Abstract
Chronic lymphocytic leukemia (CLL) is the most common hematologic malignancy in the Western Hemisphere. Despite advances in research and the development of effective treatment regimens, CLL is still largely an incurable disease. Although several prognostic factors have been identified in recent years, most of the new prognostic factors are not utilized, and treatment decisions are still based on clinical staging and limited use of cytogenetic analysis. Patients with advanced disease are treated at diagnosis, whereas others, regardless of their prognostic indicators, are offered treatment only at disease progression. Furthermore, treatment guidelines for elderly or “unfit” patients are unavailable because most CLL trials have included mostly younger, healthier patients. Given the heterogeneity of the clinical manifestations and prognosis of CLL, patients are likely to benefit from a personalized therapeutic approach. Recent advances in CLL pathobiology research, the use of high-throughput technologies, and most importantly, the introduction of novel targeted therapies with high efficacy and low toxicity are currently transforming the treatment of CLL. A personalized approach that includes early intervention in selected patients with CLL is likely to bring physicians closer to the goal of attaining cures in most patients with CLL.
Keywords: CLL, personalized medicine, targeted therapy
1. Introduction
Chronic lymphocytic leukemia (CLL), the most common leukemia in the Western Hemisphere, is characterized by gradual accumulation of mature-appearing lymphocytes co-expressing CD5, CD19, and CD23. The clinical course of the disease is extremely variable. Approximately 30% of patients have an indolent disease and their life expectancy is similar to that of age-matched healthy individuals [1] whereas other patients experience a benign course, typically lasting 5 to 10 years, that is followed by a rapidly progressive disease. In contrast, about 10% of patients with CLL have an aggressive disease with a median survival of one to three years from diagnosis [73]. Because CLL is a heterogeneous disease and its clinical course varies from patient to patient, an individualized approach is required.
A broad array of mechanisms affects the pathobiology and heterogeneity of CLL. Recognizing the essential disease-driving factors is crucial for selecting patient-specific treatment options. In this review, we describe various clinical and laboratory findings currently used to guide treatment of patients with CLL, and how recent advances in research and understanding the pathobiology of CLL are bringing us closer to the goal of individualizing therapy related decisions.
2. Prognostic stratification
A major breakthrough towards individualizing the management of CLL was the identification of cytogenetic abnormalities commonly detected by conventional cytogenetic (karyotype) analysis and fluorescence in situ hybridization (FISH) in approximately 80% of patients. Other prognostic and predictive markers have been tested in clinical trials, assuming that a differential treatment response according to any of these markers would help individualize future CLL therapy.
2.1 Common cytogenetic abnormalities
Analysis of FISH data from patients with CLL revealed that patients with the same chromosomal abnormality have similar clinical courses. This observation enabled the development of a hierarchical prognostic model and a set of rules for clinical decision making.
Approximately 50% of patients with CLL carry a monoallelic or biallelic deletion of the long arm of chromosome 13 (del[13q]). When present as a sole abnormality, del(13q) is associated with a favorable clinical outcome [26]. In contrast, deletion of the short arm of chromosome 17 (del[17p]), found in 5% to 10% of patients with CLL, is the strongest predictor of poor progression-free survival (PFS) and overall survival (OS). Patients with del(17p) have the shortest times from diagnosis to first treatment, the lowest response rates to front-line and salvage therapy, and short remission durations [5]. Deletion of the long arm of chromosome 11 (del[11q]), found in 5% to 20% of patients, is associated with extensive lymphadenopathy, progressive disease, and a short PFS [26]. Approximately 15% of patients with CLL have trisomy 12 whereas 18% have no common FISH- detectable cytogenetic abnormalities. These patients have a relatively long PFS and OS [26].
However, FISH analysis provides only a rough predictor of CLL's clinical course and treatment outcome. For example, not all patients with del(17p) have a dismal prognosis. A retrospective analysis of 99 untreated patients with CLL revealed that approximately 50% of patients with del(17p) experienced stable disease lasting for up to 6 years [97].
Guidelines developed by the International Workshop for CLL (iwCLL) recommend FISH analysis of all newly diagnosed patients using probes for 13q, T12, 11q, 6q, and 17p cytogenetic abnormalities [42]. However, with the exception of del(17p) testing, FISH analysis is not used as a guideline for follow-up or therapy-related decisions in current practice. In patients with CLL with del(17p), most clinicians would recommend early treatment intervention, preferably in a clinical trial setting [5].
2.2 Other validated prognostic factors
Other prognostic factors have been validated through the years, including the level of β2 microglobulin (β2M), the presence of somatic mutations in the variable region of the immunoglobulin heavy chain (IgVH) (unmutated IgVH is associated with a poorer prognosis) [44], the presence of the VH3-21 gene regardless of mutation status, ZAP-70 level, cell surface CD38, and the serum markers CD23 and thymidine kinase. However, in current practice, these markers do not affect the care of patients, and their routine use in treatment decision-making outside clinical trials has not been recommended by the iwCLL [42].
3. Genetic alterations detected on high-throughput whole-genome studies
High-throughput whole-genome sequencing expanded our understanding of the pathobiology of CLL and unraveled mechanism of disease heterogeneity. However, it is still unclear how these novel data would affect clinical decision making.
3.1 Genetic alterations in germ-line cells
A genome-wide association study of 1529 patients with CLL and 3115 healthy individuals identified six loci associated with CLL risk with low penetrance [25]. This association was later verified in an independent cohort of 438 patients with CLL and 328 controls [89]. However, the associated risk of developing CLL in individuals carrying these loci, as estimated by the odds ratio, was low. For example, in individuals with rs872071, the locus most strongly associated with CLL, the odds ratio was 1.54. A recent estimate of the incidence of CLL in the United States, based on the Surveillance, Epidemiology, and End Results (SEER) database for the years 2005 to 2009, is 4.2 cases per 100,000 men and women per year. Thus, individuals with a single-nucleotide polymorphism (SNP) of rs872071 would have an age-adjusted incidence of 6.5 (4.2 × 1.54) cases per 100,000 individuals per year, suggesting that probability that individuals carrying the rs872071 SNP will develop CLL is only marginally increase. It is likely that in a relatively homogeneous population with a high prevalence of CLL, such as the Ashkenazi Jewish population [83], association studies would identify a SNP with higher penetrance. Because the impact of CLL-risk loci on disease prevalence is low, the probability that these loci would provide a clinically relevant cause of CLL heterogeneity is also low.
3.2 Genetic alterations in CLL cells
Studies examining genetic alterations in CLL cells have generated clinically relevant data. Array-based genome-wide scanning of CLL peripheral blood cells from 353 untreated patients identified regions of loss of heterozygosity and copy number alterations that could not be detected by FISH. For example, a 15q15.1 deletion was found in 4% of patients. Paired analysis of CD19+ CLL cells and CD19– (nonmalignant) cells revealed copy number alterations in 88% of the CLL cells, with a mean of 1.8 copy number alterations per sample. Copy number alterations from IgVH-mutated and -unmutated CLL cells were similar, IgVH-mutated and -unmutated CLL cells had similar rates of copy number alterations and in 60% of patients with CLL, no alterations other than those detected by FISH were found. In patients with del(13q), the minimally deleted regions were found to be restricted to the DLEU1 and DLEU2 genes; in patients with del(11q), 11q minimally deleted regions were restricted to the ataxia telangiectasia mutated (ATM) locus; The dismal prognosis of patients with del (17p) is attributed to the deletion of the p53 tumor suppressor gene, and in patients with del(2p), minimally deleted regions were restricted to a 1.9-Mb fragment containing nine genes [28].
Whole-genome sequencing detected somatic mutations in CLL cells. In one study, exome sequencing of 105 CLL samples detected 1246 mutations affecting 1100 protein-coding genes, with an average of 45 mutations per patient [70]. In another study, parallel whole-genome and germline DNA sequencing of 91 CLL samples detected 1838 nonsynonymous mutations in protein-coding sequences of 1608 genes, with an average of 20 non-synonymous mutations per patient [101]. Both studies found recurrent somatic mutations in a small number of genes. The mutations in ATM and NOTCH1 had previously been identified, but other mutations,such as a spliceosome SF3B1 mutation, found in 10% to 15% of patients with CLL, were previously unknown.
3.3 Epigenetic alterations in CLL cells
Epigenetic modifications in CLL have been actively researched, and several genome-wide DNA methylation studies have been published in recent years [67, 71, 84]. These studies identified a number of genes and pathways that are aberrantly methylated in CLL. CLL cells and normal B cells had similar global CpG methylation but differed in their methylation patterns. For example, aberrant hypermethylation of HOX and Wnt signaling pathway genes was apparent in CLL cells but not in normal B cells, whereas hypomethylation of the nuclear factor of activated T cells (NFAT)-c1 P2 promoter, detected in CLL cells, correlated with upregulation of RNA transcript and protein levels of NFAT [67]. Other studies identified a correlation between validated prognostic factors and the methylation status of CLL cells [49, 71].
3.4 Clinical-genetic correlations
Fine-tuning of the current prognostication systems based on accumulating genetic data may provide a useful tool for selecting a substantial number of patients with newly diagnosed, early-stage, asymptomatic CLL who could benefit from up-front therapy. New prediction tools may arise from DNA sequencing data and from studies of mRNA or microRNAs (miRs) that are differentially expressed in low- and high-risk patients, and that may predict CLL's natural history and response to specific targeted therapies.
The CLL cell gene expression profile is easy to distinguish from that of normal B cells [79]. Using microarray technology to explore the global gene expression profile of CLL cells from patients with various prognostic factors, several investigators identified a few potential prognostic biomarkers. For example, high levels of ZAP-70 expression correlated with unmutated IgVH [79]. This is translated to higher ZAP-70 protein levels in unmutated IgVH cases, and has been used as a surrogate marker for unmutated IgVH [22]. In addition, Schweighofer et al. found that two genes, SKI and SLAMF1, predict both time to first treatment and OS better than any combination of clinical parameters does [85]. Whether a CLL gene signature will become a useful prognostic tool, like the multigene panels currently in development in other neoplasms (including breast and colon cancer) remains to be seen.
