Abstract
Impulsive delayed reward discounting (DRD) is an important behavioral process in alcohol use disorders (AUDs), reflecting incapacity to delay gratification. Recent work in neuroeconomics has begun to unravel the neural mechanisms supporting DRD, but applications of neuroeconomics in relation to AUDs have been limited. This study examined the neural mechanisms of DRD preferences in AUDs, with emphasis on dissociating activation patterns based on DRD choice type and level of cognitive conflict. Heavy drinking adult males with (n = 13) and without (n = 12) a diagnosis of an AUD completed a monetary DRD task during a functional magnetic resonance imaging scan. Participant responses were coded based on choice type (impulsive vs. restrained) and level of cognitive conflict (easy vs. hard). AUD+ participants exhibited significantly more impulsive DRD decision-making. Significant activation during DRD was found in several decision-making regions, including dorsolateral prefrontal cortex (DLPFC), insula, posterior parietal cortex (PPC), and posterior cingulate. An axis of cognitive conflict was also observed, with hard choices associated with anterior cingulate cortex and easy choices associated with activation in supplementary motor area. AUD+ individuals exhibited significant hyperactivity in regions associated with cognitive control (DLPFC) and prospective thought (PPC) and exhibited less task-related deactivation of areas associated with the brain's default network during DRD decisions. This study provides further clarification of the brain systems supporting DRD in general and in relation to AUDs.
Keywords: Alcohol use disorders, delay discounting, neuroeconomics
Introduction
A behavioral economic approach to addiction posits that substance abuse results, in part, from persistent preferences for small-immediate gains from using alcohol and other drugs (e.g., intoxication, euphoria, tension reduction) at the cost of larger-delayed gains obtained from sobriety (e.g., physical health, financial stability, interpersonal relationships) (Bickel and Marsch, 2001). From this standpoint, impulsive delayed reward discounting (DRD)—a behavioral measure of the extent to which rewards are devalued based on their delay in time—is viewed as a key behavioral process of addiction (Bickel and Johnson, 2003). Assessment of DRD is typically achieved via intertemporal choice tasks, in which individuals make dichotomous choices between smaller-sooner and larger-later rewards. The amount and delay of the rewards are systematically varied to obtain an estimate of the individual's temporal discounting function, with steeper discounting functions reflecting more precipitous devaluation of delayed rewards.
In the context of alcohol misuse, a consistent finding is that individuals with alcohol use disorders (AUDs) show steeper temporal discounting compared to healthy controls (Petry, 2001). Steeper DRD has also been reported in other samples exhibiting addictive behavior, including nicotine (Baker et al., 2003), stimulants (Coffey et al., 2003), opiates (Madden et al., 1997), and pathological gambling (Mackillop et al., 2006). A recent meta-analysis integrating findings from forty-six studies found consistent evidence of medium to large effect size differences between substance abuse samples and control groups (Mackillop et al., 2011). Finally, DRD is a prognostic indicator of future substance involvement (e.g., Audrain-McGovern et al., 2009), as well as changes over time (Krishnan-Sarin et al., 2007; MacKillop and Kahler, 2009).
Complementing the well-established behavioral literature, recent work in the field of neuroeconomics has begun to unravel the neural mechanisms underlying temporal discounting in healthy as well as substance abuse samples. Neuroeconomics integrates principles from psychology, economics, and neuroscience to understand of the neural basis for decision-making, and DRD is one of the most extensively studied processes to date. Using functional magnetic resonance imaging (fMRI), several studies have revealed a profile of brain regions that appear to be responsible for DRD choices (Ballard and Knutson, 2009; Bickel et al., 2009; Kable and Glimcher, 2007; McClure et al., 2004). A recent meta-analysis found common neural activation across DRD studies in prefrontal cortex (PFC), anterior cingulate cortex (ACC), inferior frontal gyrus (IFG), anterior insular cortex, posterior parietal cortex (PPC), posterior cingulate cortex (PCC), and subcortical activation in the ventral striatum (VS) (Carter et al., 2010). Activity in these regions is theorized to reflect the balance between two conflicting systems, one comprising motivational brain regions that are responsible for reward and incentive value (e.g., VS) and the other comprising higher cortical regions responsible for cognitive control and prospective thought (e.g., PFC, ACC, PPC) (Bickel et al., 2007; McClure et al., 2004). However, a more general single system approach has also been proposed (e.g., Kable and Glimcher, 2007; Monterosso and Luo, 2010). Parallel work in neuroeconoimcs has also examined whether brain activation can be used to predict individuals' choice behavior (Berns et al., 2012; Berns et al., 2008). For instance, one study showed that for relatively easy choices, activity in cingulate, visual, parietal and temporal cortices was predictive of participant's choice behavior (Berns et al., 2008).
The application of neuroeconomics to alcohol misuse has been relatively limited, with three fMRI studies investigating DRD to date (Boettiger et al., 2009; Boettiger et al., 2007; Claus et al., 2012). Initially, Boettiger et al. (2007) found significant differences in PFC, PPC, and hippocampal activation between individuals with alcohol dependence who were abstinent at the time of scanning and healthy controls. Activation in these regions was also positively correlated with impulsive choice across all participants. A follow up study found that administration of the opioid antagonist naltrexone significantly increased DRD-related neural activation during choice of delayed rewards in orbitofrontal cortex (OFC), among other regions, but did not affect DRD behavior in an AUD sample (Boettiger et al., 2009). Recently, Claus et al. (2012) investigated the neural mechanisms of DRD in a large, heterogeneous sample. In this case, steeper DRD was associated with increased activation in OFC, insula and angular gyrus. AUD severity was also positively associated with activation the insula, IFG, and PPC, among other regions. Abnormal processing in the insula may be especially relevant in the context of AUD pathology. The insula is suggested to be a critical component of a decision-making network that integrates neuronal processing in PFC and limbic areas, and it putatively plays a key role in processing visceral drug urges (Naqvi and Bechara, 2009; Naqvi et al., 2007). Thus, impulsive DRD may be partly attributed to dysfunction in a network of cortical and subcortical regions involved in cognitive and motivational aspects of DRD choices. These findings are similar to those from studies on stimulant (Hoffman et al., 2008; Monterosso et al., 2007) and nicotine dependence (MacKillop et al., 2012).
