Abstract
Objective
Alterations in serotonin signalling within the brain–gut axis have been implicated in the pathophysiology of irritable bowel syndrome (IBS) and is a treatment target. Acute tryptophan depletion (ATD) decreases brain serotonin (5-hydroxytryptamine; 5-HT) levels, and increases visceral perception and negative emotional bias in patients with IBS. The aim of the present study was to determine the effect of ATD on brain activity and connectivity during visceral stimuli in healthy women, and to compare the ATD-induced brain connectivity of an arousal circuit in female patients with IBS without ATD.
Methods
12 healthy females (19–25 years) were studied under placebo (PLA) conditions and ATD. Functional MRI measurements were performed during a rectal barostat protocol, consisting of random non-painful and maximal tolerable distensions. Partial least squares analyses and structural equation modelling were used to evaluate the effect of ATD on functional and effective brain connectivity during distension. Results in healthy controls under ATD were compared with the effective connectivity of brain responses to 45 mm Hg rectal distension in 14 female patients with constipation-predominant IBS (IBS-C) (24–50 years).
Results
In healthy controls, ATD resulted in increased response of an extensive brain network to balloon distension, including the amygdala and nodes of emotional arousal and homeostatic afferent networks. The effect was greater during high inflation, suggesting greater engagement of the central serotonion system with more aversive visceral stimuli. Effective connectivity analysis revealed a profound effect of ATD on coupling between emotional arousal network nodes, resulting in loss of negative feedback inhibition of the amygdala. A near-identical pattern was identified in the patients with IBS-C.
Conclusions
The findings are consistent with an ATD-induced disinhibition of and increased connectivity within an emotional arousal network during aversive stimulation. Together with the previous demonstration of ATD-induced visceral hyperalgesia in healthy controls, and the near-identical effective connectivity pattern observed in patients with IBS-C, these findings suggest that dysregulation of this brain network may play a role in central pain amplification and IBS pathophysiology.
INTRODUCTION
Preclinical and clinical evidence implicates the serotonin (5-hydroxytryptamine; 5-HT) signalling system as an important modulator of the brain–gut axis, and this signalling system may be altered in irritable bowel syndrome (IBS).1 For example, drugs aimed at modulating the activity of 5-HT selectively (selective serotonin reuptake inhibitors, 5-HT3 and 5-HT4 receptor antagonists) or both 5-HT and norepinephrine (NE) systems (non-selective reuptake inhibitors and tricyclic antidepressants) have been used in the treatment of functional gastrointestinal (GI) disorders, as well as in other chronic pain conditions and psychiatric syndromes. For example, 5-HT3 receptor (5-HT3R) antagonists have been demonstrated to be one of the most effective treatments for patients with diarrhoea-predominant IBS (IBS-D) and have shown effectiveness in co-morbid chronic pain disorders.2 Finally, the 5-HTTLPR polymorphism, and polymorphisms in 5-HT3R subunit genes have been implicated as a vulnerability factor for this group of functional pain disorders.3–5
Even though only 5% of the total 5-HT is located in the central nervous system (CNS; primarily the raphe nuclei and their widespread cortical and spinal projections), the raphe 5-HT system plays an important role in state-dependent regulation of emotional state, pain sensitivity and autonomic activity. In addition, there are close interactions of the central NE and 5-HT systems in modulating limbic brain and cortical brain regions.6 Low brain 5-HT levels have been implicated in various types of psychopathology, including depression and anxiety, and sex differences in brain 5-HT levels and 5-HT synthesis may explain the greater susceptibility of women to stress-related disorders.7,8 Tonic serotonergic input to the amygdala and locus coeruleus complex (LCC; the major noradrenergic nucleus in the brain) are thought to have predominantly inhibitory influences on regions of emotional arousal and stress circuits in the brain, and disturbances in these systems are central to major psychiatric disorders of mood and affect, and to chronic pain disorders.9,10
Acute tryptophan depletion (ATD) is a validated method to temporarily reduce 5-HT synthesis in the CNS (and presumably within the gut’s enterochromaffin cells) by decreasing the availability of its precursor tryptophan through the administration of an amino acid mixture lacking tryptophan. Following ATD, a substantial reduction of brain 5-HT synthesis as well as decreased levels of tryptophan have been demonstrated in humans.8,11 ATD increased haemodynamic brain responses to emotional words, and attentional bias towards negative stimuli was strongly associated with resting state blood flow in the right amygdala.12 ATD has been used to investigate the role of the central 5-HT system in healthy control subjects, in patients with affective disorders (depression and anxiety) and in their first-degree relatives.13–15
The reported effects of acute lowering of brain 5-HT levels using ATD on the perception of rectal distension stimuli vary between studies, with some showing increased perception of a rectal stimulus and an increased memory bias, while others showed no effect.16,17 Acute increases of synaptic 5-HT levels by administration of the 5-HT uptake inhibitor citalopram had no effect on the perception of rectal balloon distension in a mixed sample of patients with IBS-D, even though it positively affected emotional memory bias, while reducing perception of upper GI distension in healthy controls.18,19 Based on our earlier findings of enhanced visceral perception during ATD, we aimed to test the general hypotheses that (1) the intervention in healthy women results in an increased engagement of an emotional arousal circuit, including the amygdala, during experimental visceral stimulation and (2) that the induced changes are similar to those seen in female patients with IBS without any dietary interventions. To test this hypothesis, we applied functional and effective connectivity analysis to BOLD (blood oxygen level-dependent) responses acquired during low and high distension in healthy female subjects, during ATD and placebo (PLA), and compared the findings with those observed in female patients with constipation-predominant IBS (IBS-C).
