Abstract
Several lines of evidence have associated Chlamydia pneumoniae with cardiovascular disease including acceleration of atherosclerotic lesion progression in hyperlipidemic animal models by infection. Many of the proatherogenic effects of oxidized low density lipoprotein (ox-LDL) occur through the activation of the lectin-like ox-LDL receptor (LOX-1). C. pneumoniae upregulates expression of the LOX-1mRNA, promotes uptake of ox-LDL, and utilizes the LOX-1 receptor for infectivity. The overall goal of this study was to determine if C. pneumoniae organisms upregulated LOX-1 protein expression in vascular cells and whether up-regulation of pro-atherogenic factors by C. pneumoniae occurred through LOX-1. C. pneumoniae induced LOX-1 protein expression in both endothelial cells and RAW macrophages. Upregulation was prevented by preincubation of cells with LOX-1 antibody prior to infection. Similarly, C. pneumoniae upregulated protein expression of adhesion molecules, MMP-1, and MMP-3, which was mitigated by anti-LOX-1 antibody. Prior treatment of organisms with PNGase, which removes the chlamydial glycan that is N-linked to the major outer membrane, abolished C. pneumoniae up-regulation of LOX-1. These studies suggest that activation of LOX-1 expression occurs through binding of the chlamydial glycan and provide one mechanism by which C. pneumoniae infection could play a role in the pathogenesis of atherosclerosis.
Keywords: Chlamydia, LOX-1 receptor, atherosclerosis, adhesion molecules, glycan
Introduction
The association of Chlamydia pneumoniae and atherosclerosis has been documented by the detection of the organism in atherosclerotic lesions by multiple methods and by culturing the organism from atheromatous tissue (Campbell & Kuo, 2004). Importantly, a role for C. pneumoniae in atherosclerosis has been demonstrated experimentally in animal models, in which infection accelerated the development of atherosclerosis in hyperlipidemic, but not normolipidemic animals (Hu, et al., 1999, Moazed, et al., 1999, Blessing, et al., 2001, Blessing, et al., 2002). In contrast, Chlamydia trachomatis has neither been detected in atheromas, nor does C. trachomatis infection of hyperlipidemic mice accelerate atherosclerosis (Blessing, et al., 2000).
A novel receptor for oxidized-low density lipoprotein (ox-LDL) in vascular endothelial cells was discovered by Sawamura et al. and designated lectin-like ox-LDL receptor (LOX-1) (Sawamura, et al., 1997). This receptor is also expressed in macrophages and smooth muscle cells (Yoshida, et al., 1998, Draude, et al., 1999). Significantly, many of the pro-atherogenic effects of ox-LDL occur as a result of its binding to and uptake by LOX-1 (Sawamura, et al., 1997, Chen, et al., 2000). Specifically, activation of LOX-1 results in a cascade of events that are key to atherogenesis including up-regulation of multiple pro-atherogenic factors such as adhesion molecules (ICAM-1, VCAM-1, E-selectin, P-selectin), matrix metalloproteinases (MMP-1 and MMP-3), and monoctye chemoattractant protein-1 (MCP-1) (Kita, et al., 1999, Li & Mehta, 2000, Li, et al., 2003, Zhu, et al., 2005). Chlamydia pneumoniae has been shown to up-regulate LOX-1 mRNA expression in endothelial cells and promote the uptake of oxLDL (Yoshida, et al., 2006). Recently, we reported that C. pneumoniae, but not C. trachomatis, binds to the LOX-1 receptor (Campbell, et al., 2012). Interestingly, C. pneumoniae infection of endothelial cells has been shown to induce a pattern of responses similar to those observed following LOX-1 activation including up-regulation of E-selectin, MCP-1, ICAM-1 and VCAM-1 (Rodel, et al., 2003, Kim, et al., 2005). MMP-1 and MMP-3 are also up-regulated in monocytes and/or smooth muscle cells by C. pneumoniae (Rodel, et al., 2003, Kim, et al., 2005). Thus, we hypothesized that one mechanism by which C. pneumoniae may contribute to atherosclerotic processes is through binding and activation of the LOX-1 receptor. The purpose of the current study was to determine whether C. pneumoniae upregulates LOX-1 protein expression in endothelial cells and macrophages and if upregulation of the aforementioned pro-atherogenic factors occurs through activation of LOX-1.