Despite these promising initial findings, differences in DRD brain activity associated with AUDs are far from definitive. This is, in part, because of limitations and methodological differences in the behavioral paradigms used to date. Generally speaking, two types of DRD tasks have been used in previous studies. For example, Boettiger et al. (2007) used a traditional DRD task comprised of choices between smaller-immediate and larger-delayed rewards at a variety of delay lengths. The focus in that study was choices that were either impulsive (smaller short-term reward) or restrained (larger long-term reward). In contrast, the approach utilized by Claus et al. (2012) and others (Monterosso et al., 2007) used estimates of DRD behavior obtained outside of the scanner to generate a unique set of trials for each participant that ensured roughly equal numbers of trials by some choice type of interest (e.g., easy versus hard choices). Thus, in the first type, the DRD choices are identical across participants and the neural activation associated with different types of decisions can be identified, but the level of difficulty cannot. In the second type, the paradigm maximally clarifies the neural activity associated with conflict during DRD choices, but uses participant-specific stimulus sets that are not well suited to examining differences in impulsive versus restrained decision-making. In the context of AUDs, analyses based on impulsive vs. restrained choices allow for examination of impulsive decision-making profiles that are well-established as being implicated in AUDs (e.g., MacKillop et al., 2011). On the other hand, easy vs. hard comparisons provide a means to examine how DRD decision making and its neural correlates in AUDs are moderated by fluctuations in choice conflict which cannot be readily assayed in behavioral studies.
The goal of the present study was to extend the existing studies on the neuroeconomics of AUDs by examining both of these dimensions of DRD decision-making and dissociating differences attributed to different choice types and level of cognitive conflict. Participants' behavioral responses were classified in terms of impulsive vs. restrained choices and level of choice difficulty in order to provide a comprehensive assessment of the brain systems involved in DRD choices. DRD decisions were hypothesized to elicit activation in a distributed network of brain regions related to decision-making, including the MEPFC, ACC, PPC, insula, and striatum. In particular, more difficult DRD decisions were predicted to recruit greater activation in cortical regions involved in cognitive control (e.g., PFC, ACC). Finally, significant differences were predicted based on AUD status in dorsolateral and inferior PFC (Boettiger et al., 2007; Claus et al., 2012), PPC (Boettiger et al., 2007; Claus et al., 2012), and insula (Claus et al., 2012).
Materials and Methods
Participants
Twenty-six male heavy drinkers were recruited from the community via advertisements. Inclusion criteria were: 1) 21-31 years-old to minimize maturational differences in brain development; 2) alcohol consumption of 21+ drinks/week; 3) right-handed; and 4) fluency with computerized assessments. Exclusion criteria were: 1) seeking treatment for alcohol problems in the past 90 days; 2) currently taking psychotropic medications; 3) current DSM-IV substance use disorder (SUD) other than AUDs or nicotine dependence; 4) any contraindications for MRI scanning; 5) history of serious head injury; and 6) attending session with a positive breath alcohol level (i.e., BrAC > 0.00g%). One participant who completed the protocol was subsequently excluded from all data analysis due to excessive head motion during the MRI scan (i.e., greater than one voxel [3.5mm] in any direction) resulting in a final n = 25. Participant characteristics are presented in Table 1. Of the final sample, thirteen participants met DSM-IV criteria for a current AUD (alcohol dependence, n = 11; alcohol abuse, n = 2). Five participants reported smoking cigarettes, but the overall level of smoking was generally low, with only one participant reporting smoking more than one pack daily (M cigarettes/day = 11.6, SD = 11.1). Illicit drug use was also relatively limited, with four participants reporting weekly marijuana use in the past three months.
Table 1. Participant Characteristics.
| Characteristic | AUD- Mean (SD)/% | AUD+ Mean (SD)/% |
|---|---|---|
| N | 12 | 13 |
| Age | 23.08 (3.34) | 22.00 (1.58) |
| Race | 84% White; 8% Asian; 8% Mixed Race | 77% White; 8% Asian; 15% Mixed Race |
| Ethnicity | 92% Non-Hispanic | 92% Non-Hispanic |
| Education (Years) | 15.58 (1.24) | 14.65 (1.14) |
| Drinks/Week | 28.95 (8.91) | 39.17 (10.85)** |
| AUDIT | 11.67 (2.64) | 17.62 (5.82)** |
Note:
p<.01; AUD+: Met DSM-IV criteria for alcohol use disorder; AUD-: Did not meet DSM-IV criteria for alcohol use disorder; AUDIT: Alcohol Use Disorders Identification Test total score.
Assessment
Eligibility for the MRI scan was assessed using an MRI contraindication questionnaire. Participants completed a comprehensive demographics assessment. Alcohol consumption was assessed using a 28-day Timeline Follow-Back (TLFB) (Sobell and Sobell, 1992). AUD diagnosis was determined via the Structured Clinical Interview for DSM-Research Version (SCID) (First et al., 1995). A continuous measure of AUD severity was operationalized as the symptom count from both the alcohol abuse and dependence SCID modules (e.g., MacKillop et al., 2010). The Alcohol Use Disorders Identification Test (AUDIT) (Saunders et al., 1993) served as an additional index of alcohol misuse. BrAC was measured using a breathalyzer system (Intoximeters, Inc; St Louis, MO).
