SUMMARY
We examined the contribution of the amygdala to value signals within orbital (OFC) and medial (MFC) prefrontal cortex. On each trial, monkeys chose between two stimuli that were associated with different quantities of reward. In intact monkeys, as expected, neurons in both OFC and MFC signaled the reward quantity associated with stimuli. Contrasted with MFC, OFC contained a larger proportion of neurons encoding reward quantity and did so with faster response latencies. Removing the amygdala eliminated these differences, mainly by decreasing value coding in OFC. Similar decreases occurred in OFC immediately before and after reward delivery. Although the amygdala projects to both OFC and MFC, these findings show that it has its greatest influence over reward-value coding in OFC. Importantly, amygdala lesions did not abolish value coding in OFC, which shows that OFC’s representations of the value of objects, choices and outcomes depends, in large part, on other sources.
Keywords: Amygdala, reward, affect, emotion, depression, orbital prefrontal cortex, orbitofrontal cortex, ventromedial frontal cortex, medial prefrontal cortex, decision making, choices, valuation, common currency, outcome, choice–outcome learning
INTRODUCTION
The primate prefrontal cortex, especially its orbital (OFC) and medial (MFC) subdivisions, plays a key role in guiding behavior adaptively on the basis of reward value (Rushworth et al., 2007; Wallis, 2007). For example, lesions of OFC and MFC cause deficits in learning the reward value of different actions or objects (Kennerley et al., 2006; Rudebeck et al., 2008; Walton et al., 2010), as well as impairments in updating such valuations as a function of current biological needs (Izquierdo et al., 2004).
Mirroring these findings, neurons within both OFC and MFC encode the association between rewards and different stimuli (Hayden et al., 2011b; Kennerley et al., 2009; Matsumoto et al., 2003; Thorpe et al., 1983; Tremblay and Schultz, 1999). In OFC, these signals encode the subjective value of potential choices (offers), the choices made, and the outcomes that result (Padoa-Schioppa and Assad, 2006). Values are encoded both for active (instrumental) choices and passive (Pavlovian) observation, and the comparison of aversive and positive values has established that these cells encode value, as opposed to the salience of stimuli or the attention oriented toward them (Cai and Padoa-Schioppa, 2012; Morrison and Salzman, 2009; Roesch and Olson, 2004; Schoenbaum et al., 1998).
Although a role for these areas in signaling reward value is well established, little is known about the origin of value signals in OFC and MFC. One possible source is the amygdala, which is reciprocally interconnected with both areas (Carmichael and Price, 1995; Ghashghaei et al., 2007). Like neurons in OFC and MFC, neurons in the amygdala encode the value of outcomes associated with stimuli (Paton et al., 2006). Lesions of the amygdala, like lesions of OFC and MFC, disrupt the ability to update and use reward value to guide behavior (Izquierdo and Murray, 2007). In addition, interaction between OFC and the amygdala is essential for updating the value of options in the form of objects to be chosen or avoided (Baxter et al., 2000; Schoenbaum et al., 2003).
Furthermore, humans with dysfunction and degeneration of the amygdala often suffer from a loss of positive affect (Bowley et al., 2002; Der-Avakian and Markou, 2012), and diminished reward-related activity in ventromedial frontal cortex, including OFC and MFC, accompanies a variety of affective disorders linked to amygdala dysfunction (Clark et al., 2009; Keedwell et al., 2005). Delineating the sources of reward signals in OFC and MFC should advance our understanding of how the prefrontal cortex contributes to positive affect, and of the mechanisms underlying reward-related neural activity more generally.
To address whether the amygdala is critical for encoding reward value in OFC and MFC, we recorded neural activity in these areas as three monkeys performed a choice task, both before and after bilateral lesions of the amygdala.
RESULTS
Task and behavior
We trained three monkeys to perform a choice task for fluid rewards. On each trial, monkeys had to press and hold a central button and then fixate a central light spot for 0.5–1.5 s (Fig. 1A). Two visual stimuli, associated with different amounts of fluid reward, were then sequentially presented. The onset of the second stimulus (S2) followed the onset of the first (S1) by 1.0 s and, by random selection, one stimulus appeared to the left of the central spot and one appeared to the right. We presented the two stimuli for choice sequentially in an attempt to separate the valuation process of the individual items. Stimuli were randomly selected from a pool of ten stimuli (Fig. 1B) with certain restrictions (see Supplemental Information). Monkeys had learned that each of the stimuli was associated with a fixed amount of fluid—either 0.8, 0.4, 0.2, 0.1 or 0 ml of water—two stimuli for each quantity. After a variable delay of 0.0–1.5 s, the central spot brightened as a “go” signal, and the monkeys could then choose between the two stimuli by reaching to the left or right response button. The amount of fluid reward corresponding to the chosen stimulus was delivered 0.5 s later. (Further details about the behavioral methods appear in Supplemental Information).
Figure 1.
Two-choice reward-guided task and intended recording locations. A) Monkeys initiated trials by pressing and holding a central button and fixating a central fixation spot for a variable interval. Two visually distinct stimuli were then sequentially presented and, after a brief delay, monkeys were free to choose between the two stimuli by selecting the button corresponding to the desired stimulus. The amount of reward corresponding to the chosen stimulus was then dispensed after another brief delay. B) On each trial, monkeys chose between pairs of stimuli randomly selected from a pool of ten stimuli. Each stimulus was associated with a discrete amount of fluid reward (two stimuli per amount, with amounts ranging from 0.1 to 0.8 ml). C) Intended recording tracks and locations in OFC (blue) and MFC (red) plotted on T1-weighted MRI coronal sections. Numerals indicate distance in mm from the interaural plane.
All three monkeys chose the stimulus associated with the greatest amount of fluid reward on more than 95% of trials (Fig. S1A). Choice response latencies, defined as the time elapsed from the onset of the “go” signal until the release of the central button, were modulated by the amount of reward associated with the choice (ANOVA, F(3,691)=23.87, p<0.0001, Fig. S1B).
Preoperative stimulus–reward encoding
While monkeys performed the task, we recorded the activity of 280 neurons in OFC (30 in monkey H, 101 in monkey N and 149 in monkey V) and 233 neurons in MFC (116 in monkey H, 106 in monkey N and 11 in monkey V, Fig. 1C).
In agreement with previous reports, the firing rate of many neurons in OFC and MFC correlated with the amount of reward associated with the stimuli presented for choice. For example, the neuron in Fig. 2A showed systematically greater activity for stimuli associated with smaller amounts of reward, and it did so for both S1 and S2. Other neurons showed the opposite pattern, firing more in response to stimuli associated with larger amounts of fluid reward (Fig. 2B).
Figure 2.
Stimulus–reward encoding within OFC and MFC. A, B) Spike density functions and raster plots depicting the activity of two neurons recorded within OFC. The neuron in A) exhibits the highest firing rate to stimuli associated with the smallest amount of reward and the lowest firing rate to stimuli associated with greatest amount of reward, while the neuron in B) exhibits the opposite relationship. The color code shows the amount of reward associated with each stimulus. Inset figures show the relationship between each neuron’s firing rate from a 200-ms window within the stimulus-1 and −2 periods and reward value of S1 and S2 respectively. C) Percent of neurons classified by a sliding hierarchical ANOVA as encoding a factor during either the reference period (“Ref”, 1.0 s before the onset of the first stimulus), the stimulus-1 period (“Stim 1”, the initial 1.0 s after the onset of the first stimulus) or the stimulus-2 period (“Stim 2”, the initial 1.0 s after the onset of the second stimulus). Abbreviations: Dir., direction; I.D., identity; S, stimulus. D) Time course of stimulus–reward value encoding in OFC (S1: dark blue; S2: cyan) and MFC (S1: red; S2: orange) following the presentation of the first stimulus. Green and black dots at the top indicate significant differences in encoding of S1 and S2 reward value, respectively (p<0.05, Gaussian approximation test), corrected for false discovery rate, between OFC and MFC.
