Abstract
Behavioral evidence suggests that young and older adults show a benefit in source memory accuracy when processing materials in reference to the self. In the young, activity within the medial prefrontal cortex supports this source memory benefit at study. This investigation examined whether the same neural regions support this memory benefit in both age groups. Using fMRI, participants were scanned while studying and retrieving pictures of objects paired with one of three scenes (source) under self-reference and other-reference conditions. At the time of study, half of the items were presented once and half twice, allowing us to match behavioral performance between groups. Both groups showed equivalent source accuracy benefit for objects encoded self-referentially. Activity in the left dorsal medial prefrontal cortex supported subsequent source memory in both age groups for the self-referenced relative to the other-referenced items. At the time of test, source accuracy for both self- and other-referenced items was supported by a network of regions including the precuneus in both age groups. At both study and test, little in the way of age-differences emerged, suggesting that when matched on behavioral performance young and older adults engage similar regions in support of source memory when processing materials in reference to the self; however, when performance was not matched, age differences in functional recruitment were prevalent. These results suggest that by capitalizing on preserved processes (self-referential encoding), older adults can show improvement in memory for source details which typically are not well remembered relative to the young.
Keywords: self-reference, aging, source memory, medial prefrontal cortex, encoding, retrieval, social cognition
Introduction
One of the most common complaints of advancing age is difficulty remembering specific details of prior experiences. A large body of evidence has consistently shown that older adults show memory deficits for context (Johnson, Hashtroudi, & Lindsay, 1993; Spencer & Raz, 1995; Yonelinas, 2002). This deficit can appear as impoverished memory for perceptual details (e.g., the color of a presented item), associative details (e.g., what was this item paired with?), list membership (e.g., did this item appear in list A or B), among other features (Johnson et al., 1993). We refer to age-related impairments in remembering specific episodic details as source memory deficits. Interestingly, emerging evidence suggests that under certain circumstances older adults can remember source details as well as the young (May, Rahhal, Berry, & Leighton, 2005; Rahhal, May, & Hasher, 2002). In one such study young and older adults listened to true and false trivia statements spoken by either a trustworthy or an untrustworthy person. Results indicated that older participants were as accurate as the young at subsequently identifying the source of the information (i.e., whether a fact was told by the trustworthy or untrustworthy individual) (Rahhal et al., 2002), suggesting that older adults may show a source memory benefit when attending to social information.
Recent work has examined source memory benefits in both young and older adults from self-referencing—a task that emphasizes attention to social details (Dulas, Newsome, & Duarte, 2011; Hamami, Serbun, & Gutchess, 2011; Leshikar & Duarte, 2012; Serbun, Shih, & Gutchess, 2011). In some self-reference tasks, participants judge whether adjectives, such as the word “honest”, describe the self (Kuiper & Rogers, 1979; Rogers, Kuiper, & Kirker, 1977), whereas in other tasks participants evaluate whether they find stimuli pleasing (e.g., do you like this object?) (Dulas et al., 2011; Gusnard, Akbudak, Shulman, & Raichle, 2001; Raposo, Vicens, Clithero, Dobbins, & Huettel, 2010) (See Klein, 2012, for description of other self-reference tasks). In each of these tasks performance is subjective and requires stimulus processing via one’s self-schema and personal preferences. Abundant work shows that processing information in relation to the self leads to better item memory than processing information relative to “others” (e.g. Albert Einstein, the Danish Queen) (Symons & Johnson, 1997). This memory advantage has been found for both item recognition (Glisky & Marquine, 2009; Gutchess, Kensinger, Yoon, & Schacter, 2007; Mueller, Wonderlich, & Dugan, 1986; Rogers et al., 1977) as well as source memory for various kinds of details in young and older adults alike (Hamami et al., 2011; Leshikar & Duarte, 2012; Serbun et al., 2011).
In functional magnetic resonance imaging (fMRI) studies, processing materials for self-relevance leads to enhanced activity in cortical midline regions in frontal and parietal cortices (Craik et al., 1999; D’Argembeau et al., 2005; D’Argembeau et al., 2010; Gutchess, Kensinger, & Schacter, 2007; Kelley et al., 2002), including the medial prefrontal cortex (mPFC) (Gutchess, Kensinger, & Schacter, 2007; Kelley et al., 2002). In memory studies, activity in these same regions, including the mPFC, support item memory accuracy for self-referenced materials at both study and test (Fossati et al., 2004; Gutchess, Kensinger, & Schacter, 2010; Macrae, Moran, Heatherton, Banfield, & Kelley, 2004). Interestingly, Gutchess et al. (2010), reported cross-over interactions between young and older adults in several regions supporting item memory including the mPFC. These effects were driven by subsequent memory effects (hits>misses) in the older adults and subsequent forgetting effects (misses>hits) in the young, which the authors interpreted to mean that older adults performed the task in a different manner than the young. The authors of that study did not relate the cortical differences to individual performance making it unclear whether this pattern reflected compensatory or maladaptive recruitment in the old; although it should be noted that older adults performed worse than the young. Importantly, this previous study was not designed to assess source memory.
Recent work from our lab has shown that dorsal medial prefrontal cortex (dmPFC) activity supports source memory accuracy during study for self-referenced information in young adults (Leshikar & Duarte, 2012). In that study, participants encoded objects presented with scene backgrounds in either a self-reference (“do you like this object paired with this background?”) or perceptual processing condition (“is the color of the object similar to that of the background?”) and were subsequently tested on their source memory performance for both the study task and the scene. Importantly, self-referencing facilitated both item and source memory, suggesting that the self-referencing benefit extended from item memory to also include source-level contextual details. Further, successful source encoding of self-referenced events for both task and scene sources was supported by dmPFC activity, suggesting a role for this region in the encoding of source details when a self-referential strategy is employed to encode those details.
In contrast to encoding, few studies have investigated the effects of self-referential processing on retrieval related activity. The studies that have measured test phase activity have reported somewhat inconsistent findings (Fossati et al., 2004; Leshikar & Duarte, 2012). While Fossati and colleagues (2004) reported that mPFC activity supported successful item recognition for self-referenced materials, we found no link between mPFC activity and retrieval of self-referenced events. Instead we reported that activity in the posterior cingulate, another region implicated in self-referential processing (Sajonz et al., 2010), supported successful source retrieval for self-referenced events. No fMRI studies have assessed the influence of self-referential processing on both source memory encoding and retrieval activity in young and older adults. However, in an event-related potential (ERP) investigation, we found that young and older adults exhibited similar ERPs associated with accurate source retrieval of self-relevant associations (Dulas et al., 2011). Specifically, both age groups showed source memory benefits and reduced onset latencies in “old-new” ERP effects for items previously encoded in reference to the self relative to a non-self-relevant study task in which participants made judgments about object commonness. It is difficult to determine with ERPs alone, however, whether the young and older adults recruited the same brain areas to support source memory retrieval due to the poor spatial resolution of ERPs. Thus, it is not clear whether the neural regions, like the mPFC and posterior cingulate, found to support self-referential source memory accuracy in the young, also support self-referential source memory in older adults.
A recent topic of discussion in the aging and neuroscience literature is the influence of group differences in performance on functional recruitment (See Duverne, Habibi, & Rugg, 2008; Morcom, Li, & Rugg, 2007, for discussion). In most fMRI investigations of age-related changes in cognition, age comparisons contrast conditions where behavioral performance is better in the young than the older adults. This is problematic because when activation differences are evident between age groups, this could reflect the effects of age but also could be due to group differences in performance. One approach to obtain matched performance between young and older adults is to present some items once and other items multiple times (Duverne et al., 2008; Wang, Kruggel, & Rugg, 2009). By having easier (twice presented) and harder conditions (once presented), performance matching can be accomplished by comparing the harder items in the young with the easier items in the older adults. Although not a self-reference investigation, one study adopting this difficulty manipulation found substantial overlap in the regions supporting source memory accuracy between age groups (Duverne et al., 2008). In the present study we examined activity supporting source memory accuracy when performance was matched between age groups by comparing the items presented once in the young with the items presented twice in the old. We also examined activity when performance was not statistically equivalent.
In this investigation, fMRI scans were collected while young and older adults studied objects superimposed on one of three background scenes (source) under self-reference and other-reference conditions, and then again when participants made source memory decisions at test. We make several predictions in this investigation: First, we predict that self-referencing relative to other-referencing will benefit source memory to a similar degree in the young and older adults consistent with previous investigations (Dulas et al., 2011; Glisky & Marquine, 2009; Hamami et al., 2011). Second, we predict both age groups will show source accuracy activity in the hippocampus, lateral prefrontal, and lateral posterior parietal cortices at both study and test for both encoding conditions, consistent with prior investigations showing that these regions support accurate source memory regardless of stimulus or source type (Dobbins & Wagner, 2005; Eichenbaum, Yonelinas, & Ranganath, 2007; Vilberg & Rugg, 2008) or age (Dulas & Duarte, 2011, 2012; Morcom et al., 2007; Rajah, Ames, & D’Esposito, 2008). Third, we predict that study phase activity in the dmPFC will support source memory accuracy for self-referenced relative to other-referenced events in both age groups when performance is matched; however, when performance differs, we predict that age differences will appear. Alternatively, dmPFC may not support source memory accuracy in older adults. While not a memory investigation, a recent study by Moran, Jolly, and Mitchell (2012) showed that dmPFC activity is reduced with age during social processing tasks like making moral judgments about the actions of others. It is important to note, however, that the Moran study compared functional recruitment across age groups in tasks where older adults performed more poorly than the young, a limitation we will explicitly address here. Finally, it is less clear what brain regions might support source memory accuracy for self-referenced materials at the time of test, given that few studies have investigated test phase activity. Based on the extant literature, we tentatively predict that either dmPFC and/or medial parietal areas will support source retrieval success for self-referenced events for both age groups when behavioral performance is matched.
