Abstract
Background
COX inhibitors and β-blockers were recently suggested to reduce cancer progression through inhibition of tumor proliferation and growth factor secretion, induction of tumor apoptosis, and prevention of cellular immune suppression during the critical perioperative period. Here we evaluated the perioperative impact of clinically applicable drugs from these categories in the context of surgery, studying natural killer (NK) cell activity and resistance to experimental metastases.
Methods
F344 rats were treated with COX-1 inhibitors (SC560), COX-2 inhibitors (indo-methacin, etodolac, or celecoxib), a β-blocker (propranolol), or a combination of a COX-2 inhibitor and a β-blocker (etodolac and propranolol). Rats underwent laparotomy, and were inoculated intravenously with syngeneic MADB106 tumor cells for the assessment of lung tumor retention (LTR). Additionally, the impact of these drug regimens on postoperative levels of NK cytotoxicity was studied in peripheral blood and marginating-pulmonary leukocytes.
Results
Surgery increased MADB106 LTR. COX-2 inhibition, but not COX-1 inhibition, reduced postoperative LTR. Etodolac and propranolol both attenuated the deleterious impact of surgery, and their combined use abolished it. Surgery decreased NK cytotoxicity per NK cell in both immune compartments, and only the combination of etodolac and propranolol significantly attenuated these effects. Lastly, the initiation of drug treatment three days prior to surgery yielded the same beneficial effects as a single pre-operative administration, but, as discussed, prolonged treatment may be more advantageous clinically.
Conclusions
Excess prostaglandin and catecholamine release contributes to postoperative immune-suppression. Treatment combining perioperative COX-2 inhibition and β-blockade is practical in operated cancer patients, and our study suggests potential immunological and clinical benefits.
Keywords: COX-2 inhibitors, β-Adrenergic blockers, Metastases, Perioperative
COX-2 inhibitors and β-blockers are widely used in different clinical contexts. COX-2 inhibitors decrease the synthesis of prostaglandins (PG), endocrine agents that mediate pain and inflammation. β-blockers are commonly used in the treatment of cardiovascular conditions and abnormal stress responses, including social phobia and panic attacks.
These drugs were also shown to suppress cancer progression. Specifically, the beneficial effects of COX-2 inhibitors can be manifested through processes that occur both before and after excising the primary tumor. Various tumors have been shown to secrete PGs,1,2 presumably to escape destruction by suppressing the host’s cell-mediated immunity (CMI). Additionally, COX inhibitors were shown to have a preventive effect on the growth of both the primary tumor and metastases via other mechanisms, including the promotion of tumor cell apoptosis,3–5 reduction in the levels of angiogenic agents,6–8 and diminution of tumor microvascular density.3 As for β-blockers, β1 and β2 adrenoceptors are expressed by several human tumor lines, and catecholamines are potent direct stimulators of migration of various human carcinoma cell types (e.g., colon, breast, and ovary),9–12 and of secretion of proangiogenic factors by these tumors (e.g., vascular endothelial growth factor).9 Indeed, the application of a β-blocker was recently reported to abolish the direct prometastatic effect of stress and norepinephrine on human tumor cells implanted in nude mice.13
In the perioperative setting, the administration of COX-2 inhibitors and β-blockers could also prevent immune suppression, thus improving host resistance to metastatic progression. The surgical manipulation and accompanying stress responses have been shown, in animals and in humans, to be major suppressors of antimetastatic CMI activities. For example, the cytotoxic activity of natural killer (NK) cells decreases even before surgery,14 and is suppressed even further following it. Since NK cells, like other constituents of CMI, are capable of lysing circulating tumor cells and abolishing minimal residual disease (MRD), preventing the decrease in antimetastatic immune activity during the perioperative period is crucial for the prevention of MRD from establishing large progressive tumors.
The current study aims at critically evaluating the perioperative use of clinically applicable PG synthesis inhibitors and β-adrenergic blockers, alone and in combination, in attenuating postoperative suppression of CMI.
In previous studies in rats, we found that the nonselective COX inhibitor, indomethacin, and the nonselective β-adrenergic blocker, nadolol, were capable of attenuating the metastasis-promoting effects of surgery. 15 However, indomethacin is unlikely to be used clinically due to increased risk of bleeding during surgery. Thus, in the current study we tested the efficacy of different regimens of COX-2 inhibition, which can be used in the context of surgery, and compared them to COX-1 inhibition. Additionally, we used propranolol, a nonselective β-adrenergic antagonist that crosses the blood–brain-barrier and could be advantageous over nadolol, as it is widely used clinically for various conditions, including hypertension, anxiety, and excessive sympathetic responses that often characterize patients during the perioperative period.
The administration of a β-adrenergic antagonist and a COX-2 inhibitor several days before surgery could also prove useful for improving pre-operative immune competence. Catecholamines and PGs are most likely elevated even before surgery as a result of psychological distress, activation of nociceptive mechanisms, and various primary tumors that secrete PGs, presumably to evade immune destruction. Therefore, the current study also assessed the continuous use of these blockers starting three days prior to surgery.
MATERIALS AND METHODS
Animals and Counterbalancing
Fisher 344 male or female rats (Harlan laboratories, Jerusalem, Israel), 3–6 months old were used. In each experiment, the age of the rats was matched. Animals were housed four per cage with free access to food and water in a 12:12 light: dark cycle, were acclimatized to the vivarium for at least 3 weeks, and were handled four times before the experiment to reduce potential procedural stress. Body weight was measured for counterbalancing and for health records. Order of drug administration, surgery groups, and tumor injection was counterbalanced across groups in each experiment. All studies were approved by The Institutional Animal Care and Use Committee of Tel Aviv University.
Drugs and Their Administration
Celecoxib
A highly selective COX-2 inhibitor (kindly donated by Trima, Israel) was first dissolved in dimethyl sulfoxide (DMSO), then diluted 1:70 in polyethylene glycol (PEG). The drug was administered subcutaneously (7.5, 15 mg/kg, 2 ml/kg) 1 hour before surgery, and again (7.5, 15 mg/kg, 1 ml/kg) with tumor inoculation approximately 4 h later (given that its biological half-life time in rats is not known).
