage 0 = no signs of disease; Stage 1 = unilateral disease; Stage 1.5 = unilateral plus axial involvement; Stage 2 = bilateral disease, without impairment of balance; Stage 2.5 = mild bilateral disease with recovery on pull test; Stage 3 = mild to moderate bilateral disease, some postural instability, physically independent; Stage 4 = severe disability, still able to walk or stand unassisted; and Stage 5 = wheelchair bound or bedridden unless aided (Goetz et al., 2004).
On-Road Test.
All participants drove a standardized on-road test with demonstrated reliability and validity among older drivers (Justiss, Mann, Stav, & Velozo, 2006; Posse, McCarthy, & Mann, 2006). The CDRS recorded driving errors according to type and number for each of the eight categories and the total number of errors (Justiss et al., 2006). These driving errors were as follows:
Vehicle positioning (i.e., posterior or anterior position of the vehicle in relation to other vehicles, objects, or pavement markings)
Speed regulation (i.e., maintaining speed limit as well as controlled acceleration and braking); includes underspeeding (>10 mph under the posted speed limit), overspeeding (>10 mph over the posted speed limit), and other types of speeding errors (e.g., making a rolling stop instead of a complete stop).
Lane maintenance (i.e., lateral positioning of the vehicle in the lane during driving or while stopped); can include encroachment errors (steering toward the left oncoming traffic), wide errors (steering toward the shoulder of the road), and other lane errors (touching or crossing roadway lines while making turns to the right or left side).
Yielding (i.e., giving right of way to other vehicles as appropriate)
Signaling (i.e., proper use and timing of turn signals)
Visual scanning (i.e., checking blind spots and intersections)
Adjustment to stimuli (i.e., responding to driving situations such as road sign information, vehicle movements, pedestrian movements, or potential hazards)
Gap acceptance (i.e., demonstrating safe timing and spacing distance when crossing in front of oncoming traffic).
The CDRS also determined the primary on-road outcome. The global rating score (GRS) included four categorizations: 3 = pass, 2 = pass with restrictions or recommendations, 1 = fail remediable, and 0 = fail not remediable. These categories were dichotomized to pass–fail outcomes (Justiss et al., 2006).
Data Collection and Analysis
Trained research staff entered data (demographic information, clinical test scores, on-road test) into an SPSS database (Version 20; IBM Corporation, Armonk, NY). The principal investigator (Sherrilene Classen) monitored data entry and performed quality control checks to ensure data completion and accuracy. We conducted descriptive analysis (e.g., mean, standard deviation, range) for all continuous variables (e.g., demographics, driving history and habits, health-related characteristics, clinical tests, on-road test data). We reported categorical data as frequencies and percentages. Group comparisons (PD vs. HC) were completed using independent-sample t tests (controlled for unequal variance using Levene’s test) or Mann–Whitney U tests if the data were nonparametric. Chi-square or Fisher exact tests (if cell value was ≤5) were used for analyzing nominal data. To determine independent predictors of the pass–fail outcomes for the on-road test, we used logistic binomial regression. We considered p ≤ .05 as significant. We reported data as “missing” when we were unable to obtain it through follow-up calls. We used SPSS to perform the analyses.