Methods
Participants
Nineteen young (mean age: 22.4 [SD: 2.6]; range 18-27; 9 females) and nineteen older adults (mean age: 64.5 [SD: 2.8] range 61-70; 9 females) recruited from the Georgia Institute of Technology and surrounding community participated in this experiment. This sample size is equivalent to, or greater than many recent fMRI investigations studying age (Beadle, Yoon, & Gutchess, 2012; Dennis et al., 2008; Duverne et al., 2008; Giovanello, Kensinger, Wong, & Schacter, 2010; Gutchess et al., 2010; Morcom et al., 2007; St Jacques, Rubin, & Cabeza, 2010; Waring, Addis, & Kensinger, 2012). One additional older adult was recruited but not included in the analyses due to inability to perform the task. All participants were right-handed, native-English speakers. No participant reported history of psychiatric or neurological disorders (e.g., stroke, epilepsy, multiple sclerosis, etc.), vascular disease, psychoactive or vasoactive medication use. All participants had normal or corrected-to-normal vision and gave their informed, written consent in accord with the guidelines set by the Institutional Review Board at the Georgia Institute of Technology. Participants were paid $10 per hour or received extra credit for a psychology class for participating.
Neuropsychological testing
All participants were given a battery of standardized neuropsychological tests immediately following the fMRI component of the experiment. Participants who performed more than 2 standard deviations below age-appropriate norms on any task were excluded from analyses to ensure that participants were cognitively normal for their age group. These criteria also served to screen out participants showing signs of cognitive compromise. No participants were excluded based on this criterion. Tests included executive function measures, long-term memory, working memory, visuo-spatial processing, and fluency taken from the Memory Assessment Scale (Williams, 1991). Measures included trail-making A and B (Reitan & Wolfson, 1985), immediate list recall, delayed list recall, list recognition, verbal span, immediate visual recognition, delayed visual recognition and the Controlled Oral Word Association Test (“FAS”) (Benton, Hamsher, & Sivan, 1983). Group characteristics including neuropsychological test scores and demographic information are shown in Table 1.
Table 1.
Means (and standard deviations) of group characteristics shown as a function of age
| Measure | Young | Older |
|---|---|---|
| Age | 22.4 (2.6) | 64.5 (2.8)* |
| Gender | 9 females/10 males | 9 females/10 males |
| Education | 16.0 (2.4) | 17.2 (2.0) |
| Trails A | 21.5 (4.8) | 31.0 (10.5)* |
| Trails B | 41.2 (8.7) | 63.4 (20.1)* |
| List Recall - Free† | 10.7 (1.4) | 10.7 (1.2) |
| Delayed List Recall- Free† | 11.5 (0.7) | 11.1 (1.3) |
| List Recall - Cued† | 11.1 (1.0) | 10.9 (1.1) |
| Delayed Recall - Cued† | 11.6 (0.7) | 11.4 (1.0) |
| List Recognition | 12.0 (0) | 11.9 (0.2) |
| Verbal Span | 13.8 (2.6) | 12.4 (2.8) |
| MAS Visual Recognition† | 18.9 (2.7) | 17.0 (2.0)* |
| MAS Delayed Visual Recognition† | 19.0 (1.9) | 17.4 (1.5)* |
| FAS verbal fluency | 54.9 (15.3) | 55.0 (20.0) |
Note: All neuropsychological scores are reported as raw scores.
Denotes significant age difference at a threshold of p < 0.05.
Data was available for 18 of the 19 older adult participants.
Stimuli
Stimuli consisted of 356 objects and 3 scene backgrounds. During encoding, 264 objects were presented superimposed on one of 3 background scenes, the remaining 92 objects were presented as novel items at retrieval. Objects were color images of common objects (e.g., saxophone, dog, chair, etc.) taken from the Hemera Technologies ® Photo-Objects DVDs. The three background scenes were color images of a mountain, a beach, and a desert landscape obtained from Google. Across participants, objects were counterbalanced so that they appeared as studied and novel items. Objects subtended a maximum vertical and horizontal visual angle of approximately 3.7 degrees. Scenes subtended a maximum viewing angle of approximately 14.1 degrees.
Procedure
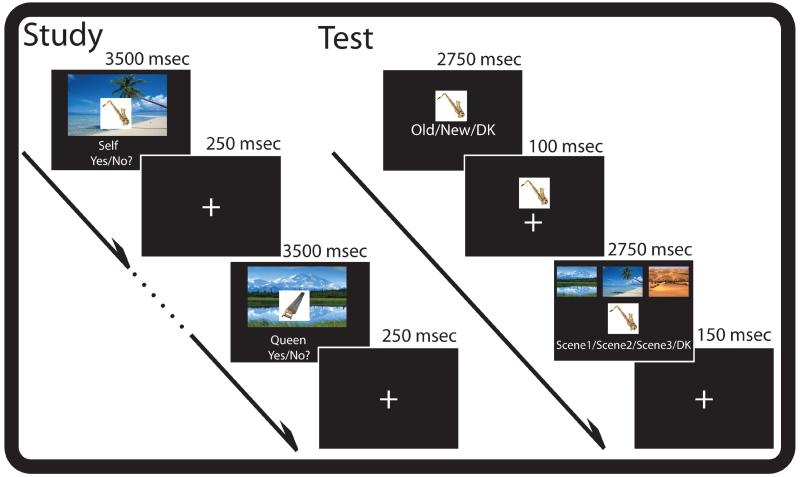
All participants completed the task protocol in one experimental session. Participants were trained on task instructions for the study and test phases of the experiment. Training included 18 practice study trials and 11 practice test trials. During training, participants verbally reported task instructions back to the experimenter to ensure task comprehension and corrective instruction was provided to the participant if necessary. After training, but prior to fMRI scanning, participants studied half of the object-scene pairings for each task (i.e. self-reference, other-reference) outside of the scanner. This was the first presentation of object-background pairs that would be shown a second time in the scanner. In preparation for the MRI scans, participants were given noise-dampening ear-plugs and headphones, a 4-button response pad in their right hand, and were instructed to minimize all movements for the duration of the experiment, especially head movements.
The study phase of the fMRI experiment was conducted over 4 scanning runs. There were 2 encoding tasks at study. In the self task, subjects judged whether they found the object-scene pairing pleasant (yes/no). In the other task, participants judged whether the Queen of England, Elizabeth II, would like the object-scene pairing (yes/no). It was important to use a non-familiar person as the other referent because evidence suggests that close-other-referencing, such as referencing via one’s best friend or mother, automatically promotes processing via one’s self (Aron, Aron, Tudor, & Nelson, 1991), and has further been shown to activate many of the same cortical regions as self-referential processing (Grigg & Grady, 2010a; Lombardo et al., 2010). Thus we wanted to avoid contamination of self-referential processing in our comparison condition. We chose Queen Elizabeth II as the “other” referent because she is someone familiar, but not personally known, to both young and older adults; we chose not to use other figures, such as American presidents, given the polarizing feeling that many of these individuals induce in some participants. Before participating in the experiment, participants read a brief biography of Elizabeth II to support participants’ ability to perform the other-reference task1.
For each study phase run, 66 trials were presented (half in each study task) for a total of 264 trials. Half of the items in each task were presented for the first time (e.g., the once presented items), the other half were presented for a second time (e.g., the twice presented items). To minimize task-switching costs especially for the older adults, trials were presented in short blocks, or “mini-blocks”, of 11 trials. Instructions before each mini-block were displayed for 6000 msec to prepare the participant to perform the designated encoding task. For each study phase trial participants had 3750 msec to encode the object-scene pair. Each object-scene pair was presented for 3500 msec followed by a 250 msec fixation interval (See Figure 1). For both encoding tasks, instructions prompted participants to consider both the object and the scene while making the pleasantness judgment (i.e., “would you/the Queen like a saxophone on the beach?”). Task instructions emphasized that there were no correct answers for this phase of the experiment. All yes/no responses were made with the index and middle fingers, respectively, of the right hand. Trials with no responses or more than one response as well as trials with response times under 200 msec were excluded from both behavioral and functional analyses.
Figure 1.
Trial schematic for the study and test phases of the experiment.
Immediately following study, the test phase of the experiment was conducted over 4 scanning runs. Each test phase run consisted of 66 studied (half from each study task) and 23 unstudied (novel) items. Trials were presented in a pseudorandom order so that no more than 5 trials of the same type (self-reference, other-reference, novel) would appear consecutively. For each trial, participants made an item recognition decision followed by a source judgment (See Figure 1). During the item recognition decision, a single object was displayed for 2750 msec. Participants judged whether the item was old or new, or whether they did not know (“don’t know”) using the index, middle, and pinky fingers of their right hand, respectively (though see counterbalancing below). While the item still on screen, the item recognition prompts were replaced with a fixation cross for 100 msec. During the source decision, all three background scenes were displayed along with the object for 2750 msec followed by a 150 msec fixation. Participants judged with which background the item was paired (using their index, middle and ring fingers), or whether they did not know (using their pinky finger). For items judged new, participants were instructed to press “don’t know” for the source decision. Overall, retrieval trials lasted a total duration of 5750 msec. For both test phase decisions the “don’t know” response option was offered to reduce potential contamination of guessing as implemented in several similar studies (Duarte, Henson, & Graham, 2008; Duarte, Henson, Knight, Emery, & Graham, 2009; Gottlieb, Uncapher, & Rugg, 2010; Leshikar & Duarte, 2012; Morcom et al., 2007; Smith, Dolan, & Rugg, 2004). For half of the participants, “don’t know” responses were always made with the pinky finger, for the other half the index finger, so that not all participants would be making don’t know responses with their weakest finger. Comparisons between the proportions of don’t know responses did not differ across counterbalanced versions, [ts < 1, p > .05]. Trials with too few or too many responses (e.g., more or less than two recognition responses) as well as trials with response times under 200 msec were excluded from both the behavioral and functional analyses. The entire experimental procedure, including practice, encoding outside of the scanner, tasks in the scanner, and debriefing, lasted a total of ~2 hours.