Delay Discounting Paradigm
Participants completed four event-related imaging runs of a DRD task consisting of dichotomous choices between two monetary rewards that were either available immediately or after a delay. Active items involved choices between a larger delayed reward (LDR; $100 after 1 day, 1 week, 1 month, 3 months, 6 months, or 1 year) and a smaller immediate reward (SIR) that was proportional to the large reward ($10, $20, $30, $40, $50, $60, $70, $80, $90, or $99) and was available today. Control items involved choices between two immediately-available monetary amounts. In total, 120 active and 32 control items were assessed using a pseudorandomized trial sequence (see Supplementary Methods).
Functional Neuroimaging Protocol
Imaging data were collected at the University of Georgia Bio-Imaging Research Center using a GE Signa HDx 3T scanner. Structural imaging used a high-resolution T1 fast spoiled gradient echo scan (voxel size 1 mm3, field of view = 25.6 cm, matrix = 256 × 256, slice thickness = 1 mm). Functional imaging used T2* echo planar imaging (EPI) scans with a gradient echo pulse sequence (TR = 2000 ms, TE = 25 ms, flip angle = 90°, field of view = 22.5 cm, matrix = 64 × 64, voxel size = 3.5 mm3, 40 contiguous 3.5 mm axial slices). Three dummy samples preceded each functional scan to establish equilibrium.
Procedure
Participants appearing eligible after a brief telephone interview were invited for an in-person screen. There, eligible participants were given an overview of the study and those interested were enrolled and provided informed consent. Enrolled participants underwent a separate testing session lasting two hours, with the MRI scan itself lasting 60 minutes. Following the scan, participants received one randomly-selected outcome from their choices made inside the scanner (e.g., Kirby et al., 1999), provided in cash either immediately or after the delay (average amount received = $88). Participants received $15/hour for their time and effort, paid by check approximately three weeks after completion of the study. All procedures were approved by the UGA Institutional Review Board.
Data Analysis
Participants' behavioral data obtained during the scan were examined for validity, consistency across runs, level of impulsive discounting exhibited and reaction times. Behavioral data from all four runs were aggregated and indifference points for each of the six delays were calculated based on each participant's array of responses (see Supplementary Methods). Mazur's (Mazur, 1987) hyperbolic discounting function (k) served as the primary index of impulsivity.
Two separate approaches were used to classify participants' behavioral responses (see Table S2). First, participants' choices were coded as “impulsive” (i.e., choosing the SIR), “restrained” (i.e., choosing the LDR), or “control” (i.e., choosing the larger immediate reward on a control trial). In a separate analysis, active trials were classified based on the putative level of choice conflict. Choices were classified as either “easy” or “hard” based on their proximity to the participant's points of indifference (Monterosso et al., 2007). For each delay interval, choices that were immediately adjacent to the indifference point +/- one choice in each direction were classified as hard choices, and all other choices were classified as easy choices. An alternative analytic approach using a 2×2 factorial design was considered, but was determined to not be viable due to relatively low numbers of items per cell for some participants. Accordingly, impulsive vs. restrained and easy vs. hard were examined in separate analyses that provided for sufficient items per choice type.
Functional imaging data processing and analysis were conducted using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). Preprocessing steps applied to the data are described in Supplementary Methods. First-level analyses of individual brain responses were performed using separate general linear models for the two choice type categorizations with three task-related regressors (impulsive/restrained/control or easy/hard/control) and seven nuisance regressors to account for invalid or missing trials and observed head motion (ΔX, ΔY, ΔZ, roll, pitch, yaw). Estimated hemodynamic response functions (HRFs) were generated for each choice type by convolving the behavioral response time series (response epoch = trial onset to behavioral response) with a canonical HRF (Cohen, 1997). Each GLM also accounted for linear, quadratic, and cubic trends. General linear tests (GLTs) were computed on the resulting β coefficients to quantify activation in each active condition after controlling for baseline visuomotor responses during control trials (e.g., active – control).
Group analysis was performed in several stages. First, β coefficients from each of the active vs. control GLTs were submitted to a voxel-wise one-sample t-test vs. 0, to identify areas of activation or deactivation in each condition. A minimum cluster size of 11 contiguous voxels (> 471 μm) and uncorrected significance threshold of p <.001 was used to assure a family-wise α <.05 (AlphaSim; Ward, 2000). Differential activation by choice type and by AUD status was examined in regions that exhibited significant activation or deactivation in the whole group analysis. Specifically, a disjunction (Boolean “OR” logic) region of interest (ROI) mask was generated that comprised regions that were significant in any of the four choice conditions (impulsive, restrained, easy, or hard). This strategy allows for characterizing the sources of group-wise differences in activation in commonly-recruited regions and been validated in previous fMRI studies of discounting (Ballard and Knutson, 2009; MacKillop et al., 2012). Hypothesis testing was then conducted in SPSS 20.0 (IBM; Armonk, NY) with mean BOLD signal per ROI per individual as the dependent variable. False discovery rate (FDR; Benjamini and Hochberg, 1995) correction was employed to correct for multiple comparisons in these analyses. Finally, for exploratory purposes, voxel-wise t tests were conducted to identify differences between AUD groups that may have been missed in the ROI analysis. Continuous associations between BOLD activity and AUD severity were also examined using zero-order correlations (Pearson's r).
Results
Behavioral Results
AUD+ participants consumed significantly more drinks/week and had significantly higher AUDIT scores (ps < .01), but AUD groups did not differ on any demographic variables assessed (Table 1). Initial screening of behavioral data suggested excellent effort and overall valid task performance. Comparisons between equivalent items across runs revealed no differences (ps > 0.65), supporting the aggregation of DRD choices. Participants provided an average of 39% impulsive responses and 61% restrained choices. In the second analysis, 34% of trials were classified as hard choices and 66% of trials were classified as easy choices.