Results
Participant Characteristics
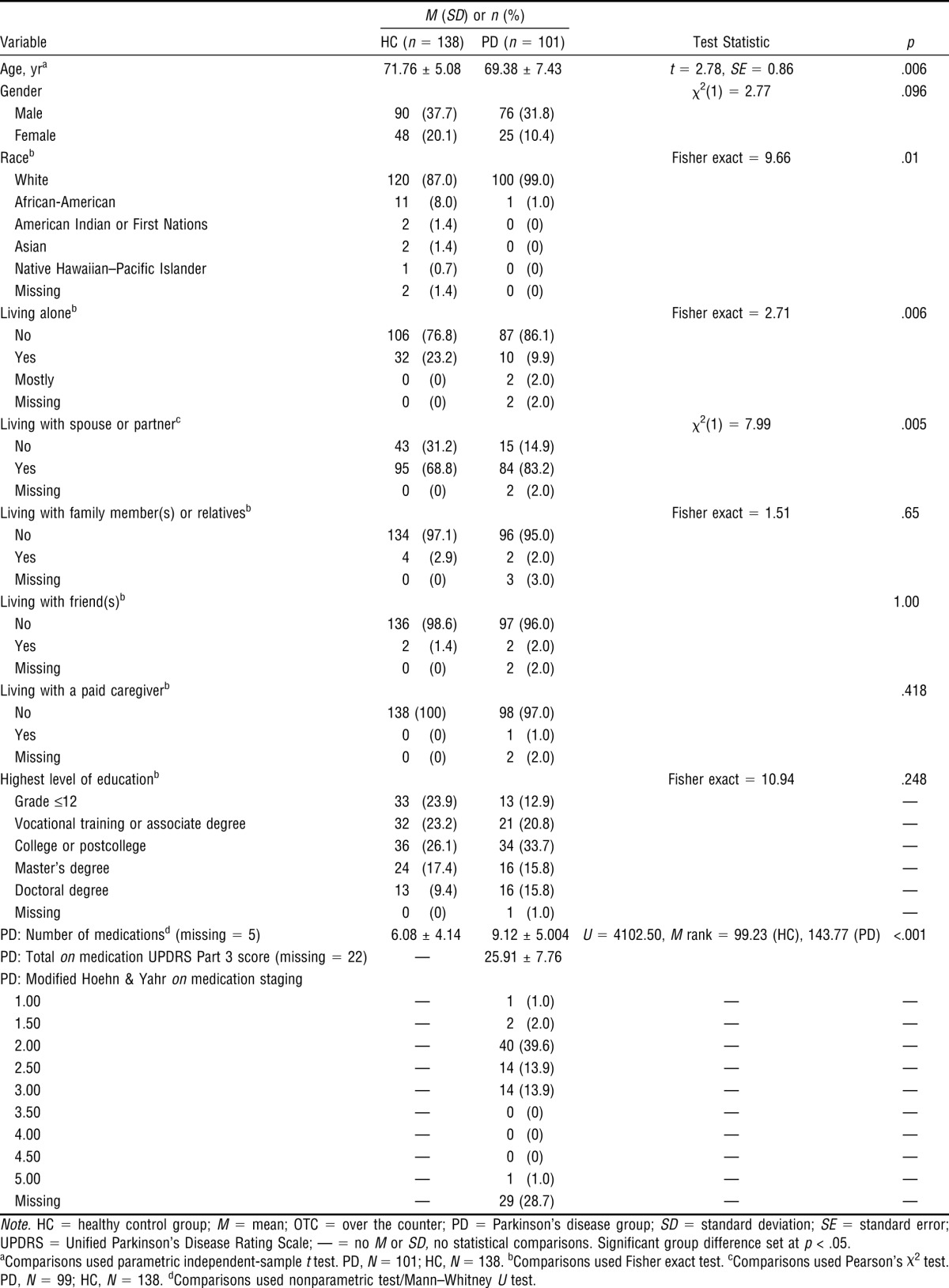
Table 1 shows that participants with PD (N = 101, M age = 69.38 ± 7.43) were significantly (p = .006) younger than the HC drivers (N = 138, M age = 71.76 ± 5.08). There were no gender differences. We detected a racial difference; all participants with PD but one were White, whereas the HC group included more participants in the non-White categories. Participants with PD were taking significantly more medications than the HC drivers. Table 1 also shows that the median modified Hoehn and Yahr on medication score was 2.00 (bilateral disease, without impairment of balance), and the total UPDRS Part 3 on medication score was 25.91 (SD ± 7.76), suggesting that the group had mild to moderate PD.
Table 1.
Descriptive Statistics and Between-Group Differences for Demographics and Medication Use of Drivers With Parkinson's Disease and Healthy Control Drivers
|
M (SD) or n (%) |
||||
| Variable | HC (n = 138) | PD (n = 101) | Test Statistic | p |
| Age, yra | 71.76 ± 5.08 | 69.38 ± 7.43 | t = 2.78, SE = 0.86 | .006 |
| Gender | χ2(1) = 2.77 | .096 | ||
| Male | 90 (37.7) | 76 (31.8) | ||
| Female | 48 (20.1) | 25 (10.4) | ||
| Raceb | Fisher exact = 9.66 | .01 | ||
| White | 120 (87.0) | 100 (99.0) | ||
| African-American | 11 (8.0) | 1 (1.0) | ||
| American Indian or First Nations | 2 (1.4) | 0 (0) | ||
| Asian | 2 (1.4) | 0 (0) | ||
| Native Hawaiian–Pacific Islander | 1 (0.7) | 0 (0) | ||
| Missing | 2 (1.4) | 0 (0) | ||
| Living aloneb | Fisher exact = 2.71 | .006 | ||
| No | 106 (76.8) | 87 (86.1) | ||
| Yes | 32 (23.2) | 10 (9.9) | ||
| Mostly | 0 (0) | 2 (2.0) | ||
| Missing | 0 (0) | 2 (2.0) | ||
| Living with spouse or partnerc | χ2(1) = 7.99 | .005 | ||
| No | 43 (31.2) | 15 (14.9) | ||
| Yes | 95 (68.8) | 84 (83.2) | ||
| Missing | 0 (0) | 2 (2.0) | ||
| Living with family member(s) or relativesb | Fisher exact = 1.51 | .65 | ||
| No | 134 (97.1) | 96 (95.0) | ||
| Yes | 4 (2.9) | 2 (2.0) | ||
| Missing | 0 (0) | 3 (3.0) | ||
| Living with friend(s)b | 1.00 | |||
| No | 136 (98.6) | 97 (96.0) | ||
| Yes | 2 (1.4) | 2 (2.0) | ||
| Missing | 0 (0) | 2 (2.0) | ||
| Living with a paid caregiverb | .418 | |||
| No | 138 (100) | 98 (97.0) | ||
| Yes | 0 (0) | 1 (1.0) | ||
| Missing | 0 (0) | 2 (2.0) | ||
| Highest level of educationb | Fisher exact = 10.94 | .248 | ||
| Grade ≤12 | 33 (23.9) | 13 (12.9) | — | |
| Vocational training or associate degree | 32 (23.2) | 21 (20.8) | — | |
| College or postcollege | 36 (26.1) | 34 (33.7) | — | |
| Master’s degree | 24 (17.4) | 16 (15.8) | — | |
| Doctoral degree | 13 (9.4) | 16 (15.8) | — | |
| Missing | 0 (0) | 1 (1.0) | — | |
| PD: Number of medicationsd (missing = 5) | 6.08 ± 4.14 | 9.12 ± 5.004 | U = 4102.50, M rank = 99.23 (HC), 143.77 (PD) | <.001 |
| PD: Total on medication UPDRS Part 3 score (missing = 22) | — | 25.91 ± 7.76 | ||
| PD: Modified Hoehn & Yahr on medication staging | ||||
| 1.00 | — | 1 (1.0) | — | — |
| 1.50 | — | 2 (2.0) | — | — |
| 2.00 | — | 40 (39.6) | — | — |
| 2.50 | — | 14 (13.9) | — | — |
| 3.00 | — | 14 (13.9) | — | — |
| 3.50 | — | 0 (0) | — | — |
| 4.00 | — | 0 (0) | — | — |
| 4.50 | — | 0 (0) | — | — |
| 5.00 | — | 1 (1.0) | — | — |
| Missing | — | 29 (28.7) | — | — |
Note. HC = healthy control group; M = mean; OTC = over the counter; PD = Parkinson's disease group; SD = standard deviation; SE = standard error; UPDRS = Unified Parkinson’s Disease Rating Scale; — = no M or SD, no statistical comparisons. Significant group difference set at p < .05.