CLL is characterized by deregulation of the miR network [12]. That differential expression of specific miRs can predict CLL risk has been reported by several investigators. A unique signature of 13 miRs was associated with known prognostic factors such as unmutated IgVH and expression of ZAP-70 [11]. Subsequent studies failed to confirm the prognostic power of that set of miRs but did identify another set of miRs that are differentially expressed according to CLL risk level [37, 57]. While overexpression of miRs such as miR-223 and miR-29c appears to be associated with an unfavorable prognosis [91], current results from microarray studies have not yet generated reproducible data. A prospective study of miR expression profiles in patients with newly diagnosed, untreated CLL is warranted.
Only a few recurrent somatic mutations have been identified in patients with untreated CLL. The majority of patients with 17p deletions have point mutations in the other TP53 allele and thus complete inactivation of p53 [110]. Mutations in TP53 have a poor prognosis regardless of the presence of 17p deletions when treated with fludarabine-based chemotherapy [110], suggesting that identifying a p53 mutation in newly diagnosed patients may provide benefits beyond those of standard FISH analysis in terms of identifying patients who are candidates for non–fludarabine-based therapy. Similarly, deletions in ATM may occur in patients with CLL who do not have del(11q) [40]. However, ATM is a big, 146-kb gene with 62 exons. Therefore, testing for mutations in the entire ATM region cannot be routinely performed with current technologies.
The transmembrane protein Notch1 acts as a transcription factor upon ligand binding. Approximately 10% of patients with CLL have a frameshift deletion mutation in NOTCH1 [70], resulting in a truncated constantly active protein. A recent report suggests that NOTCH1 mutations are frequently detected in patients with trisomy 12 and that disease course in those patients is often aggressive [81]. If these findings are confirmed in an independent patient cohort, patients with trisomy 12 harboring NOTCH1 mutations may be candidates for early therapeutic intervention.
Mutations in SF3B1 appear in 5% of newly diagnosed patients with CLL [80] but are significantly more frequent in patients with advanced disease, especially after treatment [82]. The shorter time to disease progression and lower 10-year survival rate associated with these mutations are probably fully accounted for by the mutations’ association with other unfavorable prognostic markers such as del(11q). In addition to identifying prognostically significant mutations, sequence data analysis has identified signaling pathways that are involved in the pathobiology of CLL. With the rapid development of kinase inhibitors, knowing the relationships between these pathways and CLL may be useful in selecting specific therapies on an individual basis. For example, patients with a dominant involvement of inflammatory pathways (e.g., those with a Myd88-activating mutation) may respond to anti-inflammatory targeted therapy. Similarly, patients with mutations in the cell cycle checkpoint that result in the generation of an impaired DNA double-strand break repair may respond to poly(ADP-ribose) polymerase inhibitors [103].
4. Front-line therapy
The current management of CLL at diagnosis and at time of relapse is rapidly changing. Recent years’ progress in understanding the role of cytogenetic, genetic and molecular abnormalities that affect the pathogenesis and progression of CLL are likely to change future clinical practice.
4.1 When to treat
Typically, patients with advanced CLL are candidates for treatment at the time of diagnosis. This decision is based on the Rai or Binet clinical staging systems. The Rai staging system, published in 1975 and still widely used, uses lymphadenopathy, organomegaly, and cytopenia (anemia or thrombocytopenia) to classify CLL into five prognostic groups (0, I, II, III, and IV). Patients with stage III/IV CLL who do not receive therapy have a median survival of only 19 months and therefore are considered for up-front treatment at the time of diagnosis [75]. The Binet staging system classifies CLL according to the number of involved lymph node sites and the presence of anemia and/or thrombocytopenia. CLL with lymphadenopathy with no cyopenia is classified as Binet stage A if fewer than three sites are involved and B if three or more sites are involved. CLL with anemia/thrombocytopenia is classified as Binet stage C regardless of the number of involved lymph node sites. Similar to patients with stage III/IV CLL according to the Rai system, patients with Binet stage C CLL have a median survival of 24 months with no therapy and are therefore treated early [7].
Conversely, asymptomatic patients with early-stage disease are usually not offered treatment. This practice has been established based on studies published during the 1990s that found no advantages for treatment in early-stage disease with single agent chlorambucil compared with observation [2]. However, chlorambucil is no longer a first-line treatment of choice for most patients with CLL, and none of the currently accepted regimens has yet been compared with “observation” in newly diagnosed patients. The time to first treatment for early-stage disease is likely to change as effective, relatively low-toxicity drugs, with the potential to cure CLL become available. Whereas studies comparing chlorambucil to observation only included all eligible patients with early-stage disease, in future studies, intervention at the time of diagnosis is will probably be considered only in selected patients using more stringent exclusion criteria. For example, patients with newly diagnosed CLL whose disease is not likely to progress are obviously not candidates for early intervention and should be excluded.
In an analysis of 1674 previously untreated patients with CLL from 1981 through 2004 seen at one institution, the median OS was 10.7 years. A prognostic nomogram based on six prognostic factors—advanced age, high lymphocyte count at the time of diagnosis, high levels of β2M, nodal disease in three or more regions, advanced disease stage, and male sex [105]. Because this analysis was based on data that included untreated and treated patients, it might not be sufficient for predicting treatment outcomes of asymptomatic, newly diagnosed patients and, of equal importance, for identifying which patients would do well without any therapeutic intervention.
To identify patients whose disease will progress rapidly if no treatment is given, a group at The University of Texas MD Anderson Cancer Center analyzed 930 previously untreated patients who were referred to the institution between 2004 and 2009. The model generated identified five variables that were independently associated with shorter time to first treatment: involvement of three or more lymph node sites, increased size of cervical lymph nodes, the presence of 17p or 11q deletions detected by FISH analysis, increased serum lactate dehydrogenase, and unmutated IgVH. The applicability of this model in choosing patients for up-front therapeutic intervention is still low, however. For example, even in high-risk patients with both unmutated IgVH and adverse cytogenetic abnormalities such as del(17p) and del(11q), more than 20% did not require therapy during 5 years of follow-up. However, this model appears to be better at identifying patients with low-risk disease: more than 90% of patients classified as low-risk remained event-free during 4 years of follow-up [106]. Similarly, when the MD Anderson model was applied in a cohort of 310 newly diagnosed patients in the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) database, the probability that patients in the low-risk group would not need any therapy during a 5 years of follow-up was 100% [59].
4.2 Whom to treat
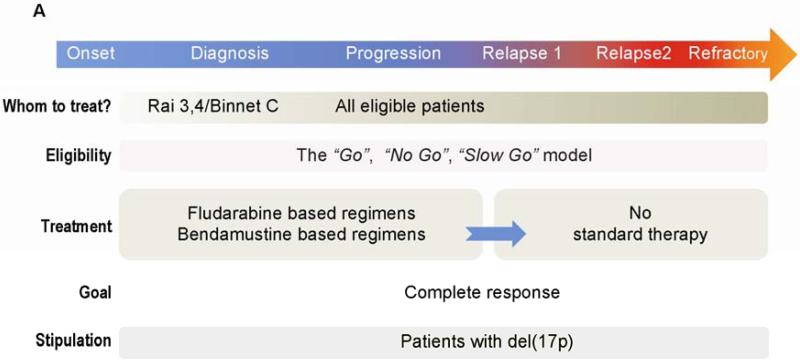
Not all patients with CLL who meet the iwCLL treatment criteria will tolerate front-line therapy. Therefore, patients with CLL have been grouped into three categories according to their performance status and comorbidities [41]. The “go go” category includes patients with a good performance status and no comorbidities. The goal of therapy in this group is to prolong PFS and OS. These patients are usually treated with the most effective standard of care regimen. The “no go” category consists of patients at the other end of the spectrum: frail patients with severe comorbidities, dependence in one or more daily activities, and a short life expectancy. These patients receive symptomatic and palliative care only. The “slow go” category consists of patients with a performance status and comorbidity severity between those of the other two groups. “Slow go” is a heterogeneous group in which therapy is tailored on an individual basis. For some patients, treatment is given to achieve response, whereas for others the main goal is some disease burden reduction with relief of symptoms and improvement in quality of life [24] (Figure 1A). In approximately 45% of patients with CLL, a major comorbidity that negatively affects response and survival is detected at the time of diagnosis, and as many as 25% of patients with CLL fail to meet the National Cancer Institute working group guidelines for participation in clinical trials. Remarkably, although comorbidities are associated with advanced age, the presence of comorbidities are less important than age in predicting prognosis of newly diagnosed patients with CLL [100].
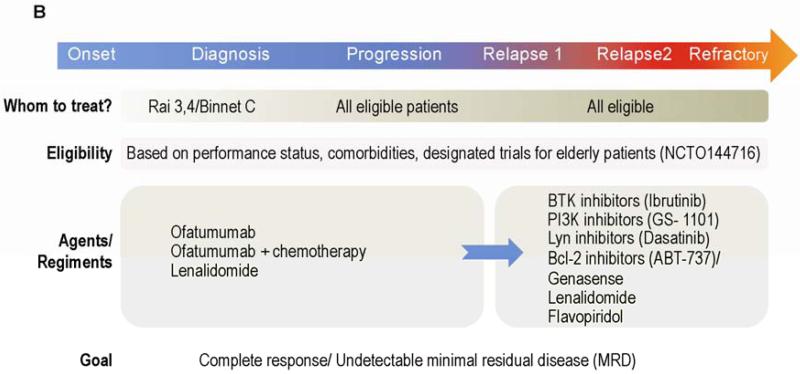
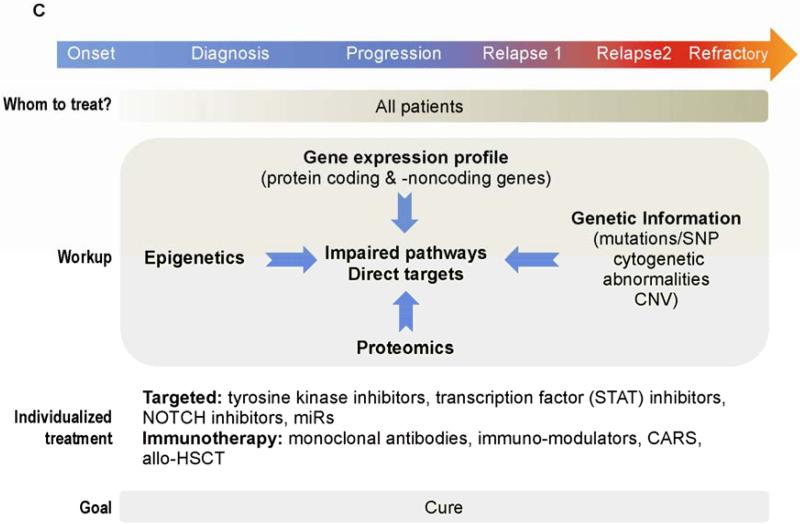
Figure 1.