Etodolac
The COX-2 specific inhibitor, etodolac, was kindly donated by Taro, Israel. For experiment 2, etodolac was first dissolved in DMSO then diluted 1:70 in PEG. For all other experiments, etodolac was dissolved in corn oil. The drug was administered subcutaneously (5, 10, 12.5, 15 mg/kg, 2 ml/kg) 1 hour before surgery. The half-life of this drug in rats was found to be 18 h.16
Indomethacin
A nonselective COX inhibitor, with a slight preference to COX-1 inhibition, was purchased from Sigma, Israel. The drug was dissolved in DMSO and PEG (as above) and administered subcutaneously (4 mg/kg, 2 ml/kg) 1 hour before surgery.
SC560
The selective COX-1 inhibitor SC-560 was obtained from Biomol (Plymouth Meeting, PA, USA) and dissolved in DMSO and PEG (as above). The drug was administered subcutaneously (3, 10, 30 mg/kg, 2 ml/kg) 1 hour prior to surgery. The intermediate dose used (10 mg/kg) is often used in rats and was found effective in blocking prostaglandin secretion in an inflammatory model.17,18
Propranolol
To block β-adrenoceptor stimulation, we used the nonselective β-adrenergic blocker, propranolol (Sigma, Israel). The drug was dissolved in phosphate buffered saline (PBS) and added to a mixture with mineral oil (Sigma, Israel) and mannide monooleate (Arlacel A, Sigma, Israel), in a 4:3:1 ratio, respectively, to create a slowly absorbed emulsion. Unpublished data from our laboratory have shown that the slow absorbance emulsion is effective for 36–48 h. One milliliter of the emulsion (1.5, 4.5 mg/kg) was administrated subcutaneously 1 hour prior to laparotomy.
Experimental Laparotomy
The laparotomy procedure has been described in detail elsewhere.19 Briefly, rats were anesthetized with 2.5–3.5% halothane and a 3.5-cm midline abdominal incision was made. The intestine was externalized, gently rubbed with a gauze pad, and kept moisturized for 50 minutes. The intestine was then returned to the abdominal cavity and the wound was sutured.
Tumor Cell Lines
MADB106
Is a selected variant cell line obtained from a pulmonary metastasis of a mammary adenocarcinoma (MADB100) chemically induced in the F344 rat. This syngeneic tumor metastasizes only to the lungs following i.v. inoculation. The MADB106 line was maintained in 5% CO2 at 37°C in monolayer cultures in complete medium (CM). Cells were removed from the culture flask with trypsin solution (0.25% in PBS), and were washed with CM and were used for the in vivo studies, as well as target cells in the in vitro assessment of NK cytotoxic activity (NKCA).
YAC-1
The standard target cell line for in vitro assessment of NKCA was used. The cell line was maintained in 5% CO2 at 37°C suspension cultures in CM.
Radiolabeling of MADB106 Tumor Cells and Assessment of Lung Tumor Retention
DNA radiolabeling of tumor cells has been described in detail elsewhere.15 For tumor cell injections, rats were lightly anesthetized with halothane, and 2 × 105 radiolabeled cells in 0.5 ml PBS (supplemented with 0.1% BSA) were injected into their tail vein. Twenty-one hours later, rats were killed with halothane, and their lungs removed and placed in a gamma counter for assessment of radioactivity. The percentage of tumor cell retention was calculated as the ratio between radioactivity measured in the lungs and the total radioactivity in the injected cell suspension. Our previous studies have demonstrated that the levels of lung radioactivity reflect the numbers of viable tumor cells in the lungs that are expected to form solid lung metastases.20–22
In Vitro Assessment of NK Cytotoxicity
Preparation of blood and marginating pulmonary effector cells
Rats were euthanized with an overdose of halothane, and their thoracic cavities were opened. To harvest leukocytes adhering to the pulmonary endothelium (MP leukocytes), we perfused the lungs by injecting heparinized PBS (30 U/ml) into the right ventricle (approximately 0.5 ml/s) and collecting 30 ml of perfusate from the left ventricle. To avoid contamination with circulating blood, we discarded the first 3 ml of perfusate. The perfusate was then centrifuged (300 g for 10 min), concentrated to approximately 0.5 ml, washed in 4 ml CM, and concentrated to a final volume of 1 ml.
Preparation of target cells
MADB106 cells were washed with CM. Both MADB106 cells and YAC-1 cells (5 × 106 of each) were incubated for 1 h with 100 μCi 51Cr (Danyel Biotech, Rehovot, Israel) in 100 μl saline, 100 μl heat-inactivated fetal calf serum (FCS), and 75 μl CM. Following incubation, cells were washed three times (300 g for 10 min) and adjusted to the concentration of 5 × 104 /ml in CM.
Cytotoxicity assay
The standard whole-blood 4 h 51Cr release assay was used to assess leukocyte antitumor cytotoxicity against standard YAC-1 target cells, as well as against syngeneic MADB106 cells. The procedure was described in detail elsewhere.15 Our previous studies indicate that cytotoxicity in this assay depends on NK cells, since their selective depletion nullified all specific killing.19,22
Flow Cytometry
The fluorescence activated cell sorter (FACS) analysis method has been described elsewhere15 and was used to identify NK cells. NK cells were identified as NKR-P1bright (CD161bright) lymphocytes using FITC-conjugated anti-NKR-P1 (Pharmingen, San Diego).