To quantify these results, we conducted a sliding hierarchical ANOVA (see Experimental Procedures) on a 2.0-s period starting at the onset of the presentation of S1. This period was divided into two parts and neurons were classified as encoding a task factor within either the S1 or S2 periods. (Note, however, that S1 remained present during the S2 period.)
Neurons in OFC were more likely than those in MFC to encode the amount of fluid reward associated with both S1 (Fig. 2C, OFC: 37%, 103/280, MFC: 19%, 45/233, χ2 = 18.07, p<0.0001) and S2 (Fig. 2C, OFC: 31%, 87/280, MFC: 11%, 25/233, χ2 = 29.65, p<0.0001). In addition, a substantial proportion of neurons in both OFC and MFC continued to encode the amount of reward associated with S1 after S2 had been presented, with OFC neurons again being more likely to encode that amount (OFC: 24%, 67/280; MFC: 15%, 35/233, χ2 = 5.79, p=0.016, Fig. 2C). A smaller proportion of neurons in both OFC and MFC encoded stimulus identity and movement direction. In OFC, neurons were more likely to encode stimulus identity than movement direction, whereas the reverse was true for MFC (both comparisons: χ2 > 6.16, p<0.013). Neurons in OFC were also likely to encode the interaction between S1, S2 reward value and movement direction. Table S1 presents the full table of effects.
It is possible that during the S2 period, neurons might not have been signaling the reward value of S1 or S2 alone. Instead, neural activity might be more closely associated with the total reward value of S1 and S2, the difference in reward value between S1 and S2 or even the difference between the chosen and unchosen value. Additional analyses of the period after S2 presentation (stimulus-2 period) revealed that a greater number of neurons in both OFC and MFC signaled the reward value of S1 and S2 as opposed to any of these alternative task factors (Supplemental Information, Tables S1 and S2).
Encoding of stimulus–reward relationships occurred earlier in OFC (328 ± 17 ms, mean ± SEM) than MFC (506 ± 33 ms; ANOVA, effect of area, F(1,147)=24.86, p<0.00001; Fig. 2D and 3A). This effect was not driven by the firing rate of individual neurons within each area (effect of or interaction involving mean firing rate, F(1,147)<3.6, p>0.05). Not only did encoding of the amount of reward associated with S1 and S2 occur earlier in OFC compared to MFC, it was also more prevalent in OFC throughout the period when the two stimuli were present (Fig. 2D, green or black circles, Gaussian approximation test, p<0.05, false discovery rate (FDR) corrected).
Figure 3.
Latency and relative encoding of reward value in OFC and MFC. A) Cumulative distribution of the latency (in 10 ms bins) with which neurons in OFC (blue, n=103) and MFC (red, n=45) were classified as signaling the reward value associated with S1. Inset bar graph shows the mean latency of encoding for OFC and MFC (same color scheme as above). B) Percent of neurons in OFC (blue) and MFC (red) classified by a sliding hierarchical ANOVA as encoding the S2 reward value, S1 relative value or interaction between these two factors during the stimulus-2 period.
Preoperative comparison of responses to S1 and S2
We found that individual neurons in OFC and MFC often signaled the amount of reward associated with both stimuli (Fig. 2A). To explore this relationship, we first compared the proportion of neurons within each area that encoded the reward value of S1 and S2. While roughly equivalent proportions of neurons in OFC encoded S1 and S2 (103 vs. 87, χ2=1.79, p>0.15), fewer neurons in MFC encoded the value of S2 in comparison to S1 (45 vs. 25, χ2=6.07, p<0.02).
Next, we determined whether neurons that encoded the amount of reward associated with S1 also encoded the amount associated with S2. In OFC, if a neuron encoded the reward value of S1, it was likely to also encode the value of S2 (68/103 cells or 24.29% of the total OFC population). By contrast, a smaller proportion of neurons in MFC showed the same effect (12/45 cells or 5.15% of the total MFC population). Binomial tests revealed that the rate at which neurons in OFC and MFC signaled the reward value of both S1 and S2 was greater than expected by chance (p<0.01). Overall, there was a significant difference between the areas (χ2=17.98, p<0.0001).
Finally, we investigated the influence of the relative value of S1 on the encoding of S2 value (i.e. whether S1 was associated with a larger or smaller quantity of reward than S2). A sliding hierarchical ANOVA (see Experimental Procedures) was conducted on a 1.0-s period starting at the onset of the presentation of S2 to examine whether the encoding of S2 value was influenced by whether S1 was larger or smaller than S2. Based on this additional analysis, OFC neurons predominantly signaled the absolute value of S2 (29%, 80/280) as opposed to the relative value of S1 or S2 value as a function of S1 value (18% and 8%, 49 and 23/280, respectively, both comparisons χ2>9.06, p<0.003, Fig. 3B), which suggests that the neurons in OFC signal the value of S1 and S2 in a largely independent manner. This was not true in MFC, where similar proportions of neurons signaled the absolute value of S2 (9% or 21/233), relative value of S1 and S2 value as a function of S1 value (10% and 5%, 23 and 11/233 respectively, both comparisons χ2<2.72, p>0.05, Fig. 3B). Taken together with the previous section, these findings suggest that whereas OFC signals the value of S1 and S2 independently, MFC preferentially signals the value of S1.
Preoperative activity near reward
Many neurons in both OFC and MFC also encoded the amount of reward that monkeys received after making a successful choice, in keeping with previous reports. For example, the neuron illustrated in Fig. 4A shows an increase in firing rate that is dependent on the amount of expected reward following a successful choice. The neuron illustrated in Fig. 4B shows an increase in firing rate later, shortly after the start of reward delivery, and this increase persists until reward delivery is completed.
Figure 4.
Expected and received reward encoding in OFC and MFC. A, B) Spike density functions and raster plots depicting the activity of A) an OFC neuron and B) an MFC neuron during the expected (−500–0 ms) and received (0–1000 ms) reward periods. Raster plots are sorted by chosen amount of reward and both spike density functions and raster plots are color coded by reward amount. C) Percent of neurons classified as significantly modulated during either the expected (−500–0 ms before reward onset) or received reward periods (0–1000 ms after reward onset). D) Time course of chosen reward value encoding in OFC (blue) and MFC (red). Green dots indicate significant differences, false discovery rate (FDR) corrected, between OFC and MFC.
To quantify this result, we conducted a sliding hierarchical ANOVA (see Experimental Procedures) on a 1.5-s period around reward delivery (from 0.5 s before until 1.0 s after the onset of reward delivery). This period was then split into either the expected-reward period or the received-reward period, with the former corresponding to the 0.5 s period prior to reward onset.