METHODS
Subjects
Twelve right-handed healthy females (19–25 years) were included in the ATD protocol, and 14 additional female patients with IBS-C (24–50 year) were studied without ATD or placebo. To ensure hormonal stability and to avoid possible effects of the menstrual cycle, the subjects who did not use oral contraceptives were studied during the follicular phase of the menstrual cycle.20 Female subjects were studied because (1) the prevalence of IBS is higher in females than in males21; (2) the rate of central 5-HT synthesis is lower in females than in males8; and (3) woman are more prone to the effect of ATD.22
Exclusion criteria in both groups comprised abdominal surgery (other than appendectomy and cholecystectomy), use of medications other than oral contraceptives within 14 days prior to testing, and lactose intolerance. In the healthy control group, a positive first-degree psychiatric family history, any history of psychiatric disease or use of psychoactive medication, or Hamilton Depression Scale (HADS) scores ≥8 were also exclusion criteria.23–25 The Medical Ethics Committee of the University Hospital Maastricht approved the study protocol and all subjects gave their informed written consent before the start of the study. Differences in HADS scores between the groups were determined using an unpaired t test.
Design
ATD study in healthy controls
The funcional MRI (fMRI)-barostat protocol comprised the measurement of brain responses to rectal non-painful stimuli and maximal tolerable stimuli in a placebo-controlled double-blind randomised crossover design under a PLA condition and an ATD condition, respectively. The two test days were separated by at least 1 week. On the fMRI test days subjects ingested an amino acid mixture at T=0 h. At T=4 h the fMRI-barostat experiment was started.
Acute tryptophan depletion
The amino acid mixtures were prepared by the department of Pharmacy of the University Hospital, as previously described.24 After an overnight fast, the subjects consumed the amino acid mixtures dissolved in 250 ml of water as quickly as possible, using a nose clamp to avoid the unpleasant smell of the mixtures. Water, decaffeinated tea and peppermint were available to neutralise the unpleasant taste. Tryptophan (TRP) and the ratio TRP/ΣLNAA (sum of the large neutral amino acids tyrosine, leucine, phenylalanine, isoleucine and valine) were measured at T<0 and T=4h, using high-performance liquid chromatography (HPLC).26
Determination of perception thresholds: barostat procedure
The determination of the non-painful distension pressures (low inflation (INF)) and maximal tolerable pressures (high INF) are described in the Supplementary data.
Rectal distension during fMRI scanning
High INF and low INF distension pressures were applied by a barostat, which was placed outside the MRI room, by means of long tubes (10 m), which were guided through the wave-guide of the MRI room wall. The rectal barostat stimulation/distension protocol consisted of six low INF distensions and six high INF distensions, respectively, in random order. Each distension lasted 30 s and was interleaved with a 30 s rest (0 mm Hg) interval. The distensions were presented in a block design. Epochs of eight scans during rectal distension were followed by eight scans without rectal distension (rest). This sequence was repeated up to the total of 202 scans.
Subjective ratings during fMRI scanning
No perceptual stimulus ratings were acquired during the MRI scanning protocol because we only applied two different rectal stimuli (high INF and low INF), which makes perceptual ratings unreliable. Moreover, it is not clear whether perceptual stimulus ratings obtained during MRI scanning are reliable, reproducible and comparable with a condition outside the MRI magnet.
fMRI data acquisition
See Supplementary data.
fMRI data processing and analyses
See Supplementary data.
Functional connectivity analysis
See Supplementary data.
Effective connectivity
Effective connectivity analysis examines neural interactions between regions simultaneously to explicitly quantify the effect brain structures exert on one another in a specific brain circuit or network and provides a means to test hypotheses regarding neural circuits/network functioning. This approach is hypotheses driven and restricts inferences to specific circuits or networks comprised of a limited number of brain regions.
Effective connectivity analysis of the ATD data was performed using path analysis within a structural equation modelling framework. Given expectations of engagement of a known emotional arousal network27–29 that includes the amygdala, rostral anterior cingulate cortex (rACC), infragenual ACC (iACC), orbital medial prefrontal cortex (omPFC) and dorsal pons/LCC (dpons/LCC),27–29 effective connectivity analysis was applied to quantify the neural activity in this specific emotional arousal network and to test for hypothesised differences induce by ATD (see figure 1). Literature searches from Pubmed (http://www.ncbi.nlm.nih.gov/entrez) and the Collations of Connectivity on the Macaque Brain (CoCoMac) database (http://www.cocomac.org) support the designated anatomical projections among the nodes of this network.30 The activity in the brain regions selected a priori and comprising the emotional arousal circuit were represented by the voxel loading most reliably on the latent variable extracted from the spatiotemporal partial least squares (st-PLS). Given the time-dependent response of brain regions across the 26 s temporal window of inflation, we chose to quantify early response in this network averaging data from the first 8 s of the response (lag 0, lag 1) for entry into the analysis. Residual variances, representing external input into the system (eg, unspecified regions, psychological characteristics, hormonal milieu), were fixed at 35% of the observed regional variances within group and condition.31 ATD-induced differences in the effective connectivity of the network were tested using multigroup tests for invariance.32 Differences in each path in the model were calculated using one degree of freedom χ2 statistics for group. Critical ratios for path coefficients, representing the coupling between regions, were interpreted as significant at p<0.05.
Figure 1.
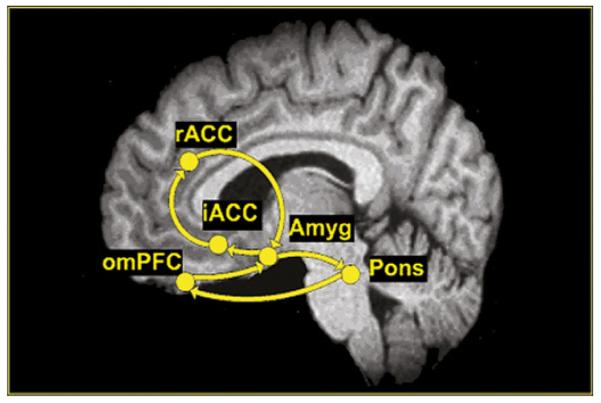
Hypothesised emotional arousal network. Anatomical projections among the nodes of the emotional arousal network are represented by unidirectional arrows. The model is based on Pezawas et al,27 Labus et al28 and Stein et al.29 Amyg, amygdala; iACC, infragenual cingulate cortex; omPFC, orbital medial prefrontal cortex; sACC. supragenual anterior cingulate cortex.