Assessment of Cytotoxicity Per NK Cell
Since we recorded the number of NK cells in each sample tested for cytotoxicity, we also empirically unified numbers of NK cells in all samples by adjusting their number to a predetermined concentration, and assessed levels of NKCA per NK cell within circulating and MP-NK cells. Because the cellular context of circulating leukocytes and MP-NK leukocytes might be different, we only compared samples within each immune compartment. We differentially diluted samples according to the FACS analysis to achieve common NK:MADB106 (for lung perfusates) or NK:YAC-1 (for whole blood) ratios, thereby assessing NKCA on a per NK cell basis. From here on, the assessment of cytotoxicity is as described above.
Statistical Analysis
One- or two-way analysis of variance (ANOVA) was used to analyze lung tumor retention. Provided significant group differences were indicated by ANOVA, Fisher’s protected least significant differences (PLSD) contrasts were used to test specific pairwise differences with respect to our hypothesized effects: surgery worsens the outcome (control saline vs. surgery saline), and each of the drug treatments (and their combination) reduces the effects of surgery (surgery saline versus surgery drug). For unplanned comparisons, Scheffé post hoc contrasts were used. NKCA was analyzed using repeated measures ANOVA (different E:T ratios as the repeated measures), and PLSDs (as above) were used to conduct pairwise comparisons, unless otherwise indicated. P<0.05 was considered significant in all studies.
PRECEDURES AND RESULTS
Experiment 1: Comparing the Efficacy of Drugs with Different COX-1/COX-2 Inhibition Selectivity
An early index of host resistance to tumor metastasis is the retention of MADB106 tumor cells in the lungs 24 h following inoculation. This index is highly predictive of the actual number of metastases that would have otherwise formed weeks later.21,23 Additionally, because the metastatic process of MADB106 is sensitive to NK activity only in the first 24 h following inoculation,21,24,25 lung tumor retention is less affected by factors unrelated to NK activity than is the number of metastases.
F344 male rats were administered with different COX inhibitors or with vehicles. The drugs used were: SC560 (selective COX-1) at doses of 3, 10, or 30 mg/kg; indomethacin (nonselective), 4 mg/kg (successfully used in our previous studies); etodolac (selective COX-2), at 12.5 mg/kg; and celecoxib (highly selective COX-2), at 7.5 or 15 mg/kg. In addition, a combination of celecoxib and SC560 was used (15 and 10 mg/kg, respectively). All the drugs and concentrations above were found to be effective in our studies or by other researchers (see “Methods” section). One hour after drug administration, rats from each group underwent laparotomy or served as nonoperated controls. Within 1 h after surgery, rats were intravenously inoculated with radiolabeled MADB106 cells. The celecoxib dose was divided into 2/3 preoperatively and 1/3 postoperatively (given simultaneously with the tumor). Twenty-one hours after tumor inoculation, animals were sacrificed, and lungs were removed for LTR assessment. The study was conducted in replicates and a total of 182 male rats were used (see Table 1 for the number of animals in each group).
TABLE 1.
Number of animals in each group in experiment 1
| Drug treatment | No surgery | Surgery |
|---|---|---|
| Vehicle | 36 | 42 |
| SC560 3 mg/kg | 3 | 8 |
| SC560 10 mg/kg | 3 | 11 |
| SC560 30 mg/kg | 3 | 4 |
| Indomethacin 4 mg/kg | 5 | 11 |
| Etodolac 12.5 mg/kg | 2 | 12 |
| Celecoxib 7.5 mg/kg | 1 | 9 |
| Celecoxib 15 mg/kg | 2 | 12 |
| Celecoxib 15 mg/kg + SC560 10 mg/kg | 7 | 11 |
Experiment 1: Results
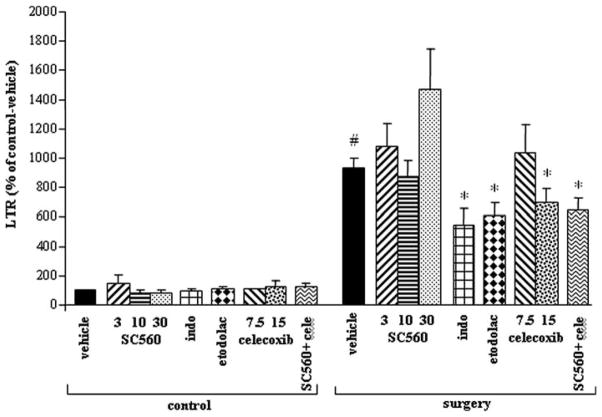
ANOVA revealed significant group differences (F(17,164) = 15.765, P<0.05). Fisher’s PLSD showed that surgery caused a significant increase in LTR compared to control rats (P<0.05). Indomethacin, etodolac, and celecoxib (15 mg/kg) all significantly attenuated the effect of surgery on LTR (by 34% to 46%) (P<0.05). No significant differences were found between the efficacies of these treatments. The selective COX-1 inhibitor, SC560, did not attenuate LTR in any of the doses tested; although this drug was effective in other studies when used in the intermediate dose of 10 mg/kg (see “Methods” section). The combination of celecoxib and SC560 decreased the effect of surgery (P<0.05) (Fig. 1), similar to the effects of celecoxib alone. In nonoperated animals, the drugs had no significant impact on LTR.
FIG. 1.
COX-2, but not COX-1 inhibition, reduced the promotion of lung tumor retention (LTR) by surgery. Data are presented as percentage of baseline (control-vehicle) (mean + SEM). Surgery significantly increased LTR (#), and drugs that inhibit COX-2 (indomethacin, etodolac, and celecoxib), but not COX-1 (SC560), significantly attenuated the effect of surgery (*).
Experiment 2: Optimal Doses of Etodolac and Propranolol for Improving In Vivo Resistance to Lung Tumor Retention
In order to achieve a dose curve for in vivo effects of etodolac, F344 rats were injected with vehicle or with 5, 10, or 15 mg/kg of etodolac. For propranolol, F344 rats were injected with a slowly absorbed emulsion containing 0, 1.5, or 4.5 mg/kg propranolol. One hour after the drug injection, rats from each group underwent laparotomy or served as nonoperated controls. Following surgery, all rats were intravenously inoculated with radiolabeled MADB106 cells. Twenty-one hours after tumor inoculation all rats were sacrificed to assess MADB106 LTR. The etodolac study was conducted in two replicates, each including animals from all experimental groups (a total of 87 animals were used in these eight groups). The propranolol study was also conducted in two replicates, each including animals from all experimental groups. A total of 88 male rats were used in these six groups (see Table 2 for n in each group).