Relative to MFC (8%, 18/233 cells), a greater percentage of neurons in OFC (21%, 60/280) encoded the amount of expected reward, a significant difference between the areas (χ2 = 15.9, p<0.001, Fig. 4C). In contrast, a similar proportion of neurons in both areas encoded the amount of received reward (31%, 88/280 cells in OFC; 28%, 66/233 cells in MFC; χ2 = 0.44, p=0.51). Both movement direction and chosen stimulus identity factors were only sparsely encoded in either OFC or MFC during this part of the trial (~5% of neurons in both OFC and MFC, Fig. 4C). Table S3 summarizes the results for all recorded neurons.
We next examined the time course of value-coding neural activity around the time of reward delivery (Fig. 4D). Encoding of the value of the chosen reward occurred significantly earlier in OFC than MFC. In OFC this signal appeared as an increase in the proportion of value-encoding cells at around the time of the choice. In MFC, the corresponding change occurred mainly after the onset of reward delivery. The greatest difference between the two areas occurred during the expected reward period (Fig. 4D). Thus, the activity of OFC neurons signaled expectation of reward quantity, whereas the activity of neurons in both areas signaled the receipt of a discrete quantity of reward.
Lesion effects on behavior
At the conclusion of the preoperative recordings, each monkey received bilateral injections of excitotoxin into the amygdala. Injections reliably eliminated both centromedial and basolateral groups of nuclei in all three of the monkeys (Fig. 5A). Based on microscope examination of Nissl-stained tissue, the injections of excitotoxins resulted in complete or nearly complete cell loss in the amygdala bilaterally (Mean: 95.5%; Range: 92.9 – 99.0%, Table S4). The extent of damage is similar to that reported in previous studies of the effects of amygdala lesions on reward-guided behavior (Izquierdo and Murray, 2007).
Figure 5.
Bilateral excitotoxic lesions of the amygdala and recording locations. A) T2-weighted MRI scans taken after bilateral injections of excitotoxin into the amygdala for each monkey (top row). Yellow arrows mark the boundaries of white hypersignal that reflects edema following injections. Nissl-stained coronal sections taken at corresponding levels (~17 mm anterior to the interaural plane) showing cell loss in the amygdala bilaterally (bottom row). B) Recording locations in OFC and MFC for each monkey plotted on ventral (OFC) and dorsal (MFC) views of the frontal lobe from a standard macaque brain. Larger symbols represent increasing numbers of neurons recorded at that location. Darker colors (OFC: blue ; MFC: red) represent preoperative recording locations, while lighter colors (OFC: cyan; MFC: orange) represent postoperative recording locations. C) Coronal T1-weighted MRI of electrode recording locations (left) and Nissl-stained sections of corresponding marking lesions (right) at three different anterior-posterior levels (+36, +32 and +28 mm anterior to the interaural plane) within OFC of monkey V. Yellow arrows denote the location of marking lesions.
Importantly, lesions of the amygdala did not alter monkey’s choice performance in the present task. Postoperatively, monkeys continued to choose the stimulus associated with the greatest amount of reward on nearly every trial (>95% of trials, Fig. S1A, ANOVA, effect of surgery, F(1,297)=0.02, p=0.29). Indeed, the present task was specifically designed, based on prior work, so that monkeys would be able to perform it adequately following amygdalectomy. While it might seem counterintuitive, this aspect of the experimental design and results aids in the interpretation of any changes in neural data. A deficit in performance following amygdalectomy would mean that changes in neural activity observed postoperatively could be attributed to altered behavior.
Choice latencies were altered postoperatively, although there was no systematic effect of the lesion; two monkeys showed a slowing, whereas one monkey exhibited a speeding of its choices (ANOVA, effect of surgery, F(1,925)=1.69, p=0.32; monkey by surgery interaction (2,925)=31.27, p<0.0001). What was clear, however, was that following lesions of the amygdala, each monkey’s response latencies remained modulated by the amount of reward that would be received on each trial (Fig. S1B, ANOVA, effect of reward size, F(3,925)=43.64, p<0.02).
We also addressed whether the amygdala lesions were behaviorally effective and altered choices based on reward value. To that end, monkeys were tested on a task virtually identical to the main choice task, but in which they had to learn new stimulus reward-value associations (Supplemental Information, Fig S2A). Three stimuli, novel at the start of each session, were used and each was associated with a different amount of fluid reward (0.0, 0.2 and 0.4 ml). On each trial monkeys were presented with two of the stimuli, randomly selected from the group of three, and were free to choose between them. Over the course of 60 trial-sessions, monkeys learned to choose the stimulus associated with the greater amount of fluid. Relative to their preoperative rates of learning, following amygdalectomy monkeys were slower to learn the quantity of reward associated with the different stimuli (effect of surgery, F(1,482)=25.73, p<0.0001, Supplemental Information, Fig S2B). Thus, lesions of the amygdala impaired monkeys’ abilities to learn and act upon reward value information in more taxing situations.
Lesion effects on stimulus–reward encoding
Postoperatively, we recorded the activity of 317 neurons in OFC (207 in monkey N and 110 in monkey V) and 237 in MFC (100 in monkey H, 137 in monkey N). There was a large degree of overlap between locations recorded pre- and postoperatively in both OFC and MFC (Fig. 5B). At the conclusion of the experiment, the locations and boundaries of OFC recordings were histologically verified (Fig. 5C).
Similar to the preoperative data, the firing rates of a large proportion of the neurons recorded in OFC and MFC were correlated with the amount of fluid reward associated with the visual stimuli. We first conducted two different tests to determine whether any alterations in firing rates postoperatively would bias our analyses to produce more type II (false-negative) errors (see Supplemental Information). The issue was that the decrease in both baseline and evoked firing rates that occurred in OFC neurons, but not in MFC neurons (Fig. S3), may have produced an excess of false-negative results for OFC. First, we inspected the residuals from the postoperative ANOVA, and we found them to be normally distributed for OFC. Second, we conducted a generalized linear model (GLM) with a Poisson distribution on a randomly selected subset of the cells and compared the results to those from an ANOVA (see Supplemental Information). These analyses indicated that the statistical tests applied to the preoperative data were also appropriate for the postoperative data.