fMRI acquisition
Structural and functional scans were acquired using a 12-channel parallel imaging head coil on a Siemens Trio 3T full body scanner (Siemens, Erlangen, Germany) at the Center for Advanced Brain Imaging located at Georgia Tech. First, T1-weighted magnetization-prepared rapid gradient echo scans (MP-RAGE; TE= 4.52 msec, 256 × 256 FOV) were acquired over 160, 1-mm thick, sagittal slices to obtain high-resolution structural images. Functional scans were acquired using a gradient echo pulse sequence (t2*-weighted, TR = 2000 msec, TE = 30msec, flip angle = 90°, 3-mm in-plane resolution), collected in 37 continuous slices (interslice gap 17.5%) aligned to the anterior-posterior commissure line covering the entire cerebrum. A total of 150 volumes were collected during each study run and 263 volumes during each test run.
fMRI analysis
Data were analyzed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology) in MATLAB R2008a (The Mathworks Inc., Natick, MA). The first five volumes of each run for each participant were discarded to allow for equilibration effects. The remaining echo planar image (EPI) volumes were corrected for differences in slice time acquisition using the middle slice of each volume as the reference slice, and then spatially realigned to the first acquired volume. Then, the structural scan of each participant was co-registered to the mean EPI image produced from the realignment step and segmented using the DARTEL toolbox (diffeomorphic anatomical registration through exponentiated lie algebra) implemented in SPM 8. DARTEL is a high dimensional warping tool that increases intra-subject registration relative to native SPM 8 registration. DARTEL generates a subject-by-subject “flow field” which allows both forward and backward structural and functional deformations (Ashburner, 2007). Using each participant’s flow field, DARTEL generates a study-specific brain template for use in the normalization step. DARTEL achieves better localization of fMRI activity than the optimized normalization procedure by treating the brain template as a deformable probability density map, comparing signal intensities of each voxel for every brain, to the distribution of intensity probabilities for that voxel. The individual realigned and resliced functional scans were then normalized to the study-specific template. The resulting normalized images were then resliced to 3mm × 3mm × 3mm resolution and spatially smoothed using an 8-mm full-width at half-maximum Gaussian kernel.
Analysis of the functional data for the study and test phases were carried out in two steps. First, neural activity was modeled as a series of two second epochs at study and four second epochs at test coinciding with onset of the various event types (i.e., source correct, source incorrect [item only hits], source don’t know [item only hits], item misses, item don’t know, for each encoding task for both once [easy] and twice [hard] presented trials, as well as correct rejections and false alarms at test), convolved with the canonical hemodynamic response function. Longer epochs at retrieval were used to capture activity for the longer retrieval phase trials. At test, two retrieval responses (item and source) were collected but activity was modeled only to the onset of the first decision prompt, given that participants were aware of both response decisions making it difficult to model activity for each retrieval decision separately. A similar procedure has been used in previous studies (Duarte et al., 2008; Dulas & Duarte, 2012). Time courses were down-sampled to the middle slice to form covariates for the General Linear Model (GLM). For each participant, 6 covariates per session representing the residual movement-related artifacts determined by the spatial realignment step were included in the first level to model residual (linear) movement artifacts. Parameter estimates for each voxel for all covariates were obtained by Restricted Maximum-Likelihood (ReML) estimation, using a high-pass temporal filter (cut-off 128 seconds) to remove low-frequency drifts. Autocorrelations within each session were corrected by applying a first-order autoregressive (AR[1]) model. Over all voxels and scans data were scaled to a grand mean of 100 (Friston, Ashburner, Kiebel, Nichols, & Penny, 2007).
Estimates for contrasts between parameters for each participant were then submitted to the second stage of analysis. ANOVAs for the study and test phase GLMs allowed us to examine common memory effects across the self and other tasks, those selective to one study task versus the other, and memory-by-task interactions. Study and test phases were modeled separately. Our contrasts of interest were between the source correct (SC) (trials subsequently associated with the correct response for the scene source decisions) and the no source (NS) trials (trials not subsequently associated with a correct source judgment) that differed between the items presented once in the young (hard trials) and the items presented twice in the older adults (easy trials). The study phase 2x2 model included factors of Task (self, other) and Memory (SC, NS). The NS trials included item-only hits, item misses, and item don’t know trials. A analogous procedure was used by Gottlieb, Uncapher, and Rugg (2010) and Leshikar and Duarte (2012). As noted by both Gottlieb et al. and Leshikar and Duarte, collapsing over item only hits and item misses allows us to draw conclusions only about source memory effects (and not item memory effects). For the test phase ANOVA model, an additional contrast (correctly rejected new items) was included. Due to insufficient responses (< 10 trials), false alarms at retrieval were not included in the ANOVA. Given that we had no a priori predictions about source memory relative to baseline and we were only interested in activity related to source memory accuracy (SC -NS), we purposefully did not include a “baseline” condition. Since all trials types occur randomly within a block, as they are based on subject-specific responses, we would argue that this introduces sufficient stochasticity to our fMRI design (Henson, 2007) that allows us to generate stable estimates for our different trial types. Importantly, inclusion of null event/fixation trials to introduce jitter and measure inter-stimulus “baseline” is not necessary in fMRI designs if the contrast of interest is between task conditions that are randomly presented, as in our study ((Henson, 2007); also see http://imaging.mrc-cbu.cam.ac.uk/imaging/DesignEfficiency). Thirty-eight covariates modeling the mean across tasks for each participant (i.e. subject effects) were also added to each model, to remove between-subject variance of no interest allowing us to examine population level effects not confounded by outlier data. Statistical Parametric Maps (SPMs) were created from the t-statistics for the various ANOVA effects of interest, using a single pooled error estimate for all contrasts, where non-sphericity was estimated using ReML as described in Friston et al. (Friston et al., 2002).
At both study and test, the primary contrasts of interest were between the SC and NS trials, allowing us to examine source memory accuracy effects. Inclusive masks were used to determine overlap between regions showing source accuracy effects associated with each task (e.g., task dependent effects) and the task-by-source memory interactions. Inclusive masking was carried out at a threshold of p < 0.01 for the mask image. Exclusive masking was used to identify regions showing source memory effects common to both encoding tasks, masking out the interactions between tasks and age groups. Exclusive masking was carried out using a liberal uncorrected threshold of p < 0.05 for the mask2. To correct for multiple comparisons, all reported results were thresholded at p < .001 with a spatial extent of 34 continuous voxels. We derived this threshold via Monte Carlo simulations to correct for both type 1 and type 2 errors (Slotnick, Moo, Segal, & Hart, 2003). The effective threshold using this approach is equivalent to p < .05, corrected. Because age comparisons were across 1 versus 2 presentations (1 presentation in young versus 2 presentations in old) we performed an additional masking procedure. Specifically, regions showing presentation effects (e.g., 1 presentation > 2 presentations; 2 presentations > 1 presentation across both tasks and both age groups; See Table 2) were exclusively masked out of all analyses to avoid confounding source memory effects with effects related to number of presentations (old/new effects, habituation, priming, repetition suppression, etc.). For all effects, peak voxels surviving the minimum statistical threshold and cluster size are reported in MNI coordinates. Neural activity for these peak maxima were plotted as the difference between the SC and NS trials. Neural activity for these peak voxels reflects the parameter estimates for the convolved regressors and is presented in arbitrary units.
Table 2.
Study phase regions showing the effect of items presented once greater than items presented twice, collapsed across age, in (A). Regions showing the effect of items presented twice greater than items presented once in (B).
| Contrast Region | Hemisphere | MNI Coordinates | BA | T-value | Cluster Size |
|---|---|---|---|---|---|
| A. 1 Presentation > 2 Presentations | |||||
|
| |||||
| Inferior Occipital | Left | −39 −78 −9 | 19 | 5.16 | 167 |
| Left | −45 −57 −12 | 37 | 3.6 | ||
| Fusiform | Left | −30 −39 −18 | 37 | 4.98 | 66 |
| Inferior Orbitofrontal | Left | −36 33 −12 | 47 | 4.48 | 37 |
|
| |||||
| B. 2 Presentation > 1 Presentation | |||||
|
| |||||
| Middle Temporal | Left | −57 −21 24 | 21 | 5.73 | 282 |
| Left | −54 −36 24 | 22 | 4.23 | ||
| Right | 63 −21 −9 | 21 | 4.65 | 64 | |
| Right | 69 −39 18 | 21 | 3.72 | 66 | |
| Cuneus | Left | −9 −66 30 | 23 | 5.38 | 76 |
| Middle Frontal | Left | −39 54 6 | 46 | 4.54 | 136 |
| Left | −42 48 18 | 45 | 3.9 | ||
| Left | −36 60 18 | 46 | 3.55 | ||
| Superior Frontal | Right | 18 6 60 | 6 | 4.38 | 185 |
| Medial Prefrontal | Left | −9 33 54 | 8 | 4.16 | |
| Supplementary Motor | Right | 3 3 57 | 6 | 4.02 | |
| Supramarginal | Right | 57 −18 21 | 48 | 3.95 | 66 |
| Right | 66 −24 27 | 2 | 3.72 | ||
| Precentral | Right | 57 3 36 | 6 | 3.94 | 76 |
| Inferior Frontal | Right | 60 12 21 | 6 | 3.84 | |
Notes: Regions are listed from highest to lowest t-value. Regions listed without a cluster size are subsumed by the larger cluster listed directly above. Regions listed without an anatomic region are identical to the region listed immediately above. BA: Brodmann’s area
Results
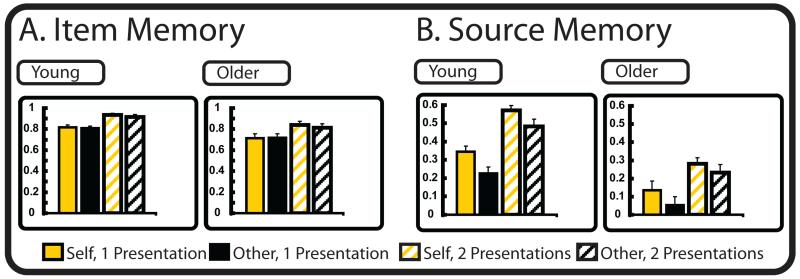
The proportion of source correct, source incorrect (item only hits), source don’t know (item only hits), item miss, and item don’t know (DK) responses for the studied items, and the proportions of correct rejections (CR) and false alarms (FA) to unstudied items are show in Table 3. Two measures of recognition memory were computed (Figure 2). First, a corrected measure of item recognition (Pr) was calculated by subtracting the proportion of FAs (e.g., unstudied items given an “old” response at test) from the proportion of item hits (e.g., studied items given an “old” response at test, regardless of source accuracy) (Snodgrass & Corwin, 1988) (See Figure 2)3. An Task (self, other) × Presentation (once presented, twice presented) × Age (young, older) ANOVA on the Pr estimates showed a main effect of Presentation [F(1, 36) = 78.1, p < .001, η2 = .68], and of Age [F(1, 36) = 6.7, p = .01, η2 = .16], without an effect of Task [F(1, 36) = 2.9, p = .10, η2 = .08], or any significant interactions [Fs < 2.0, p > .17, η2 < .05]. Pairwise comparisons showed that item recognition was better for the items presented twice relative to those presented once in both age groups [t(37)s > 7.7, p < .001, ds > 2.5], and was better in the young than the older adults [t(37)s > 2.3, p < .05, ds > 0.8].