TABLE 2.
Number of animals in each group in experiment 2
| No surgery | Surgery | ||
|---|---|---|---|
| Propranolol | 0 mg/kg | 20 | 16 |
| 1.5 mg/kg | 10 | 17 | |
| 4.5 mg/kg | 9 | 16 | |
| Etodolac | 0 mg/kg | 18 | 19 |
| 5 mg/kg | 2 | 13 | |
| 10 mg/kg | 3 | 14 | |
| 15 mg/kg | 5 | 13 |
Experiment 2: Results
For etodolac, one-way ANOVA revealed significant group differences (F(7,79) = 9.672, P<0.05). Surgery caused a large and significant increase in LTR (PLSD P<0.05) (Fig. 2A). All doses of etodolac significantly attenuated this effect. In the next experiment we tested a dose of 12.5 mg/kg, the average of the two most effective doses tested. As for propranolol, ANOVA revealed significant group differences (F(5,82) = 11.76, P<0.05). Fisher’s planned contrasts showed that the surgery caused a significant increase in LTR compared to nonoperated rats (PLSD P<0.05). Both doses of propranolol significantly attenuated this effect by more than 50% (PLSD P<0.05) (Fig. 2B). Propranolol did not impact LTR in nonoperated rats.
FIG. 2.
Propranolol and etodolac increase resistance to tumor retention to the lungs. Data are presented as percentage of baseline (control-vehicle) (mean + SEM). Surgery significantly increased LTR (#), and treatment with 5, 10, and 15 mg/kg etodolac (A) or 1.5 and 4.5 mg/kg propranolol (B) significantly attenuated the effect of surgery (*) without impacting nonoperated animals.
Experiment 3: Both Acute and Prolonged Treatment with Etodolac and Propranolol are Effective in Overcoming the Promotion of MADB106 LTR by Surgery
Rats were assigned to receive etodolac (12.5 mg/ kg/injection), propranolol (1.5 mg/kg/injection) or both drugs. Half the animals in each group began receiving the drug 3 days before surgery (injected at 72, 36, and 1 h before surgery, using the slow-release emulsion for propranolol). The other half received the drugs in a single injection 1 h before surgery. On the day of surgery, each group was subdivided to undergo laparotomy or to serve as nonoperated controls. One hour after surgery, rats were intravenously inoculated with MADB106 cells. Twenty-one hours later, all rats were sacrificed to assess MADB106 LTR. A total of 56 male rats were used (approximately seven rats per group).
Experiment 3: Results
No differences in LTR levels or in body weight were observed between rats receiving the prolonged drug treatment and those receiving only one drug injection, neither in operated nor in control groups. Therefore, for statistical analysis we considered rats receiving three or one preoperative dose (of the same drug) as belonging to the same condition. ANOVA revealed significant group differences (F(7,48) = 18.657, P< 0.05). Fisher’s PLSD showed that the surgery caused a significant increase in LTR compared to nonoperated rats (P<0.05), and propranolol and etodolac, each significantly reduced this increase by approximately 50% (Fig. 3). The combination of both drugs abolished the effect of surgery, and LTR levels decreased to the levels evident in nonoperated rats. Importantly, the drug combination was more effective compared to the propranolol treatment alone (P<0.05), and marginally significant compared to etodolac treatment alone (P = 0.058). None of the drug regimens, nor their combination, affected levels of LTR in nonoperated rats.
FIG. 3.
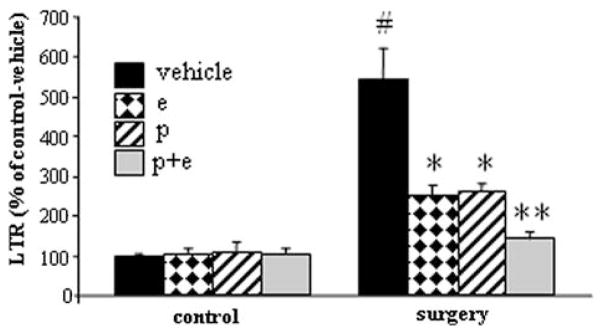
Promotion of MADB106 lung tumor cell retention by surgery, and blockade by the combined use of etodolac (e) and propranolol (p). Data are presented as percentage from baseline (control-vehicle) (mean + SEM). Surgery significantly increased LTR (#); each drug significantly attenuated the effect of surgery (*); and their combination abolished it, exhibiting a greater effect than each drug alone (**).
Experiment 4: The Effects of Surgery, Etodolac, and Propranolol on the Number and Cytotoxicity of Marginating-pulmonary (MP) and Circulating NK Cells
Here we studied the potential involvement of NK cells in mediating the above in vivo effects of surgery on MADB106 LTR, and the potential prophylactic use of etodolac and propranolol to reduce this effect. To this end, we assessed the number and activity of NK cells 12 h after surgery, as this time point is the midpoint in the time frame in which NK cells control MADB106 LTR (0 to 24 h after tumor administration). 21 We assessed NKCA in both the blood and the marginating pulmonary compartment, as we have recently reported that, per NK cell, the MP compartment exhibits markedly higher NK cytotoxicity than other compartments.15 The MP population was also found to be unique in its ability to lyse the syngeneic MADB106 tumor cells in a 4 h cytotoxicity assay.15,26 Thus, the evaluation of prophylactic treatment, as described in the current paper, should include this distinct population that seems most relevant to the in vivo immune control over MADB106 LTR.