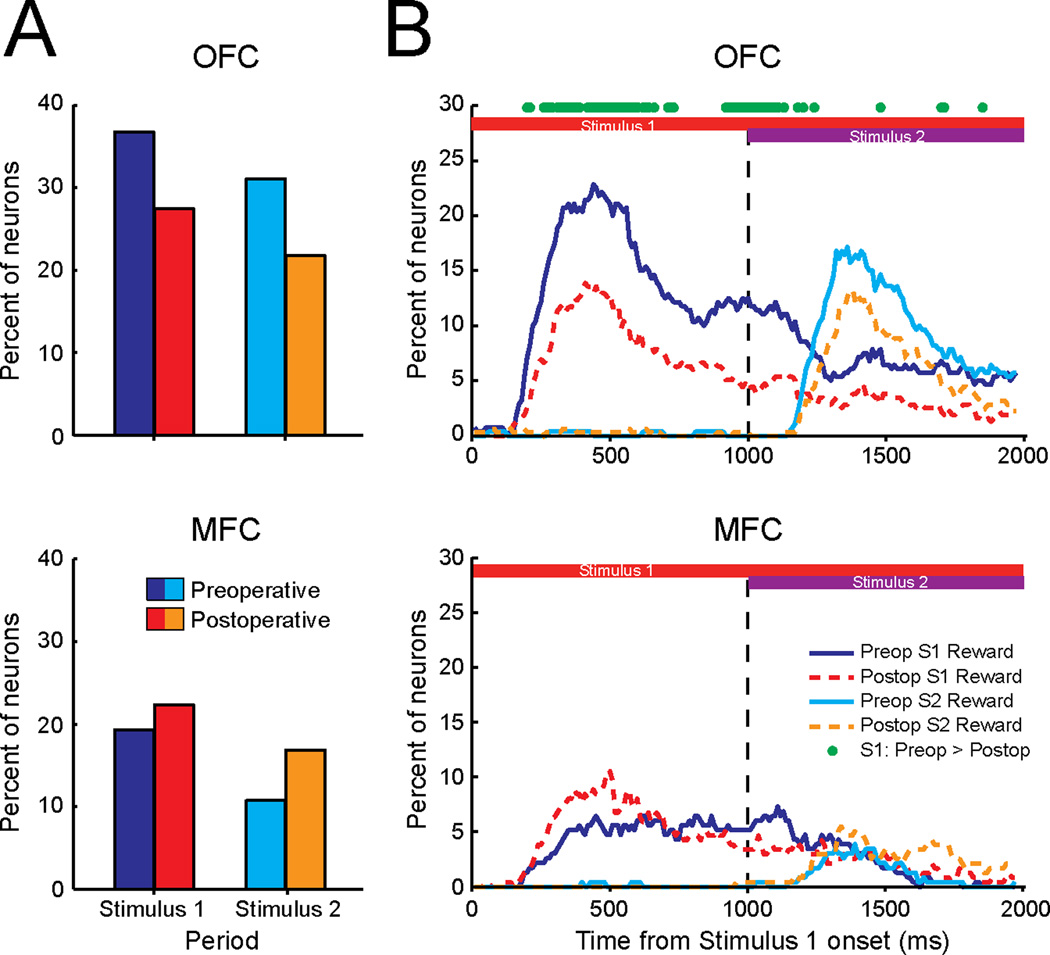
Applying the same sliding hierarchical ANOVA used for the preoperative data, we found that the proportion of OFC neurons that encoded the amount of reward associated with either S1 or S2 was reduced by amygdalectomy. Preoperatively, 37% (103/280) and 31% (87/280) of the OFC neurons recorded encoded the value of S1 and S2, respectively. Postoperatively, these percentages decreased to 27% (87/317) and 22% (69/317), respectively. Pre- and postoperative percentages differed significantly for both S1 and S2 (χ2 >5.56, p<0.018, Fig. 6A).
Figure 6.
The effect of excitotoxic lesions of the amygdala on stimulus–reward encoding in OFC and MFC. A) Percent of neurons in OFC and MFC encoding the amount of reward associated with the first (S1) or second stimulus (S2), pre- (blue/cyan) and postoperatively (red/orange). B) Time course of stimulus-reward encoding within OFC (top) and MFC (bottom) pre- (blue/cyan) and postoperatively (red/orange). Green dots indicate significant differences, FDR corrected, between pre- and postoperative proportions.
In contrast, the proportion of MFC neurons that encoded the amount of reward associated with either S1 or S2 did not decrease after amygdalectomy. Preoperative percentages of cells encoding the value of S1 and S2 were 19% (45/233) and 11% (25/233), respectively, and postoperatively these percentages were 22% (53/237) and 17% (40/237). Neither of these increases reached statistical significance (χ 2 < 3.23, p>0.07). As a result of amygdalectomy, however, the preoperative differences between OFC and MFC in encoding stimulus–reward value were eliminated (χ2 <1.75, p>0.18 for both comparisons). Amygdalectomy did not alter the proportion of neurons encoding the direction of the monkeys’ reaching movements or the identity of S1 or S2 (Table S5), indicating that the changes in neural activity in OFC and MFC following amygdala lesions are primarily associated with reward processing, at least in this task.
To further investigate this differential change in stimulus–reward encoding between the two areas, we conducted a logistic regression analysis on the proportion of neurons encoding the amount of reward associated with S1 and S2, using as factors both surgery (pre vs. post), area (OFC vs. MFC) and task period. This analysis confirmed a surgery by area interaction (χ2=8.81, p=0.003), suggesting an asymmetric change across the two areas following amygdalectomy.
We also assessed whether cumulative damage to the OFC or MFC through repeated electrode penetrations, as opposed to amygdalectomy, could account for our results. Postoperatively, there was a sharp, not a progressive, decrease in the proportion of neurons signaling reward value in OFC and a corresponding increase in MFC (Fig. S4A), indicating that amygdalectomy was the critical factor.
We then compared the time course of stimulus-reward encoding in OFC and MFC pre- and postoperatively. Postoperatively, the time course of encoding followed a similar pattern to that observed preoperatively, although fewer neurons in OFC encoded the amount of reward associated with S1 (Gaussian approximation test, p<0.05 FDR, green circles, Fig. 6B). This analysis did not reveal any significant changes in the proportion of neurons in MFC encoding the amount of reward associated with either S1 or S2.
In intact monkeys, the onset of stimulus–reward encoding was found to occur earlier in OFC than in MFC. Without amygdala input, the onset of stimulus-reward encoding was shifted slightly later in OFC (Mean ±SEM: 390.7±20.7 ms) and slightly earlier in MFC (Mean ±SEM: 433.2±28.5 ms, Fig. 7A). Comparisons of the first significant bin for all neurons, recorded pre- and postoperatively, within OFC and MFC confirmed this impression (repeated-measures ANOVA, surgery by area interaction, F(1,287)=8.76, p=0.0033, Fig. 7A). This effect could not be accounted for by the firing rate of individual neurons within each area (effect of or interaction involving mean firing rate, F(1,287)<3.62, p>0.05). Thus, amygdalectomy also altered the timing differences for S1 and S2 value encoding.
Figure 7.
Pre- and postoperative latency and relative reward value encoding in OFC and MFC. A) Cumulative distribution of the latency (in 10 ms bins) with which neurons in OFC (top) and MFC (bottom) were classified as signaling the reward value associated with S1 both pre- (blue) and postoperatively (red). Inset bar graphs show the mean latency of encoding for OFC and MFC pre- and postoperatively (same color scheme as above). B) Percent of neurons in OFC (top) and MFC (bottom) classified by a sliding hierarchical ANOVA as encoding S2 reward value, S1 relative value or interaction between these two factors during the stimulus-2 period both pre- and postoperatively. In both plots, blue bars or lines represent preoperative data and red bars or lines represent postoperative data.
Lesion effects on the comparison between responses to S1 and S2
In intact monkeys, we found that neurons in OFC were more likely than those in MFC to encode the reward value of both S1 and S2 (Fig. 3). We investigated what effect amygdala lesions would have on this property. As was the case preoperatively, there was no difference in the proportion of neurons in OFC signaling values associated with S1 and S2 (87 vs 69/317; χ2 = 2.46, p > 0.12). Contrary to the preoperative data, this was also true in MFC; the difference in the proportion of neurons in MFC signaling the value associated with S1 and S2 that was apparent preoperatively was not evident postoperatively (53 vs 40/237, χ2 = 1.9, p > 0.16).
Despite the changes in stimulus–reward encoding following lesions, other differences between the two frontal cortical regions survived amygdalectomy. For example, individual neurons in OFC were still more likely to encode the reward value of both stimuli compared to neurons in MFC (OFC: 50/87; MFC: 12/53, χ 2 =14.81, p<0.0001). Thus, postoperatively, individual OFC neurons remained more likely to encode the value of both S1 and S2 than was the case in MFC, although a comparable proportion of MFC neurons encoded both values, as noted in the previous paragraph (Fig. 6A).