Table 3.
Proportions of source correct, source incorrect (item only hits), source don’t know (item only hits), item miss, and item don’t know responses for the studied items are shown as a function of study task (self, other), presentation (one, two), and age (young, older). Proportions of false alarm and correct rejection to the unstudied items are also shown.
| Proportion means (and standard deviation) | ||||
|---|---|---|---|---|
| Young |
Older |
|||
| Response Type | ||||
| Studied in the Self task | 1 Presentation |
2 Presentations |
1 Presentation |
2 Presentations |
| Source correct | .48 (.15) | .73 (.11) | .36 (.16) | .53 (.17) |
| Source incorrect | .12 (.07) | .10 (.07) | .21 (.12) | .22 (.12) |
| Source don’t know | .24 (.12) | .12 (.09) | .26 (.21) | .20 (.21) |
| Item miss | .11 (.06) | .03 (.04) | .15 (.13) | .04 (.06) |
| Item don’t know | .05 (.07) | .02 (.02) | .02 (.03) | .01 (.02) |
| Studied in the Other | 1 | 2 | 1 | 2 |
| task | Presentation | Presentations | Presentation | Presentations |
| Source correct | .42 (.14) | .67 (.14) | .31 (.13) | .46 (.16) |
| Source incorrect | .15 (.09) | .13 (.08) | .25 (.14) | .22 (.13) |
| Source don’t know | .27 (.15) | .14 (.08) | .27 (.23) | .25 (.21) |
| Item miss | .11 (.06) | .03 (.05) | .14 (.11) | .06 (.07) |
| Item don’t know | .05 (.06) | .03 (.05) | .03 (.05) | .01 (.03) |
| Unstudied | ||||
| Correct Rejections | .91 (.08) | .83 (.20) | ||
| False Alarms | .03 (.02) | .12 (.15) | ||
| Don’t Know | .06 (.08) | .05 (.08) | ||
Figure 2.
(A) Pr estimates of item memory accuracy and (B) Psr estimates of source memory accuracy as a function of task, presentation, and age.
Second, to assess source accuracy we calculated Psr (See Duverne et al., 2008) where Psr = (p(correct) – 0.5*(1 – p(don’t know)))/(1 – (0.5*(1 – p(don’t know)))). Psr is an estimate of source recognition that removes the contribution of lucky guesses from the source recognition accuracy. A Task (self, other) × Presentation (once presented, twice presented) × Age (young, older) ANOVA on the Psr estimates yielded effects of Task [F(1, 36) = 16.9, p < .01, η2 = .32], Presentation [F(1, 36) = 87.6, p < .001, η2 = .71], and Age [F(1, 36) = 33.8, p < .01, η2 = .48], as well as a Presentation by Age interaction [F(1, 36) = 4.5, p = .04, η2 = .11]. The Task effect was driven by better source memory for the self than other task in both age groups [t(37)s > 2.9, ps < .01, ds > 1.0], and the Presentation effect resulted from better source memory for items presented twice than once [t(37)s > 7.4, ps < .01, ds > 2.4]. The Age effect was driven by better memory in the young compared to the older adults [t(36)s > 3.6, ps < .01, ds > 2.5]. The Presentation by Age interaction was driven by bigger benefit of presentation from easy (M: .29) to hard items (M: .55) in the young, relative to these same measures in the older adults (easy M: .08; hard M: .25). Given our interest in matching on behavioral performance for the imaging analyses, however, it should be noted that Psr estimates did not reliably differ when comparing the once presented items (i.e., the hard trials) in the young with the twice presented items (i.e., the easy trials) in the older adults in either the self-reference or other-reference tasks [t(36)s < 1.2, ps > .25, ds < 0.4].
fMRI results
We present three functional neuroimaging results that focused on the comparison between the hard items (one presentation) in the young with the easy items (two presentations) in the older adults where source accuracy did not statistically differ. First, we separately report study phase and test phase activity supporting task-invariant source accuracy effects using an exclusive masking procedure (see methods) followed by age differences in task-invariant source accuracy effects. Second, we report task-selective regions supporting source memory unique to the self-reference and other-reference tasks in both age groups, for study and test phases separately, and then report age differences in task-selective effects. Finally, we report age differences in task-selective regions supporting source memory for trials where there were age differences in performance—the one presentation trials.
Effects when behavioral performance is matched
Task-invariant source accuracy effects
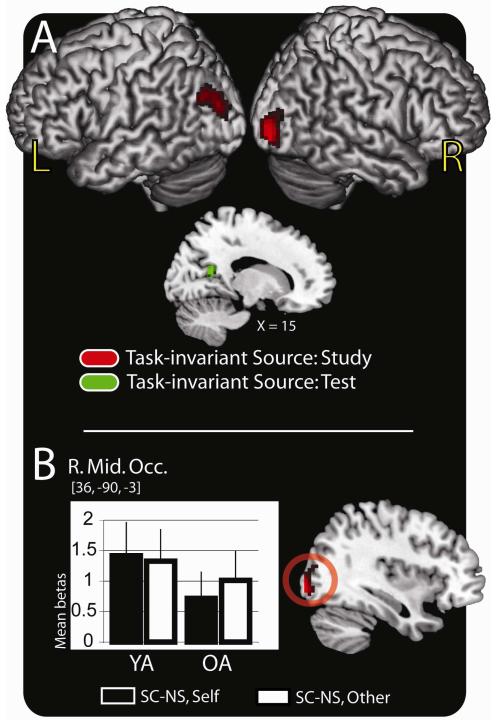
Study
The first analysis examined regions supporting accurate source memory in a task-invariant manner in both age groups. To examine these effects we exclusively masked the source accuracy effects (SC > NS) common to both study tasks and both age groups with the SPMs for the source accuracy-by-task, the source accuracy-by-group, and the 3-way interaction (see methods). We report task-invariant activity in scene processing regions of the bilateral middle occipital cortices (BA 19/39) (Table 4A; Figure 3A). Mean parameter estimates (betas) from the peak voxel of the right middle occipital are plotted in Figure 3B.
Table 4.
Regions showing task-invariant source accuracy effects and task-invariant age effects at (A) study and (B) test when behavioral performance is matched between groups.
| Region | Hemisphere | MNI Coordinates | BA | T-value | Cluster Size |
|---|---|---|---|---|---|
| A. Task-invariant, study | |||||
|
| |||||
| Middle occipital cortex | Right | 36 −90 −3 | 19 | 4.76 | 111 |
| Right | 42 −84 −9 | 3.42 | |||
| Middle occipital cortex | Left | −33 −84 21 | 19 | 4.08 | 113 |
| Left | −39 −72 27 | 39 | 3.98 | ||
|
| |||||
| Young > older, study | |||||
|
| |||||
| None | |||||
|
| |||||
| Older > young, study | |||||
|
| |||||
| None | |||||
|
| |||||
| B. Task-invariant, test | |||||
|
| |||||
| Retrosplenial cortex | Right | 15 −54 18 | 18 | 4.44 | 120 |
|
| |||||
| Calcarine cortex | Left | −6 −51 6 | 30 | 4.17 | |
|
| |||||
| Precuneus | Left | −3 −54 18 | 30 | 3.89 | |
|
| |||||
| Young > older, test | |||||
|
| |||||
| None | |||||
|
| |||||
| Older > young, test | |||||
|
| |||||
| Cerebellum | Left | −30 −48 −33 | NA | 4.19 | 70 |
Notes: Task-invariant regions were defined by exclusively masking source accuracy regions (SC > NS) with regions showing memory by task, group by memory, and 3-way interactions. Age effects were defined by inclusively masking age differences in source memory accuracy (e.g., [SC > NS, young] > [SC > NS, older]) withthe source memory effects for the relevant group (e.g., SC > NS, young). The right middle occipital cortex region depicted in bold is shown in Figure 3. Regions are listed for each cluster from highest to lowest t-value. Regions listed without a cluster size are subsumed by the larger cluster listed directly above. Regions listed without an anatomic region are identical to the region listed immediately above. BA: Brodmann’s area; SC = source correct; NS = no source
Figure 3.