Treatment algorithms in CLL. A. Standard of care. Although several prognostic factors predict the outcome of patients with newly diagnosed CLL, only clinical staging is commonly used to decide which patients should be treated at time of diagnosis. In contrast, even patients with RAI stage 0 to 2 are usually treated when disease has progressed. About 30% of CLL patients never require treatment. Clinical evaluation for fitness is used to decide whether a patient is eligible for high dose chemoimmunotherapy (CIT) (“Go”), palliative care (“No-Go”) or for moderate treatment (“Slow Go”). Cytogenetic analysis and FISH are used to identify patients with del (17p). Because standard CIT in patients with del(17p) yields inferior results compared to those obtained in patients with other cytogenetic abnormalities, other therapeutic modalities have been offered to patients with del(17p). B. Clinical trials. Promising results with novel agents in patients with relapsed/refractory disease led to clinical trials investigating the activity of these agents in previously untreated patients. In addition to assessment of response by standard criteria, the clinical significance of undetectable MRD is also being investigated. C. Future therapies. Extensive diagnostic work-up detecting of abnormally activated pathways and potential molecular targets will soon be available. A treatment plan that includes targeted therapies as single agent or in combination will soon be offered to all patients including the elderly and the unfit. Cure is becoming a reasonable goal, especially since combinations of induction with maintenance therapy strategies to maintain long term remissions are under development. Patients with refractory disease could still be salvage using immunotherapy based approaches (eg: CARS/allo-SCT).
CLL is a disease of the elderly. The median age at diagnosis ranges from 65 to 70 years [88], and the incidence of CLL increases exponentially with age. In the United States, the annual incidences of CLL per 100,000 people younger than 50, 50 to 64, 65 to 74, and at least 75 years old are 2.2, 7.2, 19.1, and 30.6, respectively (SEER database). Unfortunately, elderly patients are significantly underrepresented in the clinical trials that established the current treatment recommendations. For example, the phase III randomized trial comparing fludarabine and cyclophosphamide (FC) with fludarabine, cyclophosphamide, and rituximab (FCR) included only 81 patients older than 70 years in a group of 817 (10%). Patients older than 81 years were automatically excluded, and most patients older than 70 years were excluded because of low performance status, severe comorbidities as defined by the Cumulative Illness Rating Scale, or low creatinine clearance. Nonetheless, patients 65 years old or older with good physical fitness who were included in the trial tolerated both the FC and the FCR regimens and showed improved outcomes similar to those of younger patients [43]. Similarly, the phase III trial comparing bendamustine with chlorambucil included only patients younger than 75 years [53]. While some elderly patients are successfully treated with chemoimmunotherapy outside clinical trials, most are considered ineligible and are usually treated with milder modalities such as chlorambucil, single-agent bendamustine, or dose-reduced FCR (“FCR-Lite”).
The availability of agents with better safety profiles provides effective treatment options for “slow go” or “no go” patients. Currently, these agents are available only to patients who are enrolled in clinical trials. In contrast to “go go” patients, “slow go” patients lack established evidence-based treatment recommendations. The introduction of novel, less toxic, effective agents will likely change this reality.
4.3 Choosing first-line treatment
The FCR regimen, a combination that was developed at The University of Texas MD Anderson Cancer Center [96], is the current standard of care for first-line treatment for patients with CLL who require treatment, with the exception of those with del(17p), for whom there is currently no consensus regarding front-line therapy (discussed in detail below). A series of phase III randomized trials demonstrated a gradual improvement in treatment outcomes until FCR was found to yield the best results. First, chlorambucil was compared with fludarabine and was found to yield better outcomes [74], then fludarabine was compared with the FC combination [14, 29, 32]. Finally, the superiority of FCR compared with FC was confirmed by the German CLL study group in the CLL8 trial [43].
For almost 40 years, the standard of care for CLL was single agent chlorambucil. The overall response (OR) rate was 33%, the complete remission (CR) rate was only 4%, and the median PFS was 14 months [74]. In contrast, treatment with FCR yields an OR rate of 95%, a CR rate of 44%, and a PFS time of 52 months [43]. The introduction of fludarabine-based regimens resulted in a survival benefit; 31% of patients treated with single-agent fludarabine but only 19% of those treated with single-agent chlorambucil were alive 8 years after treatment [72]. The follow-up periods of the most recent clinical trials are too short for assessment of survival rates; nevertheless, early reports showed survival benefits for combination therapies. In the CLL4 trial, FC improved OS compared with single-agent fludarabine in patients who did not harbor del(17p) or p53 mutation [93]. Similarly, in CLL8, at a median follow–up time of 37.7 months, the median OS times were 84.1 months in the FCR group and 79 months in the FC group (p = 0.01) [43]. A long-term follow-up of 300 patients treated with FCR identified eight patients who developed therapy related myeloid neoplasms (t-MN) [96]. Therefore, whether the alkylating agent cyclophosphamide should be removed from the FCR regimen has been debated. During a median follow-up of 117 months of 104 patients treated with fludarabine and rituximab (FR), either sequentially or concurrently, no documented t-MN were reported. Whereas the results in the sequential arm were less favorable than FCR, a 90% OR rate and a 47% CR rate in the concurrent arm are similar to those achieved with FCR regiment [109].
For patients with a poor performance status, fludarabine-based therapy has been individualized. Among the regimens available for such patients are FCR-Lite, which consists of a low dose of fludarabine and a low dose of cyclophosphamide [33], low-dose FC; and oral fludarabine [34].
Bendamustine provides an alternative to fludarabine-based regimens for patients with newly diagnosed CLL. Bendamustine is a bifunctional drug that comprises an alkylating group with a side chain similar to that of chlorambucil and a central ring system that resembles those of purine analogues [55]. A phase III randomized trial comparing chlorambucil with bendamustine demonstrated the superiority of bendamustine, with an OR rate of 68% vs. 31%, a CR rate of 31% vs. 2%, and a medianPFS time of 22 vs. 8 months [53]. A favorable outcome, albeit with a higher rate of infectious complications, was recorded in a single-arm phase II trial of bendamustine combined with rituximab in patients with previously untreated CLL [31]. A phase III trial comparing FCR with BR is currently undergoing (CLL10: NCT00769522). Until the results from this trial become available there is no definite way to prefer either approach: While outcomes in FCR trials seem slightly superior, efficacy cannot be adequately compared because of differences in patients’ characteristics. Finally, survival advantage and estimated risk for development of secondary malignancies are only available for patients who were treated with fludarabine based regiments because of a longer follow-up.
A consensus on front-line treatment for patients with del(17p) has not been reached. Overall, these patients’ long-term response to chemotherapy is poor and their survival rate low. In the 21 patients with del(17p) in the CLL8 trial, the OR rate was 71% and the CR rate was only 5%, significantly lower than the rates observed in all other patients in the trial [43]. Because of those disappointing results, other therapeutic options have been studied for patients with del(17p), including the anti-CD52 monoclonal antibody alemtuzumab (Campath) as a salvage [51] or front-line therapy. In a small randomized phase III trial comparing alemtuzumab with chlorambucil in patients with del(17p), the OR rate was 64% for alemtuzumab. This result led to a recommendation for using alemtuzumab in front-line therapy for patients with del(17p) and no bulky disease [45]. The addition of chemotherapy or high-dose steroids to alemtuzumab improved response rate but increased toxicity [68].
4.4 Hematopoeitic stem cell transplantation in patients with an unfavorable prognosis
Hematopoietic stem cell transplantation has been offered to selected patients with CLL. Several studies demonstrated a graft-versus-leukemia effect in patients with CLL undergoing allogeneic stem cell transplantation (allo-SCT) [6]. Allo-SCT has been associated with high morbidity and mortality rates and therefore is not recommended as a front-line therapy for most patients with CLL [5]. However, because the expected PFS in patients with del(17p) is short, younger patients with del(17p) and good performance status are candidates for allo-SCT in first remission, and the European Group for Blood and Marrow Transplantation (EBMT) recommended that del(17p) be considered an indicator for allo-SCT in first remission [27]. Encouraging data from the CLL3X trial by the German CLL study group showed better outcomes for patients who had received fewer than three prior treatments, supporting the recommendation of the EBMT for early consideration of allo-SCT in patients with del(17p). In this multicenter prospective phase II study, high-risk patients received a reduced-intensity conditioning regimen followed by allo-SCT. The 4-year non-relapse mortality, event-free survival, and OS rates were 23%, 42%, and 65%, respectively. Remarkably, the outcomes of allo-SCT in patients with del(17p) (n = 13) were similar to the outcomes of allo-SCT in patients with other cytogenetic abnormalities [27].
5. Treatment of patients with relapsed/refractory disease
Whereas an identical front-line regimen is usually administered to most patients, not a single universally accepted treatment exists for patients with relapsed or refractory disease. Furthermore, the success rate of currently available regimens for treatment of relapsed/refractory disease is inferior to that of currently available regimens for front-line therapy. Therefore, patients with relapsed/refractory disease, particularly those with refractory disease, should be enrolled in clinical trials (Figure 1B).
Several nonchemotherapy agents are currently being investigated, mainly in the setting of relapsed or refractory CLL. These novel agents target specific pathways that are activated in CLL and have a relatively low toxicity profile. Encouraging results in patients with progressive disease suggest that these drugs are highly effective, even in patients with adverse prognostic factors such as unmutated IgVH or adverse chromosomal abnormalities. Some of these agents may radically change the current goals of therapy for CLL by providing an option for cure in at least a subset of patients.