Finally, we conducted the same sliding window ANOVA on the period after the presentation of S2 to examine the influence of S1 on S2 value encoding. Before lesions, we found that neurons in the OFC predominantly signaled the value of S2 independently of S1, whereas similar proportions of neurons in MFC signaled the value of S2 as well as the relative value of S1. As was the case before amygdala lesions, neurons in the OFC principally signaled the value of S2 (22%, 70/317) as opposed to the relative value of S1 or S2 as a function of S1 following amygdalectomy (10% and 6%, 31 and 18/317 respectively, both comparisons, χ2>17.01, p<0.0001, Fig. 7B). Mirroring the previously observed changes after amygdalectomy, more MFC neurons signaled the value of S2 (16%, 38/237) compared to the relative value of S1, or S2 value as a function of S1 (8% and 5%, 18 and 11/237 respectively, both comparisons χ2>731, p<0.01, Fig. 7B). Any differences between OFC and MFC were, similar to the aforementioned analyses, abolished postoperatively (all comparisons p>0.5). Thus, in contrast to before amygdalectomy, encoding of the value of S1 and S2 is signaled in a largely independent manner after in both OFC and MFC.
Lesion effects on activity near reward
Later in each trial, amygdalectomy caused a sizable change in the proportion of neurons that encoded the expected- and received-reward quantity, and OFC and MFC again differed in this respect. Using the same sliding-window hierarchical ANOVA as before, we found that, compared to the preoperative data, significantly fewer neurons in OFC encoded the amount of expected reward after amygdala lesions (21%, 60/280, preoperatively vs. 13%, 39/317, postoperatively; χ2 =8.3, p<0.005). As illustrated in Fig. 8, amygdalectomy caused no such change in MFC (8% both pre- and postoperatively; 18/233 vs. 19/237, χ2=0, p=1). Amygdalectomy abolished the difference between the two areas in the proportion of neurons encoding the quantity of expected reward (χ2 =2.22, p>0.13).
Figure 8.
The effect of excitotoxic lesions of the amygdala on expected and received reward encoding in OFC and MFC. A) Percent of neurons encoding the expected and received reward value in OFC (top) and MFC (bottom) pre- and postoperatively. After removal of the amygdala, there was a significant reduction in the proportion of neurons encoding the value of expected and received reward in OFC. B) Time course of encoding of chosen reward value in OFC (top) and MFC (bottom), pre- and postoperatively. Green dots indicate significant differences, FDR corrected, between pre- and postoperative proportions of neurons. In all plots blue bars or lines denote preoperative data and red bars or lines denote postoperative data.
There was also a postoperative decrease in the proportion of OFC neurons encoding the amount of received reward (31%, 88/280, preoperatively vs. 22%, 70/317, postoperatively; χ2 =6.02, p=0.013), whereas no such change occurred in MFC (28%, 66/233, preoperatively vs. 25%, 60/237, postoperatively; χ2 =0.17, p>0.68). Encoding of identity of the chosen stimulus and movement direction were largely unchanged postoperatively in both OFC and MFC (Table S6). The decrease in the proportion of neurons signaling reward value within either the expected or received periods could not be attributed to cumulative damage within OFC. Following amygdalectomy, there was a sharp, not a progressive, decrease in encoding of reward value (Fig. S4B).
To further explore the effect of amygdala lesions on reward encoding across OFC and MFC, we compared the time course of encoding near the time of reward delivery for neurons classified as encoding chosen reward value (Fig. 8B). In OFC, there was a consistent and pervasive decrease in the encoding of the amount of both the expected and received reward (Gaussian approximation test, p<0.05, FDR corrected). By contrast, no such change occurred in MFC. Although there was a slight decrease in the proportion of MFC neurons encoding received reward ~900 ms following reward delivery, no comparisons survived correction for the FDR. Taken together, these data indicate that the encoding of expected and received reward was affected by amygdalectomy in OFC, but not in MFC.
DISCUSSION
OFC and MFC both play a crucial role in signaling the emotional or rewarding value of stimuli and actions (Rushworth et al., 2007), and disruptions of these signals are associated with a variety of psychological disorders (Clark et al., 2009; Murray et al., 2011). The origins of these prefrontal reward-value signals, however, remain unknown. To investigate the contribution of the amygdala to affective valuations in the prefrontal cortex, we recorded from neurons in OFC and MFC of macaques before and after amygdala lesions.
Preoperatively, as expected from previous neurophysiological studies, neurons in both OFC and MFC encoded the amount of reward associated with the two stimuli presented for choice (Fig. 2C, D). The properties of the two areas differed, however: (1) more OFC than MFC neurons encoded the amount of reward associated with stimuli (Fig. 2C); (2) OFC tended to encode the value of both stimuli, whereas MFC preferentially encoded the value of S1 (Fig. 3B); and (3) OFC encoded the amount of reward after the choice but prior to reward onset, whereas MFC did so mainly during and after reward delivery (Fig. 4C, D).
Lesions of the amygdala affected value encoding appreciably more in OFC than in MFC. Removing inputs from the amygdala significantly reduced the encoding of reward value in OFC, in both the stimulus period and around the time of reward delivery (Figs. 6B and 8B). The same lesion, however, had no significant impact on value encoding in MFC (Figs. 6B and 8B). As a consequence of its differential effects on the two areas, amygdalectomy abolished most of the preoperative differences between OFC and MFC in terms of value coding. These data provide the first evidence for a causal contribution of the amygdala to neural activity related to reward valuations within the macaque prefrontal cortex, and they reveal that the amygdala makes its most important contribution to reward valuations in OFC, as opposed to MFC.
Recordings within the MFC were, however, limited to one subdivision of this part of frontal cortex that receives projections from the amygdala (Ghashghaei et al., 2007). It is possible that recording in other parts of the MFC, notably the ventral bank of the cingulate sulcus, might have produced different results to those reported here (Cai and Padoa-Schioppa, 2012).
It is important, however, not to overstate the effect of the amygdala lesions on value coding in prefrontal cortex. Amygdalectomy reduced the proportion of value-encoding cells but did not abolish encoding of reward value completely. Thus, the prefrontal cortex, in general, and OFC, in particular, appears to have both amygdala-dependent and amygdala-independent reward encoding networks. We can only speculate on the amygdala-independent sources of valuation signals. One possibility involves direct projections from the dopaminergic neurons of the midbrain (Williams and Goldman-Rakic, 1998). Thalamo-cortical projections that relay inputs from the substantia nigra, basal forebrain (including substantia innominata and ventral pallidum) and brainstem might also contribute (Russchen et al., 1987).
Interpretational issues
In addition to the possible statistical anomalies caused by different activity rates pre- and postoperatively, which we addressed in the Results and Supplemental Information, our experimental design raises some inherent interpretational issues. Ideally, an examination of amygdala contributions to reward value coding would involve the study of the activity of individual neurons before, during and after recovery from reversible inactivation of the amygdala. In this initial study, however, we adopted a different approach. We recorded from separate neural populations before and after amygdalectomy. This experimental design raises several questions: (1) was the amygdala lesion complete?; (2) was the neuronal sample taken from the same regions and types of neurons pre- and postoperatively?; (3) was the decrease in reward value signals observed simply due to cumulative damage to the OFC and MFC?