Study in IBS-C
Brain responses to 5, 25 and 45 mm Hg rectal distension were assessed by fMRI. Subjects completed four runs comprising five 45 mm Hg inflations, five 25 mm Hg inflations and five 5 mm Hg inflations presented in semi-random order. Each trial consisted of 18 s before balloon inflation, followed by 15 s of inflation at the designated pressure. The inflations were cued by a light 3–5 s before inflation. The tube length was ~10 m.
fMRI data acquisition
See Supplementary data.
Effective connectivity
As described above, path analysis was used to characterise the effective connectivity of the previously described emotional arousal network during the 45 mm Hg inflation in patients with IBS. Voxels or nodes of the network best representing the regions comprising the network (amygdala, rACC, iACC and omPFC) were identified from the results of both a task and seed PLS. See Supplementary data.
RESULTS
Subject characteristics
Twelve healthy female volunteers (mean age 22.7±0.5 years) participated in the ATD study, and 14 female patients with IBS-C (mean age 36.1±8.1 years) were also studied. In the ATD study, nine subjects were on oral contraceptives. In the IBS-C study, one subject was postmenopausal and five were on oral contraceptives. HADS anxiety (mean±SD) for the IBS subjects was: 7.4±3.1. HADS depression for the IBS subjects was: 3.4±2.6. The IBS group had three subjects with a score above 8 on HADS anxiety. HADS anxiety for the healthy subjects was: 2.3±2.2. HADS depression for the healthy subjects was: 0.3±0.7. The differences between the groups were significant for both anxiety and depression (p<0.01).
ATD study
Rectal distension pressures
Individual distension pressures required to generate a subjective sensation of urge to defecate (low INF) or maximal tolerable pressure (high INF) are shown in figure 2. The mean values for low and high INF were 19.6±6.6 and 52.1±12.2 mm Hg, respectively.
Figure 2.
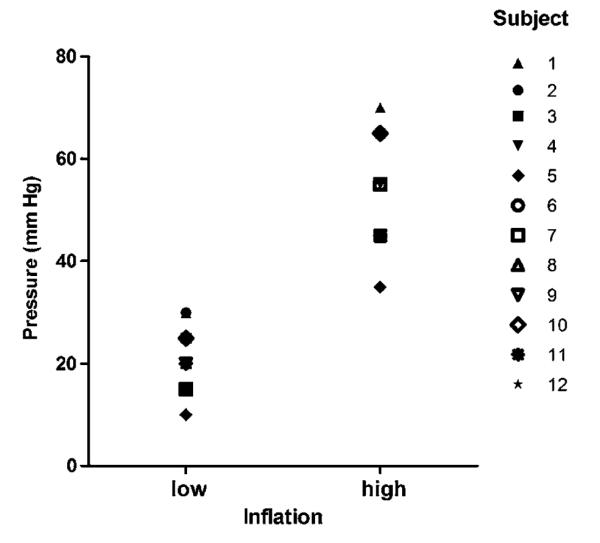
Individual rectal pressure values required to produce a sensation of mild urge (low) and maximal tolerable (high) during controlled rectal distension. Despite interindividual variation of individual thresholds, there was no overlap between low and high ratings.
Validation of ATD
No significant baseline differences between ATD and PLA were found for plasma tryptophan levels (34.4±1.3 and 37.3±1.8 μmol/l, respectively, p=0.21) and the ratio TRP/ΣLNAAs (0.15±0.02 and 0.16±0.05, respectively, p=0.17). The amino acid analysis showed that the depletion was successful. During ATD, plasma tryptophan levels (14.4±1.1 μmol/l) and the TRP/ΣLNAAs ratio (0.02±0.01) decreased significantly (58% and 85%, p<0.001 and p<0.001, respectively), compared with baseline values. Values are given as the mean±SEM.
Effect of ATD on brain responses to rectal distension
Functional connectivity
St-PLS revealed a significant (p<0.01, 73% variance) network (pattern) of brain regions, which showed an interaction between group (ATD, PLA) and condition (low, high INF) (figure 3). Although during both PLA and ATD there were differences in brain activity due to the level of balloon inflation, these distension-related differences were significantly enhanced during ATD.
Figure 3.
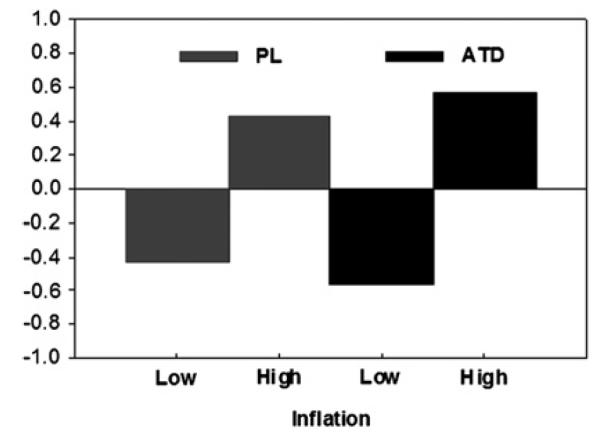
Plot of the linear contrast weights (or design saliences) for the significant latent variable of the brain network. The y-axis represents the numerical weights for the contrast. Inflation conditions for placebo (PL) and during acute tryptophan depletion (ATD) are shown on the x-axis. The design saliences indicate that the network is characterised by an interaction between group (ATD, PL) and condition (low, high inflation). Although differences in brain activity due to the level of balloon inflation were observed in both groups, these differences were greater during ATD compared with PL. For details of the analysis, see the Methods section.