Task-invariant activity at study and test. (A) Source memory regions (SC > NS) exhibiting task-invariant activation common to both younger and older adults at the time of study (red colors) and at the time of test (green colors) are rendered on a standard brain in MNI space. (B) Anatomic overlays and graphs depict mean parameter estimates (betas) from the peak voxel exhibiting task-invariant activation at the time of study. Bars represent, from left to right, the difference between the SC and NS trials in the self-reference task and the difference between the SC and NS trials in the other-reference task in the young adults (YA) followed by the same values in the older adults (OA). Bars are plotted in arbitrary units with the error bars depicting the SEM. Statistical threshold: p < .001, 34 contiguous voxels, corrected for multiple comparisons. MNI = Montreal Neurological Institute; SC = source correct; NS = no source; R. Mid. Occ. = right middle occipital cortex; SEM = standard error of the mean
We then examined regions showing age effects in task-invariant source accuracy. To conduct this analysis we inclusively masked age differences in source memory accuracy (e.g., [SC > NS, young] > [SC > NS, older]) with the source memory effects for the relevant group (e.g., SC > NS, young). No regions exhibited greater source accuracy activity for the young than the older adults or for the older adults over the young.
Test
Using an identical masking procedure to that of study, we report task-invariant source memory effects in both age groups in several occipital regions including the left precuneus (BA 30) and right retrosplenial cortex (BA 18) (Table 4B; Figure 3A).
We then examined regions showing age effects in task-invariant source accuracy. We found no evidence of age effects in cortical regions.
Task-selective source accuracy effects
Study
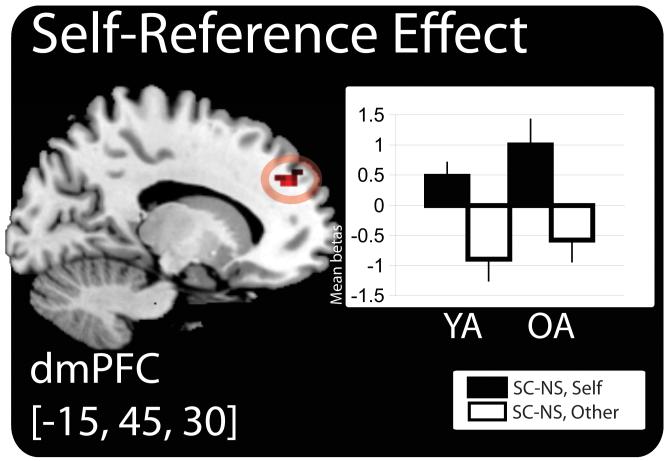
The second analysis examined brain regions that uniquely supported source accuracy for one study task versus the other. To conduct this analysis we inclusively masked (see methods) the interaction where the source accuracy effect was larger for one condition relative to the other (e.g., [SC > NS, self-reference] > [SC > NS, other-reference]) with the source accuracy effect for the relevant condition (e.g., SC > NS, self-reference). No regions survived this contrast. Given our a priori interest in dmPFC, we further analyzed this contrast at a reduced threshold of p < 0.001, uncorrected with a minimum cluster size of 5 contiguous voxels, as performed by others (Gutchess et al., 2005; Leclerc & Kensinger, 2010; Park & Rugg, 2011). Self-reference-selective activity was found in two cortical regions—the left dmPFC (x = −15, y = 45, z = 30; BA 32; peak t-value: 3.97; cluster size: 19), and the left inferior frontal gyrus (x = −57, y = 30, z = 12; BA 45; peak t-value: 3.49; cluster size: 6). Mean beta estimates from the peak voxel of the dmPFC region are plotted in Figure 4. No other-reference-selective source accuracy effects were observed at study.
Figure 4.
Task-selective source memory effect at study. The anatomic overlay and graph depicts the mean parameter estimates (betas) from the peak voxel exhibiting self mean parameter estimates (betas) from the peak voxel exhibiting self-reference-selective source memory effects in both young and older adults in both young and older adults. Bars represent, from left to right, the di fference between the SC and NS t rials in the self rials in the self-reference task and the difference between the SC and NS trials in the other-reference task in the young adults (YA), followed by the same values in the older adults (OA). Bars are plotted in arbitrary units with the e Bars are plotted in arbitrary units with the error bars depicting the SEM. Statistical threshold: p < .001, uncorrected, 5 contiguous voxels. MNI = Montreal Neurological Institute; SC = source correct; NS = no source; dmPFC = dorsal medial prefrontal cortex; SEM = standard error of the mean
We also examined task-selective source accuracy regions that differed as a function of age. To perform this analysis we inclusively masked the 3-way interaction showing larger task-selective effects for one age group relative to other (e.g., ([task-selective, self-reference] > [task-task-selective, other-reference], [young > older adults]) with the relevant condition effect (e.g., SC > NS, self-reference, young)). No regions survived this contrast.
Test
No regions showed task-selective effects or age effects at test for either the self- or other-reference task.
Age effects when behavioral performance is not matched
Thus far, functional analyses have been reported for trials for which behavioral source memory accuracy is matched across age groups. Given that most aging studies report imaging comparisons where behavioral differences exist between young and older adults, we analyzed source memory effects for trials where source accuracy was better in the young than in the older adult. To conduct this analysis, only the data from the once presented items were analyzed.
Study
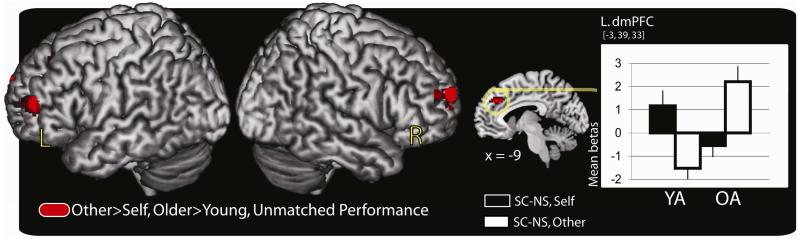
Many regions showed age-effects for task-selective regions. There were no regions showing greater task-selective effects for the young adults relative to the older adults (Table 5A), however, other-reference-selective source effects were larger in the older adults than the young in numerous frontal regions including the left dorsal medial prefrontal cortex (BA 32), the left middle frontal gyrus (BA 46), and the right superior frontal gyrus (BA 10) (Table 5B; Figure 5). These results indicate numerous age-related source accuracy differences when source memory performance differs across age.
Table 5.
Age differences in task-selective source accuracy effects at the time of study when behavioral performance is unmatched between groups. Regions showing greater self-selective effects for the young relative to the older adults are shown in (A) and those showing greater other-selective effects for the older relative to the young adults are show in (B).
| Region | Hemisphere | MNI Coordinates | BA | T-value | Cluster Size |
|---|---|---|---|---|---|
| A. Self-reference > other-reference, young > older, study | |||||
|
| |||||
| None | |||||
|
| |||||
| B. Other-reference > self-reference, older > young, study | |||||
|
| |||||
| Superior frontal gyrus | Right | 24 66 18 36 54 21 |
10 10 |
5.16 3.84 |
87 |
|
| |||||
| Middle frontal gyrus | Left | −33 51 15 | 46 | 4.58 | 51 |
|
| |||||
| Superior frontal gyrus | Left | −27 63 12 | 11 | 3.4 | |
|
| |||||
| Dorsal medial PFC | Left | −3 39 33 | 32 | 4.34 | 81 |
|
| |||||
| Superior frontal gyrus | Left | −12 36 42 | 32 | 4.01 | |
Notes: Trials in this contrast were the once presented trials for the young and older adults where source memory performance was better in the young. The dorsal medial PFC region depicted in bold is shown in Figure 5. Regions are listed from highest to lowest t-value. Regions listed without a cluster size are subsumed by the larger cluster listed directly above. Regions listed without an anatomic region are identical to the region listed immediately above. BA: Brodmann’s area; PFC = prefrontal cortex
Figure 5.
Regions show study phase age differences in task-selective source memory accuracy when behavioral performance is unmatched between age groups. All regions are displayed on a standard surface-rendered brain in MNI space. The anatomic overlay and graph depicts the mean parameter estimates (betas) from the peak voxel in the left dmPFC exhibiting age effects. The bars in the graph represent, from left to right, the difference between the SC and NS trials in the self-reference task and the difference between the SC and NS trials in the other-reference task in the young adults (YA), followed by the same values in the older adults (OA). Bars are plotted in arbitrary units with the error bars depicting the SEM. Statistical threshold: p < .001, 34 contiguous voxels, corrected for multiple comparisons. MNI = Montreal Neurological Institute; SC = source correct; NS = no source; L. dmPFC = left dorsolateral prefrontal cortex; SEM = standard error of the mean
Test
There were no age-effects for task-selective regions at test.
Discussion
The purpose of this study was to investigate the effects of self-referencing on source memory performance and associated neural activity in young and older adults. We report four primary findings: First, both age groups showed a similar source memory benefit from self-referencing, consistent with previous behavioral investigations. Second, task-invariant activity supported source memory in both age groups in several regions implicated in scene processing in bilateral occipital cortices. Third, study phase activity in the dmPFC supported source memory accuracy for self-referenced materials similarly in both age groups. Fourth, age differences in task-selective source memory activity in the dorsal and lateral prefrontal cortex were evident when performance differed between groups. Overall, we show little evidence of functional recruitment differences across age, and instead report patterns of relatively similar cortical recruitment when comparing functional activity where both groups are matched on behavioral performance. It is only when we examine age differences on trials for which performance differs that we observed extensive differences between age groups.