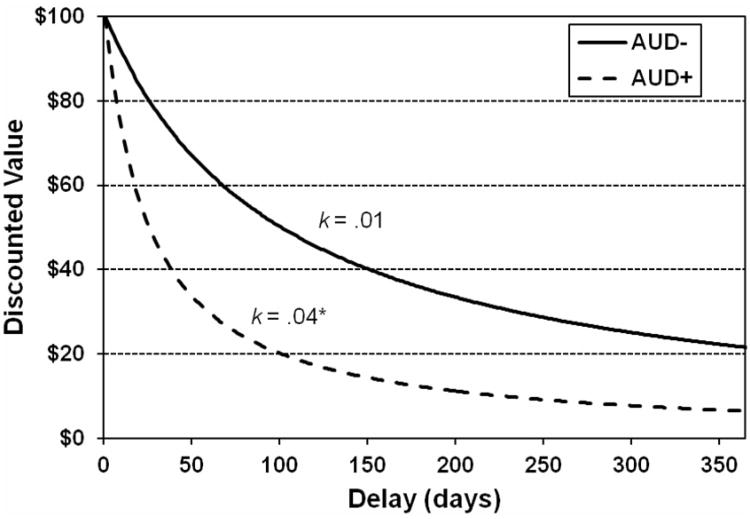
The hyperbolic discounting function provided an excellent fit to the data (median R2 = .92, inter-quartile range = .88 - .96). Compared to the AUD- group, AUD+ participants' discounting rate was significantly steeper, reflecting greater impulsivity, t(23) = 3.15, p < .01 (Figure 1). Discounting rate and AUDIT were significantly correlated (r = .41, p <.05). Impulsive choices were significantly slower than restrained choices and both were slower than control choices (ps < .05). Hard choices were significantly slower than easy choices and control choices were once again the fastest (ps < .001). No significant effects of AUD status on response times were found (ps > .82) (see Figure S2).
Figure 1.
Delay Discounting by AUD Status. Hyperbolic temporal discounting curves corresponding to the average k values for AUD+ (solid line) and AUD- (dashed line) groups. Note: *p < .05; AUD = alcohol use disorder.
Brain Activity during Delay Discounting
Neural activation during the control choices constituted the active baseline. Control trials were associated with activation in a distributed network of decision making and sensorimotor regions, including bilateral PPC, medial frontal gyrus, DLPFC, PCC, insula, and thalamus (Figure 2A; Table S3).
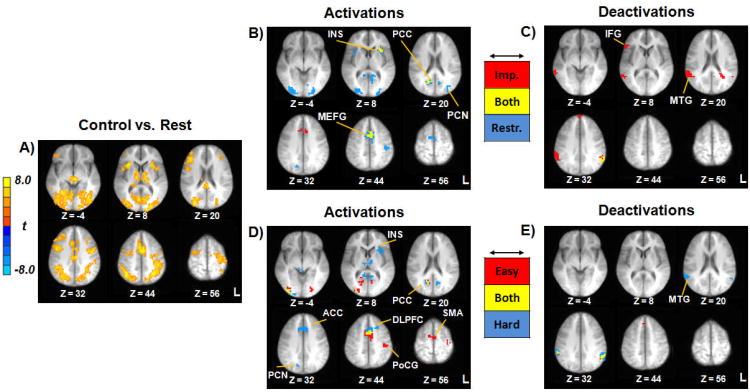
Figure 2.
Neurocognitive Correlates of Delay Discounting. Panel A depicts activation during control trials versus rest. Panels B and C reflect activations and deactivations, respectively, for the impulsive/restrained categorization (red = impulsive choices; blue = restrained choices; yellow = both impulsive and restrained choices). Panels D and E reflect activations and deactivations, respectively, for the easy/hard categorization (red = easy choices; blue = hard choices; yellow = both easy and hard choices). Images thresholded at p < .001; cluster extent 11 voxels. All images shown in radiological convention (Left = Right) with corresponding Z coordinates given in Talairach space. Note: ACC = anterior cingulate; DLPFC = dorsolateral prefrontal cortex; IFG = inferior frontal gyrus; INS = insula; MEFG = medial frontal gyrus; MTG = middle temporal gyrus; PCC = posterior cingulate; PCN = precuneus; PoCG = postcentral gyrus; SMA = supplementary motor area; L = left; R = right.
Activation profiles for each of the four active choice types are depicted in Figure 2. For impulsive/restrained choices, bilateral activation across choice types was present in medial frontal gyrus, anterior insula, and PCC (Figure 2B). Impulsive choices were associated with activation in anterior cingulate cortex (ACC) and restrained choices were associated with bilateral activation in bilateral cuneus/precuneus, postcentral gyrus, and supplementary motor area (SMA) (Figure 2B). Significant deactivation was present in left supramarginal gyrus for both types as well as right IFG, anterior medial frontal gyrus, and bilateral middle temporal gyrus (MTG) on impulsive choices (Figure 2C). Easy choices were associated with bilateral activation in SMA, left postcentral gyrus, and PCC, whereas hard choices were associated with activation in ACC, bilateral DLPFC, right precuneus, bilateral thalamus, and left anterior insula. Hard choices were associated with bilateral deactivation in middle temporal gyrus (Figure 2E).