Materials and Methods
Chlamydial organisms and cell lines
C. pneumoniae AR-39 and C. trachomatis E/UW-5/Cx were grown in HL (human line) cells and HeLa cells, respectively. Organisms were purified by Hypaque gradient centrifugation. Purified organisms were suspended in sucrose-phosphate-glutamic acid (SPG) buffer and stored at −70°C in aliquots until used. Human micro-vascular endothelial cells (HMEC-1) were originally obtained from E.W. Ades (Centers for Disease Control and Prevention, Atlanta). Human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics (San Diego) and the murine macrophage RAW 264.7 cell line was obtained from (ATCC, Manassas, VA). Endothelial cells were grown in EMB™, Endothelial Basal Medium to which EGM™, Endothelial Growth Medium, Single QuOTS ™ were added according to the directions of the manufacturer (Lonnza, Walkersville, MD) and RAW cells were grown in Dulbecco modified Eagle medium (Gibco BRL, Langley, OK) containing 10% fetal bovine serum, 100 U/ml vancomycin, and 100 μg/ml streptomycin.
Reagents
The polyclonal antibodies used were against: LOX-1 (ab60178, Abcam); ICAM-1 (sc7891 and sc1511, Santa Cruz, Biotechnology); E-selectin (sc14011, Santa Cruz Biotechnology) and B-actin (ab75186, Abcam). The monoclonal antibodies used included: LOX-1(AF1789, R & D Systems); MMP-1 (MAB901, R & D Systems); MMP-3 (1B4, Santa Cruz, Biotechnology); and VCAM-1 (BBA6, R & D). The concentrations were used as recommended by the manufacturer. Secondary antibodies were conjugated to horseradish peroxidase and included: rabbit anti-mouse IgG (315-005-044); goat anti-rabbit IgG (112-005-167); and rabbit anti-goat IgG (305-005-003). All were obtained from Jackson Labs and were used at dilutions of 1:500-1:1000.
Protein Analysis
Endothelial cells and macrophages were plated in 24 well plates to a confluency of 4 × 105 cells. The host cells were incubated with 0.2 ml of the appropriate antibody dilution of anti-LOX-1 antibody or SPG (or PBS) for 2 hr at 37 °C with gentle rocking prior to infection. The host cells were washed with PBS, infected for 2 hr with gentle rocking at 37°C, washed, and analyzed at 24 hr post-infection. In some experiments, C. pneumoniae organisms were exposed to N-glycanase for 3 hr at 37° in a CO2 incubator and then washed with PBS to remove N-glycanase prior to infection. For analysis of the expression of pro-atherogenic factors, host cell lysates were prepared at 24 hr post infection. Protein concentration was determined using an Eppendorf biophotometer. Proteins were separated on 5–15% polyacrylamide followed by immunoblotting using the appropriate primary antibody to the respective factors followed by the addition of secondary antibody conjugated to horseradish peroxidase. Pierce ECL Western blotting Substrate (Thermo Scientific) was used for visualization. Protein expression was normalized to B-actin. TNF-α or LPS were used as positive controls for demonstrating up-regulation of LOX-1 (Mehta & Li, 2002). Minor adjustments in contrast were occasionally made using Photoshop Elements.