Behavioral results
Consistent with previous findings (Dulas et al., 2011; Hamami et al., 2011; Leshikar & Duarte, 2012; Serbun et al., 2011), we report that young and older adults showed a source memory benefit from self-referencing. A common finding in the aging and memory literature is that aging is associated with deficits in remembering specific contextual details of prior episodes but the majority of these previous studies have not assessed memory for socially relevant information (for review see, Mitchell & Johnson, 2009). Our data, however, are consistent with a growing body of evidence that suggests that older adults show a context memory benefit for materials that are social in nature (May et al., 2005; Rahhal et al., 2002), suggesting preserved memory for at least certain types of materials/events. Previous investigations have shown source memory benefits for materials processed in reference to the self in older adults (Dulas et al., 2011; Glisky & Marquine, 2009; Hamami et al., 2011). For example, our previous work has shown a source memory benefit for objects processed self-referentially (e.g., “Do I like this object?”) versus a control condition (e.g., “Is this a common object?”) in older adults (Dulas et al., 2011). Thus, processing information for self-relevance appears to be an effective strategy for enhancing memory for context in the young and older adults alike. Interestingly and in contrast to prior neuroimaging studies (Gutchess et al., 2010; Gutchess, Kensinger, Yoon, et al., 2007), item recognition did not statistically benefit from self-referencing in either age group. While not predicted, it should be noted that previous behavioral studies have sometimes failed to show a self-reference effects in item memory (experiment 2, Bower & Gilligan, 1979; Kuiper & Rogers, 1979), but those studies often involve a highly familiar other such as one’s mother in the comparison condition. While speculative, because participants knew that a source memory test would follow study, they may have focused more on source features than on the item itself, which in turn, could have resulted in the self-reference effect for source memory but not for item memory. Future work will be necessary to explore this possibility.
Evidence suggests that processing information in relation to the self boosts recognition memory relative to non-self-referential tasks such as semantic encoding in the young (Rogers et al., 1977; Symons & Johnson, 1997). This self-reference effect is theorized to result from enhanced elaboration and organization of newly learned information when processed for personal meaning (Klein & Kihlstrom, 1986; Rogers et al., 1977). Older adults show self-reference memory benefits equal to that of the young (Glisky & Marquine, 2009; Rosa & Gutchess, 2011), which may reflect the relative stability of the self as a mental representation across age (Terracciano, McCrae, & Costa, 2010). Indeed, older adults may especially benefit from self-referencing given that older participants tend to rely on internally generated thoughts and feelings when making source memory judgments (Comblain, D’Argembeau, Van der Linden, & Aldenhoff, 2004; Hashtroudi, Johnson, & Chrosniak, 1990). Thus, self-referential encoding tasks may capitalize on the fact that self-relevant, internally generated information is readily encoded and subsequently retrieved in older adults. In addition, the older adults might have further benefitted from our use of an other-referent that was similar in age to them. Prior work has shown that when information is seen as more personally meaningful to older adults, such as encountering someone of a similar age, they are more motivated to process that information deeply (Hess, 2005). Yet, it seems that if there were more overlap between self and other for the older adults in our study, due to similar age, older adults would have shown reduced task-specific activity than the young. This was not the case in this study.
While both age groups showed a self-referencing benefit, young adults still exhibited better memory performance than the older adults. Our difficulty manipulation, however, resulted in statistically equivalent source memory performance for the items presented once in the young and the items presented twice in the older adults. Critically, this design allowed us to compare functional results across trials where we could remove the confounding influence of performance differences, and more accurately measure age-effects in cortical recruitment, while also allowing us to examine functional recruitment where performance differed, consonant with many published aging investigations.
fMRI results4
Effects when behavioral performance is matched
Task-Invariant source accuracy effects
When behavioral performance was matched between age groups, we identified several areas that supported source memory accuracy across tasks and age groups. Specifically, task-invariant activity was seen in several visual processing areas in bilateral middle occipital cortices. These regions have often been implicated in object and scene perception and memory. For instance, prior investigations have reported occipital activity in support of object-scene memory (Goh et al., 2004; Grill-Spector, Kourtzi, & Kanwisher, 2001; Hayes, Nadel, & Ryan, 2007). Here we show that occipital activity supported source memory for the visually complex scenes. We have previously shown that bilateral occipital activity supports accurate subsequent memory of both scene and conceptual source details (i.e., “Did I see this object in the self or control task?”) (Leshikar & Duarte, 2012).
Other areas supporting source accuracy included the precuneus and the retrosplenial cortex. While not memory investigations per se, a few studies have identified activity in posterior parietal activity in support of both self- and other-focused processes (Grigg & Grady, 2010b; Heatherton et al., 2006; Lombardo et al., 2010; Ochsner et al., 2004). These studies suggest that thinking about the self and thinking about others recruit precuneus in conjunction with other cortical midline regions including the vmPFC (Lombardo et al., 2010). Interestingly, one recent investigation found highly correlated activity between posterior parietal cortex and the vmPFC during both self- and other-reference engagement (Grigg & Grady, 2010b), offering further support that overlapping regions support thinking about oneself as well as thinking about others.
Interestingly, we found no evidence of source accuracy effects in the hippocampus. Task and material-invariant activity in the hippocampus has frequently been reported in episodic memory studies (Badre & Wagner, 2007; Eichenbaum et al., 2007; Gottlieb et al., 2010; Uncapher & Rugg, 2009) and is suggested to reflect the binding of information into a retrievable memory trace (for reviews see Cohen et al., 1999; Eichenbaum, 2000). While null findings should be interpreted cautiously, there is limited but growing evidence in the socio-cognitive literature suggesting reduced hippocampal engagement in memory tasks involving social materials. The strongest evidence to date supporting this notion comes from Todorov and Olson (2008), who found preserved learning of face-trait associations in an amnesic with hippocampal damage; a surprising finding given the necessary role of the hippocampus in paired associate learning (Cohen et al., 1999). This work suggests that memory for materials social in nature may be hippocampal independent (See also Grilli & Glisky, 2010). Future work will be necessary to fully determine the role that the hippocampus plays in episodic memory for socially relevant events.
Relative to prior investigations, few age group differences emerged in the analysis of source memory effects at study or test, suggesting that when matched on behavioral performance both young and older adults show similar cortical recruitment in service of memory, but nonetheless some age differences did emerge. Interestingly, many of these regions over-recruited by older adults, like the dmPFC, abutted regions that were commonly recruited by both age groups. We (Dulas & Duarte, 2012) and others (Morcom et al., 2007) have identified a similar pattern during source retrieval and have suggested it may reflect a decline in neural efficiency in older adults with enhanced activity in a common network necessary to support equivalent memory performance to that of the young. Reduced efficiency leads older adults to recruit the same regions as the young, but to additionally recruit adjacent cortex in order to meet the task demands. This finding is in accord with other work suggesting reduced neural specialization with age—or dedifferentiation—as has been reported by many (Goh, Suzuki, & Park, 2010; Park et al., 2004; Voss et al., 2008). The current data lend support to this hypothesis.
Task-selective source accuracy effects
We sought to test our prediction that dmPFC would support accurate source memories for self-referenced materials in young and older adults alike, at least when performance was matched. Our results confirmed this prediction. Previous work has shown that medial prefrontal cortex supports memory for a range of socially-processed materials (Amodio & Frith, 2006). The present finding of self-selective source memory accuracy in dmPFC activity is similar to that described by Leshikar & Duarte (2012). In that study of young adults, we observed activity in the left dmPFC predictive of subsequent source accuracy for multiple source features (scene and task) encoded in reference to the self. A similar effect in the dmPFC was observed in this study in both young and older adults. One important difference between the current design and that of our previous study is that the non-self-referential task in our previous experiment (i.e. “Is the object color present in the scene?”) was arguably more shallow than our other-referential task (i.e. “Would the Queen like the object-scene pairing?”). Thus, we could not conclusively rule out the possibility that the self-selective source accuracy effects in the dmPFC in our previous study could be explained by depth of encoding rather than a self-reference effect, per se (though it should be noted that dmPFC activity is not typically associated with depth of processing effects) (Kausler, 1994). In this investigation, we are more confident that we controlled for processing depth between the self-reference and the other-reference condition which allows us to conclude that dmPFC supported the encoding of self-relevant source details in both age groups. Importantly, across both the current and our previous study, dmPFC activity supported source accuracy for an event detail (scene) that is not in and of itself self-relevant. In most, if not all previous self-reference investigations, medial prefrontal activity has supported item memory for explicitly self-relevant adjectives (Gutchess et al., 2010; Macrae et al., 2004) or for the self-reference encoding task performed (e.g., did you encounter this object in the self-reference condition?) (Leshikar & Duarte, 2012). What our data suggest is that self-referencing is an effective strategy for binding various kinds of episodic details into memory and facilitating successful retrieval of those details. Similar to our previous investigation, test phase activity in dmPFC did not support source accuracy. Finding dmPFC activity only at study may suggest that this region plays a role in establishing the memory representation for item features studied self-referentially, but that at the time of test other cortical regions utilize this representation in service of accurate source memory.
No task-selective source memory effects were observed for the other-referencing condition at study or at test. One reason for this may have been the lower source memory performance for the other- relative to the self-reference task for both age groups. We have previously observed task-selective source memory effects for the non-self-referential task (Leshikar & Duarte, 2012) making it unlikely that performance differences can fully account for the absence of other-reference source effects here. Another reason why we did not observe other-reference memory effect may be due to functional and neural overlap between the two referencing tasks. That is, other-referencing may have relied on many of the same processes and associated regions as self-referencing, such as the vmPFC (see Denny, Kober, Wager, & Ochsner, 2012, which showed mPFC in both self and other-referencing), but self-referencing engaged additional regions, most notably the dmPFC in service of memory. Indeed, prior work has established that vmPFC regions are recruited when thinking about close others (Mitchell, Macrae, & Banaji, 2006; Moran, Lee, & Gabrieli, 2011). By analogy, we have previously found a similar effect in a paired associate learning task where we observed task-selective activity for visual imagery but not for sentence generation (Leshikar, Duarte, & Hertzog, 2012). In that investigation, we argued that both tasks engaged similar semantic processes recruiting largely overlapping regions, but that visual imagery engaged additional imagistic operations and associated brain regions. As described earlier, activity in the precuneus, a regions implicated in social cognition, supported source memory accuracy for both self- and other-reference encoding, supporting the hypothesis that both tasks rely on many of the same operations.