aComparisons used parametric independent-sample t test. PD, N = 101; HC, N = 138. bComparisons used Fisher exact test. cComparisons used Pearson’s χ2 test. PD, N = 99; HC, N = 138. dComparisons used nonparametric test/Mann–Whitney U test.
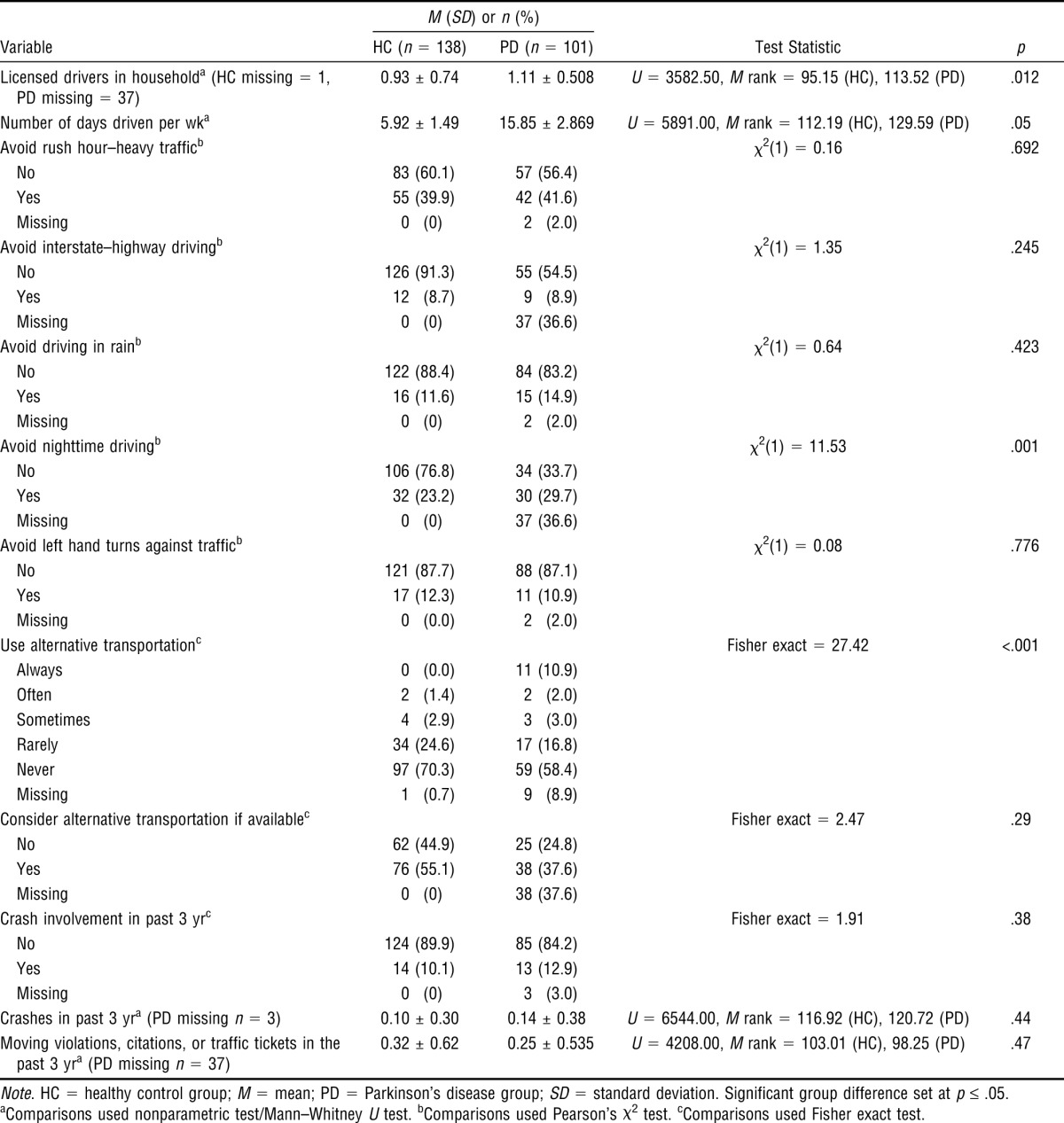
Although participants with PD drove more days than drivers in the HC group, we observed no differences for self-reported crashes and violations. Compared with HC participants, participants with PD displayed more self-reported avoidance behavior related to nighttime driving only. A larger percentage of participants with PD reported using alternative transportation compared with HC participants (Table 2).
Table 2.