Results
Previously, Yoshida et al. demonstrated that C. pneumoniae infection enhanced LOX-1 mRNA expression in human umbilical vein endothelial cells (HUVEC) (Yoshida, et al., 2006). Additionally, C. pneumoniae infection promoted uptake of oxLDL, but not acetylated LDL, which is characteristic of the pattern of ligand recognition by the LOX-1 receptor. Subsequently, we have demonstrated that C. pneumoniae binds to the LOX-1 receptor and C. trachomatis does not (Campbell, et al., 2012). Thus, we investigated the effect of C. pneumoniae infection on protein expression of LOX-1 and proatherogenic factors that are up-regulated following activation of LOX-1 in endothelial cells (HMEC and/or HUVEC) and RAW cells. C. pneumoniae infection upregulated LOX-1 protein expression in endothelial cells as early as 6 hrpost-infection (p.i.) and up to 72 hr p.i. (Fig. 1, Panel A). Importantly, pre-exposure of cells with anti-LOX-1 antibody (Ab) prior to infection negated this effect (Fig. 1, Panel A). Similar results were observed in C. pneumoniae infected RAW macrophages as C. pneumoniae enhanced protein expression was first observed at 6 hr pi, the second time point measured (Fig. 1, Panel B). Likewise, upregulation of LOX-1 by C. pneumoniae infection was inhibited by incubation of RAW cells with LOX Ab prior to infection.
Figure 1.
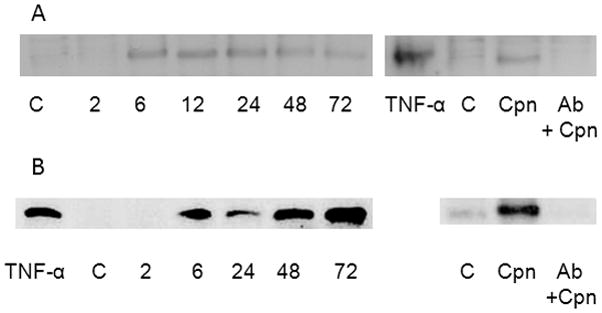
Time course of LOX-1 protein expression upregulation by Chlamydia pneumoniae. Endothelial cells (HUVEC, Panel A) and RAW macrophages (Panel B) were plated in 24 well plates to a confluency of 4 × 105 cells and infected with C. pneumoniae at a MOI of 10. At various times post-infection, infected cells were harvested and cell lysates prepared. LOX-1 protein expression was determined in uninfected TNF-α stimulated cells (positive control), uninfected cells (base level control, labeled as C), infected cells (Cpn), and cells pretreated with anti-LOX-1 antibody for 2 hrs at 37 °C with gentle rocking prior to infection (Cpn+ Ab). C. pneumoniae upregulated LOX-1 expression in both cell types in comparison to uninfected cells, which was negated by preincubation of cells with anti-LOX-1 antibody prior to infection.
As we have previously reported that C. trachomatis did not bind to the LOX-1 receptor (Campbell, et al., 2012), we predicted that C. trachomatis infection would not have an effect on expression of the LOX-1 receptor. As shown in Figs. 2A and 2B, in contrast to C. pneumoniae, C. trachomatis did not enhance LOX-1 protein expression in either HMEC or RAW cells and pre-incubation with anti-LOX-1 antibodies prior to infection did not have any effect on LOX-1 expression in comparison with antibody treated uninfected cells.
Figure 2.