The extents of the amygdala lesions were confirmed by histological analysis. The lesions were largely as intended (Fig. 5A, Table S4). All nuclei in the amygdaloid complex suffered complete or nearly complete damage bilaterally, including the basal and accessory basal nuclei, the main source of amygdala projections to the OFC (Ghashghaei et al., 2007). Inadvertent damage was, by contrast, variable in extent and asymmetric between hemispheres across subjects.
In both OFC and MFC, locations of the recorded cells varied little between the pre- vs. postoperative samples (Fig. 5B). Although we cannot rule out any difference, it seems unlikely that a systematic bias in the location of cells within either area contributed to our conclusions about the effects of amygdalectomy.
Neuronal sampling bias, toward larger and more active units, needs to be considered in any study of this kind. Size bias should have little impact because we do not expect any significant change in the distribution of neuron sizes postoperatively. Activity is a more likely source of bias (Fig. S3). Our neuron search criteria minimized this potential source by selecting and isolating neurons when the monkey had yet to begin task performance, so this source also seems unlikely to have influenced our principal results.
By recording in the same monkeys and areas both pre- and postoperatively, it is possible that the effects reported might simply be due to cumulative damage from repeated electrode penetrations. There are two reasons to think that this is not the case. First, despite a similar number of penetrations in MFC, we did not observe a decrement in the proportion of neurons signaling reward value over time or postoperatively. Instead, in certain parts of the trial we actually found an increase in signaling in MFC (Fig 6A). Second, we directly assessed how the proportion of neurons signaling reward value changed over time. We found that amygdalectomy essentially produced step-function changes in the proportion of neurons signaling reward value (Fig S4).
Relation to previous studies
Our preoperative finding that OFC and MFC differentially encode the reward value associated with distinctive visual stimuli in intact monkeys agrees with several previous reports (Cai and Padoa-Schioppa, 2012; Matsumoto et al., 2003; Padoa-Schioppa and Assad, 2006; Roesch and Olson, 2004; Tremblay and Schultz, 1999). We could not confirm, however, the finding that the encoding of reward value is more prevalent within MFC than within OFC (Kennerley et al., 2009). Perhaps this difference results from the relative simplicity of our experimental design. Unlike the monkeys studied by Kennerley et al., ours never had to consider decision variables such as effort, delay or probability of reward.
Emerging evidence suggests that neurons in OFC encode the reward value of different options in a predominantly invariant (e.g., absolute or “menu-independent”) way (Padoa-Schioppa and Assad, 2008). In our preoperative data, OFC cells tended to encode the value of both stimuli, whereas MFC encoded S1 preferentially. This finding makes it unlikely that MFC played an important role in the comparison between the two options, which cannot occur until S2 onset. OFC could contribute to this comparison, however, a finding consistent with the results of lesion studies of OFC (Rudebeck and Murray, 2011b; Walton et al., 2010). The reward-value signals for S1 and S2 could be compared within OFC or transmitted to other parts of the brain for comparison, choice and action (Boorman et al., 2009; Noonan et al., 2010; Rudebeck and Murray, 2011a).
In accord with previous reports, neurons in OFC and MFC encoded the amount of reward after a choice and near the time of reward (Cai and Padoa-Schioppa, 2012; Kennerley and Wallis, 2009; Padoa-Schioppa and Assad, 2006). By delaying the delivery of reward, we could compare the encoding of expected and received reward. In our preoperative data, OFC neurons encoded value before reward delivery and this value-related activity began at about the time of the monkeys’ choices. Neurons in MFC, in contrast, predominantly encoded the amount of reward after reward delivery (Fig. 4D). Note that “reward expectancy”, in this sense, occurs after the monkeys’ decisions, choices and actions have been made, thus extending the previously reported role of OFC in signaling reward expectancy in order to make decisions or choices (Holland and Gallagher, 2004; Schoenbaum et al., 2009). The present data also extend previous findings by showing that such reward or outcome expectancy signals are largely absent in monkey MFC, either before or after the choice. If representative of the entire MFC, our results support the idea that MFC has a larger role in the evaluation of choices after an outcome has occurred, or, at least, has begun (Hayden et al., 2011a; Ito et al., 2003; Walton et al., 2004).
Only two previous studies have investigated the impact of amygdala lesions on reward-related neural activity within frontal cortex, one in humans and one in rats (Hampton et al., 2007; Schoenbaum et al., 2003). The present study is the first of its kind in monkeys and the first to record both before and after lesions of the amygdala in multiple parts of the frontal lobe.
Our findings complement and extend the aforementioned studies in rats and humans. There are, however, some key differences. First, the present data show that both expected and received reward signals are attenuated in OFC following amygdala lesions. By contrast, the previous work in rats and humans reported that expected-reward, but not received-reward, signals were reduced in OFC (the ventromedial frontal cortex in humans) of subjects without an amygdala. One possibility is that this discrepancy may reflect differences in experimental design. Lesions were made prior to testing in the previous studies, whereas the present study recorded neural activity pre- and postoperatively. Studying the same subjects before and after lesions is important as it controls for inter-subject variability. Alternatively, these divergent results could reflect variation in task structure. In the human study, subjects had to learn and reverse the association between stimuli and rewards (outcomes) during the task. In the present study, the association between stimuli and reward amount was well learned and never changed. In the study by Schoenbaum and colleagues in rats, it was reported that amygdala lesions resulted in an increase in the proportion of neurons signaling stimulus-specific information in OFC. We did not see such an increase following lesions. This divergence in findings could again be the result of different paradigms; the rat study involved learning whereas in the present experiment, performance was based on previously learned stimulus–reward associations. Alternatively, the divergent results may reflect differences in task structure. The stimulus–reward task used by Schoenbaum et al. (2003) included a reversal phase in which the association between two stimuli and two distinct outcomes changed. The present task used neither reversals nor distinct types of outcomes. A change in the mappings between stimuli and rewards may have heightened differences in stimulus-specific encoding between controls and rats with amygdala lesions. Finally, the discrepant results could stem from species differences. The homology between rat and human OFC remains much less certain than that between OFC of monkeys and humans (Passingham and Wise, 2012).
Amygdala contributions to prefrontal reward value encoding
Interaction between the amygdala and the prefrontal cortex is critical for emotion and reward guided behavior (Holland and Gallagher, 2004). In this report, we have described changes in reward coding caused by amygdalectomy in a primate, studying two areas that have clear homologues among Old World monkeys, apes and humans. These lesion-induced encoding changes suggest that signals from the amygdala provide a specific type of reward-related information to OFC (but not MFC), potentially signaling the value of specific sensory features of predicted outcomes (Balleine and Killcross, 2006; Baxter and Murray, 2002), the relative reward value of various choice options, and updating the value of choice options in terms of current needs (Baxter et al., 2000).
The differential effect of amygdala lesions on OFC and MFC suggests that OFC–amygdala and MFC–amygdala pathways may be functionally distinct. Lesions of MFC in monkeys do not affect the ability to update the value of rewarded objects or actions (Chudasama et al., 2012), and interaction between MFC and amygdala is not critical for signaling or updating reward value (Coutureau et al., 2009). Interaction between MFC and amygdala may be important for assessing the energetic costs associated with obtaining food rewards (Floresco and Ghods-Sharifi, 2007). In summary, whereas interactions between OFC and the amygdala may be critical for computations of reward value (benefits), interaction between MFC and the amygdala may be more important for assessing the cost of acting (Rushworth et al., 2007).