Descriptive Statistics and Between-Group Differences for Driving History and Habits of Drivers With Parkinson's Disease and Healthy Control Drivers
|
M (SD) or n (%) |
||||
| Variable | HC (n = 138) | PD (n = 101) | Test Statistic | p |
| Licensed drivers in householda (HC missing = 1, PD missing = 37) | 0.93 ± 0.74 | 1.11 ± 0.508 | U = 3582.50, M rank = 95.15 (HC), 113.52 (PD) | .012 |
| Number of days driven per wka | 5.92 ± 1.49 | 15.85 ± 2.869 | U = 5891.00, M rank = 112.19 (HC), 129.59 (PD) | .05 |
| Avoid rush hour–heavy trafficb | χ2(1) = 0.16 | .692 | ||
| No | 83 (60.1) | 57 (56.4) | ||
| Yes | 55 (39.9) | 42 (41.6) | ||
| Missing | 0 (0) | 2 (2.0) | ||
| Avoid interstate–highway drivingb | χ2(1) = 1.35 | .245 | ||
| No | 126 (91.3) | 55 (54.5) | ||
| Yes | 12 (8.7) | 9 (8.9) | ||
| Missing | 0 (0) | 37 (36.6) | ||
| Avoid driving in rainb | χ2(1) = 0.64 | .423 | ||
| No | 122 (88.4) | 84 (83.2) | ||
| Yes | 16 (11.6) | 15 (14.9) | ||
| Missing | 0 (0) | 2 (2.0) | ||
| Avoid nighttime drivingb | χ2(1) = 11.53 | .001 | ||
| No | 106 (76.8) | 34 (33.7) | ||
| Yes | 32 (23.2) | 30 (29.7) | ||
| Missing | 0 (0) | 37 (36.6) | ||
| Avoid left hand turns against trafficb | χ2(1) = 0.08 | .776 | ||
| No | 121 (87.7) | 88 (87.1) | ||
| Yes | 17 (12.3) | 11 (10.9) | ||
| Missing | 0 (0.0) | 2 (2.0) | ||
| Use alternative transportationc | Fisher exact = 27.42 | <.001 | ||
| Always | 0 (0.0) | 11 (10.9) | ||
| Often | 2 (1.4) | 2 (2.0) | ||
| Sometimes | 4 (2.9) | 3 (3.0) | ||
| Rarely | 34 (24.6) | 17 (16.8) | ||
| Never | 97 (70.3) | 59 (58.4) | ||
| Missing | 1 (0.7) | 9 (8.9) | ||
| Consider alternative transportation if availablec | Fisher exact = 2.47 | .29 | ||
| No | 62 (44.9) | 25 (24.8) | ||
| Yes | 76 (55.1) | 38 (37.6) | ||
| Missing | 0 (0) | 38 (37.6) | ||
| Crash involvement in past 3 yrc | Fisher exact = 1.91 | .38 | ||
| No | 124 (89.9) | 85 (84.2) | ||
| Yes | 14 (10.1) | 13 (12.9) | ||
| Missing | 0 (0) | 3 (3.0) | ||
| Crashes in past 3 yra (PD missing n = 3) | 0.10 ± 0.30 | 0.14 ± 0.38 | U = 6544.00, M rank = 116.92 (HC), 120.72 (PD) | .44 |
| Moving violations, citations, or traffic tickets in the past 3 yra (PD missing n = 37) | 0.32 ± 0.62 | 0.25 ± 0.535 | U = 4208.00, M rank = 103.01 (HC), 98.25 (PD) | .47 |
Note. HC = healthy control group; M = mean; PD = Parkinson's disease group; SD = standard deviation. Significant group difference set at p ≤ .05.
aComparisons used nonparametric test/Mann–Whitney U test. bComparisons used Pearson’s χ2 test. cComparisons used Fisher exact test.
On-Road Test
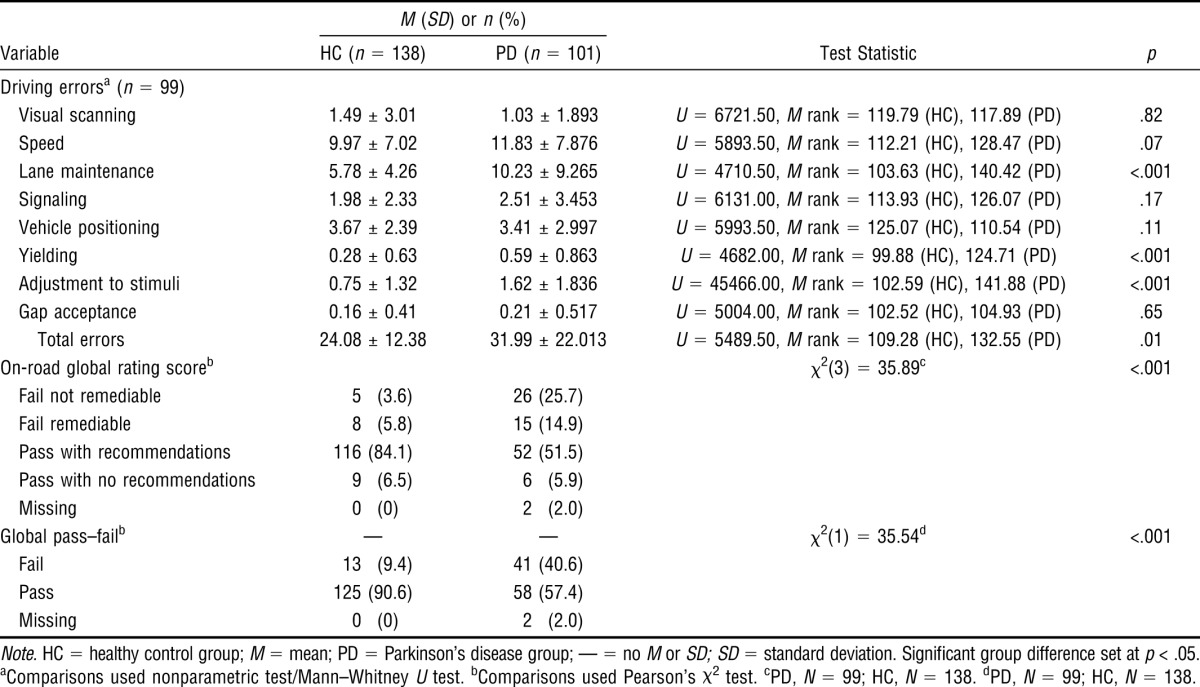
Participants with PD made more lane maintenance, yielding, adjustment to stimuli, and total driving errors than the HC drivers. The GRS for participants with PD and HC participants was significantly different: Drivers with PD did worse than the HC drivers. Participants with PD (41%) failed the on-road test to a greater extent than HC (9%; Table 3).