Because ATD resulted in strong effects in a large number of brain regions during INF, we summarise the main results below with a focus on the homeostatic afferent, emotional arousal, attentional and cognitive modulatory regions hypothesised to be key regions in response to interoceptive stimuli.33 A detailed cluster report summarising the latent variable is presented in Table 1 in the Supplementary data.
In general, during high INF (compared with low INF), the ATD-sensitive network comprised statistically reliable activations within regions of the homeostatic afferent network (dorsal ACC subregions (rACC and mid-cingulate cortex (MCC)), insula subregions (anterior and posterior insula (a/p INS) and thalamus) and the emotional arousal network (pons/LCC), amygdala). Additional regions included the cerebellum and the substantia nigra/ventral tegmental area. Less activity in the ATD-sensitive network was observed during high INF as compared with low INF in prefrontal regions (dorsolateral PFC (BA 9), omPFC (BA 11)), and in regions of the default network (posterior cingulate cortex (PCC) and ACC) (Table 1, Supplement).
ATD affects brain responses preferentially during high INF
While most regions generally demonstrated sustained activity, higher activity during ATD as compared with PLA was observed transiently in the amygdala, a key brain region in the emotional arousal network, and the thalamus (which is part of the homeostatic afferent rather than the emotional arousal network). As this transient activation was only observed during the first 4 s (first scan) of the brain’s response to balloon inflation, we examined the effective connectivity of the emotional arousal network only during the initial part of the response (first two scans, ~8 s). Activity for the regions comprising the emotional arousal network was extracted from the most reliable (ie, representative) voxels contributing to the latent variable extracted by the task st-PLS (table 1).
Table 1.
Spatial location of voxels in MNI space of the voxel identified from the the spatiotemporal partial least squares and considered as representative nodes for the emotional arousal network regions
| Network node | X | Y | Z |
|---|---|---|---|
| Amyg | −16 | 2 | −20 |
| Pons | −6 | −32 | −18 |
| rACC | −4 | 20 | 30 |
| iACC | −2 | 20 | −6 |
| omPFC | −16 | 54 | −16 |
Amyg, amygdala; iACC, infragenual anterior cingulate cortex; omPFC, orbital medial prefrontal cortex; rACC, rostral anterior cingulate cortex.
Effective connectivity
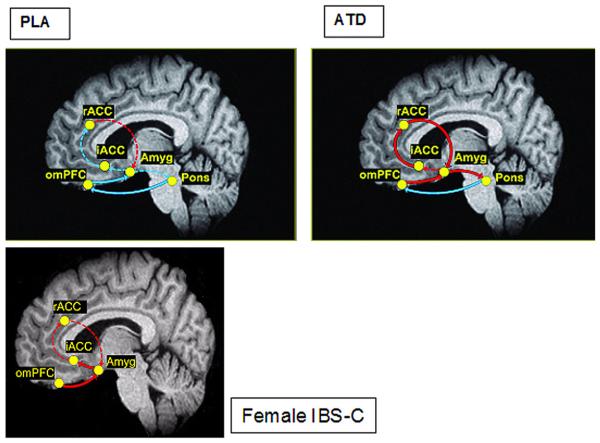
Effective connectivity analyses revealed that during high INF, subjects receiving ATD (compared with PLA) exhibited a stronger engagement of the emotional arousal circuitry (table 2, figure 4). For example, during PLA, a significant negative connectivity from the pons to the omPFC (β=−2.44), and from the omPFC to the amygdala (β=−0.23) was observed. The connectivity between these regions was altered during ATD—for example, the negative connectivity from the pons to the omPFC was greatly reduced (β=−0.61, Δχ2=5.6, p<0.05), and that from the omPFC to the amygdala became positive (β=0.17, Δχ2=5.6, p<0.01). In addition, during ATD, many more nodes of the emotional arousal circuitry were engaged, including: amygdala to the pons (β=0.90), rACC to the amygdala (β=1.20), and pons and iACC to the rACC (β=0.43). χ2 Tests for differences between PLA and ATD during high INF indicated that connectivity was significantly altered in all but one connection (amygdala to iACC). Specifically, the emotional arousal circuitry was significantly and more positively engaged during ATD (iACC to rACC, Δχ2=4.0, p<0.05, rACC to amygdala, Δχ2=6.3, p<0.05, and amygdala to pons, Δχ2=6.8, p<0.01).
Table 2.
Results of the effective connectivity analysis for the ATD study
| PLA |
ATD |
||||||
|---|---|---|---|---|---|---|---|
| Emotional arousal circuits | β | SE | β | SE | Δ χ 2 | ||
| omPFC | → | Amyg | −0.23 | 0.11 | 0.17 | 0.08 | 5.6 |
| Amyg | → | iACC | −0.04 | 0.17 | 0.07 | 0.11 | 0.2 |
| iACC | → | rACC | −0.15 | 0.25 | 0.43 | 0.15 | 4.0 |
| rACC | → | Amyg | 0.27 | 0.31 | 1.20 | 0.21 | 6.0 |
| Amyg | → | Pons | −0.75 | 0.44 | 0.90 | 0.10 | 6.8 |
| Pons | → | omPFC | −2.44 | 0.66 | −0.61 | 0.19 | 6.3 |
β Values (path coefficients) represent coupling between network regions and demonstrate differences in the effective connectivity between PLA and ATD. The χ2 difference statistic quantifies the differences in connectivity between PLA and ATD. χ2 difference values in bold are significant at p<0.05. Path coefficients were interpreted as significant at p<0.05 and are in bold in the table.
Amyg, amygdala; ATD, acute dietary tryptophan depletion; iACC, infragenual anterior cingulate cortex; omPFC, orbital medial prefrontal cortex; PLA, placebo; rACC, rostral anterior cingulate cortex; Δχ2, χ2 difference statistic.