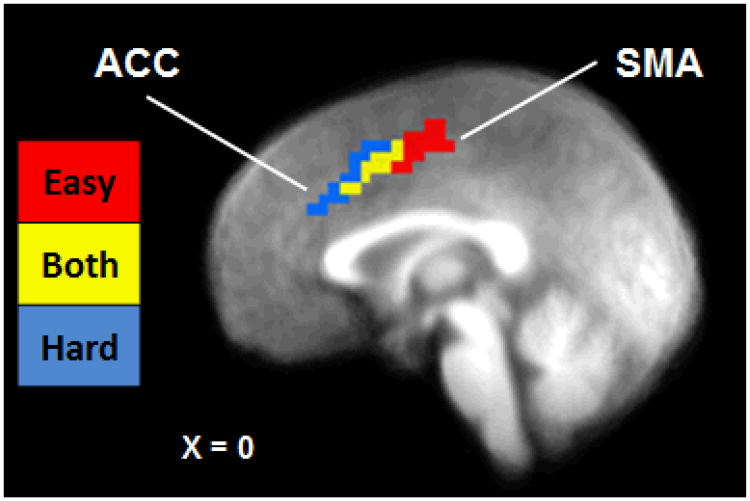
In order to characterize differences between DRD choice types, an empirically-defined ROI analysis was conducted using a disjunction mask comprised of 19 empirically-defined ROIs (see Figure S3 and Table 2). Comparisons between mean BOLD signal per ROI revealed a number of significant differences by choice type (Table 2). Regions that showed significantly greater activation in hard compared to easy choices included anterior insula and bilateral DLPFC, whereas postcentral gyrus was significantly more active during easy choices. Closer examination of the activation observed in bilateral medial frontal gyrus revealed an axis of activation from posterior-dorsal SMA to anterior-ventral ACC (Figure 3). This activation pattern scaled spatially to the level of cognitive conflict, with easy choices associated with SMA activation, both easy and hard choices associated with the intermediate voxels, and hard choices associated with ACC activation. For the impulsive-restrained categorization, the only statistically significant difference between choice types was for inferior parietal lobule, in which greater deactivation was present during impulsive compared to restrained choices. Several other regions showed increased activation during restrained relative to impulsive choices, including precuneus, DLPFC, and middle occipital gyrus, though these differences were not significant after FDR correction.
Table 2. Empirically-defined ROI Analysis by Choice Type.
| Impulsive | Restrained | R vs. I | Easy | Hard | H vs. E | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
||||||||
| Region | BA | Vox | X, Y, Z | M | SE | M | SE | t(23) | M | SE | M | SE | t(23) |
| 1. Medial frontal gyrus | 32 | 257 | 0, 7, 43 | 0.21 | 0.04 | 0.22 | 0.03 | 0.42 | 0.19 | 0.04 | 0.25 | 0.04 | 2.14 |
| 2. Inferior parietal lobule (R) | 40 | 155 | 58, -44, 21 | -0.32 | 0.04 | -0.18 | 0.04 | 3.73a | -0.17 | 0.04 | -0.30 | 0.04 | -3.39a |
| 3. Posterior cingulate (R) | 30 | 116 | 14, -58, 17 | 0.22 | 0.05 | 0.27 | 0.05 | 1.29 | 0.24 | 0.05 | 0.26 | 0.05 | 0.54 |
| 4. Middle occipital gyrus (R) | 18 | 109 | 28, -84, 2 | 0.23 | 0.06 | 0.33 | 0.05 | 2.21 | 0.30 | 0.06 | 0.25 | 0.05 | -0.98 |
| 5. Posterior cingulate (L) | 30 | 84 | -9, -56, 11 | 0.24 | 0.05 | 0.29 | 0.05 | 1.34 | 0.25 | 0.06 | 0.29 | 0.05 | 0.90 |
| 6. Supramarginal gyrus (L) | 40 | 71 | -58, -48, 31 | -0.31 | 0.04 | -0.26 | 0.04 | 1.42 | -0.24 | 0.05 | -0.33 | 0.04 | -2.11 |
| 7. Postcentral gyrus (L) | 3 | 63 | -39, -24, 49 | 0.18 | 0.05 | 0.24 | 0.05 | 1.37 | 0.29 | 0.05 | 0.13 | 0.06 | -3.18a |
| 8. Middle occipital gyrus (L) | 18 | 57 | 23, 84, 0 | 0.17 | 0.05 | 0.28 | 0.05 | 2.56 | 0.24 | 0.05 | 0.21 | 0.05 | -0.57 |
| 9. Anterior insula (L) | 13 | 47 | -29, 15, 10 | 0.22 | 0.04 | 0.22 | 0.04 | -0.04 | 0.16 | 0.04 | 0.27 | 0.04 | 2.71a |
| 10. Brainstem | — | 34 | 2, -30, -11 | 0.17 | 0.04 | 0.13 | 0.04 | -1.46 | 0.11 | 0.04 | 0.18 | 0.02 | 1.96 |
| 11. Thalamus | — | 34 | 5, -14, 7 | 0.16 | 0.03 | 0.15 | 0.03 | -0.53 | 0.13 | 0.04 | 0.17 | 0.03 | 1.78 |
| 12. Anterior insula (R) | 13 | 31 | 33, 13, 10 | 0.19 | 0.04 | 0.21 | 0.04 | 1.09 | 0.17 | 0.04 | 0.22 | 0.04 | 1.42 |
| 13. Inferior frontal gyrus (R) | 45 | 26 | 51, 24, 7 | -0.33 | 0.05 | -0.20 | 0.06 | 2.14 | -0.26 | 0.06 | -0.24 | 0.05 | 0.22 |
| 14. Posterior cingulate (L) | 31 | 22 | -23, -47, 20 | -0.10 | 0.02 | -0.05 | 0.02 | 2.04 | -0.05 | 0.02 | -0.09 | 0.02 | -1.53 |
| 15. Precuneus (L) | 19 | 22 | 30, 75, 24 | 0.15 | 0.05 | 0.24 | 0.04 | 2.18 | 0.19 | 0.05 | 0.20 | 0.05 | 0.30 |
| 16. DLPFC (R) | 8 | 18 | 22, 21, 39 | 0.12 | 0.03 | 0.18 | 0.04 | 2.39 | 0.12 | 0.03 | 0.20 | 0.03 | 3.32a |
| 17. DLPFC (L) | 8 | 18 | -20, 17, 44 | 0.14 | 0.04 | 0.18 | 0.04 | 1.22 | 0.11 | 0.04 | 0.24 | 0.04 | 3.71a |
| 18. Medial frontal gyrus (R) | 8 | 17 | 4, 36, 39 | -0.17 | 0.05 | -0.17 | 0.04 | 0.09 | -0.20 | 0.04 | -0.13 | 0.04 | 1.40 |
| 19. Superior frontal gyrus (R) | 9 | 12 | 2, 56, 33 | -0.84 | 0.13 | -0.51 | 0.15 | 2.26 | -0.58 | 0.16 | -0.62 | 0.12 | -0.26 |
Note.