Table 3.
Descriptive Statistics and Between-Groups Differences for Driving Errors of Drivers With Parkinson's Disease and Healthy Control Drivers
|
M (SD) or n (%) |
||||
| Variable | HC (n = 138) | PD (n = 101) | Test Statistic | p |
| Driving errorsa (n = 99) | ||||
| Visual scanning | 1.49 ± 3.01 | 1.03 ± 1.893 | U = 6721.50, M rank = 119.79 (HC), 117.89 (PD) | .82 |
| Speed | 9.97 ± 7.02 | 11.83 ± 7.876 | U = 5893.50, M rank = 112.21 (HC), 128.47 (PD) | .07 |
| Lane maintenance | 5.78 ± 4.26 | 10.23 ± 9.265 | U = 4710.50, M rank = 103.63 (HC), 140.42 (PD) | <.001 |
| Signaling | 1.98 ± 2.33 | 2.51 ± 3.453 | U = 6131.00, M rank = 113.93 (HC), 126.07 (PD) | .17 |
| Vehicle positioning | 3.67 ± 2.39 | 3.41 ± 2.997 | U = 5993.50, M rank = 125.07 (HC), 110.54 (PD) | .11 |
| Yielding | 0.28 ± 0.63 | 0.59 ± 0.863 | U = 4682.00, M rank = 99.88 (HC), 124.71 (PD) | <.001 |
| Adjustment to stimuli | 0.75 ± 1.32 | 1.62 ± 1.836 | U = 45466.00, M rank = 102.59 (HC), 141.88 (PD) | <.001 |
| Gap acceptance | 0.16 ± 0.41 | 0.21 ± 0.517 | U = 5004.00, M rank = 102.52 (HC), 104.93 (PD) | .65 |
| Total errors | 24.08 ± 12.38 | 31.99 ± 22.013 | U = 5489.50, M rank = 109.28 (HC), 132.55 (PD) | .01 |
| On-road global rating scoreb | χ2(3) = 35.89c | <.001 | ||
| Fail not remediable | 5 (3.6) | 26 (25.7) | ||
| Fail remediable | 8 (5.8) | 15 (14.9) | ||
| Pass with recommendations | 116 (84.1) | 52 (51.5) | ||
| Pass with no recommendations | 9 (6.5) | 6 (5.9) | ||
| Missing | 0 (0) | 2 (2.0) | ||
| Global pass–failb | — | — | χ2(1) = 35.54d | <.001 |
| Fail | 13 (9.4) | 41 (40.6) | ||
| Pass | 125 (90.6) | 58 (57.4) | ||
| Missing | 0 (0) | 2 (2.0) | ||
Note. HC = healthy control group; M = mean; PD = Parkinson's disease group; — = no M or SD; SD = standard deviation. Significant group difference set at p < .05.
aComparisons used nonparametric test/Mann–Whitney U test. bComparisons used Pearson’s χ2 test. cPD, N = 99; HC, N = 138. dPD, N = 99; HC, N = 138.
Logistic Regression
The five greatest predictors, in order, of on-road pass–fail outcomes in participants with PD were visual scanning, signaling, vehicle positioning, speeding, and total errors (Table 4). A one-sample t-test subanalysis revealed that participants with PD made more speed regulation errors (overspeeding M = 0.86, SD ± 1.49; underspeeding M = 4.07, SD ± 4.57); t(61) = 7.004, p < .001.
Table 4.