After completing treatment cycle, all patients should undergo a standardized response assessment. The desired goal of treatment is CR, defined as the absence of constitutional symptoms, normalization of several surrogate markers of disease burden such as blood lymphocyte counts and spleen and lymph node size, and the absence of a clonal population of lymphocytes in the peripheral blood. Outcome assessment in patients enrolled in research protocols may differ from that in standard clinical practice. For example, patients undergoing CLL treatment who have persistent anemia, thrombocytopenia, or neutropenia may be considered to be in CR with incomplete recovery of the bone marrow, likely because of drug toxicity [42]. Some novel agents that are currently being tested in CLL induce mobilization of tumor cells from lymph nodes into the peripheral blood, resulting in transient lymphocytosis. Patients receiving such agents otherwise attained dramatic responses and were considered non-responders according to International Workshop for CLL criteria. To address this issue, a Lymphoma Research Foundation–sponsored workshop recommended that the response criteria for CLL be modified and the term “nodal response”, describing “CR with transient lymphocytosis”, be added [19].
Most patients who attain a CR with the currently available regimens eventually experience relapse. However, time to next treatment differs significantly between individuals. Quantitation of minimal residual disease (MRD) at the end of treatment has been suggested as a means of predicting response duration [61]. Real-time quantitative PCR (RQ-PCR) to detect the unique VDJ rearrangement in the IGHV [gene and four-color flow cytometry are the technologies most used to quantify MRD. Each technique can detect 1 CLL cell in 10,000 to 100,000 (10−4 to 10−5) leukocytes [39].
Because low MRD levels during and after treatment has been associated with longer PFS and OS, MRD could be used as a surrogate marker for assessing treatment efficacy in randomized trials [8]. Quantitative MRD testing, although not reflective of lymph node, marrow, or visceral residual disease, could be used in clinical trials of consolidation and maintenance therapy strategies in patients with positive MRD. Furthermore, with the introduction of novel therapies that may cure a fraction of patients with CLL, MRD could be used as a monitoring tool, as is now the common clinical practice in patients with chronic myelogenous leukemia treated with tyrosine kinase inhibitors.
In CLL, relapsed disease has been defined as disease progression 6 months or more after attaining CR or partial response , refractory disease has been defined as a failure to achieve CR or partial response, and progressive disease has been defined as disease progression within 6 months of the completion a given therapy [42]. With current chemotherapy, immunotherapy, and chemoimmunotherapy regimens, the treatment outcomes of refractory disease are dismal; the current median survival times of patients who are refractory to purine analogues are often less than 1 year. Treatment outcomes of patients with progressive disease are significantly better than those of patients with refractory disease. Arguably, refractory disease and progressive disease should be considered separately. Nevertheless, most trials of salvage therapy for CLL have grouped patients with these two types of disease together [60].
Evidently, treatment of relapsed disease should be personalized, and factors such as response duration, cytogenetic abnormalities, and performance status should be considered (Figure 1C). Response duration after initial therapy may usefully guide second-line treatment. In patients with prolonged PFS, retreatment with the regimen used in the front-line treatment is a reasonable option, whereas in patients with a short PFS, another treatment option should be considered. Indeed, patients who attained a short PFS with a fludarabine-based therapy had a substantial response to a bendamustine-based regimen [31].
Cytogenetic abnormalities constitute another useful guide for second-line therapy. For example, patients with del(11q) who were initially treated with fludarabine-based therapy typically responded to the same regimen as second-line therapy [46]. Other considerations when treating relapsed/refractory disease are performance status, biological age, concurrent illnesses, and the availability of experimental therapies.
Although long-term disease-free remissions have been observed in some patients treated with FCR [50], most previously treated patients with CLL eventually relapse and will develop with time refractory disease [60]. Currently available data on treatment of refractory disease are of patients with disease refractory to fludarabine-based combinations. Notably, CLL that is refractory to one regimen may respond to a different drug or regimen. For example, in a study comparing chlorambucil with fludarabine, 46% of the patients who did not respond to chlorambucil responded to fludarabine, and 7% of those who did not respond to fludarabine and were switched to chlorambucil and eventually responded [74]. Moreover, even though cross-reactivity between purine analogues is well established, some patients who do not respond to fludarabine-based therapy may respond to pentostatin combined with cyclophosphamide [102]. High-dose steroid therapy has been shown to induce responses in patients with refractory disease. In a trial of 25 patients with refractory CLL, half of whom had a 17p abnormality, 19 (77%) responded to high-dose methylprednisolone [99].
Other options for patients with refractory CLL include alemtuzumab-based regimens, and for a minority of patients with good performance status, no comorbidities, and an HLA matched donor, allo-SCT should be considered. Because high treatment-related mortality rates (30% to 40%) were reported with myeloablative conditioning [66], current transplants generally use reduced-intensity conditioning. In a series of 86 patients with relapsed/refractory disease treated with nonmyeloablative regimens at MD Anderson, the estimated 5-year survival rate was 51%, and only 3% died within 100 days of the transplant [52]. Similarly, in 82 patients with fludarabine-refractory CLL, nonmyeloablative allo-SCT resulted in a 5-year OS rate of 50% [90].
On the other end of the spectrum is a heterogeneous group of patients with refractory CLL who are not candidates for aggressive therapies. Some are elderly and frail, and others, although in relatively good health, are heavily pretreated. Palliative care for these patients has traditionally included radiation treatment. Splenic irradiation has been indicated for painful splenic enlargement or severe hypersplenism-induced cytopenia [20], and irradiation of a bulky lymphoid mass may alleviate compression symptoms [47]. Some patients receiving palliative care only may live for several years. For example, the median OS time of five such patients treated with palliative splenic radiation was 45 months [54]. Nevertheless, the best option is enrollment in clinical trials because several of the currently used novel agents are effective, with generally tolerable side effects.
6. Novel agents in the treatment of CLL
Traditionally, the efficacy of new drugs has been explored in patients with relapsed/refractory disease. In accordance with that tradition, novel agents with promising activity in CLL are currently investigated in clinical trials enrolling patients with advanced disease.
6.1 Tyrosine kinase inhibitors for CLL
The tyrosine kinase inhibitors currently being investigated in CLL have been shown to induce impressive responses in patients with relapsed/refractory disease [107]. Because B cell receptor (BCR) signaling plays a pivotal role in the pathogenesis of CLL, the tyrosine kinase inhibitors being investigated for treatment of CLL have been collectively referred to as BCR inhibitors; however, this name is misleading because these molecules inhibit other intracellular pathways activated by toll-like receptors(TLRs), cytokines, chemokines, integrins, CD40, and B cell activating factor(BAFF) [107].
6.1.1 Ibrutinib (PCI-37265)
Ibrutinib is primarily an irreversible inhibitor of the Bruton tyrosine kinase. Like other kinase inhibitors tested in patients with CLL, ibrutinib inhibits several signaling pathways, including BCR, toll-like receptors (TLR), B cell activating factor (BAFF) and CD40. Hence, ibrutinib not only reduces BCR signaling, but also interfere in the cross-talk between CLL and stromal cells [107]. In a phase Ib/II trial of 116 patients with relapsed or refractory disease, ibrutinib induced durable remissions in all subsets of patients, including elderly patients and patients with high-risk disease. Remarkably, transient lymphocytosis, observed in most patients in the trial, was associated with response. In addition, partial restoration of humoral immune deficiency, which is found with advanced disease, was also reported, with a sustained increase in levels of IgA [48].
GS-1101 (CAL-101/Idelalisib)
GS-1101 inhibits primarily the P13Kδ kinase. In a phase I study that included 54 patients with refractory/relapsed, 80% had a reduction in lymphadenopathy of ≥ 50%, and a median PFS had not been reached at 11 months. Similar to what was observed in patients treated with ibrutinib, transient lymphocytosis was associated with response, and the clinical effect was independent of traditional prognostic factors, most notably the IgVH mutation status [107].
6.2 Lenalidomide (Revlimid)
Lenalidomide is a 3-aminothalidomide derivative of thalidomide with immune-modulatory potency. Compared with thalidomide, lenalidomide has reduced neurotoxicity, has reduced neurosedative toxicity, and is associated with a reduced risk of venous thromboembolism [13]. Several phase II clinical trials for CLL demonstrated the efficacy of lenalidomide alone or in combination with anti-CD20 antibodies, with OR rates ranging from 32% to 65% [4, 15, 17, 30]. Patients with unfavorable cytogenetic abnormalities, particularly elderly patients, also seem to benefit from lenalidomide [30]. Tumor flare reaction is an adverse effect of lenalidomide that is observed only in patients with CLL. This effect is characterized by a painful swelling of the lymph nodes and/or splenomegaly accompanied by rash, low-grade fever, and occasionally a rise in peripheral white blood cell counts [16]. Non-steroidal anti-inflammatory drugs and low-dose steroid therapy alleviate the symptoms but not the frequency of tumor flare reaction. Whether the appearance of tumor flare is associated with long-term clinical response is not yet clear [16].
6.3 Flavopiridol (Alvocidib)
Flavopiridol is a synthetic flavone that has cyclin-dependent kinase inhibitor properties. This drug was shown to induce apoptosis of CLL cells independently of p53 status. In a phase II study of 64 patients previously treated with purine analogues with a median of four previous treatments, 53% responded to flavopiridol, including 21 with del(17p) and 14 with del(11q) [56]. A longer follow-up showed that the patients with del(17p) or del(11q) had PFS and OS similar to those of the patients without those abnormalities [108].
6.4 Inhibition of B-cell lymphoma-2 (Bcl-2)
Several B-cell lymphoma-2 (Bcl-2) family members known to regulate the intrinsic/mitochondrial apoptotic pathway are overexpressed in CLL cells. Bcl-2 and its related proteins Bcl-XL and Mcl-1 provide CLL cells with a partial protection from apoptosis and their high levels were found to be associated with an aggressive clinical course, chemotherapy resistance, and a short survival rate [78, 98]. Several compounds designed to inhibit Bcl-2 and Bcl-2-related proteins are currently investigated in the laboratory and in clinical trials.
6.4.1 BH3 mimetic
All Members of the Bcl-2 family share conserved Bcl-2 homology (BH) domains termed BH1, BH2, BH3, and BH4. Most Bcl-2 family members have a multi domain structure that, function as inhibitors of apoptosis by binding to and inhibiting BAX and BAK. Bcl-2 family proteins members with BH3 domain only, referred to as “BH3 only domain” are apoptotic activators, and they exert their pro-apoptotic effect either by direct activation of BAX and BAK or by releasing these proteins from their binding to apoptotic inhibitors [92].