Paired samples test is significant after FDR correction; DLPFC, dorsolateral prefrontal cortex; BA, Brodmann area; Vox, number of voxels in the cluster; L, left hemisphere; R, right hemisphere.; M/SE, mean and standard error BOLD signal per condition; XYZ coordinates correspond to center of mass in Talairach space.
Figure 3.
Neural Axis of Cognitive Conflict during Easy and Hard Intertemporal Choice Decisions. Sagittal image depicting dissociation of medial prefrontal cortex region identified in the easy/hard analysis (red = easy choices; blue = hard choices; yellow = both easy and hard choices). Image thresholded at p < .001, cluster extent = 11 voxels. X coordinate given in Talairach space. Note: ACC = anterior cingulate cortex; SMA = supplementary motor area.
Differences between AUD+ and AUD- Individuals
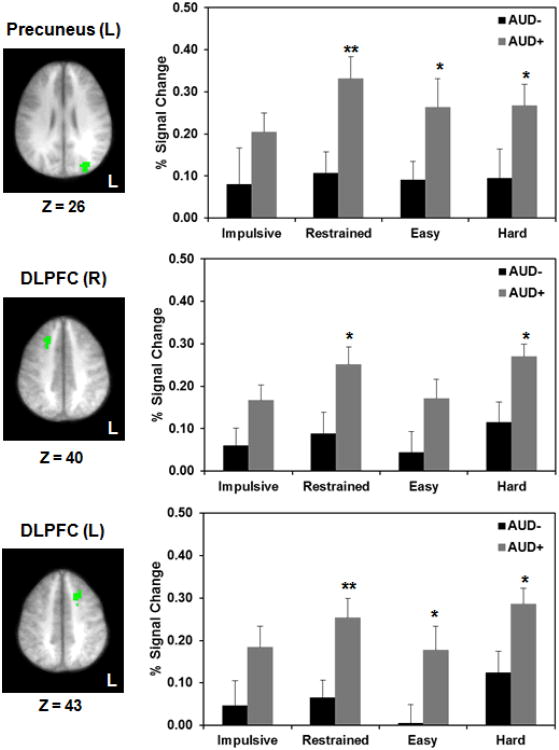
We then conducted group comparisons between AUD+/AUD- individuals in those ROIs that showed task-related differential activation in the whole group analysis (see Table S4). AUD+ individuals exhibited significant hyperactivity compared to AUD- in DLPFC and precuneus on restrained, easy and hard choices, but not impulsive choices (Figure 4). AUD- individuals, on the other hand, had significantly greater deactivation in PCC on impulsive choices and anterior SFG on hard choices.
Figure 4.
Hyperactivity in AUD+ Individuals. Significant differences between AUD groups were found in precuneus (top row), right DLPFC (middle row), and left DLPFC (bottom row). Axial slices depict the location of each region in radiological convention (Left = Right) with corresponding Z coordinates given in Talairach space. Bar graphs depict corresponding mean BOLD activation (+ standard error) per condition for AUD- (black bars) and AUD+ (gray bars). Note. *p<.05; **p<.01; AUD = alcohol use disorder; L = left.
Exploratory voxel-wise comparisons between AUD groups confirmed the significant hyperactivity in DLPFC in AUD+ participants (see Table S5). However, additional clusters of significant AUD-related hyperactivity were found in posterior insula, putamen, and precentral gyrus, among other regions (see Table S5). Significant correlations were found between AUD severity and IFG on impulsive choices (r = .41, p < .05) and IPL on restrained (r = .43, p < .05) and hard choices (r = .51, p < .05). Lower AUD severity was associated with greater deactivation in each of these regions.
Discussion
The primary goal of this study was to clarify the neural basis of impulsive DRD decision-making in relation to alcohol misuse. This is (to our knowledge) the first neuroeconomic study to examine both choice type and cognitive conflict within a single imaging session. The findings provide a number of novel insights into the brain mechanisms responsible for intertemporal choice preferences and differences based on AUD status. By categorizing participants' choices both in terms of their choice type and level of cognitive conflict, we were able to ascertain the profile of brain activity associated with intertemporal choice in general and examine differences based on AUD status.
Neural Mechanisms of DRD
One unique aspect of this study was the use of a DRD paradigm that allowed for dual categorization of participants' choices. Several interesting dissociations between choice types based on delay and difficulty emerged. A profile of brain areas was implicated across all choice types, including a large cluster in medial frontal gyrus and bilateral PCC. Overlapping activation was also observed in right anterior insula. Activation in these regions has been consistently reported in previous DRD studies (Carter et al., 2010) and these results support the notion that these areas are implicated in general aspects of intertemporal choices. Several interesting choice-specific patterns were also found. For instance, hard choices elicited significantly greater activation in DLPFC, as has been reported in previous research (Claus et al., 2012; McClure et al., 2004; Monterosso et al., 2007). DLPFC activation was also increased during restrained choices, although this difference was not statistically significant after application of FDR correction. DLPFC serves a variety of cognitive functions, ranging from executive control to working memory (Miller and Cohen, 2001). In the context of DRD, DLPFC may support the recruitment of working memory to actively maintain and juxtapose information during contemplation between multiple reward alternatives and the exertion of cognitive control over the drives for immediate gratification. However, DLPFC is also implicated in a variety of other cognitive functions that may contribute to DRD behavior, such as selective attention, planning, and coordinating multiple cognitive processes (Stuss and Knight, 2002).