Logistic Regression to Determine Driving Errors as Predictors of On-Road Pass–Fail Outcomes
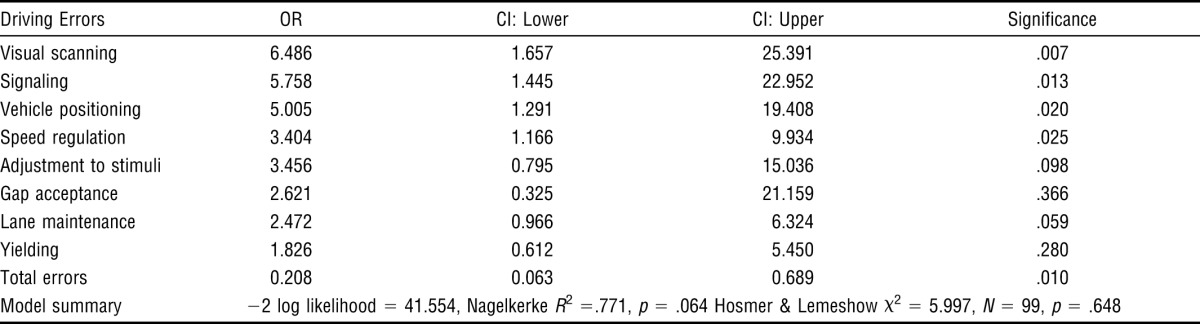
| Driving Errors | OR | CI: Lower | CI: Upper | Significance |
| Visual scanning | 6.486 | 1.657 | 25.391 | .007 |
| Signaling | 5.758 | 1.445 | 22.952 | .013 |
| Vehicle positioning | 5.005 | 1.291 | 19.408 | .020 |
| Speed regulation | 3.404 | 1.166 | 9.934 | .025 |
| Adjustment to stimuli | 3.456 | 0.795 | 15.036 | .098 |
| Gap acceptance | 2.621 | 0.325 | 21.159 | .366 |
| Lane maintenance | 2.472 | 0.966 | 6.324 | .059 |
| Yielding | 1.826 | 0.612 | 5.450 | .280 |
| Total errors | 0.208 | 0.063 | 0.689 | .010 |
| Model summary | −2 log likelihood = 41.554, Nagelkerke R2 =.771, p = .064 Hosmer & Lemeshow χ2 = 5.997, N = 99, p = .648 | |||
Note. CI = 95% confidence interval; OR = odds ratio.
Discussion
This study determined, in a large sample of drivers with PD and HC drivers, that group differences existed for demographics, number of medications used, driving history and habits, and driving errors. Most prominently, the study quantified specific driving errors predictive of the on-road pass–fail outcome.
Not surprisingly, he PD group reported taking more medications than the HC group, but drivers with PD reported driving more days than the HC drivers. A partial explanation for the increased driving frequency may be that participants with PD were younger, were more active, and had potentially more health care appointments or, alternatively, chose to spread trips out over several days, but this difference needs to be clarified in future studies. From a crash and traffic violation perspective and consistent with current literature (Heikkilä et al., 1998; Radford et al., 2004; Singh et al., 2007), participants with PD were as safe as HC participants. However, of all the possible avoidance behaviors, participants with PD reported only less nighttime driving. Uc and colleagues (Uc et al., 2005; Uc, Rizzo, Anderson, et al., 2009) reported issues with visual dysfunction and visual deficits in participants with PD under low-contrast conditions. This finding, observed in mild to moderate PD, may partially explain the avoidance of night driving in our group. An interesting finding and new to the PD literature was that a larger percentage of participants with PD reported using alternative transportation compared with HC participants. This finding may imply that some at-risk drivers with PD self-regulate to take measures for risk reduction, such as using alternative transportation options.
Participants with PD made more on-road lane maintenance, yielding, adjustment to stimuli, underspeeding, and total driving errors compared with HC participants. Many different types of driving errors (route finding, traffic sign identification, traffic signals, curves, speed adjustment, impaired braking distance, T junctions, and use of mirrors) are reported in the literature, and researchers also consistently report that participants with PD make more errors than HC participants (Classen et al., 2011; Cordell et al., 2008; Grace et al., 2005; Scally et al., 2011; Stolwyk et al., 2006; Uc et al., 2006a, 2007, 2011). In this study, participants with PD also received more pass with recommendations, fail with recommendations, and fail outcomes than HC participants, indicating that drivers with PD are a high-risk group who may potentially benefit from interventions to improve or preserve fitness to drive. Consistent with current literature, participants with PD failed the on-road test significantly more often than drivers in the HC group (Classen et al., 2011).
The driving errors that predicted on-road pass–fail outcomes in PD were visual scanning, signaling, vehicle positioning, and speeding (underspeeding). Although the mechanisms underlying these driving errors remain unclear, we postulate that these errors occur as a result of visual, cognitive, and attentional deficits, as manifested by problems with visual scanning, underspeeding, signaling (dual-task divided or selective attention), and vehicle positioning (visual–spatial). Further studies such as functional imaging may help elucidate the neuroanatomical correlates associated with these specific deficits in PD.
Rehabilitation strategies (compensatory strategies, adaptive equipment, or both) can be used for clients with PD in driving rehabilitation clinics. For example, visual scanning errors such as not checking the blind spot during a lane change may be mitigated with mirror aids. Mirrors reduce the number of areas to visually scan and thus reduce the driver’s divided attention demands. Signaling errors, a potential result of impaired divided and selective attention, may be reduced by providing cues through advanced driver assistance systems. The driver who underspeeds may be taught to increase following distance as an adaptive strategy and, as such, may mitigate the potential effects of slow processing speed while traveling at the pace of traffic. Vehicle positioning errors, potentially caused by visual–spatial deficits, may be reduced by teaching a reference point for stopping behind a vehicle (where the rear tires of the vehicle in front are in view) and at stop lines (where the line on the pavement intersects at a specific location on the driver’s vehicle).
Such strategies have not been tested in a controlled fashion. This study therefore opens plausible research and clinical opportunities to DRSs and neurologists to examine the effectiveness of driving rehabilitation interventions in improving fitness to drive in people with mild to moderate PD. Through such inquiry, the evidence base of the DRSs working with clients with PD will be supported and advanced.
Limitations
Beyond the limitations of this study already discussed (participants with PD being younger than HC participants and disproportionately White, self-reported diagnoses, and missing data), other limitations include the possibility of selection bias (better drivers may have enrolled in the study) and evaluator bias (knowing which participants had PD may have made the evaluators more critical in their assessment approach). The study did not control for the effects of medications, daytime sleepiness, or depression on the driving performance of participants with PD. Finally, drivers with more severe PD were not represented in this study, and the correlates and predictors of driving fitness in that group may differ.