Amygdala, and prefrontal contributions to emotion and mental health
A long line of research has implicated the primate amygdala in reward valuation processes and emotion (Baxter and Murray, 2002). Because of its role in regulating emotion, dysfunction of the amygdala is thought to play a central role in psychological disorders, including depression, obsessive-compulsive disorder and post-traumatic stress disorder (Clark et al., 2009; Davis and Whalen, 2001). For example, degeneration has been reported in the amygdala of patients suffering from major depressive disorder (Bowley et al., 2002). Our data suggest that any effect of dysfunction or degeneration within the amygdala would primarily affect the processing of emotion and reward value within OFC. The close homology between OFC in monkeys and humans, mentioned above, provides further support for this conclusion.
In keeping with this idea, reduced reward related activity in ventromedial prefrontal cortex, which is usually taken to include the OFC, has been reported in patients suffering from depression (Keedwell et al., 2005). Such a correspondence in findings adds further weight to the idea that dysfunction within a network that includes OFC and the amygdala may contribute to symptoms such as anhedonia. Dysfunction of this network is thought to underlie a number of psychological disorders, including those mentioned above (Der-Avakian and Markou, 2012; Murray et al., 2011).
Our results demonstrate the value of combining recordings and lesions within the same subjects to probe specific, anatomically grounded contributions to reward valuation and emotion. The present results therefore provide a rare insight into the amygdala’s contributions to value encoding in the brain structures, such as OFC, that are heavily implicated in psychological disorders.
EXPERIMENTAL PROCEDURES
Subjects
Three adult male rhesus macaques (Macaca mulatta), monkeys H, N and V, served as subjects; they weighed 8.5, 8.0 and 8.4 kg, respectively, at the beginning of training. Animals were pair housed when possible, kept on a 12-h light dark cycle and had access to food 24 hours a day. Throughout training and testing each monkey’s access to water was controlled for 6 days per week. All procedures were reviewed and approved by the NIMH Animal Care and Use Committee.
Apparatus
Monkeys were trained to perform a two-choice visually guided task for water reward. All trial events and timing were controlled using the open source program NIMH Cortex (ftp://helix.nih.gov/lsn/cortex/). Eye position and pupil size were monitored and acquired at 60 frames per second with an infrared occulometer (Arrington Research, Scottsdale, AZ).
During training and testing monkeys sat in a primate chair with their heads restrained. Directly in front of the chair, three buttons were spaced horizontally 7 cm apart (center to center). These buttons had embedded infrared sensors to detect contact.
Surgical procedures, neural recordings, imaging and histological reconstruction
For detailed information on surgical procedures, see Supplemental Information. In brief, each monkey was implanted with a titanium head restraint device and then, in a separate surgery, a plastic recording chamber was placed over the exposed dura mater of the left frontal lobe. After the preoperative recordings were completed, MRI-guided bilateral excitotoxic lesions of the amygdala were made in each monkey.
Potentials from single neurons were isolated with tungsten microelectrodes (FHC, Inc. or Alpha Omega, 0.5–1.5 MΩ at 1 KHz) advanced by an 8-channel micromanipulator (NAN instruments, Nazareth, Israel) attached to the recording chamber. Spikes from putative single neurons were isolated online using a Plexon Multichannel Acquisition Processor and later verified with Plexon Off Line Sorter on the basis of principal-component analysis, visually differentiated waveforms and interspike intervals. Neurons were isolated before monkeys were engaged in any task. Other than the quality of isolation, there were no selection criteria for neurons.
OFC recordings were made on the ventral surface of the frontal lobe between the lateral and medial orbital sulci, roughly corresponding to Walker’s (1940) areas 11 and 13. All OFC recordings were between 27 and 38 mm anterior to the interaural plane. Neurons in MFC were primarily recorded in the dorsal bank of the cingulate sulcus (areas 9 and 24), although some sites were in the ventral part of the fundus of the cingulate sulcus. MFC neurons were recorded between the anterior tip of the cingulate sulcus (approximately +38 mm) and +24 mm.
Both before and after lesions of the amygdala, recordings were made in overlapping regions in each of the three monkeys (Fig. 5B). Recording sites were verified by T1-weighted MRI imaging of electrodes after recording sessions and by placing electrolytic marking lesions (15 µA direct current for 25 seconds, electrode positive) at selected locations in OFC after recordings had been completed (Fig. 5C). At the conclusion of the study, monkeys were deeply anesthetized and transcardially perfused with saline (0.9%) followed by formalin. The brains were removed, sectioned in the coronal plane, Nissl-stained and mounted onto glass slides for visual inspection. Marking lesions were clearly visible in both posterior and anterior parts of OFC, confirming the boundaries of our recording zone in OFC (Fig. 5C).
The extent and location of the amygdala lesions was assessed using T2-weighted MRI conducted within one week of each surgery (Fig. 5A, top row). Lesion volume was then confirmed from histology (Fig. 5A, bottom row and Table S4). The locations and extents of the lesions were largely as intended. There was near complete cell loss in all nuclei in the amygdaloid complex (mean = 95.5%). Inadvertent damage was evident in the entorhinal and perirhinal cortex (see Table S4). In addition, portions of the ventral claustrum and anterior hippocampus sustained slight cell loss. Importantly, with the possible exception of the entorhinal cortex, this unintended damage was slight (e.g., extending less that 2 mm in antero-posterior extent) and asymmetric between the hemispheres. Finally, one monkey (Monkey N) sustained an infarction in the dorsal striatum, bilaterally. Overall, damage in all three monkeys consistently centered on the amygdala, bilaterally.
Data analysis
Behavioral data
Correct trials were defined as those involving a choice of the stimulus associated with the greatest amount of reward. Choice response latency was defined as the interval from the onset of the “go” signal until release of the central button. Both measures were calculated for each monkey and analyzed using ANOVA with factors of surgery (2 levels), monkey (3 levels, random effect), sessions (52–118, random effect nested below monkey) and reward size (4 levels, choice response latency analysis only). For the task in which monkeys learned the association between novel stimuli and reward quantity (Supplemental Information, Fig. 2), data were analyzed using ANOVA with factors of surgery (2 levels, fixed effect), block (four levels, fixed effect), monkey (3 levels, random effect) and session (22–25 levels, random effect nested below monkey).
Neural data
To identify task-related neurons, all trials on which monkeys chose one of the two stimuli were analyzed (i.e., all trials on which monkeys did not break fixation or release the central button early, and arrived on one of the two choice buttons within the permitted time). Within those trials, two separate periods were analyzed: a stimulus period 0–2000 ms from the presentation of stimulus 1 and a reward period −500 ms to +1000 ms from reward delivery onset.
A sliding hierarchical ANOVA model was fitted to the firing rates of each neuron (200 ms bins starting at 0 ms, advanced in 10-ms increments) in each of these two periods. These parameters for the sliding window analysis were selected based on previous work in the field (for example, Kennerley et al., 2009). Changing the size of the bins, either ±50 ms, did not alter the results. For the stimulus period, the model included factors of stimulus-1 reward amount (5 levels), stimulus-2 reward amount (5 levels), stimulus-1 identity (10 levels), stimulus-2 identity (10 levels) and movement direction (2 levels). Factors of stimulus-1 and −2 identity were nested within stimulus-1 and −2 reward amount respectively.