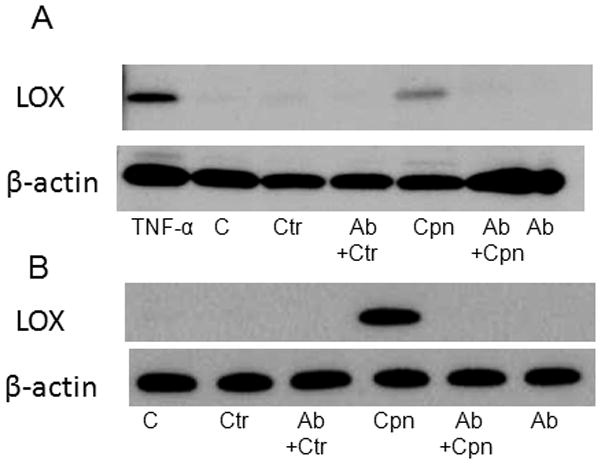
Chlamydia trachomatis does not up regulate LOX-1 protein expression. Endothelial cells (HMEC, Panel A) and RAW macrophages (Panel B) were plated in 24 well plates to a confluency of 4 × 105 cells and infected with C. trachomatis (Ctr) or C. pneumoniae (Cpn) at a MOI of 10. LOX-1 protein expression was determined in uninfected TNF-α stimulated cells (positive control), uninfected cells (base level control, labeled as C), infected cells, and infected cells pretreated with anti-LOX-1 antibody for 2 hrs at 37 °C with gentle rocking prior to infection (Ctr+Ab or Cpn+Ab). LOX-1 protein expression was visualized by immunoblot using anti-LOX-1 specific antibodies and normalized to cellular B-actin expression.
Studies using flow cytometry or analysis of mRNA have reported that C. pneumoniae infection up-regulated several factors associated with the pathogenesis of atherosclerosis (Kita, et al., 1999, Li & Mehta, 2000, Li, et al., 2003, Zhu, et al., 2005). To determine whether C. pneumoniae upregulation of these factors occurred through activation of the LOX-1 receptor, protein expression was compared in uninfected cells and infected cells either pre-exposed to anti-LOX-1 antibody or SPG. As shown in Fig. 3 (Panels A and B), C. pneumoniae infection augmented the expression of pro-atherogenic factors (ICAM-1, VCAM-1, E-selectin, MMP-1, MMP-3) in endothelial cells, although upregulation of MMP-1 expression was slight in HMEC in comparison to HUVEC. Enhanced expression of these proatherogenic factors by C. pneumoniae was also observed in RAW macrophages (Panel C) in comparison to uninfected cells (lane labeled as C in all panels). TNF-α or LPS were used as a positive control to induce expression of the pro-atherogenic factors in uninfected cells.
Figure 3.
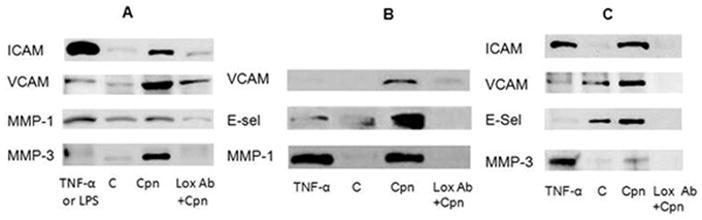
Upregulation of protein expression of proatherogenic factors by Chlamydia pneumoniae occurs through LOX-1 activation. Endothelial cells (HMEC, Panel A and HUVEC, Panel B) and RAW macrophages (Panel C) were plated in 24 well plates to a confluency of 4 × 105 cells and infected with C. pneumoniae (Cpn) at a MOI of 10. Protein expression was determined through immunoblot analysis using specific antibodies against Intercellular Adhesion Molecule-1 (ICAM), Vascular Cellular Adhesion Molecule-1 (VCAM), E-selectin (E-Sel), matrix metalloproteinase (MMP-1) and matrix metalloproteinase 3 (MMP-3). Protein expression was determined in uninfected TNF- α or LPS stimulated cells (positive control), uninfected cells (base level control, labeled as C), infected cells (Cpn), and cells pretreated with anti-LOX-1 antibody for 2 hrs at 37°C with gentle rocking prior to infection (Cpn+Ab). C. pneumoniae upregulation of adhesion molecule protein expression was prevented by prior treatment of cells with anti-LOX-1 antibody.