Implications for Occupational Therapy Practice
The results of this study have the following implications for occupational therapy practice:
Drivers with Parkinson's disease are more likely than healthy drivers to have impaired fitness to drive.
The driving errors predicting on-road pass–fail outcomes in PD are visual scanning, signaling, vehicle positioning, speed regulation (mainly underspeeding), and total errors.
By understanding the types of driving errors made by drivers with PD and the client and contextual factors underlying those errors, occupational therapy practitioners have the opportunity to provide tailored intervention strategies.
Conclusion
This study confirms and adds to previous driving studies in the PD literature by identifying main group differences between the PD and HC groups. However, new information emerged; that is, drivers with PD avoided night driving to a greater extent, used alternative transportation more, and made more and different driving errors compared with HC drivers. Additionally, we identified specific errors predictive of failing an on-road test. Our suggested mitigation strategies require empirical testing.
Acknowledgment
This research project was funded by the National Parkinson Foundation and the National Institute on Aging (R21) PAR-06-247 (Sherrilene Classen, principal investigator).
Footnotes
The Florida state statute requirements for visual acuity are as follows: Each or both eyes without correction must be at least 20/40; if acuity is 20/50 or less, the applicant is referred to an eye specialist for possible improvement. Each or both eyes with correction must be at least 20/70; the worse eye must be better than 20/200. If one eye is blind, the other eye, with or without correction, must be 20/40. The absolute visual acuity minimum is 20/70. Bioptic telescopes are not allowed. For visual fields, the minimum field requirement is 130° horizontal.
The on medication state is defined as 1 hour after medication intake and patient report of having optimal perceived benefit. The CDRS tested the participants with PD only during their on state.
Contributor Information
Sherrilene Classen, Sherrilene Classen, PhD, MPH, OTR/L, is Professor and Director, School of Occupational Therapy, Elborn College, Room 2555B, 1201 Western Road, Western University, London, Ontario N6G 1H1 Canada. At the time of the study, she was Director, Institute for Mobility, Activity and Participation, and Associate Professor, Department of Occupational Therapy, College of Public Health and Health Professions, University of Florida, Gainesville; sclassen@uwo.ca.
Babette Brumback, Babette Brumback, PhD, is Professor and Program Director, Department of Biostatistics, University of Florida, Gainesville.
Miriam Monahan, Miriam Monahan, MS OT, CDRS, is Occupational Therapist and Certified Driving Rehabilitation Specialist, Department of Occupational Therapy and Institute for Mobility, Activity and Participation, University of Florida, Gainesville.
Irene I. Malaty, Irene I. Malaty, MD, is Assistant Professor, Center for Movement Disorders and Neurorestoration, Department of Neurology, University of Florida, Gainesville
Ramon L. Rodriguez, Ramon L. Rodriguez, MD, is Director, Movement Disorders Clinic, Center for Movement Disorders and Neurorestoration, Department of Neurology, University of Florida, Gainesville
Michael S. Okun, Michael S. Okun, MD, is Co-Director, Center for Movement Disorders and Neurorestoration, Department of Neurology, University of Florida, Gainesville
Nikolaus R. McFarland, Nikolaus R. McFarland, MD, PhD, is Assistant Professor, Department of Neurology, University of Florida, Gainesville
References
- Classen S., McCarthy D. P., Shechtman O., Awadzi K. D., Lanford D. N., Okun M. S., …, Fernandez H. H. Useful Field of View as a reliable screening measure of driving performance in people with Parkinson’s disease: Results of a pilot study. Traffic Injury Prevention. 2009;10:593–598. doi: 10.1080/15389580903179901. http://dx.doi.org/10.1080/15389580903179901 . [DOI] [PubMed] [Google Scholar]
- Classen S., Witter D. P., Lanford D. N., Okun M. S., Rodriguez R. L., Romrell J., …, Fernandez H. H. Usefulness of screening tools for predicting driving performance in people with Parkinson’s disease. American Journal of Occupational Therapy. 2011;65:579–588. doi: 10.5014/ajot.2011.001073. http://dx.doi.org/10.5014/ajot.2011.001073 . [DOI] [PubMed] [Google Scholar]
- Cordell R., Lee H. C., Granger A., Vieira B., Lee A. H. Driving assessment in Parkinson’s disease—A novel predictor of performance. Movement Disorders. 2008;23:1217–1222. doi: 10.1002/mds.21762. http://dx.doi.org/10.1002/mds.21762 . [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goetz C. G., Poewe W., Rascol O., Sampaio C., Stebbins G. T., Counsell C., …, Seidl L., Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Movement Disorders. 2004;19:1020–1028. doi: 10.1002/mds.20213. http://dx.doi.org/10.1002/mds.20213 . [DOI] [PubMed] [Google Scholar]