Figure 4.
Estimated effective connectivity of the initial response in the hypothesised emotional arousal circuit during high inflation pressure with placebo (PLA) and acute tryptophan depletion (ATD) and in patients with constipation-predominant irritable bowel syndrome (IBS-C). The β coefficients (calculated in effective connectivity analysis) are depicted by the style and colour of the arrows. Solid arrows represent a parameter estimate that was considered statistically significantly different from zero, whereas dashed lines represent non-significant coefficients. Red arrows represent positive coupling whereas blue arrows represent negative coupling. The magnitude of the coefficients associated with each thickness is depicted. Amyg, amygdala; iACC, infragenual cingulate cortex; omPFC, orbital medial prefrontal cortex; sACC, supragenual anterior cingulate cortex. For details of the analysis, see the Methods section. For actual values of β coefficients, see tables 2 and 4.
Distension study in patients with IBS
Representative voxels of the emotional arousal network identified in the PLS analyses are presented in table 3. Effective connectivity analysis of brain activity during 45 mm Hg distension in patients with IBS-C revealed a similar pattern of connectivity to healthy controls receiving ATD (table 4, figure 4). For example, nodes positively engaged during ATD were also positively engaged in IBS-C, including: omPFC to amygdala (β=0.19) and amygdala to iACC (β=0.49).
Table 3.
Spatial location of voxels in MNI space identified from the partial least squares analysis of patients with IBS-C and considered as representative nodes for the emotional arousal network regions
| Networknode | X | Y | Z |
|---|---|---|---|
| Amyg | 24 | −8 | −20 |
| rACC | 10 | 34 | 32 |
| iACC | 4 | 30 | −6 |
| omPFC | 4 | 32 | −22 |
Amyg, amygdala; iACC, infragenual anterior cingulate cortex; IBS-C, constipation-predominant irritable bowel syndrome; omPFC, orbital medial prefrontal cortex; rACC, rostral anterior cingulate cortex.
Table 4.
Results of the effective connectivity analysis for patients with IBS-C
| IBS-C |
||||
|---|---|---|---|---|
| Emotional arousal circuits | β | SE | ||
| omPFC | → | Amyg | 0.19 | 0.08 |
| Amyg | → | iACC | 0.49 | 0.21 |
| iACC | → | rACC | 0.17 | 0.11 |
| rACC | → | Amyg | 0.174 | 0.28 |
β values (path coefficients) representing the coupling between regions were considered significant at p<0.05 and are in bold in the table.
Amyg, amygdala; ATD, acute dietary tryptophan depletion; iACC, infragenual anterior cingulate cortex; IBS-C, constipation-predominant irritable bowel syndrome; omPFC, orbital medial prefrontal cortex; PLA, placebo; rACC, rostral anterior cingulate cortex; Δχ2, χ2 difference statistic.
DISCUSSION
During controlled rectal balloon distension, acute lowering of central and peripheral 5-HT concentrations by ATD in healthy women without any personal or family history of psychiatric or GI symptoms resulted in an increased response of an extensive network of brain regions, all of which receive serotonergic projections from the dorsal and medial raphe nuclei.34,35 Using an identical ATD protocol, we have previously shown that acute lowering of 5-HT can enhance visceral perception in healthy women, which may be in part influenced by an ATD-mediated increase in emotional response bias.16
In the current study, the observed ATD effect was greater during an inflation pressure close to the tolerance threshold (high INF), suggesting a greater engagement of the central 5-HT system with more aversive visceral stimuli. Effective connectivity analysis revealed a profound effect of ATD on the coupling between nodes of an emotional arousal network, consistent with a loss of feedback inhibition of the amygdala, and an ATD-related disinhibition of the entire network. Despite slightly different experimental conditions and analytical techniques, nearly identical changes in the same circuit were observed in female patients with IBS-C, without ATD. Together, these findings suggest that alterations in serotonergic modulation of an emotional arousal circuit may play a role in centrally mediated visceral hypersensitivity in IBS.
In the following, we will discuss these findings in the context of the ATD literature, and in regards to the concept of increased activity of emotional arousal circuits in the pathophysiology of IBS.
ATD effect on functional connectivity of brain regions during visceral stimulation
Using multivariate analysis techniques, we identified an extensive network of brain regions which differentiated low and high INF conditions, with greater engagement of this network during ATD, compared with PLA. The ATD-sensitive network included (but was not limited to) brain regions involved in the processing of interoceptive information, including INS, dACC and thalamus, as well as key regions of an emotional arousal circuit, including the amygdala and ACC subregions. These findings are consistent with a recent report by Roiser et al showing differentially affected BOLD responses in healthy controls in an ‘extended visceromotor network’ which participates in the processing of emotional information.12,36 The fact that the ATD effect was more pronounced during the high INF condition suggests that in the healthy brain, ascending 5-HT pathways arising from pontine serotonergic nuclei show greater engagement with more painful signals from the viscera.