Previously, we demonstrated that the Chlamydia MOMP is glycosylated and that the N-linked glycan is a high mannose oligosaccharide (Kuo, et al., 1996). However, C. pneumoniae was shown to bind to the mannose 6-phosphate receptor while C. trachomatis utilized the mannose receptor, suggesting subtle differences in the glycan between these two species (Kuo, et al., 2002, Puolakkainen, et al., 2005). Because the LOX-1 receptor is a C-type lectin that can bind carbohydrates, we determined whether the chlamydial glycan played a role in activation of LOX-1 expression. As shown in Fig. 4, pre-exposure of organisms to peptide-N-glycosidase F (PNGase), which cleaves N-linked glycans, but not O-linked glycans, prevented up-regulation of LOX-1 expression in both endothelial cells (panel A) and RAW macrophages (panel B), suggesting that the chlamydial glycan plays a role in activation of the LOX-1 receptor.
Figure 4.
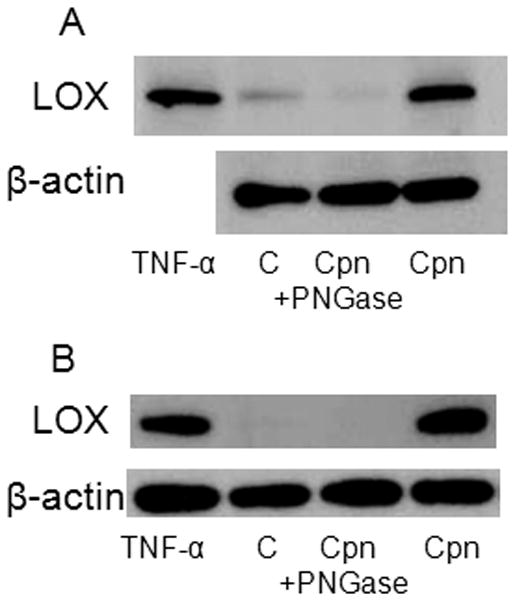
Upregulation of LOX-1 by C. pneumoniae does not occur when the chlamydial glycan is removed by N-glycanase treatment. Endothelial cells (HMEC, Panel A) and RAW macrophages (Panel B) were plated in 24 well plates to a confluency of 4 × 105 cells and infected with C. pneumoniae (Cpn) at a MOI of 10. LOX-1 protein expression was determined in uninfected TNF-α stimulated cells (positive control), uninfected cells (base level control, labeled as C), infected cells, and infected cells exposed to N-glycanase (Cpn+) PNGase with anti-LOX-1 antibody for 2 hrs at 37 °C with gentle rocking prior to infection (Cpn+Ab). LOX-1 protein expression was visualized by immunoblot using anti-LOX-1 specific antibodies and normalized to cellular B-actin expression.
Discussion
The role of LOX-1 in atherogenesis is well established as activation of the LOX-1 receptor is associated with a plethora of events that contribute to the atherosclerotic process (Ogura, et al., 2009, Yoshimoto, et al., 2011). Activation of endothelial LOX-1 results in endothelial dysfunction, cell injury and apoptosis (Ogura, et al., 2009). Moreover, it has been demonstrated that LOX-1 expression is increased in hyperlipidemia and atherosclerotic lesions (Ogura, et al., 2009, Yoshimoto, et al., 2011). Various pro-inflammatory and pro-oxidative stimuli as well as other stimuli known to be involved in the pathogenesis of cardiovascular disease are known to increase the expression of LOX-1 (Ogura, et al., 2009). In addition to oxLDL, LOX-1 recognizes diverse ligands under the categories of polyanionic chemicals, polysaccharides, phospholipids, and bacteria. In this current report, we demonstrated that C. pneumoniae upregulates LOX-1 protein expression in endothelial cells and macrophages and that upregulation of ICAM-1, VCAM-1, E-selectin, and MMP-1 and MMP-3 protein expression by C. pneumoniae occurs through activation of the LOX-1 receptor as prior incubation with anti-LOX-1 antibody prevents C. pneumoniae upregulation not only of LOX-1, but also expression of adhesion molecules and matrix metalloproteinase. As predicted from our previous studies demonstrating that, in contrast to C. pneumoniae, C. trachomatis did not bind to the LOX-1 receptor, C. trachomatis did not up-regulate LOX-1 protein expression (Fig. 2). Our preliminary in vivo studies correlate with the species-specific differences observed in vitro. Specifically, we have demonstrated that intranasal inoculation of mice with C. pneumoniae induces LOX-1 mRNA expression in both the lung and aorta (Campbell et al. Proceedings of the 12th International Meeting on Human Chlamydial Infections) and C. trachomatis does not (unpublished data). Importantly, C. trachomatis does not accelerate atherosclerosis in hyperlipidemic animal models of atherosclerosis nor has the organism been detected in atherosclerotic lesions in humans (Hu, et al., 1999, Moazed, et al., 1999, Blessing, et al., 2000, Blessing, et al., 2001).