Age effects when behavioral performance is unmatched
Few regions showed age differences when source accuracy performance did not significantly differ between age groups. When age comparisons were conducted on trials for which performance differed across age (the once presented items), however, a different pattern emerged with age effects surfacing in numerous regions. While no regions showed greater recruitment in the younger adults, older adults exhibited greater other-selective source memory recruitment than the young in several frontal regions, including the dmPFC. Finding greater recruitment in dmPFC stands in contrast to numerous investigations that have reported under-recruitment of dmPFC in older adults (Leclerc & Kensinger, 2008; Moran et al., 2012; Park et al., 2012). It is worth noting, however, that the additional recruitment in dmPFC in our data occurred when behavioral performance was not matched. Yet, it is difficult to tell from these data alone whether this response was adaptive and helped performance in the face of a challenging task, or whether it was maladaptive. Overall, finding age differences only when performance differed may indicate an over-estimation of age-related effects in memory tasks in previous investigations which have allowed performance to vary between groups. In the present study, older adults performed more poorly than the young adults when the task demands were higher. It may be that age differences for unmatched trials reflect differences in how the older adults encoded materials in the more difficult condition. It seems likely that when age differences occur, they reflect both the biological effects of aging as well as differences due to performance.
Limitations
One limitation of our current design is our comparison of the items presented once in the young and twice in the older adults. With this design, it is possible that there could be processing differences related to old/new effects, repetition suppression, priming, etc., that would differ across trial types. While certainly a possibility, we think this outcome is unlikely to have dramatically affected our results given our masking procedure to remove the effects of presentation. Indeed, no regions were masked out using the masking procedure, suggesting that our effects of interest were not susceptible to the effects of presentation. Another limitation of our design is the use of the “mini-block” design at study. While the mini-block design is particularly ideal to reduce task-switching costs, especially in the older adults, it is possible that that such a design reduced our ability to say with certainty whether task-specific effects occurred on particular trials or during the block in general. Yet, given that our self-referential source encoding effects (i.e., dmPFC) are consistent with those reported in prior investigations, we think this possible confound did not unduly impact our results.
Conclusion
In this investigation, we found that self-referencing supported source accuracy in both young and older adults and further, that activity in the dmPFC supported this memory benefit in both age groups. This suggests that the cognitive processes engaged while self-referencing were similar in both young and older adults. Given prior findings of age reduction in memory for episodic details, our data implicate self-referential processing as a beneficial mnemonic strategy that facilitates memory for contextual details in the young and older adults alike. Critically, few age differences emerged when performance was statistically equivalent across groups, but when performance was unmatched, numerous regions showed age effects, suggesting the importance of accounting for age differences in performance in memory investigations. Overall, these data suggest that by utilizing preserved cognitive processes such as self-referential encoding, older adults can show source memory improvements for associations not typically well-remembered with advancing age.
Acknowledgements
The authors would like to thank Yashu Jiang, Michael Dulas, and Rose Donohue for their contributions to this project. E.D.L. was supported by NIH grants T32-AG000175 and T3-AG000204 while working on this project and A.D. was supported by The American Federation for Aging Research.
Footnotes
Prior to participating in the experiment, participants read the following brief biography of the Queen: “Elizabeth II became Queen of the United Kingdom (Britain) on February 6, 1952, after the death of her father, George VI. Having reigned for 58 years, she is the third-longest-reigning monarch in British history. She and her husband, Prince Phillip, have eight grandchildren, and four children, including her oldest son and heir apparent, Prince Charles. As a constitutional monarch, she takes only a limited direct part in government. Her duties include appointing a Prime Minister, dissolving Parliament, and bestowing honors, among others.”
A liberal threshold for an exclusive mask is more conservative in excluding regions from the masked SPM. The procedure of exclusively masking main effects by their interaction is formally equivalent to the original definition of a “cognitive conjunction” (Price & Friston, 1997).
As a part of our interrogation of our behavioral data we examined the signal detection measure of bias (C) (Snodgrass & Corwin, 1988) in both age groups. We found that neither age group showed strong influences of bias when making the old/new decisions (YA: −.11, OA: .17; where −1 reflects a perfect bias to call items “old” and 1 reflects a perfect bias to call items “new”). This is compared to other aging investigations that have reported equivalent or higher estimates of response bias in younger and older adults (Dodson & Schacter, 2002; Morcom, Good, Frackowiak, & Rugg, 2003).
It should be noted that at least some of the source encoding related activity for items presented twice could reflect memory of the first presentation that occurred outside of the scanner rather than encoding per se. As discussed in the methods, we addressed this confound by masking out regions sensitive to the number of presentations (e.g., 1 presentation > 2 presentations; 2 presentations > 1 presentation across both tasks and both age groups) from all our contrasts of interest. These regions sensitive to presentation number are shown in Table 2. None of these regions were masked out of our source accuracy contrasts, shedding doubt on the possibility that a substantial amount of the encoding activations reflected memory for prior presentation.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Aron A, Aron EN, Tudor M, Nelson G. Close relationships as including other in the self. Journal of Personality and Social Psychology. 1991;60(2):241. [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. doi: S1053-8119(07)00584-8 [pii] 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Beadle JN, Yoon C, Gutchess AH. Age-related neural differences in affiliation and isolation. Cognitive, Affective, & Behavioral Neuroscience. 2012:1–11. doi: 10.3758/s13415-012-0085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Hamsher S. K. d., Sivan AB. Multilingual aplasia examination. 2nd ed. AJA Associates; Iowa City: 1983. [Google Scholar]
- Bower GH, Gilligan SG. Remembering information related to one’s self. Journal of research in personality. 1979;13(4):420–432. [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9(1):83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Comblain C, D’Argembeau A, Van der Linden M, Aldenhoff L. The effect of ageing on the recollection of emotional and neutral pictures. Memory. 2004;12(6):673–684. doi: 10.1080/09658210344000477. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, Kapur S. In search of the self: A positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Salmon E. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25(2):616–624. doi: 10.1016/j.neuroimage.2004.11.048. doi: S1053-8119(04)00712-8 [pii] 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Stawarczyk D, Majerus S, Collette F, Van der Linden M, Salmon E. Modulation of medial prefrontal and inferior parietal cortices when thinking about past, present, and future selves. Soc Neurosci. 2010;5(2):187–200. doi: 10.1080/17470910903233562. doi: 915135845 [pii] 10.1080/17470910903233562. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol Learn Mem Cogn. 2008;34(4):791–808. doi: 10.1037/0278-7393.34.4.791. doi: 2008-08549-007 [pii] 10.1037/0278-7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self-and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24(8):1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cereb Cortex. 2005;15(11):1768–1778. doi: 10.1093/cercor/bhi054. doi: bhi054 [pii] 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Dodson CS, Schacter DL. Aging and strategic retrieval processes: Reducing false memories with a distinctiveness heuristic. Psychology and Aging. 2002;17(3):405. doi: 10.1037//0882-7974.17.3.405. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. The effects of aging on the neural correlates of subjective and objective recollection. Cereb Cortex. 2008;18(9):2169–2180. doi: 10.1093/cercor/bhm243. doi: bhm243 [pii] 10.1093/cercor/bhm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Knight RT, Emery T, Graham KS. Orbito-frontal Cortex is Necessary for Temporal Context Memory. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21316. doi: 10.1162/jocn.2009.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. The effects of aging on material-independent and material-dependent neural correlates of contextual binding. Neuroimage. 2011;57(3):1192–1204. doi: 10.1016/j.neuroimage.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. The effects of aging on material-independent and material-dependent neural correlates of source memory retrieval. Cerebral Cortex. 2012;22(1):37–50. doi: 10.1093/cercor/bhr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulas MR, Newsome RN, Duarte A. The effects of aging on ERP correlates of source memory retrieval for self-referential information. Brain Res. 2011;1377:84–100. doi: 10.1016/j.brainres.2010.12.087. doi: S0006-8993(11)00008-4 [pii] 10.1016/j.brainres.2010.12.087. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg M. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiology of Aging. 2008;29(12):1902–1916. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nature Reviews Neuroscience. 2000;1(1):41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The Medial Temporal Lobe and Recognition Memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Lepage M, Graham SJ, Grady C, Keightley ML, Mayberg H. Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22(4):1596–1604. doi: 10.1016/j.neuroimage.2004.03.034. doi: 10.1016/j.neuroimage.2004.03.034 S1053811904001909 [pii] [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Kiebel S, Nichols TE, Penny WD, editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Elsevier; 2007. [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J. Classical and Bayesian inference in neuroimaging: applications. Neuroimage. 2002;16(2):484–512. doi: 10.1006/nimg.2002.1091. doi: 10.1006/nimg.2002.1091 S1053811902910918 [pii] [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Kensinger EA, Wong AT, Schacter DL. Age-related neural changes during memory conjunction errors. Journal of Cognitive Neuroscience. 2010;22(7):1348–1361. doi: 10.1162/jocn.2009.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Marquine MJ. Semantic and self-referential processing of positive and negative trait adjectives in older adults. Memory. 2009;17(2):144–157. doi: 10.1080/09658210802077405. doi: 792992980 [pii] 10.1080/09658210802077405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JO, Siong SC, Park D, Gutchess A, Hebrank A, Chee MW. Cortical areas involved in object, background, and object-background processing revealed with functional magnetic resonance adaptation. J Neurosci. 