The drug treatments and the experimental design (4 × 2 × 2) were exactly as in the previous study (experiment 3). Twelve hours after surgery, MP leukocytes were harvested from the vasculature of the lungs, and their cytotoxicity was assessed in vitro against the MADB106 tumor line. Additionally, we assessed cytotoxicity of circulating leukocytes against YAC-1 target cells (as no appreciable NKCA against MADB106 is achieved in this compartment15). FACS analysis was used to identify and count NK cells. A total of 139 male rats were used in three replicates, each including all experimental groups (a minimum number of eight animals per group was used).
Experiment 4: Results
For both operated and nonoperated groups, no differences were observed between rats receiving the prolonged drug treatments and those receiving only one administration before surgery. Therefore, for statistical analyses we considered the two drug regimens as one condition.
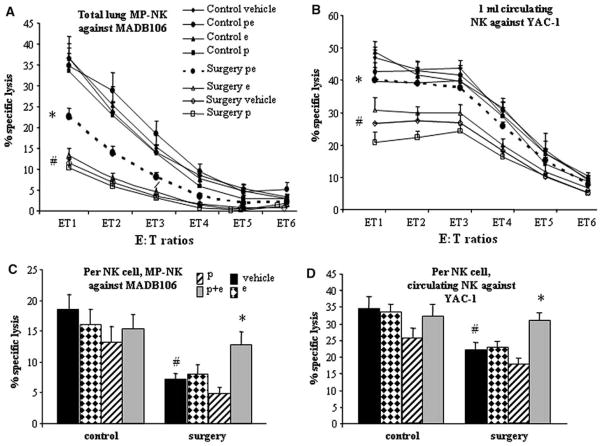
NK cytotoxicity per MP compartment
MP-NK cells exhibited marked NKCA against MADB106 target cells, increasing from 3% to 36% along the different E:T ratios in the control group (Fig. 4A). ANOVA indicated significant group differences (F(7,131)= 19.11, P<0.05). Surgery significantly suppressed this cytotoxicity (PLSD, P<0.05), and the combined administration of etodolac and propranolol significantly reduced this suppression (PLSD, P<0.05), but did not abolish it. None of the drug treatments alone reduced the effect of surgery, and no treatment affected nonoperated animals.
FIG. 4.
The effects of surgery, etodolac (e) and propranolol (p) on the number and cytotoxicity of marginating-pulmonary (MP) and circulating NK cells. Data is shown as mean + SEM. (A) MP-NK cytotoxicity per lung against MADB106 cells, and (B) circulating NK cytotoxicity per ml blood against YAC-1 target cells: surgery significantly reduced lysis by NK cells in both compartments (#), and only the combined p + e treatment attenuated this effect (*). (C and D) cytotoxicity per NK cell in the MP compartment (C) and in the circulation (D), against the MADB106 and YAC-1 target lines, respectively (average of the highest E:T ratios). Numbers of NK cells were standardized in all samples tested for cytotoxicity to achieve measures per NK cell. Surgery significantly reduced lysis per NK cell in both compartments (#), and only the combined p + e treatment attenuated this effect (*).
NK cytotoxicity per milliter of blood
Blood leukocytes exhibited marked NKCA against the YAC-1 cell line, increasing from 9% to 46% along the different E:T ratios in the control group (Fig. 4B). ANOVA indicated significant group differences (F(7,101) =9.248, P<0.05). Surgery markedly and significantly suppressed this cytotoxicity (PLSD P<0.05), and the simultaneous administration of etodolac and propranolol significantly abolished this effect (P<0.05), yielding cytotoxicity levels very similar to those seen in nonoperated animals. Each drug alone had no effects in operated animals, and no treatment affected nonoperated animals.
NK cytotoxicity per MP-NK cell
Because significant lysis levels were evident only in the two highest E:T ratios (8–30%), we averaged these E:T ratios and used this index for further analysis (Fig. 4C). ANOVA indicated significant group differences (F(7,77) = 6.657, P<0.05). Surgery caused a significant decrease in MP-NK cytotoxicity per NK cell against MADB106 (PLSD P<0.05). Each drug alone did not attenuate this effect. The combination of both drugs significantly reduced the effect of surgery (PLSD P<0.05). Post hoc Scheffé comparison did not indicate drug effects in nonoperated animals.
NK cytotoxicity per circulating NK cell
Since significant lysis levels were evident only in the three highest E:T ratios (14–28%), we averaged these E:T ratios and used this index for further analysis (Fig. 4D). ANOVA indicated significant group differences (F(7,94) = 5.879, P<0.05). Surgery caused a significant decrease in NKCA against YAC-1 target cells (PLSD P<0.05). The combination of both drugs significantly reduced this effect (P<0.05), whereas administration of each drug alone had no impact. Scheffé contrasts for unplanned comparison revealed no other significant group differences.
Number of circulating- and MP-NK cells
ANOVA did not reveal significant group differences in the number of circulating NK cells (Table 3). As for MP-NK cell numbers, group differences were significant (F(7,101) = 4.023, P<0.05). A 2 × 4 ANOVA indicated that surgery had a main effect, reducing the number of MP-NK cells (F(1,105) = 15.237, P<0.05). Drug treatment also had a main effect (F(3,103) = 4.786, P<0.05), and Scheffé post hoc analysis indicated that the combination of drugs increased numbers of MP-NK cells compared to the vehicle condition. No interaction was observed.
TABLE 3.
Numbers of NK cells in the marginating pulmonary (MP) compartment and in the circulation in the different experimental groups (mean±SEM)
| MP-NK cells
|
Circulating NK cells
|
|||
|---|---|---|---|---|
| Control | Surgerya | Control | Surgery | |
| Vehicle | 459.858 ± 56 | 317.36 ± 38.4 | 567.563 ± 63.9 | 487.737 ± 30.4 |
| Etodolac | 580.42 ± 107.15 | 383.356 ± 58.8 | 524.333 ± 39.9 | 443.176 ± 38.7 |
| Propranolol | 468.857 ± 75.16 | 342.15 ± 26.72 | 549.333 ± 85.7 | 529.5 ± 31.5 |
| Etodolac + propranolol | 784.4 ± 119.45b | 470.912 ± 82.8b | 489.222 ± 68.8 | 470.8 ± 31.6 |
significant difference between surgery and control (main effect).
significant difference between propranolol + etodolac treatment and vehicle (main effect).