In addition to the LOX-1 receptor, we have reported that C. pneumoniae also binds to the mannose 6-phosphate receptor on endothelial cells through the chlamydial glycan (Puolakkainen, et al., 2005). Our cumulative studies have demonstrated that the chlamydial glycan plays a role in attachment and infectivity (Kuo, et al., 1996, Kuo, et al., 2004, Puolakkainen, et al., 2005, Campbell, et al., 2006, Kuo, et al., 2007). Significantly, removal of the glycan by exposure of organisms to N-glycanase significantly decreases infectivity both in vitro in cell culture and in vivo in mouse models of Chlamydia infection (Kuo, et al., 2004, Campbell, et al., 2006). Because the LOX-1 receptor is a member of the C-type lectin family and contains a carbohydrate binding domain, we determined whether N-glycanase exposure of organisms had any effect on C. pneumoniae upregulation of the LOX-1 receptor. The resulting lack of upregulation of LOX-1 protein expression by C. pneumoniae (Fig. 4, panel A and B) following glycan removal, indicated that the C. pneumoniae glycan plays a role in attachment to/activation of the LOX-1 receptor. Heat shock protein 60 (HSP60) has also been shown to be a ligand of the LOX-1 receptor. Recently, Lin et al. investigated whether recombinant C. pneumoniae GroEL1, a chlamydial HSP60, upregulated LOX-1 expression in vitro in coronary artery endothelial cells and in vivo in a hyperlipidemic rabbit model of atherosclerosis (Lin, et al., 2011). Their results demonstrated that recombinant GroEL1 protein induced LOX-1 expression and oxLDL uptake in endothelial cells through TLR-4 in vitro. Importantly, intravenous administration of the C. pneumoniae recombinant Hsp60 induced inflammatory responses as measured by elevated erythrocyte sedimentation rate and serum C-reactivate protein, enhanced LOX-1 protein expression in the abdominal aorta endothelium, and augmented atherosclerotic lesion formation in rabbits fed an atherogenic diet, but had no effect on normocholesterolemic animals. In conjunction with our earlier studies demonstrating that the organism can bind to the LOX-1 receptor, the cumulative results suggest alternative pathways for LOX-1 activation by C. pneumoniae, one which would be dependent on TLR4 through GroEL1 and the other by direct activation of LOX-1 through interaction with the chlamydial glycan. However, it should be noted that our studies were done with C. pneumoniae organisms rather than with a purified ligand. In conclusion, the findings that C. pneumoniae binds to the LOX-1 receptor and activates LOX-1 expression resulting in the induction of various pro-atherogenic factors suggest that one mechanism by which C. pneumoniae infection contributes to the atherosclerotic processes is through activating the LOX-1 receptor.
Acknowledgments
This study was supported by funding from the National Institutes of Health (AI43060).
Footnotes
The authors have no conflicts of interest to declare.