Similar to the natural “BH3 only domain” proteins, BH3 mimetics possess a pro-apoptotic activity by binding to anti apoptotic proteins and preventing them from sequestering the apoptotic activators BAX and BAK.[76]. BH3 mimetics may be active as single agents and may also have synergistic activity when combined with chemotherapeutic agents [58]. ABT-737 and its analogue ABT-263 (navitoclax) exhibited single agent activity in cell lines expressing high levels of Bcl-2 or Bcl-XL [65, 94, 95]. Preliminary results of a Phase I trial of ABT-737 in patients with fludarabine refractory CLL revealed a drop in lymphocyte counts by more than 50% in 19 of 21 patients. PR was documented in 35% of patients but no CR was observed [77].
6.4.2 BCL-2 antisense
Antisense DNA oligonucleotides are single stranded DNA sequences that prevent translation of targeted mRNA by specific hybridization, thereby inducing the enzymatic cleavage of the target massager RNA [23]. Oblimersen sodium (Genasense) is a synthetic 18-base single stranded DNA designed to down-regulate Bcl-2 mRNA expression by selective hybridization to the first six codons in the open reading frame that encode the Bcl-2 protein. The RNA/antisense duplex recruits RNase H which degrades the RNA and releases the antisense construct to hybridize with another mRNA molecule [18]. As a single agent, Genasense had a modest response in patients with refractory or relapsed CLL [64]. However, the combination of fludarabine, cyclophsophamide and Genasense resulted in higher rates of CR [62] that was translated to five years OS survival benefit [63].
7. Immunotherapy
A novel personalized immunotherapy approach uses autologous T cells expressing chimeric antigen receptors (CARs) directed against specific tumor-associated antigens. A typical CAR is a humanized monoclonal antibody that recognizes tumor-associated antigens and initiates an antitumor response. There have been several clinical reports on the use of CARs targeting CD19 in patients with refractory CLL. In one case, autologous T cells from a patient with refractory CLL were transfected with a lentiviral vector expressing CARs with specificity for CD19 coupled with CD137 (a costimulatory receptor) and CD3-ζ (a signal transduction component of T cells). This patient experienced a complete remission that lasted 10 months after treatment. The most alarming toxic effect, which was also reported in other cases, was a life-threatening tumor lysis syndrome [69]. In a report of four patients with bulky CLL and chemotherapy-refractory disease, CAR+ T cells were infused after prior conditioning with cyclophosphamide. One patient developed hypotension, dyspnea, and renal failure 4 days after administration of the T cells. Postmortem studies did not attribute the cause of death to the infused CAR+ T cells [9]. However, if not overcome, such devastating side effects may limit the applicability of this approach.
The success of non-chemotherapy approaches will no doubt revolutionize treatment of patients with refractory CLL in the foreseeable future, and such approaches will likely gain a role in front-line therapy. Most promising are the agents with high efficacy and low toxicity, which will help overcome the traditional barriers such as age and comorbidities.
8. Conclusions
The huge clinical heterogeneity in the natural history, treatment outcomes, and overall prognosis of CLL suggests that a personalized approach should be used to treat CLL. Individualizing care is becoming increasingly complex, and expertise in CLL management has been shown to substantially influence treatment outcomes. Patients treated by a hematologist specializing in CLL had longer OS than patients treated by a general hematologist even when both groups were treated at the same center [87]. A critical decision is when to initiate therapy. Symptomatic patients are considered for treatment; however, the formal indications for treatment leave a large amount of leeway for the treating physician. Even patients with clear indications for treatment should be evaluated for eligibility. Because most patients with CLL are elderly, many are defined as “slow go” or “no go” according to physical condition and comorbidities and are not candidates for high-dose chemotherapy.
Unlike the current decision-making criteria, which are based solely on clinical indications, future decision-making criteria to initiate therapy should integrate knowledge of the biology of the disease. Thus, new subsets of patients could be offered treatment at the time of diagnosis. Early interventions with nonchemotherapy agents that target specific deregulated pathways in the neoplastic cell or its environment may prove useful in patients whose predicted response to standard therapy is poor. Treating these patients when their disease burden is still low may induce cure in at least some and may prevent clonal evolution as the disease becomes increasingly resistant to therapies introduced later on.
Attaining a long-lasting CR or cure would probably require a combination of two or more novel agents targeting different pathways. Such agents are likely to gradually replace chemotherapy as first-line therapy in most patients and to be offered to some patients who are not eligible for chemotherapy.
The goal of personalized treatment is to design patient-specific plans based on both pathobiologic and clinical disease characteristics. At diagnosis, screening for somatic mutations and limited proteomics and transcriptome analysis can provide a useful patient-specific map of deregulated pathways that are susceptible to therapeutic interventions. Progress in unraveling the biology of CLL and translating this understanding into effective therapies is rapidly changing the way patients with CLL are treated and may provide tools that can cure many patients with this disease.
Acknowledgments
We thank Sarah Bronson for editing our manuscript.
Grant Support
This work was supported by a grant from CLL Global Research Foundation. The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant CA16672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest Statement
None
References
- 1.Natural history of stage A chronic lymphocytic leukaemia untreated patients. French Cooperative Group on Chronic Lymphocytic Leukaemia. Br J Haematol. 1990;76:45–57. doi: 10.1111/j.1365-2141.1990.tb07835.x. [DOI] [PubMed] [Google Scholar]
- 2.Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists’ Collaborative Group. J Natl Cancer Inst. 1999;91:861–868. doi: 10.1093/jnci/91.10.861. [DOI] [PubMed] [Google Scholar]
- 3.Amrein PC, Attar EC, Takvorian T, Hochberg EP, Ballen KK, Leahy KM, Fisher DC, Lacasce AS, Jacobsen ED, Armand P, Hasserjian RP, Werner L, Neuberg D, Brown JR. Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia. Clin Cancer Res. 2011;17:2977–2986. doi: 10.1158/1078-0432.CCR-10-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badoux XC, Keating MJ, Wen S, Lee BN, Sivina M, Reuben J, Wierda WG, O'Brien SM, Faderl S, Kornblau SM, Burger JA, Ferrajoli A. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood. 2011;118:3489–3498. doi: 10.1182/blood-2011-03-339077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badoux XC, Keating MJ, Wierda WG. What is the best frontline therapy for patients with CLL and 17p deletion? Curr Hematol Malig Rep. 2011;6:36–46. doi: 10.1007/s11899-010-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Bassat I, Raanani P, Gale RP. Graft-versus-leukemia in chronic lymphocytic leukemia. Bone Marrow Transplant. 2007;39:441–446. doi: 10.1038/sj.bmt.1705619. [DOI] [PubMed] [Google Scholar]
- 7.Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, Vaugier G, Potron G, Colona P, Oberling F, Thomas M, Tchernia G, Jacquillat C, Boivin P, Lesty C, Duault MT, Monconduit M, Belabbes S, Gremy F. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–206. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Bottcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch RM, Fingerle-Rowson G, Fink AM, Buhler A, Zenz T, Wenger MK, Mendila M, Wendtner CM, Eichhorst BF, Dohner H, Hallek MJ, Kneba M. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30:980–988. doi: 10.1200/JCO.2011.36.9348. [DOI] [PubMed] [Google Scholar]
- 9.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, Olszewska M, Bernal Y, Pegram H, Przybylowski M, Hollyman D, Usachenko Y, Pirraglia D, Hosey J, Santos E, Halton E, Maslak P, Scheinberg D, Jurcic J, Heaney M, Heller G, Frattini M, Sadelain M. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF, O'Brien S. Targeting BTK with Ibrutinib in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2013 doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, Iuliano R, Palumbo T, Pichiorri F, Roldo C, Garzon R, Sevignani C, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 12.Calin GA, Liu CG, Sevignani C, Ferracin M, Felli N, Dumitru CD, Shimizu M, Cimmino A, Zupo S, Dono M, Dell'Aquila ML, Alder H, Rassenti L, Kipps TJ, Bullrich F, Negrini M, Croce CM. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci U S A. 2004;101:11755–11760. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carballido E, Veliz M, Komrokji R, Pinilla-Ibarz J. Immunomodulatory drugs and active immunotherapy for chronic lymphocytic leukemia. Cancer Control. 2012;19:54–67. doi: 10.1177/107327481201900106. [DOI] [PubMed] [Google Scholar]