Another important finding from the present study was the distinction between easy and hard choices that was observed in the SMA and ACC. Cognitively demanding hard choices were associated with activation in ACC whereas easy choices were associated with activation in a dorsal posterior region proximal to the SMA. The ACC have been is implicated in monitoring and conflict processing (Kerns et al., 2004), suggesting that, in the context of DRD, this region may be responsible for resolving the inner struggle experienced when making choices between two similarly-valued rewards. The SMA, on the other hand, may be involved in more basic processes, such as motor planning and automatic behavior (Tanji and Shima, 1994) rather than the higher order DRD-related conflict processing per se (McClure et al., 2004). This MEPFC dissociation is particularly interesting when considered along with the results of the impulsive-restrained analysis. In the latter case, similar ACC voxels were found to be primarily activated during impulsive choices whereas SMA voxels were recruited when making restrained choices. This apparent discrepancy has potentially important implications for neuroeconomics studies because it suggests that using only one response coding strategy (i.e., either easy-hard or impulsive-restrained, but not both) may lead to an incomplete picture of brain-behavior relationships. While previous discounting studies have reported activation in a similar ACC-SMA region associated with hard choices (Monterosso et al., 2007), this is the first neuroeconomics study to show the transition from easy to hard along an apparent axis of cognitive conflict.
Significant activation was not observed in the VS as was found in some previous neuroeconoimcs studies (e.g., Carter et al., 2010; McClure et al., 2004). However, not all neuroeconomics studies have observed VS activation during DRD (Boettiger et al., 2009; Hoffman et al., 2008; Monterosso et al., 2007), while other studies suggest that VS activation may be associated with properties of the stimuli themselves (Claus et al., 2011). Finally, ventral portions of the brain are particularly prone to susceptibility artifacts in the MRI signal (e.g., Wilson et al., 2002) and this may have resulted in limited detection power for VS.
Inefficient Neural Processing in AUD+ Individuals
The second primary contribution of the present study is the findings concerning differential activation as a function of AUD diagnosis. Several differences in neural response between AUD groups were found across restrained, easy, and hard choices. As hypothesized, AUD+ individuals showed hyperactivity in regions associated with cognitive control and behavioral inhibition (e.g., DLPFC) and prospective thought (e.g., precuneus). The hypothesized differences in insular or IFG activation were not supported, although anterior insula did differentiate between choice types in the whole-group analysis. These results are consistent with the findings of existing neuroeconomics studies in AUDs (Boettiger et al., 2009; Boettiger et al., 2007). The location of the bilateral DLPFC clusters in this study is similar to the location of the PFC cluster in the Boettiger et al. studies, further underscoring the importance of this region as a consistent locus of AUD-related impairments. The present study extends the work of Boettiger et al. by further clarifying the conditions under which DLPFC activity differs between AUD groups. These results suggest that DLPFC hyperactivity may be most prominent when DRD choices require increasing levels of cognitive control.
Group differences in PPC in the present study are also consistent with Boettiger et al. (2007); however, the location of PPC cluster in this study differs somewhat from previous research. The PPC region in the present study was in a more medial/posterior area near the precuneus—a region that has been consistently implicated in prospective thought and forecasting the future (e.g., Schacter et al., 2007). One interpretation of this result is that AUD+ individuals may be engaging prospective processes to support DRD choices to a greater extent than their non-AUD counterparts. Taken together, these results support the notion that AUD-related hyperactivity may reflect inefficient neural processing in these individuals. Specifically, when behavior was equated across groups (i.e., when the outcome or type of choice was the same), AUD+ participants required additional neural resources to make these decisions. An inefficiency interpretation is also consistent with the findings in previous neuroeconoimcs studies in addiction samples (Hoffman et al., 2008; Monterosso et al., 2007).
Interestingly, the hyperactivity in parietal and prefrontal regions in AUD+ individuals was accompanied by less deactivation relative to AUD- in regions that are part of the brain's so-called “default network” (Buckner et al., 2008). These included clusters in anterior PFC and PCC in the group comparisons and IPL in the continuous analyses. The default network is most active when individuals are not actively engaged in tasks with external demands, and several studies have found these regions to be deactivated during active task-related processing (for a review, see Buckner et al., 2008). One interpretation of the present results is that individuals with AUDs may have an underlying inability to fully disengage certain portions of the default network during DRD choices. As such, they may require additional task-related neural processing (i.e., hyperactivation of prefrontal and parietal cortices) to offset the heightened background activity. Aberrant functional organization of the default network has been found in addiction samples (Ma et al., 2011), which underscores the need for future empirical investigation of this preliminary interpretation.
More broadly, from a clinical standpoint, the evidence of generally greater neural activity during DRD converges with an increasing appreciation of deficits in executive function in alcoholism and other forms of addiction (for a review, see Jarmolowicz et al., in press). Rather than general deficits, however, it appears that specific impulsivity-related aspects of executive function are impaired (e.g., Bickel et al., 2012).
Methodological Considerations
The current findings should be interpreted in light of this study's strengths, limitations and other considerations. First, as noted above, the DRD paradigm used was a strength of the study, but a complete 2×2 factorial analysis of the fMRI data was not feasible due to a limited number of items per cell in a subset of participants. The use of separate analyses for impulsive vs. restrained and easy vs. hard precluded direct comparison between these two categorization schemes. Future neuroeconomic studies may benefit from using a behavioral paradigm that balances high-resolution assessment of the same DRD items across participants with idiosyncratic DRD items that are based on individual's discounting functions (e.g., Monterroso et al. 2007). The former will provide adequate power for comparisons by choice type, whereas the latter will ensure adequate trials for conflict-based analyses.
A second strength of this study was the use of an AUD- group that was very similar to the AUD+ group demographically and was comprised of individuals who were themselves very heavy drinkers. Compared to a non-drinking control group, this active control provided more stringent specifications associated with AUD status. However, in the absence of non-drinking or light drinking control groups, potential differences that might be present between both of the current groups and those lower severity samples cannot be addressed. Participants were also all male and were predominately white, educated young adults. The sample size in this study was relatively small, which may have resulted in decreased statistical power to detect significant differences between choice types and AUD groups, particularly after applying FDR correction. As such, caution is warranted when interpreting these findings and replication of these results in larger AUD samples is of critical importance. Future studies would benefit from including multiple strata of drinkers (e.g., abstinent, light drinkers, heavy drinkers with or without AUDs) to map the continuum of alcohol problems and from also including larger, more diverse samples.