2004;24(45):10223–10228. doi: 10.1523/JNEUROSCI.3373-04.2004. doi: 24/45/10223 [pii] 10.1523/JNEUROSCI.3373-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JO, Suzuki A, Park DC. Reduced neural selectivity increases fMRI adaptation with age during face discrimination. Neuroimage. 2010;51(1):336–344. doi: 10.1016/j.neuroimage.2010.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb LJ, Uncapher MR, Rugg MD. Dissociation of the neural correlates of visual and auditory contextual encoding. Neuropsychologia. 2010;48(1):137–144. doi: 10.1016/j.neuropsychologia.2009.08.019. doi: S0028-3932(09)00338-8 [pii] 10.1016/j.neuropsychologia.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg O, Grady CL. The default network and processing of personally relevant information: converging evidence from task-related modulations and functional connectivity. Neuropsychologia. 2010a;48(13):3815. doi: 10.1016/j.neuropsychologia.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg O, Grady CL. Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PloS one. 2010b;5(10):e13311. doi: 10.1371/journal.pone.0013311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41(10-11):1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Grilli MD, Glisky EL. Self-imagining enhances recognition memory in memory-impaired individuals with neurological damage. Neuropsychology. 2010;24(6):698. doi: 10.1037/a0020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. doi: 10.1073/pnas.071043098 071043098 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Soc Neurosci. 2007;2(2):117–133. doi: 10.1080/17470910701399029. doi: 778890605 [pii] 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Functional neuroimaging of self-referential encoding with age. Neuropsychologia. 2010;48(1):211–219. doi: 10.1016/j.neuropsychologia.2009.09.006. doi: S0028-3932(09)00360-1 [pii] 10.1016/j.neuropsychologia.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Yoon C, Schacter DL. Ageing and the self-reference effect in memory. Memory. 2007;15(8):822–837. doi: 10.1080/09658210701701394. doi: 783624081 [pii] 10.1080/09658210701701394. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17(1):84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hamami A, Serbun SJ, Gutchess AH. Self-referencing enhances memory specificity with age. Psychology and Aging. 2011;26(3):636. doi: 10.1037/a0022626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashtroudi S, Johnson MK, Chrosniak LD. Aging and qualitative characteristics of memories for perceived and imagined complex events. Psychol Aging. 1990;5(1):119–126. doi: 10.1037//0882-7974.5.1.119. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Nadel L, Ryan L. The effect of scene context on episodic object recognition: Parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus. 2007;17(9):873–889. doi: 10.1002/hipo.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Soc Cogn Affect Neurosci. 2006;1(1):18–25. doi: 10.1093/scan/nsl001. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R. Efficient experimental design for fMRI. Statistical parametric mapping. The analysis of functional brain images. 2007:193–210. [Google Scholar]
- Hess TM. Memory and aging in context. Psychological Bulletin. 2005;131(3):383. doi: 10.1037/0033-2909.131.3.383. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Pschol. Rev. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Kausler DH. Learning and memory in normal aging. Academic Press; 1994. [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–794. doi: 10.1162/08989290260138672. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Klein SB. Self, Memory, and the Self-Reference Effect An Examination of Conceptual and Methodological Issues. Personality and Social Psychology Review. 2012;16(3):283–300. doi: 10.1177/1088868311434214. [DOI] [PubMed] [Google Scholar]
- Klein SB, Kihlstrom JF. Elaboration, organization, and the self-reference effect in memory. J Exp Psychol Gen. 1986;115(1):26–38. doi: 10.1037//0096-3445.115.1.26. [DOI] [PubMed] [Google Scholar]
- Kuiper NA, Rogers TB. Encoding of personal information: Self-other differences. Journal of Personality and Social Psychology. 1979;37(4):499. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cognitive, Affective, & Behavioral Neuroscience. 2008;8(2):153–164. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related valence-based reversal in recruitment of medial prefrontal cortex on a visual search task. Social Neuroscience. 2010;5(5-6):560–576. doi: 10.1080/17470910903512296. [DOI] [PubMed] [Google Scholar]
- Leshikar ED, Duarte A. Medial prefrontal cortex supports source memory accuracy for self-referenced items. Social Neuroscience. 2012;7(2):126–145. doi: 10.1080/17470919.2011.585242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshikar ED, Duarte A, Hertzog C. Task-Selective Memory Effects for Successfully Implemented Encoding Strategies. PloS one. 2012;7(5):e38160. doi: 10.1371/journal.pone.0038160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, Baron-Cohen S. Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience. 2010;22(7):1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14(6):647–654. doi: 10.1093/cercor/bhh025. doi: 10.1093/cercor/bhh025 bhh025 [pii] [DOI] [PubMed] [Google Scholar]
- May CP, Rahhal T, Berry EM, Leighton EA. Aging, source memory, and emotion. Psychol Aging. 2005;20(4):571–578. doi: 10.1037/0882-7974.20.4.571. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychol Bull. 2009;135(4):638–677. doi: 10.1037/a0015849. doi: 2009-09537-007 [pii] 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Jolly E, Mitchell JP. Social-cognitive deficits in normal aging. The Journal of Neuroscience. 2012;32(16):5553–5561. doi: 10.1523/JNEUROSCI.5511-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Lee SM, Gabrieli JD. Dissociable neural systems supporting knowledge about human character and appearance in ourselves and others. Journal of Cognitive Neuroscience. 2011;23(9):2222–2230. doi: 10.1162/jocn.2010.21580. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126(Pt 1):213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age Effects on the Neural Correlates of Episodic Retrieval: Increased Cortical Recruitment with Matched Performance. Cereb Cortex. 2007 doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Mueller JH, Wonderlich S, Dugan K. Self-referent processing of age-specific material. Psychol Aging. 1986;1(4):293–299. doi: 10.1037//0882-7974.1.4.293. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16(10):1746–1772. doi: 10.1162/0898929042947829. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(35):13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Rugg MD. Neural correlates of encoding within-and across-domain inter-item associations. Journal of Cognitive Neuroscience. 2011;23(9):2533–2543. doi: 10.1162/jocn.2011.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Carp J, Kennedy KM, Rodrigue KM, Bischof GN, Huang C-M, Park DC. Neural broadening or neural attenuation? Investigating age-related dedifferentiation in the face network in a large lifespan sample. The Journal of Neuroscience. 2012;32(6):2154–2158. doi: 10.1523/JNEUROSCI.4494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. Neuroimage. 1997;5(4 Pt 1):261–270. doi: 10.1006/nimg.1997.0269. doi: S1053-8119(97)90269-X [pii] 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Rahhal TA, May CP, Hasher L. Truth and character: sources that older adults can remember. Psychol Sci. 2002;13(2):101–105. doi: 10.1111/1467-9280.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, Ames B, D’Esposito M. Prefrontal contributions to domain-general executive control processes during temporal context retrieval. Neuropsychologia. 2008;46(4):1088–1103. doi: 10.1016/j.neuropsychologia.2007.10.023. doi: S0028-3932(07)00370-3 [pii] 10.1016/j.neuropsychologia.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo A, Vicens L, Clithero JA, Dobbins IG, Huettel SA. Contributions of frontopolar cortex to judgments about self, others and relations. Social Cognitive and Affective Neuroscience. 2010 doi: 10.1093/scan/nsq033. doi: nsq033 [pii] 10.1093/scan/nsq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Therapy and clinical assessment. Neuropsychological Press; Tucson, AZ: 1985. [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. J Pers Soc Psychol. 1977;35(9):677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Rosa NM, Gutchess AH. Source memory for action in young and older adults: Self vs. close or unknown others. Psychology and Aging. 2011;26(3):625. doi: 10.1037/a0022827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajonz B, Kahnt T, Margulies DS, Park SQ, Wittmann A, Stoy M, Bermpohl F. Delineating self-referential processing from episodic memory retrieval: common and dissociable networks. Neuroimage. 2010;50(4):1606–1617. doi: 10.1016/j.neuroimage.2010.01.087. doi: S1053-8119(10)00110-2 [pii] 10.1016/j.neuroimage.2010.01.087. [DOI] [PubMed] [Google Scholar]
- Serbun SJ, Shih JY, Gutchess AH. Memory for details with self-referencing. Memory. 2011;19(8):1004–1014. doi: 10.1080/09658211.2011.626429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J., Jr. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res. 2003;17(1):75–82. doi: 10.1016/s0926-6410(03)00082-x. doi: S092664100300082X [pii] [DOI] [PubMed] [Google Scholar]
- Smith AP, Dolan RJ, Rugg MD. Event-related potential correlates of the retrieval of emotional and nonemotional context. J Cogn Neurosci. 2004;16(5):760–775. doi: 10.1162/089892904970816. doi: 10.1162/089892904970816. [DOI] [PubMed] [Google Scholar]
- Snodgrass J, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. Journal of Experimental Psychology. 1988;116:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychol Aging. 1995;10(4):527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Rubin DC, Cabeza R. Age-related effects on the neural correlates of autobiographical memory retrieval. Neurobiology of Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons CS, Johnson BT. The self-reference effect in memory: a meta-analysis. Psychol Bull. 1997;121(3):371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- Terracciano A, McCrae RR, Costa PT. Intra-individual Change in Personality Stability and Age. J Res Pers. 2010;44(1):31–37. doi: 10.1016/j.jrp.2009.09.006. doi: 10.1016/j.jrp.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Olson IR. Robust learning of affective trait associations with faces when the hippocampus is damaged, but not when the amygdala and temporal pole are damaged. Social Cognitive and Affective Neuroscience. 2008;3(3):195–203. doi: 10.1093/scan/nsn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Rugg MD. Selecting for memory? The influence of selective attention on the mnemonic binding of contextual information. J Neurosci. 2009;29(25):8270–8279. doi: 10.1523/JNEUROSCI.1043-09.2009. doi: 29/25/8270 [pii] 10.1523/JNEUROSCI.1043-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia. 2008;46(7):1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. doi: S0028-3932(08)00015-8 [pii] 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Chaddock L, Prakash RS, Colcombe SJ, Morris KS, Kramer AF. Dedifferentiation in the visual cortex: an fMRI investigation of individual differences in older adults. Brain Research. 2008;1244:121–131. doi: 10.1016/j.brainres.2008.09.051. [DOI] [PubMed] [Google Scholar]
- Wang TH, Kruggel F, Rugg MD. Effects of advanced aging on the neural correlates of successful recognition memory. Neuropsychologia. 2009;47:1352–1361. doi: 10.1016/j.neuropsychologia.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring JD, Addis DR, Kensinger EA. Effects of aging on neural connectivity underlying selective memory for emotional scenes. Neurobiology of Aging. 2012 doi: 10.1016/j.neurobiolaging.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. Memory assessment scales professional manual. Odessa: Psychological Assessment Resources. 1991 [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]