- Grace J., Amick M. M., D’Abreu A., Festa E. K., Heindel W. C., Ott B. R. Neuropsychological deficits associated with driving performance in Parkinson’s and Alzheimer’s disease. Journal of the International Neuropsychological Society. 2005;11:766–775. doi: 10.1017/S1355617705050848. http://dx.doi.org/10.1017/S1355617705050848 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä V. M., Turkka J., Korpelainen J., Kallanranta T., Summala H. Decreased driving ability in people with Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1998;64:325–330. doi: 10.1136/jnnp.64.3.325. http://dx.doi.org/10.1136/jnnp.64.3.325 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. J., Daniel S. E., Blankson S., Lees A. J. A clinicopathologic study of 100 cases of Parkinson’s disease. Archives of Neurology. 1993;50:140–148. doi: 10.1001/archneur.1993.00540020018011. http://dx.doi.org/10.1001/archneur.1993.00540020018011 . [DOI] [PubMed] [Google Scholar]
- Justiss M. D., Mann W. C., Stav W. B., Velozo C. A. Development of a behind-the-wheel driving performance assessment for older adults: The older driver, Part 2. Topics in Geriatric Rehabilitation. 2006;22:121–128. [Google Scholar]
- Michon J. A. A critical view of driver behavior models: What do we know, what should we do? In: Evans E. L., Schwing R., editors. Human behavior and traffic safety. New York: Plenum; 1985. pp. 485–520. [Google Scholar]
- Posse C., McCarthy D. P., Mann W. C. A pilot study of interrater reliability of the Assessment of Driving-related Skills: Older driver screening tool. Topics in Geriatric Rehabilitation. 2006;22:113–120. [Google Scholar]
- Radford K. A., Lincoln N. B., Lennox G. The effects of cognitive abilities on driving in people with Parkinson’s disease. Disability and Rehabilitation. 2004;26:65–70. doi: 10.1080/09638280310001629633. http://dx.doi.org/10.1080/09638280310001629633 . [DOI] [PubMed] [Google Scholar]
- Ramaker C., Marinus J., Stiggelbout A. M., Van Hilten B. J. Systematic evaluation of rating scales for impairment and disability in Parkinson’s disease: A systematic review. Movement Disorders. 2002;17:867–876. doi: 10.1002/mds.10248. [DOI] [PubMed] [Google Scholar]
- Scally K., Charlton J. L., Iansek R., Bradshaw J. L., Moss S., Georgiou-Karistianis N. Impact of external cue validity on driving performance in Parkinson’s disease. Parkinson’s Disease. 2011;2011:1–10. doi: 10.4061/2011/159621. Retrieved from http://dx.doi.org/10.4061/2011/159621 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Pentland B., Hunter J., Provan F. Parkinson’s disease and driving ability. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:363–366. doi: 10.1136/jnnp.2006.103440. http://dx.doi.org/10.1136/jnnp.2006.103440 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolwyk R. J., Triggs T. J., Charlton J. L., Moss S., Iansek R., Bradshaw J. L. Effect of a concurrent task on driving performance in people with Parkinson’s disease. Movement Disorders. 2006;21:2096–2100. doi: 10.1002/mds.21115. http://dx.doi.org/10.1002/mds.21115 . [DOI] [PubMed] [Google Scholar]
- Uc E. Y., Rizzo M., Anderson S. W., Dastrup E., Sparks J. D., Dawson J. D. Driving under low-contrast visibility conditions in Parkinson disease. Neurology. 2009;73:1103–1110. doi: 10.1212/WNL.0b013e3181bacf6e. http://dx.doi.org/10.1212/WNL.0b013e3181bacf6e . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uc E. Y., Rizzo M., Anderson S. W., Qian S., Rodnitzky R. L., Dawson J. D. Visual dysfunction in Parkinson disease without dementia. Neurology. 2005;65:1907–1913. doi: 10.1212/01.wnl.0000191565.11065.11. http://dx.doi.org/10.1212/01.wnl.0000191565.11065.11 . [DOI] [PubMed] [Google Scholar]
- Uc E. Y., Rizzo M., Anderson S. W., Sparks J. D., Rodnitzky R. L., Dawson J. D. Driving with distraction in Parkinson disease. Neurology. 2006a;67:1774–1780. doi: 10.1212/01.wnl.0000245086.32787.61. http://dx.doi.org/10.1212/01.wnl.0000245086.32787.61 . [DOI] [PubMed] [Google Scholar]
- Uc E. Y., Rizzo M., Anderson S. W., Sparks J., Rodnitzky R. L., Dawson J. D. Impaired visual search in drivers with Parkinson’s disease. Annals of Neurology. 2006b;60:407–413. doi: 10.1002/ana.20958. http://dx.doi.org/10.1002/ana.20958 . [DOI] [PubMed] [Google Scholar]
- Uc E. Y., Rizzo M., Anderson S. W., Sparks J. D., Rodnitzky R. L., Dawson J. D. Impaired navigation in drivers with Parkinson’s disease. Brain. 2007;130:2433–2440. doi: 10.1093/brain/awm178. http://dx.doi.org/10.1093/brain/awm178 . [DOI] [PubMed] [Google Scholar]
- Uc E. Y., Rizzo M., Johnson A. M., Dastrup E., Anderson S. W., Dawson J. D. Road safety in drivers with Parkinson disease. Neurology. 2009;73:2112–2119. doi: 10.1212/WNL.0b013e3181c67b77. http://dx.doi.org/10.1212/WNL.0b013e3181c67b77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uc E. Y., Rizzo M., Johnson A. M., Emerson J. L., Liu D., Mills E. D., …, Dawson J. D. Real-life driving outcomes in Parkinson disease. Neurology. 2011;76:1894–1902. doi: 10.1212/WNL.0b013e31821d74fa. http://dx.doi.org/10.1212/WNL.0b013e31821d74fa . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M., Worringham C., Kerr G., Mallon K., Silburn P. Quantitative assessment of driving performance in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:176–180. doi: 10.1136/jnnp.2004.047118. http://dx.doi.org/10.1136/jnnp.2004.047118 . [DOI] [PMC free article] [PubMed] [Google Scholar]