DISCUSSION
This study demonstrates that treatment combining the β-blocker propranolol and the COX-2 inhibitor etodolac can efficiently prevent immunosuppression following surgery. Importantly, whereas none of the blockers alone attenuated the suppression of circulating or pulmonary NKCA, their combined use was markedly effective, indicating synergistic impact of the blockers. This synergism may be attributed to the fact that both CAs and PGs can independently suppress NKCA by activating their respective membrane receptors on NK cells,27–31 causing intracellular elevation of cAMP levels.28,32,33 Thus, when both CAs and PGs are in excess, only their simultaneous blockade can prevent NK suppression through this mechanism.
PGs and CAs can also affect CMI levels through their known modulatory impact on cytokine levels. Both compounds were shown to inhibit the production of CMI-enhancing cytokines (e.g., IL-12),34–37 and to increase levels of Th2 cytokines, which are believed to suppress CMI.36–38 Taken together, blocking excessive effects of CAs and PGs during the perioperative period could prove effective in preventing CMI suppression.
Noteworthy is that the postoperative decrease in NKCA and its attenuation by the drug treatment occurred in both the blood and the MP compartments. We recently reported that MP-NK cells are uniquely potent and are the only known NK cell population that can effectively kill the syngeneic MADB106 tumor cells15,26,39 which were used here in both the in vivo studies and in assessing ex vivo MP-NKCA. Therefore, alterations in MP-NK activity against MADB106 may predict alterations in host susceptibility to metastasis of the MADB106 tumor and potentially other autologous tumor cells.
Importantly, our findings directly demonstrate that suppression of NK activity and its attenuation by the drug treatment occurred on a per-NK-cell basis, rather than through alteration in the number of NK cells. Alterations in cytotoxicity per NK cell are attributable to immunomodulation through cellular mechanisms as detailed above, and are likely to occur in all NK cells that are exposed to a similar humoral milieu. Therefore, our findings suggest the generalizability of these effects to immune compartments other than the blood and the MP compartment. Irrespective of preventing suppression per NK cell, the combined drug treatment also increased the numbers of MP-NK cells in operated and in nonoperated animals. This effect further contributed to the total increase in NK activity per the entire MP compartment, and may have clinical ramifications given the potential of MP-NK cells to prevent lung metastasis and eradicate circulating tumor cells.
The findings that each blocker alone succeeded in reducing postoperative levels of LTR are not in contradiction with their inability to prevent suppression of NKCA when used alone. The measure of LTR reflects the rat’s cumulative resistance to MADB106 throughout the entire 21 h period since tumor inoculation, whereas NKCA reflects immune competence at the time at which immunocytes were harvested – 12 h postoperatively in the current study. At other time points, either CAs or PGs may be predominant, single-handedly impacting NK cells, and through them affecting LTR. Thus, at certain periods, solitary blockade of each receptor system may suffice to reduce LTR. Alternatively, mechanisms other than NK cells are likely to impact LTR, and could be affected separately by CAs and PGs. Regardless of the specific mechanisms, the data for both NKCA and LTR demonstrate that the combination of blockers is more efficacious in abolishing the deleterious effects of surgery than each drug alone.
In this study, preoperative treatment with both propranolol and etodolac began 3 days before surgery, and was compared to a single administration of the drugs 1 h before surgery (experiments 3 and 4). No adverse effects of prolonged administration were observed in the studied immunological measures, and the same beneficial impacts were noted postoperatively. Given that these drugs are used clinically for prolonged periods for other medical conditions, and given the current results, the preoperative use of these drugs in the clinical setting prior to tumor excision seems feasible, and may prove advantageous.
Specifically, the beneficial effects of COX-2 inhibitors can be manifested through several processes that occur both pre- and postoperatively. Primary tumors have been shown to secrete PGs, presumably to suppress CMI and escape destruction. Additionally, COX inhibitors were shown to have a preventive effect on the growth of both the primary tumor and metastases via other mechanisms, such as promotion of tumor apoptosis,3,4,40,41 reduction in the levels of angiogenic agents,4,6–8 and diminution of tumor microvascular density.3 Moreover, a recent study reported that two COX inhibitors, indomethacin and celecoxib, downregulated expression levels of MHC-I molecules on murine mammary tumor cells. Decreased expression of MHC-I is a known triggering signal for NK cells to lyse such tumor cells.42 Lastly, decreasing excess release of PGs is expected to enhance CMI by shifting the cytokine balance towards Th1 dominance and IFNγ secretion by NK cells.29 Taken together, administration of COX-2 inhibitors perioperatively could halt tumor progression and enhance immune competence before and after surgery.