- 14.Catovsky D, Richards S, Matutes E, Oscier D, Dyer MJ, Bezares RF, Pettitt AR, Hamblin T, Milligan DW, Child JA, Hamilton MS, Dearden CE, Smith AG, Bosanquet AG, Davis Z, Brito-Babapulle V, Else M, Wade R, Hillmen P. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370:230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 15.Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, Porter CW, Goodrich DW, Bernstein ZP, Wallace P, Spaner D, Mohr A, Byrne C, Hernandez-Ilizaliturri F, Chrystal C, Starostik P, Czuczman MS. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24:5343–5349. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- 16.Chanan-Khan AA, Cheson BD. Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol. 2008;26:1544–1552. doi: 10.1200/JCO.2007.14.5367. [DOI] [PubMed] [Google Scholar]
- 17.Chen CI, Bergsagel PL, Paul H, Xu W, Lau A, Dave N, Kukreti V, Wei E, Leung-Hagesteijn C, Li ZH, Brandwein J, Pantoja M, Johnston J, Gibson S, Hernandez T, Spaner D, Trudel S. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. J Clin Oncol. 2011;29:1175–1181. doi: 10.1200/JCO.2010.29.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson BD. Oblimersen for the treatment of patients with chronic lymphocytic leukemia. Therapeutics and clinical risk management. 2007;3:855–870. [PMC free article] [PubMed] [Google Scholar]
- 19.Cheson BD, Byrd JC, Rai KR, Kay NE, O'Brien SM, Flinn IW, Wiestner A, Kipps TJ. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol. 2012;30:2820–2822. doi: 10.1200/JCO.2012.43.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chisesi T, Capnist G, Dal Fior S. Splenic irradiation in chronic lymphocytic leukemia. Eur J Haematol. 1991;46:202–204. doi: 10.1111/j.1600-0609.1991.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 21.Coiffier B, Lepretre S, Pedersen LM, Gadeberg O, Fredriksen H, van Oers MH, Wooldridge J, Kloczko J, Holowiecki J, Hellmann A, Walewski J, Flensburg M, Petersen J, Robak T. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1-2 study. Blood. 2008;111:1094–1100. doi: 10.1182/blood-2007-09-111781. [DOI] [PubMed] [Google Scholar]
- 22.Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, Marce S, Lopez- Guillermo A, Campo E, Montserrat E. ZAP-70 expression as a surrogate for immunoglobulin-variable- region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 23.Curcio LD, Bouffard DY, Scanlon KJ. Oligonucleotides as modulators of cancer gene expression. Pharmacology & therapeutics. 1997;74:317–332. doi: 10.1016/s0163-7258(97)00005-3. [DOI] [PubMed] [Google Scholar]
- 24.Del Giudice I, Mauro FR, Foa R. Chronic lymphocytic leukemia in less fit patients: “slow-go”. Leuk Lymphoma. 2011;52:2207–2216. doi: 10.3109/10428194.2011.606386. [DOI] [PubMed] [Google Scholar]
- 25.Di Bernardo MC, Crowther-Swanepoel D, Broderick P, Webb E, Sellick G, Wild R, Sullivan K, Vijayakrishnan J, Wang Y, Pittman AM, Sunter NJ, Hall AG, Dyer MJ, Matutes E, Dearden C, Mainou-Fowler T, Jackson GH, Summerfield G, Harris RJ, Pettitt AR, Hillmen P, Allsup DJ, Bailey JR, Pratt G, Pepper C, Fegan C, Allan JM, Catovsky D, Houlston RS. A genome-wide association study identifies six susceptibility loci for chronic lymphocytic leukemia. Nat Genet. 2008;40:1204–1210. doi: 10.1038/ng.219. [DOI] [PubMed] [Google Scholar]
- 26.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, Dohner K, Bentz M, Lichter P. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 27.Dreger P, Corradini P, Kimby E, Michallet M, Milligan D, Schetelig J, Wiktor-Jedrzejczak W, Niederwieser D, Hallek M, Montserrat E. Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia. 2007;21:12–17. doi: 10.1038/sj.leu.2404441. [DOI] [PubMed] [Google Scholar]
- 28.Edelmann J, Holzmann K, Miller F, Winkler D, Buhler A, Zenz T, Bullinger L, Kuhn MW, Gerhardinger A, Bloehdorn J, Radtke I, Su X, Ma J, Pounds S, Hallek M, Lichter P, Korbel J, Busch R, Mertens D, Downing JR, Stilgenbauer S, Dohner H. High-resolution genomic profiling of chronic lymphocytic leukemia reveals new recurrent genomic alterations. Blood. 2012;120:4783–4794. doi: 10.1182/blood-2012-04-423517. [DOI] [PubMed] [Google Scholar]
- 29.Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C, Siehl S, Jager U, Bergmann M, Stilgenbauer S, Schweighofer C, Wendtner CM, Dohner H, Brittinger G, Emmerich B, Hallek M. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–891. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- 30.Ferrajoli A, Lee BN, Schlette EJ, O'Brien SM, Gao H, Wen S, Wierda WG, Estrov Z, Faderl S, Cohen EN, Li C, Reuben JM, Keating MJ. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer K, Cramer P, Busch R, Stilgenbauer S, Bahlo J, Schweighofer CD, Bottcher S, Staib P, Kiehl M, Eckart MJ, Kranz G, Goede V, Elter T, Buhler A, Winkler D, Kneba M, Dohner H, Eichhorst BF, Hallek M, Wendtner CM. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29:3559–3566. doi: 10.1200/JCO.2010.33.8061. [DOI] [PubMed] [Google Scholar]
- 32.Flinn IW, Neuberg DS, Grever MR, Dewald GW, Bennett JM, Paietta EM, Hussein MA, Appelbaum FR, Larson RA, Moore DF, Jr., Tallman MS. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25:793–798. doi: 10.1200/JCO.2006.08.0762. [DOI] [PubMed] [Google Scholar]
- 33.Foon KA, Boyiadzis M, Land SR, Marks S, Raptis A, Pietragallo L, Meisner D, Laman A, Sulecki M, Butchko A, Schaefer P, Lenzer D, Tarhini A. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:498–503. doi: 10.1200/JCO.2008.17.2619. [DOI] [PubMed] [Google Scholar]
- 34.Forconi F, Fabbri A, Lenoci M, Sozzi E, Gozzetti A, Tassi M, Raspadori D, Lauria F. Low-dose oral fludarabine plus cyclophosphamide in elderly patients with untreated and relapsed or refractory chronic lymphocytic Leukaemia. Hematol Oncol. 2008;26:247–251. doi: 10.1002/hon.868. [DOI] [PubMed] [Google Scholar]
- 35.Fraser G, Smith CA, Imrie K, Meyer R. C. Hematology Disease Site Groupof Cancer Care Ontario's Program in Evidence-Based, Alemtuzumab in chronic lymphocytic leukemia. Current oncology. 2007;14:96–109. doi: 10.3747/co.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, Schaefer-Cutillo J, De Vos S, Sinha R, Leonard JP, Cripe LD, Gregory SA, Sterba MP, Lowe AM, Levy R, Shipp MA. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fulci V, Chiaretti S, Goldoni M, Azzalin G, Carucci N, Tavolaro S, Castellano L, Magrelli A, Citarella F, Messina M, Maggio R, Peragine N, Santangelo S, Mauro FR, Landgraf P, Tuschl T, Weir DB, Chien M, Russo JJ, Ju J, Sheridan R, Sander C, Zavolan M, Guarini A, Foa R, Macino G. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood. 2007;109:4944–4951. doi: 10.1182/blood-2006-12-062398. [DOI] [PubMed] [Google Scholar]
- 38.Furman RR, Byrd JC, Brown JR, Coutre SE, Benson DM, Wagner-Johnston ND, Flinn IW, Kahl BS, Spurgeon SE, Lannutti B, Giese NA, Webb HK, Ulrich RG, Peterman S, Holes LM, Yu AS. CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110 delta, Demonstrates Clinical Activity and Pharmacodynamic Effects In patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. Blood. 2010;116:31–31. [Google Scholar]
- 39.Ghia P. A look into the future: can minimal residual disease guide therapy and predict prognosis in chronic lymphocytic leukemia? Hematology Am Soc Hematol Educ Program. 2012;2012:97–104. doi: 10.1182/asheducation-2012.1.97. [DOI] [PubMed] [Google Scholar]
- 40.Guarini A, Marinelli M, Tavolaro S, Bellacchio E, Magliozzi M, Chiaretti S, De Propris MS, Peragine N, Santangelo S, Paoloni F, Nanni M, Del Giudice I, Mauro FR, Torrente I, Foa R. ATM gene alterations in chronic lymphocytic leukemia patients induce a distinct gene expression profile and predict disease progression. Haematologica. 2012;97:47–55. doi: 10.3324/haematol.2011.049270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hallek M. State-of-the-art treatment of chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2009:440–449. doi: 10.1182/asheducation-2009.1.440. [DOI] [PubMed] [Google Scholar]
- 42.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, Hensel M, Hopfinger G, Hess G, von Grunhagen U, Bergmann M, Catalano J, Zinzani PL, Caligaris-Cappio F, Seymour JF, Berrebi A, Jager U, Cazin B, Trneny M, Westermann A, Wendtner CM, Eichhorst BF, Staib P, Buhler A, Winkler D, Zenz T, Bottcher S, Ritgen M, Mendila M, Kneba M, Dohner H, Stilgenbauer S. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 44.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 45.Hillmen P, Skotnicki AB, Robak T, Jaksic B, Dmoszynska A, Wu J, Sirard C, Mayer J. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–5623. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 46.X.L.T. Jain P, Benjamini O, Lerner S, Wang X, Ferrajoli A, Burger JA, Estrov Z, Wierda W, Kantargian H, O'Brien S, Abruzzo LV, Keating MJ. Deletion 11q Abnormality in Patients with Chronic Lymphocytic Leukemia (CLL) May Not Have Poor Clinical Outcomes and Bulky Disease (clinical and radiological) At Presentation – Clinical Characteristics of (n=172) Previously Untreated Patients with CLL and del11q Cytogenetic Abnormality. American Society of Hematology; Atlanta, GA: 2013. [Google Scholar]
- 47.Johannsson J, Specht L, Mejer J, Jensen BA. Phase II study of palliative low-dose local radiotherapy in disseminated indolent non-Hodgkin's lymphoma and chronic lymphocytic leukemia. Int J Radiat Oncol Biol Phys. 2002;54:1466–1470. doi: 10.1016/s0360-3016(02)03050-x. [DOI] [PubMed] [Google Scholar]