References
- Blessing E, Campbell LA, Rosenfeld ME, Kuo CC. Chlamydia pneumoniae and hyperlipidemia are co-risk factors for atherosclerosis: infection prior to induction of hyperlipidemia does not accelerate development of atherosclerotic lesions in C57BL/6J mice. Infect Immun. 2002;70:5332–5334. doi: 10.1128/IAI.70.9.5332-5334.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing E, Nagano S, Campbell LA, Rosenfeld ME, Kuo CC. Effect of Chlamydia trachomatis infection on atherosclerosis in apolipoprotein E-deficient mice. Infect Immun. 2000;68:7195–7197. doi: 10.1128/iai.68.12.7195-7197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing E, Campbell LA, Rosenfeld ME, Chough N, Kuo CC. Chlamydia pneumoniae infection accelerates hyperlipidemia induced atherosclerotic lesion development in C57BL/6J mice. Atherosclerosis. 2001;158:13–17. doi: 10.1016/s0021-9150(00)00758-9. [DOI] [PubMed] [Google Scholar]
- Campbell LA, Kuo CC. Chlamydia pneumoniae--an infectious risk factor for atherosclerosis? Nat Rev Microbiol. 2004;2:23–32. doi: 10.1038/nrmicro796. [DOI] [PubMed] [Google Scholar]
- Campbell LA, Lee A, Kuo CC. Cleavage of the N-linked oligosaccharide from the surfaces of Chlamydia species affects infectivity in the mouse model of lung infection. Infect Immun. 2006;74:3027–3029. doi: 10.1128/IAI.74.5.3027-3029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LA, Puolakkainen M, Lee A, Rosenfeld ME, Garrigues HJ, Kuo CC. Chlamydia pneumoniae binds to the lectin-like oxidized LDL receptor for infection of endothelial cells. Microbes Infect. 2012;14:43–49. doi: 10.1016/j.micinf.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Kakutani M, Minami M, et al. Increased expression of lectin-like oxidized low density lipoprotein receptor-1 in initial atherosclerotic lesions of Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol. 2000;20:1107–1115. doi: 10.1161/01.atv.20.4.1107. [DOI] [PubMed] [Google Scholar]
- Draude G, Hrboticky N, Lorenz RL. The expression of the lectin-like oxidized low-density lipoprotein receptor (LOX-1) on human vascular smooth muscle cells and monocytes and its down-regulation by lovastatin. Biochem Pharmacol. 1999;57:383–386. doi: 10.1016/s0006-2952(98)00313-x. [DOI] [PubMed] [Google Scholar]
- Hu H, Pierce GN, Zhong G. The atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. J Clin Invest. 1999;103:747–753. doi: 10.1172/JCI4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MP, Gaydos CA, Wood BJ, Hardick JP, Zhang Y, Wahl LM. Chlamydia pneumoniae enhances cytokine-stimulated human monocyte matrix metalloproteinases through a prostaglandin E2-dependent mechanism. Infect Immun. 2005;73:632–634. doi: 10.1128/IAI.73.1.632-634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Kume N, Ishii K, Horiuchi H, Arai H, Yokode M. Oxidized LDL and expression of monocyte adhesion molecules. Diabetes Res Clin Pract. 1999;45:123–126. doi: 10.1016/s0168-8227(99)00041-8. [DOI] [PubMed] [Google Scholar]
- Kuo C, Takahashi N, Swanson AF, Ozeki Y, Hakomori S. An N-linked high-mannose type oligosaccharide, expressed at the major outer membrane protein of Chlamydia trachomatis, mediates attachment and infectivity of the microorganism to HeLa cells. J Clin Invest. 1996;98:2813–2818. doi: 10.1172/JCI119109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Lee A, Campbell LA. Cleavage of the N-linked oligosaccharide from the surfaces of Chlamydia species affects attachment and infectivity of the organisms in human epithelial and endothelial cells. Infect Immun. 2004;72:6699–6701. doi: 10.1128/IAI.72.11.6699-6701.