The approach of acutely reducing the activity of the brain 5-HT signalling system by diet-induced tryptophan depletion is a well-established technique to study the role of 5-HT signalling in the brain. It has been used previously in healthy controls, in patients with major depression and in patients with IBS during different types of experimental stimuli.9,16,17,37,38 ATD has been validated by demonstrating that the technique in healthy controls was associated with a marked reduction in 5-HT synthesis and a reduction of 5-HT metabolites in the cerebrospinal fluid (CSF) consistent with lowering of brain 5-HT concentrations.8,11 Similarly, as already demonstrated previously by others, we showed in the current study that the dietary manipulation resulted in a significant lowering of plasma tryptophan levels, and the TRP/ΣLNAA ratio, the best peripheral index of tryptophan availability to the brain for 5-HT synthesis.12,16,39
In general, biological effects of ATD, including those on brain activity and on neurotransmitters in the CSF, have been more consistent than effects on subjective measures, such as mood, perceptual or pain ratings. For example, a recent fMRI study in healthy women showed that ATD greatly increased brain haemodynamic responses to emotional words even though no effect on subjective mood ratings was observed.12 One of the most consistent findings in healthy controls, and in patients with depression and those with IBS has been the increase in negative emotional bias.12,16,40 Even though perceptual responses were not measured in the current study, greater ATD-induced responses within interoceptive brain regions, in particular the aINS, during an aversive rectal distension would be expected to be associated with increased perceptual ratings of such a stimulus. In support of this expectation, we have previously shown that ATD in healthy controls resulted in increased pain perception at higher distension pressures, making them more similar to ratings by patients with IBS.16
ATD affects effective connectivity within an emotional arousal network
Consistent with the close interaction of the 5-HT system with central stress circuits, ATD resulted in higher corticotrophin-releasing factor (CRF) levels compared with a control condition, without an associated change in subjective mood.9 Positive feedback regulation between the pontine noradrenergic LCC and the amygdala plays an important role in central CRF release, and both LCC and amygdala activity are modulated by 5-HT pathways.6,41,42 For example, 5-HT3Rs, the target of a novel, effective IBS drug, are found presynaptically and postsynaptically, and activation can facilitate the release of a variety of excitatory and inhibitory neurotransmitters.43 We have previously demonstrated reductions in amygdala and brainstem activity during 5-HT3R antagonism in patients with IBS during expectation of an aversive rectal stimulus, consistent with a role for these receptors in mediating the release of an excitatory neurotransmitter in these brain regions.44 It has been suggested that the connectivity within an emotional arousal network including the amygdala, and infragenual and supragenual subregions of the ACC may be in part determined by 5-HT mechanisms, since healthy controls who are s carriers of the 5-HTTLPR gene polymorphism showed evidence for structural changes within these regions, and alterations in functional connectivity between two nodes of this circuit.27,5 In the current study, ATD resulted in a profound change in nearly all connections between the nodes of this arousal circuit during the initial stages of the rectal distension, in particular in the pathways from omPFC and rACC which under normal conditions provide inhibitory input to the amygdala. Inhibitory input from these regions was either lost or weakened (became more positive) by ATD in healthy controls. These changes would be expected to result in greater amygdala activation, as observed during the high INF condition. The pattern of connectivity observed in healthy controls after ATD were nearly identical to the pattern of connectivity observed in a group of women with IBS-C, who were studied without ATD or PLA. These findings are consistent with the hypothesis that the increased engagement of this circuit in patients with IBS is in part related to reduced serotonergic modulation.
Limitations of the study
No simultaneous recording of perceptual ratings during MRI scanning were made because we only used two different distension pressures (high INF and low INF) which makes determination of differences in perceptual ratings unreliable. Moreover, litte is known about the reproducibility of perceptual ratings during MRI scanning. However, we have previously demonstrated the effect of ATD on perceptual ratings of the same rectal stimulus in healthy women. We showed mild but significant effects of ATD in the highest distension pressures (painful distensions).16 Individual distension pressures to elicit mild urge and tolerance, respectively, were determined in an interval between 2 and 14 days prior to the fMRI procedure. It is conceivable that perceptual sensitivity may have changed during the time of the threshold determination and the fMRI study. However, perception thresholds in healthy controls are fairly stable between two distension sessions and, given the lack of overlap of distension pressures for the low and high INF condition, it is highly unlikely that individuals had changed their subjective ratings sufficiently to be given an inappropriate stimulus intensity during the two conditions.45 Our subjects were not characterised in terms of trait anxiety, and no genetic information on the serotonin transporter gene (SERT) polymorphism in participants was obtained. Given the limited sample size, it is conceivable that over-representation of one of these subgroups may have influenced our results. A possible effect of ATD on mechanoelastic properties of the rectum cannot be ruled out, which in turn might indirectly affect brain responses to rectal distension. An increase in 5-hydroxyindoleacetic acid metabolites with the ATD procedure has previously been reported, consistent with a lowering of peripheral 5-HT concentrations. Such peripheral effects would be expected to affect the gut-based 5-HT signalling system, which plays an important role in mediating and modulating intestinal reflexes and motility, in addition to providing a major input to subsets of vagal afferents to the brain.1 However, we have previously demonstrated that pressure–volume relationships before and after ATD were not different in healthy subjects.16 These findings are consistent with the known greater effect of ATD on the availability of tryptophan in the brain, in particular the cortex, compared with peripheral tissues.46
The two groups (healthy controls and patients with IBS) were studied under slightly different experimental conditions and analysed by slightly different approaches. However, despite these differences, findings in the two groups are remarkably similar, and consistent with earlier results using the connectivity model.28 Finally, functional connectivity can be modulated by experimental conditions and it is unknown how the observed differences in connectivity during rectal distension relate to potential differences in resting state connectivity.
Summary and implications for IBS pathophysiology
Acute lowering of central (and peripheral) 5-HT availability in healthy women, which has previously been shown to result in enhanced visceral perception, is associated with changes in a large number of brain regions, including an emotional arousal circuit during rectal distension. Nearly identical changes were seen in a group of female patients with IBS-C. Female patients with IBS have previously been shown to have greater amygdala activation and greater engagement of the emotional arousal circuit during the expectation of an aversive rectal stimulus when compared with male patients,28,47 and the BOLD responses to expectation of such a stimulus in emotional arousal regions were correlated with altered brain responses to the actual distension.48 Furthermore, differences in rectal distension-induced brain responses between female patients with IBS and healthy controls were highly correlated with anxiety ratings.49 Pharmacological reduction in the activity of nodes of the emotional arousal circuit with a 5-HT3R antagonist, which has anxiolytic properties, has previously been shown to be associated with IBS symptom reduction.44 Hence, altered processing and perception of visceral afferent signals in patients with IBS appears to be closely correlated with central mechanisms of anxiety. When viewed together, our findings suggest that acute lowering of central 5-HT concentrations in healthy women has an IBS-like effect both on brain responsiveness in emotional arousal regions and on the perception of visceral stimuli.