Additional benefits of using selective COX-2 inhibitors during the perioperative period are related to two major concerns in cancer treatment, namely pain alleviation and the use of morphine. On the one hand, pain was shown to suppress NKCA43 by causing the release of endogenous opioids and inducing various stress responses. Pain was also shown to promote tumor development,44 and to increase postoperative metastasis in animal models.20 On the other hand, the use of morphine and other opiates (e.g., fentanyl) for pain alleviation is problematic, as these drugs were also shown to decrease NKCA and promote tumor metastasis.45,46 Studies in humans reported compelling evidence suggesting similar deleterious effects of opiates in the perioperative context.47,48 Morphine was also reported to directly promote tumor proliferation.49 Lastly, opiates exert numerous side effects, including nausea, vomiting, and respiratory depression. For all these reasons, the use of morphine should be minimized, especially in cancer patients. COX-2 inhibitors act to reduce pain through the prevention of PG release, and the use of COX-2 inhibitors as adjuvants to morphine were shown to reduce pain and the need for postoperative morphine.50
The clinical perioperative use of β-blockers for the prevention of metastatic development could be beneficial through different mechanisms. The direct effects involving the inhibition of tumor cells were described in the introduction. Additionally, propranolol is commonly used to treat generalized anxiety and post-traumatic stress disorders,51,52 and can be employed to reduce stress and anxiety levels in patients awaiting surgery. In cancer patients, distress was associated with impaired CMI, including impaired NKCA,53 which could contribute to tumor progression.54
In conclusion, controlling exaggerated inflammatory reactions and physiological and psychological sympathetic responses is suggested by the current and by other studies. The advantages of the specific drug regimen used herein (etodolac and propranolol) are its evident promising outcomes in an animal model, minimal or no side effects, and relatively easy and safe use of established and inexpensive drugs. These drugs have been used clinically during surgery for other purposes. Such perioperative treatment may benefit patients through immunological and nonimmunological mechanisms, both before and following surgery. Thus, we suggest that the time has come to initiate clinical studies employing this drug regimen in operated cancer patients.
Acknowledgments
Support was provided by NIH/NCI CA125456 grant (S. B-E) and by a grant from the Israel-USA Binational Science Foundation (S. B-E).
References
- 1.Menetrier-Caux C, Bain C, Favrot MC, et al. Renal cell carcinoma induces interleukin 10 and prostaglandin E2 production by monocytes. Br J Cancer. 1999;79:119–30. doi: 10.1038/sj.bjc.6690021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wojtowicz-Praga S. Reversal of tumor-induced immunosuppression: a new approach to cancer therapy. J Immunother. 1997;20:165–77. [PubMed] [Google Scholar]
- 3.Roche-Nagle G, Connolly EM, Eng M, et al. Antimetastatic activity of a cyclooxygenase-2 inhibitor. Br J Cancer. 2004;91:359–65. doi: 10.1038/sj.bjc.6601967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinicrope FA, Gill S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metastasis Rev. 2004;23:63–75. doi: 10.1023/a:1025863029529. [DOI] [PubMed] [Google Scholar]
- 5.Kern MA, Haugg AM, Koch AF, et al. Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res. 2006;66:7059–66. doi: 10.1158/0008-5472.CAN-06-0325. [DOI] [PubMed] [Google Scholar]
- 6.Wei D, Wang L, He Y, et al. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004;64:2030–8. doi: 10.1158/0008-5472.can-03-1945. [DOI] [PubMed] [Google Scholar]
- 7.Jones MK, Wang H, Peskar BM, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999;5:1418–23. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 8.Rozic JG, Chakraborty C, Lala PK. Cyclooxygenase inhibitors retard murine mammary tumor progression by reducing tumor cell migration, invasiveness and angiogenesis. Int J Cancer. 2001;93:497–506. doi: 10.1002/ijc.1376. [DOI] [PubMed] [Google Scholar]
- 9.Yang EV, Sood AK, Chen M, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357–64. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 10.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 11.Masur K, Niggemann B, Zanker KS, et al. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61:2866–9. [PubMed] [Google Scholar]
- 12.Lutgendorf SK, Cole S, Costanzo E, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9:4514–21. [PubMed] [Google Scholar]
- 13.Sood AK, Bhatty R, Kamat AA, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–75. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenfeld K, Avraham R, Benish M, et al. Immune suppression while awaiting surgery and following it: dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav Immun. 2007;21:503–13. doi: 10.1016/j.bbi.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Melamed R, Rosenne E, Shakhar K, et al. Marginating pulmonary- NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19:114–26. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Shi JM, Lai SG, Xu CJ, et al. Pharmacokinetic difference between S-(+)- and R-(−)-etodolac in rats. Acta Pharmacol Sin. 2004;25:996–9. [PubMed] [Google Scholar]
- 17.Hetu PO, Riendeau D. Cyclo-oxygenase-2 contributes to constitutive prostanoid production in rat kidney and brain. Biochem J. 2005;391:561–6. doi: 10.1042/BJ20050451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adami M, Coppelli G, Guaita E, et al. Effects of cyclooxygenase- 1 and -2 inhibition on gastric acid secretion and cardiovascular functions in rats. Pharmacology. 2006;76:84–92. doi: 10.1159/000089834. [DOI] [PubMed] [Google Scholar]
- 19.Page GG, Ben-Eliyahu S, Liebeskind JC. The role of LGL/NK cells in surgery-induced promotion of metastasis and its attenuation by morphine. Brain Behav Immun. 1994;8:241–50. doi: 10.1006/brbi.1994.1022. [DOI] [PubMed] [Google Scholar]
- 20.Bar-Yosef S, Melamed R, Page GG, et al. Attenuation of the tumor-promoting effect of surgery by spinal blockade in rats. Anesthesiology. 2001;94:1066–73. doi: 10.1097/00000542-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Eliyahu S, Page GG. In vivo assessment of natural killer cell activity in rats. Prog Neuroendocrineimmunol. 1992;5:199–214. [Google Scholar]
- 22.Ben-Eliyahu S, Page GG, Yirmiya R, et al. Acute alcohol intoxication suppresses natural killer cell activity and promotes tumor metastasis. Nat Med. 1996;2:457–60. doi: 10.1038/nm0496-457. [DOI] [PubMed] [Google Scholar]