- 48.Byrd R.R.F. John C., Coutre Steven, Flinn Ian W., Burger Jan A., Blum Kristie A., Sharman Jeff P., Grant Barbara, Jones Jeffrey A., Wierda William G., Zhao Weiqiang, Heerema Nyla A., Johnson Amy J., Tran Anh, Clow Fong, Kunkel Lori, James Danelle F., O'Brien Susan. The Bruton's Tyrosine Kinase (BTK) Inhibitor Ibrutinib (PCI-32765) Promotes High Response Rate, Durable Remissions, and Is Tolerable in Treatment Naïve (TN) and Relapsed or Refractory (RR) Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) Patients Including Patients with High-Risk (HR) Disease: New and Updated Results of 116 Patients in a Phase Ib/II Study. American Society of Hematology; Atlanta, Georgia: 2012. [Google Scholar]
- 49.Kanduri M, Cahill N, Goransson H, Enstrom C, Ryan F, Isaksson A, Rosenquist R. Differential genome-wide array-based methylation profiles in prognostic subsets of chronic lymphocytic leukemia. Blood. 2010;115:296–305. doi: 10.1182/blood-2009-07-232868. [DOI] [PubMed] [Google Scholar]
- 50.T.C.S. Keating MJ, Wierda WG, O'Brien S, Thomas DA, Cortes JE, Lerner S, Plunkett W. American Society of Hematology. Atlanta, GA: 2012. Is Chronic Lymphocytic Leukemia Still Incurable? [Google Scholar]
- 51.Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, Albitar M, Brettman L, Santabarbara P, Wacker B, Rai KR. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 52.Khouri IF, Bassett R, Poindexter N, O'Brien S, Bueso-Ramos CE, Hsu Y, Ferrajoli A, Keating MJ, Champlin R, Fernandez-Vina M. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer. 2011;117:4679–4688. doi: 10.1002/cncr.26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knauf WU, Lissichkov T, Aldaoud A, Liberati A, Loscertales J, Herbrecht R, Juliusson G, Postner G, Gercheva L, Goranov S, Becker M, Fricke HJ, Huguet F, Del Giudice I, Klein P, Tremmel L, Merkle K, Montillo M. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4378–4384. doi: 10.1200/JCO.2008.20.8389. [DOI] [PubMed] [Google Scholar]
- 54.Lavrenkov K, Krepel-Volsky S, Levi I, Ariad S. Low dose palliative radiotherapy for splenomegaly in hematologic disorders. Leuk Lymphoma. 2012;53:430–434. doi: 10.3109/10428194.2011.614708. [DOI] [PubMed] [Google Scholar]
- 55.Leoni LM. Bendamustine: rescue of an effective antineoplastic agent from the mid-twentieth century. Seminars in hematology. 2011;48(Suppl 1):S4–11. doi: 10.1053/j.seminhematol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Lin TS, Ruppert AS, Johnson AJ, Fischer B, Heerema NA, Andritsos LA, Blum KA, Flynn JM, Jones JA, Hu W, Moran ME, Mitchell SM, Smith LL, Wagner AJ, Raymond CA, Schaaf LJ, Phelps MA, Villalona-Calero MA, Grever MR, Byrd JC. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–6018. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marton S, Garcia MR, Robello C, Persson H, Trajtenberg F, Pritsch O, Rovira C, Naya H, Dighiero G, Cayota A. Small RNAs analysis in CLL reveals a deregulation of miRNA expression and novel miRNA candidates of putative relevance in CLL pathogenesis. Leukemia. 2008;22:330–338. doi: 10.1038/sj.leu.2405022. [DOI] [PubMed] [Google Scholar]
- 58.Mason KD, Khaw SL, Rayeroux KC, Chew E, Lee EF, Fairlie WD, Grigg AP, Seymour JF, Szer J, Huang DC, Roberts AW. The BH3 mimetic compound, ABT-737, synergizes with a range of cytotoxic chemotherapy agents in chronic lymphocytic leukemia. Leukemia. 2009;23:2034–2041. doi: 10.1038/leu.2009.151. [DOI] [PubMed] [Google Scholar]
- 59.Molica S, Mauro FR, Callea V, Giannarelli D, Lauria F, Rotoli B, Cortelezzi A, Liso V, Foa R. The utility of a prognostic index for predicting time to first treatment in early chronic lymphocytic leukemia: the GIMEMA experience. Haematologica. 2010;95:464–469. doi: 10.3324/haematol.2009.011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montserrat E, Moreno C, Esteve J, Urbano-Ispizua A, Gine E, Bosch F. How I treat refractory CLL. Blood. 2006;107:1276–1283. doi: 10.1182/blood-2005-02-0819. [DOI] [PubMed] [Google Scholar]
- 61.Moreton P, Kennedy B, Lucas G, Leach M, Rassam SM, Haynes A, Tighe J, Oscier D, Fegan C, Rawstron A, Hillmen P. Eradication of minimal residual disease in B-cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. J Clin Oncol. 2005;23:2971–2979. doi: 10.1200/JCO.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 62.O'Brien S, Moore JO, Boyd TE, Larratt LM, Skotnicki A, Koziner B, Chanan-Khan AA, Seymour JF, Bociek RG, Pavletic S, Rai KR. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2007;25:1114–1120. doi: 10.1200/JCO.2006.07.1191. [DOI] [PubMed] [Google Scholar]
- 63.O'Brien S, Moore JO, Boyd TE, Larratt LM, Skotnicki AB, Koziner B, Chanan-Khan AA, Seymour JF, Gribben J, Itri LM, Rai KR. 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. J Clin Oncol. 2009;27:5208–5212. doi: 10.1200/JCO.2009.22.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Brien SM, Cunningham CC, Golenkov AK, Turkina AG, Novick SC, Rai KR. Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23:7697–7702. doi: 10.1200/JCO.2005.02.4364. [DOI] [PubMed] [Google Scholar]
- 65.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O'Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 66.Pavletic SZ, Khouri IF, Haagenson M, King RJ, Bierman PJ, Bishop MR, Carston M, Giralt S, Molina A, Copelan EA, Ringden O, Roy V, Ballen K, Adkins DR, McCarthy P, Weisdorf D, Montserrat E, Anasetti C. Unrelated donor marrow transplantation for B-cell chronic lymphocytic leukemia after using myeloablative conditioning: results from the Center for International Blood and Marrow Transplant research. J Clin Oncol. 2005;23:5788–5794. doi: 10.1200/JCO.2005.03.962. [DOI] [PubMed] [Google Scholar]
- 67.Pei L, Choi JH, Liu J, Lee EJ, McCarthy B, Wilson JM, Speir E, Awan F, Tae H, Arthur G, Schnabel JL, Taylor KH, Wang X, Xu D, Ding HF, Munn DH, Caldwell C, Shi H. Genome-wide DNA methylation analysis reveals novel epigenetic changes in chronic lymphocytic leukemia. Epigenetics. 2012;7:567–578. doi: 10.4161/epi.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.M.E. Pettitt AR, Dearden C. Results of the phase II NCRI CLL206 trial of alemtuzumab in combination with high-dose methylprednisolone for high-risk (17p-) CLL. European Hematology Association; Berlin, Germany: 2009. [Google Scholar]
- 69.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quesada V, Conde L, Villamor N, Ordonez GR, Jares P, Bassaganyas L, Ramsay AJ, Bea S, Pinyol M, Martinez-Trillos A, Lopez-Guerra M, Colomer D, Navarro A, Baumann T, Aymerich M, Rozman M, Delgado J, Gine E, Hernandez JM, Gonzalez-Diaz M, Puente DA, Velasco G, Freije JM, Tubio JM, Royo R, Gelpi JL, Orozco M, Pisano DG, Zamora J, Vazquez M, Valencia A, Himmelbauer H, Bayes M, Heath S, Gut M, Gut I, Estivill X, Lopez-Guillermo A, Puente XS, Campo E, Lopez-Otin C. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet. 2012;44:47–52. doi: 10.1038/ng.1032. [DOI] [PubMed] [Google Scholar]
- 71.Rahmatpanah FB, Carstens S, Hooshmand SI, Welsh EC, Sjahputera O, Taylor KH, Bennett LB, Shi H, Davis JW, Arthur GL, Shanafelt TD, Kay NE, Wooldridge JE, Caldwell CW. Large-scale analysis of DNA methylation in chronic lymphocytic leukemia. Epigenomics. 2009;1:39–61. doi: 10.2217/epi.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.P.B.L. Rai KR, Appelbaum FR, Tallman MS, Belch A, Morrison VA, Larson R. Long-Term Survival Analysis of the North American Intergroup Study C9011 Comparing Fludarabine (F) and Chlorambucil (C) in Previously Untreated Patients with Chronic Lymphocytic Leukemia (CLL) American Society of Hematology; New-Orleans, LA: 2009. [Google Scholar]
- 73.K.M.J. Rai KR. Staging and prognosis of chronic lymphcoytie leukemia. In: BD R, editor. Uptodate. Waltham, MA: 2005. p. 2012. [Google Scholar]
- 74.Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L, Hines J, Threatte GA, Larson RA, Cheson BD, Schiffer CA. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343:1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 75.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 76.Richardson A, Kaye SB. Pharmacological inhibition of the Bcl-2 family of apoptosis regulators as cancer therapy. Current molecular pharmacology. 2008;1:244–254. doi: 10.2174/1874467210801030244. [DOI] [PubMed] [Google Scholar]
- 77.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, Carney DA, He SZ, Huang DC, Xiong H, Cui Y, Busman TA, McKeegan EM, Krivoshik AP, Enschede SH, Humerickhouse R. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robertson LE, Plunkett W, McConnell K, Keating MJ, McDonnell TJ. Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia. 1996;10:456–459. [PubMed] [Google Scholar]
- 79.Rosenwald A, Alizadeh AA, Widhopf G, Simon R, Davis RE, Yu X, Yang L, Pickeral OK, Rassenti LZ, Powell J, Botstein D, Byrd JC, Grever MR, Cheson BD, Chiorazzi N, Wilson WH, Kipps TJ, Brown PO, Staudt LM. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med. 2001;194:1639–1647. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rossi D, Bruscaggin A, Spina V, Rasi S, Khiabanian H, Messina M, Fangazio M, Vaisitti T, Monti S, Chiaretti S, Guarini A, Del Giudice I, Cerri M, Cresta S, Deambrogi C, Gargiulo E, Gattei V, Forconi F, Bertoni F, Deaglio S, Rabadan R, Pasqualucci L, Foa R, Dalla-Favera R, Gaidano G. Mutations of the SF3B1 splicing factor in chronic lymphocytic leukemia: association with progression and fludarabine-refractoriness. Blood. 2011;118:6904–6908. doi: 10.1182/blood-2011-08-373159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rossi D, Rasi S, Fabbri G, Spina V, Fangazio M, Forconi F, Marasca R, Laurenti L, Bruscaggin A, Cerri M, Monti S, Cresta S, Fama R, De Paoli L, Bulian P, Gattei V, Guarini A, Deaglio S, Capello D, Rabadan R, Pasqualucci L, Dalla-Favera R, Foa R, Gaidano G. Mutations of NOTCH1 are an independent predictor of survival in chronic lymphocytic leukemia. Blood. 2012;119:521–529. doi: 10.1182/blood-2011-09-379966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rozovski U, Keating M, Estrov Z. The significance of spliceosome mutations in chronic lymphocytic leukemia. Leuk Lymphoma. 2012 doi: 10.3109/10428194.2012.742528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruchlemer R, Polliack A. Geography, ethnicity and “roots” in chronic lymphocytic leukemia. Leuk Lymphoma. 2012 doi: 10.3109/10428194.2012.740670. [DOI] [PubMed] [Google Scholar]