One may speculate that acute or chronic variations in central 5-HT concentrations may play a role in IBS symptom generation/exacerbation, in particular in female patients. Given the lower brain 5-HT synthesis that has been described in women, and the lower serotonin transporter expression that has been postulated in carriers of the s-allele of the 5-HTTLPR polymorphism, female s carriers may be particularly susceptible to developing IBS symptoms under conditions of low 5-HT availability.8
Supplementary Material
Significance of this study.
What is already known about this subject?
▶ The serotonin (5-hydroxytryptamine; 5-HT) signalling system plays an important role in modulating brain–gut interactions.
▶ Pharmacological manipulation of the 5-HT signalling system has a beneficial effect on irritable bowel syndrome (IBS) symptoms.
▶ Acute tryptophan depletion (ATD) is a validated technique to acutely lower central and peripheral 5-HT concentrations.
▶ ATD in healthy women is associated with an increased visceral sensitivity and negative emotional bias.
What are the new findings?
▶ ATD in healthy women results in an increased response of an extensive network of brain regions to an aversive visceral stimulus, including increased responses of the amygdala.
▶ ATD alters the coupling within an emotional arousal circuit, resulting in reduced feedback inhibition of the amygdala during an aversive visceral stimulus. An identical pattern of effective connectivity within the emotional arousal network was observed in female patients with IBS-C.
How might it impact on clinical practice in the foreseeable future?
▶ Reduced central 5-HT levels as seen in women, s carriers of the 5-HTTLPR gene polymorphism, sleep deprivation, depression and chronic stress may be responsible for the increased IBS symptom prevalence in these populations and conditions.
▶ Pharmacological interventions in populations and conditions with reduced central 5-HT levels aimed at normalising 5-HT signalling may be of therapeutic value.
Acknowledgments
Funding Profileringsfonds University Hospital Maastricht.
Footnotes
Competing interests None.
Patient consent Obtained.
Ethics approval This study was conducted with the approval of the Ethics Committee of the University Hospital Maastricht.
Contributors JL, analysis and interpretation of data, statistical analysis, drafting of the manuscript; EAM, analysis and interpretation of data, drafting of the manuscript; JJ, analysis and interpretation of data; LAK, analysis and interpretation of data; TOCK, acquisition of data, technical support; EATE, technical support; WHB, technical support, study concept and design; RJMB, study concept and design; MAvN study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Mayer EA, Bradesi S. Alosetron and irritable bowel syndrome. Expert Opin Pharmacother. 2003;4:2089–98. doi: 10.1517/14656566.4.11.2089. [DOI] [PubMed] [Google Scholar]
- 3.Niesler B, Kapeller J, Fell C, et al. 5-HTTLPR and STin2 polymorphisms in the serotonin transporter gene and irritable bowel syndrome: effect of bowel habit and sex. Eur J Gastroenterol Hepatol. 2010;22:856–61. doi: 10.1097/MEG.0b013e32832e9d6b. [DOI] [PubMed] [Google Scholar]
- 4.Mawe GM, Coates MD, Moses PL. Review article: intestinal serotonin signaling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1067–76. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- 5.Fukudo S, Kanazawa M, Mizuno T, et al. Impact of serotonin transporter gene polymorphism on brain activation by colorectal distention. Neuroimage. 2009;47:946–51. doi: 10.1016/j.neuroimage.2009.04.083. [DOI] [PubMed] [Google Scholar]
- 6.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–23. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 7.Brewerton TD. Toward a unified theory of serotonin dysregulation in eating and related disorders. Psychoneuroendocrinology. 1995;20:561–90. doi: 10.1016/0306-4530(95)00001-5. [DOI] [PubMed] [Google Scholar]
- 8.Nishizawa S, Benkelfat C, Young SN, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci USA. 1997;94:5308–13. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyrka AR, Carpenter LL, McDougle CJ, et al. Increased cerebrospinal fluid corticotrophin releasing factor concentrations during tryptophan depletion in healthy adults. Biol Psychiatry. 2004;56:531–4. doi: 10.1016/j.biopsych.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Van der Veen FM, Evers EA, Deutz NE, et al. Effects of acute tryptophan depletion on mood and facial emotion perception related brain activation and performance in healthy women with and without a family history of depression. Neuropsychopharmacology. 2007;32:216–24. doi: 10.1038/sj.npp.1301212. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter LL, Anderson GM, Pelton GH, et al. Tryptophan depletion during continuous CSF sampling in healthy human subjects. Neuropsychopharmacology. 1998;19:26–35. doi: 10.1016/S0893-133X(97)00198-X. [DOI] [PubMed] [Google Scholar]
- 12.Roiser JP, Levy J, Fromm SJ, et al. The effects of tryptophan depletion on neural responses to emotional words in remitted depression. Biol Psychiatry. 2009;66:441–50. doi: 10.1016/j.biopsych.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van der Does AJ. The effects of tryptophan depletion on mood and psychiatric symptoms. J Affect Disord. 2001;64:107–19. doi: 10.1016/s0165-0327(00)00209-3. [DOI] [PubMed] [Google Scholar]
- 14.Bell C, Abrams J, Nutt D. Tryptophan depletion and its implications for psychiatry. Br J Psychiatry. 2001;178:399–405. doi: 10.1192/bjp.178.5.399. [DOI] [PubMed] [Google Scholar]
- 15.Young SN, Smith SE, Pihl RO, et al. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology (Berl) 1985;87:173–7. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]