Conclusions
The current study further clarifies the brain systems supporting intertemporal choice behavior in general and in relation to AUDs. The findings suggest that the way that DRD choices are categorized influences the activation profiles obtained and, ultimately, the conclusions drawn about how brain activity relates to DRD behavior. Furthermore, the results indicated that AUDs were associated with significantly greater neural activity in most types of DRD decision-making. This suggests that the capacity to delay gratification may be more cognitively taxing for these individuals. The present investigation provides further support for the neuroeconomic approach to studying addiction. Future work to extend these findings may help clarify the neural deficits underlying impulsive DRD in addicted individuals.
Supplementary Material
Acknowledgments
This study was partially supported by the National Institutes of Health (grants: K23 AA016936, P30 DA027827), the University of Georgia Research Foundation, and a graduate fellowship from the John & Mary Franklin Foundation. Funding sources did not have any role in this study other than financial support. The authors are grateful to Kim Mason, RT(R)(MR) for her assistance with MRI data collection and also contributions by the research assistants and staff of the UGA Experimental & Clinical Psychopharmacology Laboratory.
Footnotes
This work was conducted at the Experimental and Clinical Psychopharmacology Laboratory and the Bio-Imaging Research Center at the University of Georgia, Athens, GA 30602.
Author Contributions: All authors contributed to the study concept and design. MA, JA, and CLB contributed to the data collection. MA performed the analysis of behavioral and fMRI data. JM and LHS assisted with data analysis and interpretation of findings. MA and JM drafted the manuscript. All authors critically reviewed content and approved final version for publication.
References
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend. 2009 doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Berns GS, Bell E, Capra CM, Prietula MJ, Moore S, Anderson B, Ginges J, Atran S. The price of your soul: neural evidence for the non-utilitarian representation of sacred values. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2012;367:754–762. doi: 10.1098/rstb.2011.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, Capra CM, Chappelow J, Moore S, Noussair C. Nonlinear neurobiological probability weighting functions for aversive outcomes. Neuroimage. 2008;39:2047–2057. doi: 10.1016/j.neuroimage.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, McClure SM. Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology (Berl) 2012;221:361–387. doi: 10.1007/s00213-012-2689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Johnson MW. Delay discounting: A fundamental behavioral process of drug dependence. In: Loewenstein G, Read D, Baumeister R, editors. Time and Deision: Economic and Psychological Perspectives on Intertemporal Choice. Russell Sage Foundation; New York: 2003. [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90(1):S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Pitcock JA, Yi R, Angtuaco EJ. Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. J Neurosci. 2009;29:8839–8846. doi: 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Kelley EA, Mitchell JM, D'Esposito M, Fields HL. Now or Later? An fMRI study of the effects of endogenous opioid blockade on a decision-making network. Pharmacol Biochem Behav. 2009;93:291–299. doi: 10.1016/j.pbb.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, Fields HL. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27:14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Carter RM, Meyer JR, Huettel SA. Functional neuroimaging of intertemporal choice models: A review. Journal of Neuroecience, Psychology, and Economics. 2010;3:27–45. [Google Scholar]
- Claus ED, Kiehl KA, Hutchison KE. Neural and behavioral mechanisms of impulsive choice in alcohol use disorder. Alcohol Clin Exp Res. 2012;35:1209–1219. doi: 10.1111/j.1530-0277.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J. User's guide for the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I, Version 2.0.) New York State Psychiatric Institute, Biometrics Research Department; New York: 1995. [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, Mitchell SH. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology (Berl) 2008;201:183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarmolowicz DP, Mueller ET, Koffarnus MN, Carter AE, Gatchalian KM, Bickel W. Executive dysfunction in addiction. In: MacKillop J, de Wit H, editors. The Wily-Blackwell Handbook of Addiction Psychopharmacology. Wiley-Blackwell; Oxford, UK: in press. [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Cavallo DA, Carroll KM, Potenza MN. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Fu XM, Li N, Wang CX, Zhang H, Qian RB, Xu HS, Hu X, Zhang DR. Abnormal brain default-mode network functional connectivity in drug addicts. PLoS One. 2011;6:e16560. doi: 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Wier L, David SP, Ray LA, Bickel WK, Sweet LH. The neuroeconomics of nicotine dependence: A preliminary study of delay discounting of monetary and cigarette rewards in smokers using fMRI. Psychiatry Research: Neuroimaging. 2012;1:20–29. doi: 10.1016/j.pscychresns.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Anderson EJ, Castelda BA, Mattson RE, Donovick PJ. Convergent validity of measures of cognitive distortions, impulsivity, and time perspective with pathological gambling. Psychol Addict Behav. 2006;20:75–79. doi: 10.1037/0893-164X.20.1.75. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug Alcohol Depend. 2009;104:197–203. doi: 10.1016/j.drugalcdep.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Miranda R, Jr, Monti PM, Ray LA, Murphy JG, Rohsenow DJ, McGeary JE, Swift RM, Tidey JW, Gwaltney CJ. Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. Journal of Abnormal Psychology. 2010;119:106–114. doi: 10.1037/a0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analysis of Behavior: Vol 5 The Effects of Delay and Intervening Events on Reinforcement Value. Erlbaum; Hillsdale: 1987. pp. 55–73. [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Luo S. An argument against dual valuation system competition: Cognitive capacities supporting future orientation mediate rather than compete with visceral motivations. Journal of Neuroecience, Psychology, and Economics. 2010;3:1–14. doi: 10.1037/a0016827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford University Press; Oxford; 2002. [Google Scholar]
- Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371:413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000 http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
- Wilson JL, Jenkinson M, de Araujo I, Kringelbach ML, Rolls ET, Jezzard P. Fast, fully automated global and local magnetic field optimization for fMRI of the human brain. Neuroimage. 2002;17:967–976. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.