For the ‘reward’ period, the model included factors of chosen reward amount (4 levels), chosen stimulus identity (8 levels) and movement direction (2 levels). Chosen reward amount only included 4 levels because the monkeys almost never chose the stimuli associated with no fluid reward (0.0 ml in Fig. 1B). Chosen stimulus identity was nested within chosen reward amount. Main factors and first-order interactions were included in both models.
Two additional analyses were conducted on the stimulus-2 period, 1000–2000 ms from the presentation of S1. As above, we fitted a sliding hierarchical ANOVA model to the firing rates of each neuron (200 ms bins starting at 0 ms, advanced in 10-ms increments) in this period. In the first of these analyses, the sum of the reward value of S1 and S2 (10 levels), the difference in value between S1 and S2 (11 levels) as well as the difference in value between the chosen and unchosen stimulus (6 levels) were included in the model with movement direction (2 levels). For the second, the model included factors of stimulus-2 reward amount (5 levels), relative value of S1 compared to S2 (2 levels) and the interaction between these two factors.
For the analysis of the stimulus, reward and stimulus-2 periods, neurons were classified as encoding a factor or interaction if four consecutive bins of the sliding window analysis had p values below 0.0005. These criteria were chosen based on analyses of a 1000-ms reference period, which occurred prior to the onset of the first stimulus, using the same sliding window ANOVA analysis conducted on the stimulus and reward periods. The criteria of p<0.0005 and four consecutive bins at this p level produced less than 3% positive results for main factors on the reference-period activity, which we take to be type I errors. We also assessed these criteria on the stimulus-period using a permutation test (1,000 repetitions). This additional analysis revealed that the threshold of 4 consecutive bins at p<0.0005 produced type-1 errors at a rate of 5.6%. Differences in the numbers of neurons in OFC and MFC classified as encoding a factor were subsequently assessed using chi-square tests.
Time course analyses plotted the proportion of neurons in OFC and MFC at 10 ms intervals that were classified as encoding a particular factor (e.g., Fig. 2D, S1 reward). Comparisons between the proportion of neurons in OFC and MFC over time used the Gaussian approximation (Zar, 1999), corrected for multiple comparisons using a false discovery rate (FDR) correction (Benjamini and Yekutieli, 2001).
Timing analyses were also conducted to assess when neurons in either OFC or MFC encoded a particular factor. The latency was defined as the 10-ms bin in the sliding window analysis at which each neuron was classified as significant (i.e., the fourth bin below p<0.0005). The latency of each neuron was then square root transformed to stabilize the variance for data sets with different means. Differences between OFC and MFC in the onset of neural signals were then determined using repeated-measures ANOVA with factors of surgery (2 levels) and area (2 levels) where appropriate for the dataset (pre- vs postoperative). A continuous variable of mean firing rate for each neuron was also included to account for differences in signal strength between areas.
Additional analyses were conducted to assess whether the hierarchical ANOVA models applied to the preoperative data were appropriate to apply to the postoperative data given slight alterations in firing rate following lesions of the amygdala. As explained in the Supplemental Information, two different approaches were taken and each indicated that applying the same ANOVA to the pre- and postoperative data was appropriate for the present data set.
Supplementary Material
HIGHLIGHTS.
Neurons in OFC and MFC differentially encode the reward value of stimuli.
Lesions of the amygdala reduce reward coding in OFC, but not in MFC.
Amygdala lesions abolish differences between OFC and MFC in reward coding.
Amygdala input is not necessary for reward coding in either OFC or MFC.
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the National Institute of Mental Health. We thank Kevin Blomstrom, Kevin Fomalont and Joshua Ripple for assistance with data collection, and James Fellows, Ping Yu Chen and David Yu for help with surgery and histology. We are indebted to Steven Wise and Bruno Averbeck for comments on analyses and an earlier version of the manuscript. The authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: None
REFERENCES
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. Journal of Neuroscience. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annals of Statistics. 2001;29:1165–1188. [Google Scholar]
- Boorman ED, Behrens TE, Woolrich MW, Rushworth MF. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62:733–743. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Cai X, Padoa-Schioppa C. Neuronal encoding of subjective value in dorsal and ventral anterior cingulate cortex. J Neurosci. 2012;32:3791–3808. doi: 10.1523/JNEUROSCI.3864-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Daniels TE, Gorrin DP, Rhodes SE, Rudebeck PH, Murray EA. The role of the anterior cingulate cortex in choices based on reward value and reward contingency. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: implications for treatment. Annu Rev Neurosci. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Coutureau E, Marchand AR, Di Scala G. Goal-directed responding is sensitive to lesions to the prelimbic cortex or basolateral nucleus of the amygdala but not to their disconnection. Behav Neurosci. 2009;123:443–448. doi: 10.1037/a0014818. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb Cortex. 2007;17:251–260. doi: 10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, Adolphs R, Tyszka MJ, O'Doherty JP. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55:545–555. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Heilbronner SR, Pearson JM, Platt ML. Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. J Neurosci. 2011a;31:4178–4187. doi: 10.1523/JNEUROSCI.4652-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Pearson JM, Platt ML. Neuronal basis of sequential foraging decisions in a patchy environment. Nat Neurosci. 2011b;14:933–939. doi: 10.1038/nn.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci. 2007;27:1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Dahmubed AF, Lara AH, Wallis JD. Neurons in the frontal lobe encode the value of multiple decision variables. J Cogn Neurosci. 2009;21:1162–1178. doi: 10.1162/jocn.2009.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Wallis JD. Evaluating choices by single neurons in the frontal lobe: outcome value encoded across multiple decision variables. Eur J Neurosci. 2009;29:2061–2073. doi: 10.1111/j.1460-9568.2009.06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Suzuki W, Tanaka K. Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science. 2003;301:229–232. doi: 10.1126/science.1084204. [DOI] [PubMed] [Google Scholar]
- Morrison SE, Salzman CD. The convergence of information about rewarding and aversive stimuli in single neurons. J Neurosci. 2009;29:11471–11483. doi: 10.1523/JNEUROSCI.1815-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Wise SP, Drevets WC. Localization of dysfunction in major depressive disorder: prefrontal cortex and amygdala. Biol Psychiatry. 2011;69:e43–e54. doi: 10.1016/j.biopsych.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Walton ME, Behrens TE, Sallet J, Buckley MJ, Rushworth MF. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci USA. 2010;107:20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nat Neurosci. 2008;11:95–102. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE, Wise SP. The Neurobiology of the Prefrontal Cortex: Anatomy, Evolution, and the Origin of Insight. 1 edn. Oxford University Press; USA: 2012. [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MF. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28:13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Balkanizing the primate orbitofrontal cortex: Distinct subregions for comparing and contrasting values. Ann N Y Acad Sci. 2011a;1239:1–13. doi: 10.1111/j.1749-6632.2011.06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J Neurosci. 2011b;31:10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Amaral DG, Price JL. The afferent input to the magnocellular division of the mediodorsal thalamic nucleus in the monkey, Macaca fascicularis. J Comp Neurol. 1987;256:175–210. doi: 10.1002/cne.902560202. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: neuronal activity in the behaving monkey. Exp Brain Res. 1983;49:93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Walker A. A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol. 1940;73:59–86. [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Walton ME, Behrens TE, Buckley MJ, Rudebeck PH, Rushworth MF. Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron. 2010;65:927–939. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. 2004;7:1259–1265. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Zar J. Biostatistical Analysis. 4th edn. New Jersey: Prentice Hall; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.