- 16.Kilkens TO, Honig A, van Nieuwenhoven MA, et al. Acute tryptophan depletion affects brain–gut responses in irritable bowel syndrome patients and controls. Gut. 2004;53:1794–800. doi: 10.1136/gut.2004.041657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shufflebotham J, Hood S, Hendry J, et al. Acute tryptophan depletions alters gastrointestinal and anxiety symptoms in iRRITABLE bOWEL sYNdrome. Am J Gastroenterol. 2006;101:2582–7. doi: 10.1111/j.1572-0241.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 18.Kilkens TO, Honig A, Fekkes D, et al. The effects of an acute serotonergic challenge on brain–gut responses in irritable bowel syndrome patients and controls. Aliment Pharmacol Ther. 2005;22:865–74. doi: 10.1111/j.1365-2036.2005.02660.x. [DOI] [PubMed] [Google Scholar]
- 19.Tack J, Sarnelli G. Serotonergic modulation of visceral sensation: upper gastrointestinal tract. Gut. 2002;51(Suppl 1):i77–80. doi: 10.1136/gut.51.suppl_1.i77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houghton LA, Lea R, Jackson N, et al. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471–4. doi: 10.1136/gut.50.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang L, Toner BB, Fukudo S, et al. Gender, age, society, culture, and the patient’s perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435–46. doi: 10.1053/j.gastro.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 22.Smith KA, Clifford EM, Hockney RA, et al. Effect of tryptophan depletion on mood in male and female volunteers: a pilot study. Hum Psychopharmacol. 1997;12:111–17. [Google Scholar]
- 23.Moore P, Landolt HP, Seifritz E, et al. Clinical and physiological consequences of rapid tryptophan depletion. Neuropsychopharmacology. 2000;23:601–22. doi: 10.1016/S0893-133X(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 24.Sobczak S, Riedel WJ, Booij I, et al. Cognition following acute tryptophan depletion: difference between first-degree relatives of bipolar disorder patients and matched healthy control volunteers. Psychol Med. 2002;32:503–15. doi: 10.1017/s0033291702005342. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 26.Van Eijk HM, Rooyakkers DR, Deutz NE. Rapid routine determination of amino acids in plasma by high-performance liquid chromatography with a 2–3 microns Spherisorb ODS II column. J Chromatogr. 1993;620:143–8. doi: 10.1016/0378-4347(93)80062-9. [DOI] [PubMed] [Google Scholar]
- 27.Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate–amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 28.Labus JS, Naliboff BN, Fallon J, et al. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–43. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–45. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Kotter R. Online retrieval, processing, and visualization of primate connectivity data from the CoCoMac database. Neuroinformatics. 2004;2:127–44. doi: 10.1385/NI:2:2:127. [DOI] [PubMed] [Google Scholar]
- 31.McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23(Suppl 1):S250–63. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Joreskog KG. Simultaneous factor analysis in several populations. Psychometrika. 1971;36:409–26. [Google Scholar]
- 33.Mayer EA, Naliboff BN, Craig AD. Neuroimaging of the brain–gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–42. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 35.Varnäs K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 2004;22:246–60. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev. 2002;39:107–40. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 37.Moreno FA, McGavin C, Malan TP, et al. Tryptophan depletion selectively reduces CSF 5-HT metabolites in healthy young men: results from single lumbar puncture sampling technique. Int J Neuropsychopharmacol. 2000;3:277–83. doi: 10.1017/S1461145700002133. [DOI] [PubMed] [Google Scholar]
- 38.Moreno FA, Rowe DC, Kaiser B, et al. Association between a serotonin transporter promoter region polymorphism and mood response during tryptophan depletion. Mol Psychiatry. 2002;7:213–16. doi: 10.1038/sj.mp.4000962. [DOI] [PubMed] [Google Scholar]
- 39.Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological regulation by plasma neutral amino acids. Science. 1972;178:414–16. doi: 10.1126/science.178.4059.414. [DOI] [PubMed] [Google Scholar]
- 40.Klaassen T, Riedel WJ, Deutz NE, et al. Mood congruent memory bias induced by tryptophan depletion. Psychol Med. 2002;32:167–72. doi: 10.1017/s003329170100438x. [DOI] [PubMed] [Google Scholar]
- 41.Koob GF. Corticotropin releasing factor, norepinephrine and stress. Biol Psychiatry. 1999;46:1167–80. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 42.Blier P. Crosstalk between the norepinephrine and serotonin systems and its role in the antidepressant response. J Psychiatry Neurosci. 2001;26:S3–10. [PMC free article] [PubMed] [Google Scholar]
- 43.Chameau P, van Hooft JA. Serotonin 5-HT(3) receptors in the central nervous system. Cell Tissue Res. 2006;326:573–81. doi: 10.1007/s00441-006-0255-8. [DOI] [PubMed] [Google Scholar]
- 44.Berman SM, Chang L, Suyenobu B, et al. Condition-specific deactivation of brain regions by 5-HT3 receptor antagonist alosetron. Gastroenterology. 2002;123:969–77. doi: 10.1053/gast.2002.35990. [DOI] [PubMed] [Google Scholar]
- 45.Naliboff BD, Berman S, Derbyshire SWG, et al. Longitudinal changes in perceptual and brain responses to visceral stimulation in irritable bowel syndrome patients. Gastroenterology. 2006;131:352–65. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Carpenter LL, Anderson GM, Siniscalchi JM, et al. Acute changes in cerebrospinal fluid 5-HIAA following oral paroxetine challenge in healthy humans. Neuropsychopharmacology. 2003;28:339–47. doi: 10.1038/sj.npp.1300025. [DOI] [PubMed] [Google Scholar]
- 47.Naliboff BD, Berman S, Chang L, et al. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–47. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 48.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–59. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elsenbruch S, Rosenberger C, Enck P, et al. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2010;59:489–95. doi: 10.1136/gut.2008.175000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.