- 84.Rush LJ, Raval A, Funchain P, Johnson AJ, Smith L, Lucas DM, Bembea M, Liu TH, Heerema NA, Rassenti L, Liyanarachchi S, Davuluri R, Byrd JC, Plass C. Epigenetic profiling in chronic lymphocytic leukemia reveals novel methylation targets. Cancer Res. 2004;64:2424–2433. doi: 10.1158/0008-5472.can-03-2870. [DOI] [PubMed] [Google Scholar]
- 85.Schweighofer CD, Coombes KR, Barron LL, Diao L, Newman RJ, Ferrajoli A, O'Brien S, Wierda WG, Luthra R, Medeiros LJ, Keating MJ, Abruzzo LV. A two-gene signature, SKI and SLAMF1, predicts time-to-treatment in previously untreated patients with chronic lymphocytic leukemia. PLoS One. 2011;6:e28277. doi: 10.1371/journal.pone.0028277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sehn LH, Assouline SE, Stewart DA, Mangel J, Gascoyne RD, Fine G, Frances-Lasserre S, Carlile DJ, Crump M. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012;119:5118–5125. doi: 10.1182/blood-2012-02-408773. [DOI] [PubMed] [Google Scholar]
- 87.Shanafelt TD, Kay NE, Rabe KG, Inwards DJ, Zent CS, Leis JF, Schwager SM, Thompson CA, Bowen DA, Witzig TE, Slager SL, Call TG. Hematologist/oncologist disease-specific expertise and survival: lessons from chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) Cancer. 2012;118:1827–1837. doi: 10.1002/cncr.26474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shanafelt TD, Rabe KG, Kay NE, Zent CS, Jelinek DF, Reinalda MS, Schwager SM, Bowen DA, Slager SL, Hanson CA, Call TG. Age at diagnosis and the utility of prognostic testing in patients with chronic lymphocytic leukemia. Cancer. 2010;116:4777–4787. doi: 10.1002/cncr.25292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Slager SL, Goldin LR, Strom SS, Lanasa MC, Spector LG, Rassenti L, Leis JF, Camp NJ, Kay NE, Vachon CM, Glenn M, Weinberg JB, Rabe KG, Cunningham JM, Achenbach SJ, Hanson CA, Marti GE, Call TG, Caporaso NE, Cerhan JR. Genetic susceptibility variants for chronic lymphocytic leukemia, Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research. cosponsored by the American Society of Preventive Oncology. 2010;19:1098–1102. doi: 10.1158/1055-9965.EPI-09-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sorror ML, Maris MB, Sandmaier BM, Storer BE, Stuart MJ, Hegenbart U, Agura E, Chauncey TR, Leis J, Pulsipher M, McSweeney P, Radich JP, Bredeson C, Bruno B, Langston A, Loken MR, Al-Ali H, Blume KG, Storb R, Maloney DG. Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23:3819–3829. doi: 10.1200/JCO.2005.04.569. [DOI] [PubMed] [Google Scholar]
- 91.Stamatopoulos B, Meuleman N, Haibe-Kains B, Saussoy P, Van Den Neste E, Michaux L, Heimann P, Martiat P, Bron D, Lagneaux L. microRNA-29c and microRNA-223 down-regulation has in vivo significance in chronic lymphocytic leukemia and improves disease risk stratification. Blood. 2009;113:5237–5245. doi: 10.1182/blood-2008-11-189407. [DOI] [PubMed] [Google Scholar]
- 92.Stamelos VA, Redman CW, Richardson A. Understanding sensitivity to BH3 mimetics: ABT-737 as a case study to foresee the complexities of personalized medicine. Journal of molecular signaling. 2012;7:12. doi: 10.1186/1750-2187-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.E.F.B. Stilgenbauer S, Busch R, Zenz T, Winkler D. Biologic and Clinical Markers for Outcome after Fludarabine (F) or F Plus Cyclophosphamide (FC) - Comprehensive Analysis of the CLL4 Trial of the GCLLSG. American Society of Hematology; San Francisco, Ca: 2008. [Google Scholar]
- 94.Tahir SK, Wass J, Joseph MK, Devanarayan V, Hessler P, Zhang H, Elmore SW, Kroeger PE, Tse C, Rosenberg SH, Anderson MG. Identification of expression signatures predictive of sensitivity to the Bcl-2 family member inhibitor ABT-263 in small cell lung carcinoma and leukemia/lymphoma cell lines. Molecular cancer therapeutics. 2010;9:545–557. doi: 10.1158/1535-7163.MCT-09-0651. [DOI] [PubMed] [Google Scholar]
- 95.Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, Warner RB, Ng SC, Fesik SW, Elmore SW, Rosenberg SH, Tse C. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–1183. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 96.Tam CS, O'Brien S, Wierda W, Kantarjian H, Wen S, Do KA, Thomas DA, Cortes J, Lerner S, Keating MJ. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tam CS, Shanafelt TD, Wierda WG, Abruzzo LV, Van Dyke DL, O'Brien S, Ferrajoli A, Lerner SA, Lynn A, Kay NE, Keating MJ. De novo deletion 17p13.1 chronic lymphocytic leukemia shows significant clinical heterogeneity: the M. D. Anderson and Mayo Clinic experience. Blood. 2009;114:957–964. doi: 10.1182/blood-2009-03-210591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thomas A, Pepper C, Hoy T, Bentley P. Bryostatin induces protein kinase C modulation, Mcl-1 up- regulation and phosphorylation of Bcl-2 resulting in cellular differentiation and resistance to drug- induced apoptosis in B-cell chronic lymphocytic leukemia cells. Leuk Lymphoma. 2004;45:997–1008. doi: 10.1080/10428190310001639470. [DOI] [PubMed] [Google Scholar]
- 99.Thornton PD, Matutes E, Bosanquet AG, Lakhani AK, Grech H, Ropner JE, Joshi R, Mackie PH, Douglas ID, Bowcock SJ, Catovsky D. High dose methylprednisolone can induce remissions in CLL patients with p53 abnormalities. Annals of hematology. 2003;82:759–765. doi: 10.1007/s00277-003-0710-5. [DOI] [PubMed] [Google Scholar]
- 100.Thurmes P, Call T, Slager S, Zent C, Jenkins G, Schwager S, Bowen D, Kay N, Shanafelt T. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49:49–56. doi: 10.1080/10428190701724785. [DOI] [PubMed] [Google Scholar]
- 101.Wang L, Lawrence MS, Wan Y, Stojanov P, Sougnez C, Stevenson K, Werner L, Sivachenko A, DeLuca DS, Zhang L, Zhang W, Vartanov AR, Fernandes SM, Goldstein NR, Folco EG, Cibulskis K, Tesar B, Sievers QL, Shefler E, Gabriel S, Hacohen N, Reed R, Meyerson M, Golub TR, Lander ES, Neuberg D, Brown JR, Getz G, Wu CJ. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011;365:2497–2506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weiss MA, Maslak PG, Jurcic JG, Scheinberg DA, Aliff TB, Lamanna N, Frankel SR, Kossman SE, Horgan D. Pentostatin and cyclophosphamide: an effective new regimen in previously treated patients with chronic lymphocytic leukemia. J Clin Oncol. 2003;21:1278–1284. doi: 10.1200/JCO.2003.08.100. [DOI] [PubMed] [Google Scholar]
- 103.Weston VJ, Oldreive CE, Skowronska A, Oscier DG, Pratt G, Dyer MJ, Smith G, Powell JE, Rudzki Z, Kearns P, Moss PA, Taylor AM, Stankovic T. The PARP inhibitor olaparib induces significant killing of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood. 2010;116:4578–4587. doi: 10.1182/blood-2010-01-265769. [DOI] [PubMed] [Google Scholar]
- 104.Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A, Robak T, Furman RR, Hillmen P, Trneny M, Dyer MJ, Padmanabhan S, Piotrowska M, Kozak T, Chan G, Davis R, Losic N, Wilms J, Russell CA, Osterborg A, Hx CDSI. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wierda WG, O'Brien S, Wang X, Faderl S, Ferrajoli A, Do KA, Cortes J, Thomas D, Garcia-Manero G, Koller C, Beran M, Giles F, Ravandi F, Lerner S, Kantarjian H, Keating M. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109:4679–4685. doi: 10.1182/blood-2005-12-051458. [DOI] [PubMed] [Google Scholar]
- 106.Wierda WG, O'Brien S, Wang X, Faderl S, Ferrajoli A, Do KA, Garcia-Manero G, Cortes J, Thomas D, Koller CA, Burger JA, Lerner S, Schlette E, Abruzzo L, Kantarjian HM, Keating MJ. Multivariable model for time to first treatment in patients with chronic lymphocytic leukemia. J Clin Oncol. 2011;29:4088–4095. doi: 10.1200/JCO.2010.33.9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wiestner A. Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Blood. 2012;120:4684–4691. doi: 10.1182/blood-2012-05-423194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Woyach JA, Lozanski G, Ruppert AS, Lozanski A, Blum KA, Jones JA, Flynn JM, Johnson AJ, Grever MR, Heerema NA, Byrd JC. Outcome of patients with relapsed or refractory chronic lymphocytic leukemia treated with flavopiridol: impact of genetic features. Leukemia. 2012;26:1442–1444. doi: 10.1038/leu.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Woyach JA, Ruppert AS, Heerema NA, Peterson BL, Gribben JG, Morrison VA, Rai KR, Larson RA, Byrd JC. Chemoimmunotherapy with fludarabine and rituximab produces extended overall survival and progression-free survival in chronic lymphocytic leukemia: long-term follow-up of CALGB study 9712. J Clin Oncol. 2011;29:1349–1355. doi: 10.1200/JCO.2010.31.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zenz T, Eichhorst B, Busch R, Denzel T, Habe S, Winkler D, Buhler A, Edelmann J, Bergmann M, Hopfinger G, Hensel M, Hallek M, Dohner H, Stilgenbauer S. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28:4473–4479. doi: 10.1200/JCO.2009.27.8762. [DOI] [PubMed] [Google Scholar]