- 23.Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160:3251–8. [PubMed] [Google Scholar]
- 24.Barlozzari T, Reynolds CW, Herberman RB. In vivo role of natural killer cells: involvement of large granular lymphocytes in the clearance of tumor cells in anti-asialo GM1-treated rats. J Immunol. 1983;131:1024–7. [PubMed] [Google Scholar]
- 25.Barlozzari T, Leonhardt J, Wiltrout RH, et al. Direct evidence for the role of LGL in the inhibition of experimental tumor metastases. J Immunol. 1985;134:2783–9. [PubMed] [Google Scholar]
- 26.Shakhar G, Abudarham N, Melamed R, et al. Amelioration of operation-induced suppression of marginating pulmonary NK activity using poly IC: a potential approach to reduce postoperative metastasis. Ann Surg Oncol. 2007;14:841–52. doi: 10.1245/s10434-006-9078-9. [DOI] [PubMed] [Google Scholar]
- 27.Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79:1093–104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- 28.Whalen MM, Bankhurst AD. Effects of beta-adrenergic receptor activation, cholera toxin and forskolin on human natural killer cell function. Biochem J. 1990;272:327–31. doi: 10.1042/bj2720327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker W, Rotondo D. Prostaglandin E2 is a potent regulator of interleukin-12- and interleukin-18-induced natural killer cell interferon-gamma synthesis. Immunology. 2004;111:298–305. doi: 10.1111/j.1365-2567.2004.01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malygin AM, Meri S, Timonen T. Regulation of natural killer cell activity by transforming growth factor-beta and prostaglandin E2. Scand J Immunol. 1993;37:71–6. doi: 10.1111/j.1365-3083.1993.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 31.Chouaib S, Welte K, Mertelsmann R, et al. Prostaglandin E2 acts at two distinct pathways of T lymphocyte activation: inhibition of interleukin 2 production and down-regulation of transferrin receptor expression. J Immunol. 1985;135:1172–9. [PubMed] [Google Scholar]
- 32.Tamir A, Isakov N. Cyclic AMP inhibits phosphatidylinositol-coupled and -uncoupled mitogenic signals in T lymphocytes. Evidence that cAMP alters PKC-induced transcription regulation of members of the jun and fos family of genes. J Immunol. 1994;152:3391–9. [PubMed] [Google Scholar]
- 33.Torgersen KM, Vaage JT, Levy FO, et al. Selective activation of cAMP-dependent protein kinase type I inhibits rat natural killer cell cytotoxicity. J Biol Chem. 1997;272:5495–500. doi: 10.1074/jbc.272.9.5495. [DOI] [PubMed] [Google Scholar]
- 34.Stolina M, Sharma S, Lin Y, et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–70. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 35.van der Pouw Kraan TC, Boeije LC, Smeenk RJ, et al. Prostaglandin- E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–9. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elenkov IJ, Chrousos GP, Wilder RL. Neuroendocrine regulation of IL-12 and TNF-alpha/IL-10 balance. Clinical implications. Ann NY Acad Sci. 2000;917:94–105. doi: 10.1111/j.1749-6632.2000.tb05374.x. [DOI] [PubMed] [Google Scholar]
- 37.Elenkov IJ, Papanicolaou DA, Wilder RL, et al. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians. 1996;108:374–81. [PubMed] [Google Scholar]
- 38.Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg. 2004;389:475–84. doi: 10.1007/s00423-004-0472-0. [DOI] [PubMed] [Google Scholar]
- 39.Rosenne E, Shakhar G, Melamed R, et al. Inducing a mode of NK-resistance to suppression by stress and surgery: a potential approach based on low dose of poly I-C to reduce postoperative cancer metastasis. Brain Behav Immun. 2007;21:395–408. doi: 10.1016/j.bbi.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao Y, Pearman AT, Zimmerman GA, et al. Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci USA. 2000;97:11280–5. doi: 10.1073/pnas.200367597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zha S, Yegnasubramanian V, Nelson WG, et al. Cyclooxygenases in cancer: progress and perspective. Cancer Lett. 2004;215:1–20. doi: 10.1016/j.canlet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Kundu N, Walser TC, Ma X, et al. Cyclooxygenase inhibitors modulate NK activities that control metastatic disease. Cancer Immunol Immunother. 2005;54:981–7. doi: 10.1007/s00262-005-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacerdote P, Manfredi B, Bianchi M, et al. Intermittent but not continuous inescapable footshock stress affects immune responses and immunocyte beta-endorphin concentrations in the rat. Brain Behav Immun. 1994;8:251–60. doi: 10.1006/brbi.1994.1023. [DOI] [PubMed] [Google Scholar]
- 44.Lewis JW, Shavit Y, Terman GW, et al. Apparent involvement of opioid peptides in stress-induced enhancement of tumor growth. Peptides. 1983;4:635–8. doi: 10.1016/0196-9781(83)90010-4. [DOI] [PubMed] [Google Scholar]
- 45.Shavit Y, Martin FC. Opioids, stress, and immunity: animal studies. Ann Behav Med. 1987;9:11–15. [Google Scholar]
- 46.Shavit Y, Ben-Eliyahu S, Zeidel A, et al. Effects of fentanyl on natural killer cell activity and on resistance to tumor metastasis in rats. Dose and timing study. Neuroimmunomodulation. 2004;11:255–60. doi: 10.1159/000078444. [DOI] [PubMed] [Google Scholar]
- 47.Exadaktylos AK, Buggy DJ, Moriarty DC, et al. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–4. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beilin B, Shavit Y, Hart J, et al. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesth Analg. 1996;82:492–7. doi: 10.1097/00000539-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Gupta K, Kshirsagar S, Chang L, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–8. [PubMed] [Google Scholar]
- 50.Reuben SS, Connelly NR. Postoperative analgesic effects of celecoxib or rofecoxib after spinal fusion surgery. Anesth Analg. 2000;91:1221–5. doi: 10.1097/00000539-200011000-00032. [DOI] [PubMed] [Google Scholar]
- 51.Pitman RK, Sanders KM, Zusman RM, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–92. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 52.Vaiva G, Ducrocq F, Jezequel K, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54:947–9. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 53.Lutgendorf SK, Sood AK, Anderson B, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23:7105–13. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Spiegel D, Sephton SE, Terr AI, et al. Effects of psychosocial treatment in prolonging cancer survival may be mediated by neuroimmune pathways. Ann NY Acad Sci. 1998;840:674–83. doi: 10.1111/j.1749-6632.1998.tb09606.x. [DOI] [PubMed] [Google Scholar]