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Puolakkainen M, Lin TM, Witte M, Campbell LA. Mannose-receptor positive and negative mouse macrophages differ in their susceptibility to infection by Chlamydia species. Microb Pathog. 2002;32:43–48. doi: 10.1006/mpat.2001.0479. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Lee A, Jiang SJ, Yaraei K, Campbell LA. Inoculation of Chlamydia pneumoniae or Chlamydia trachomatis with ligands that inhibit attachment to host cells reduces infectivity in the mouse model of lung infection: implication for anti-adhesive therapy. Microbes Infect. 2007;9:1139–1141. doi: 10.1016/j.micinf.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Li D, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101:2889–2895. doi: 10.1161/01.cir.101.25.2889. [DOI] [PubMed] [Google Scholar]
- Li D, Liu L, Chen H, Sawamura T, Ranganathan S, Mehta JL. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation. 2003;107:612–617. doi: 10.1161/01.cir.0000047276.52039.fb. [DOI] [PubMed] [Google Scholar]
- Lin FY, Lin YW, Huang CY, et al. GroEL1, a heat shock protein 60 of Chlamydia pneumoniae, induces lectin-like oxidized low-density lipoprotein receptor 1 expression in endothelial cells and enhances atherogenesis in hypercholesterolemic rabbits. J Immunol. 2011;186:4405–4414. doi: 10.4049/jimmunol.1003116. [DOI] [PubMed] [Google Scholar]
- Mehta JL, Li D. Identification, regulation and function of a novel lectin-like oxidized low-density lipoprotein receptor. J Am Coll Cardiol. 2002;39:1429–1435. doi: 10.1016/s0735-1097(02)01803-x. [DOI] [PubMed] [Google Scholar]
- Moazed TC, Campbell LA, Rosenfeld ME, Grayston JT, Kuo CC. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. J Infect Dis. 1999;180:238–241. doi: 10.1086/314855. [DOI] [PubMed] [Google Scholar]
- Ogura S, Kakino A, Sato Y, et al. Lox-1: the multifunctional receptor underlying cardiovascular dysfunction. Circ J. 2009;73:1993–1999. doi: 10.1253/circj.cj-09-0587. [DOI] [PubMed] [Google Scholar]
- Puolakkainen M, Kuo CC, Campbell LA. Chlamydia pneumoniae uses the mannose 6-phosphate/insulin-like growth factor 2 receptor for infection of endothelial cells. Infect Immun. 2005;73:4620–4625. doi: 10.1128/IAI.73.8.4620-4625.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodel J, Prochnau D, Prager K, Pentcheva E, Hartmann M, Straube E. Increased production of matrix metalloproteinases 1 and 3 by smooth muscle cells upon infection with Chlamydia pneumoniae. FEMS Immunol Med Microbiol. 2003;38:159–164. doi: 10.1016/S0928-8244(03)00126-3. [DOI] [PubMed] [Google Scholar]
- Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kondratenko N, Green S, Steinberg D, Quehenberger O. Identification of the lectin-like receptor for oxidized low-density lipoprotein in human macrophages and its potential role as a scavenger receptor. Biochem J. 1998;334 ( Pt 1):9–13. doi: 10.1042/bj3340009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Koide N, Mori I, Ito H, Yokochi T. Chlamydia pneumoniae infection enhances lectin-like oxidized low-density lipoprotein receptor (LOX-1) expression on human endothelial cells. FEMS Microbiol Lett. 2006;260:17–22. doi: 10.1111/j.1574-6968.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- Yoshimoto R, Fujita Y, Kakino A, Iwamoto S, Takaya T, Sawamura T. The discovery of LOX-1, its ligands and clinical significance. Cardiovasc Drugs Ther. 2011;25:379–391. doi: 10.1007/s10557-011-6324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Xia M, Hou M, Tang Z, Li Y, Ma J, Ling W. Ox-LDL plays dual effect in modulating expression of inflammatory molecules through LOX-1 pathway in human umbilical vein endothelial cells. Front Biosci. 2005;10:2585–2594. doi: 10.2741/1722. [DOI] [PubMed] [Google Scholar]


