Summary
Recent development of molecular genetic techniques are rapidly advancing understanding of the functional role of brain circuits in behavior. Critical to this approach is the ability to target specific neuron populations and circuits. The collection of over 250 BAC Cre-recombinase driver lines produced by the GENSAT project provides a resource for such studies. Here we provide characterization of GENSAT BAC-Cre driver lines with expression in specific neuroanatomical pathways within the cerebral cortex and basal ganglia.
INTRODUCTION
Advances in molecular genetic techniques are revealing new details of the neuronatomical organization of brain circuitry and the functional role of these circuits in behavior (Luo et al., 2008; Huang and Zeng, 2013). Engineered viral vector constructs have been developed to label axonal projections of targeted neurons with unprecedented clarity, while others allow for retrograde transynaptic labeling of neurons providing inputs or anterograde transynaptic labeling of post-synatpic targets of axonal projections. Development of optogenetic and DREADD techniques provide the ability to functionally manipulate neural circuits to study their role in behavior while calcium indicators provide the ability to analyze the physiologic activity in targeted neuron populations (Alexander et al. 2009; Fenno et al, 2011). Together these approaches are providing new insights into the functional organization of neural circuits. For example, optogenetic studies, using light activation of Channel Rhodopsin (ChR), have demonstrated the ability to functionally manipulate specific neural pathways to determine their role in behaviors including fear memory (Ehrlich et al., 2009), anxiety (Tye et al., 2011), feeding (Atasoy et al., 2012), reward (Kravitz et al, 2012) and movement (Kravitz et al., 2010). The analytic potential of these approaches is enhanced by the ability to target specific neuron populations, which are defined components of neural circuits. One approach involves the use of transgenic Cre-driver mouse lines in which Cre-recombinase is expressed under the control of gene-specific promoters. In this study we characterize BAC-Cre driver lines from the GENSAT project (Gong et al., 2007) that allow for targeting components of the neural circuits of the cerebral cortex and basal ganglia.
The adaptation of bacterial artificial chromosomes (BACs) to express enhanced green fluorescent protein (EGFP) with specified gene promoters has proven to be an efficient strategy to generate transgenic mice with EGFP expression targeted to specific neuron populations (Heintz, 2001; Gong et al., 2003). This approach was modified in the GENSAT project to generate over 250 BAC-Cre driver lines, which display expression of Cre-recombinase in specific neuron and glial populations throughout the brain (Gong et al., 2007). Characterization of Cre-expression in these lines was determined by crossing BAC-Cre driver lines with a Rosa26-EGFP reporter line, and visualizing EGFP labeling in coronal and sagittal brain sections, which are presented on the GENSAT website (http://www.gensat.org). While the GENSAT collection provides a large number of BAC-Cre driver lines the nominal characterization provided has limited their usefulness for the research community. For example, while there are over 50 BAC-Cre lines with expression in specific cortical layers or areas, determination of specific cortical neuron subtypes requires information concerning their axonal projections. Moreover, the characterization of Cre expression obtained with the Rosa26-EGFP reporter line in some cases does not provide an accurate description of functional Cre-expression in the adult animal. In this study we provide additional characterization of 100 GENSAT BAC-Cre driver lines. Additional characterization utilizes injection of viral vectors that provide for Cre-dependent labeling of axonal projections (Luo et al., 2008; Madisen et al, 2010). This axonal tracing data is critical for distinguishing between subtypes of neuronal populations based on their connections. These data are presented on a website: (http://GENSATcreBrains.biolucida.net) that provides the ability to view high resolution images of the neuroanatomical data in the context of the functional organization of cerebral cortical and basal ganglia circuits.
RESULTS
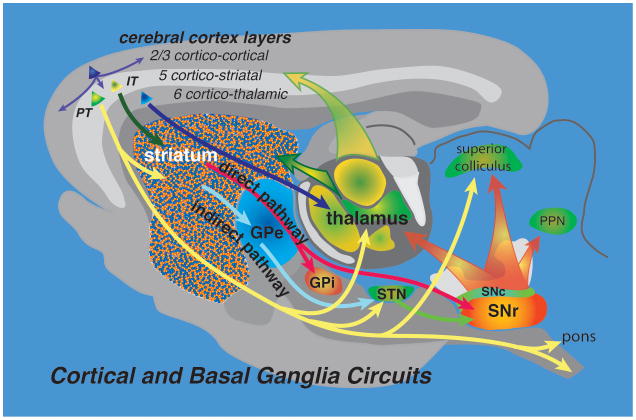
The major connections of the cerebral cortex and basal ganglia (Gerfen and Wilson, 1996) are diagrammed in Figure 1. Pyramidal neurons in different layers of the cerebral cortex are distinguished their axonal projections patterns, with layer 2/3 neurons providing intracortical connections, layer 5 neurons providing projections to subcortical structures including the striatum, and layer 6 neurons providing projections to the thalamus. The principal neuron of the striatum is the spiny projection neuron. One subtype, the direct pathway neuron, projects directly to the output nuclei of the basal ganglia in the internal segment of the globus pallidus (GPi) and substantia nigra pars reticulata (SNr). The other subtype, the indirect striatal pathway neurons project only to the external segment of the globus pallidus (GPe) whose projections to the subthalamic nucleus and SNr provide indirect connections to the basal ganglia output nuclei. The output of the basal ganglia is directed to the thalamus, superior colliculus and other midbrain structures. Within each of these general classifications of neuron types there are subtypes distinguished by their axonal projections. In this study, AAV-cre dependent expression vectors are used to identify specific neuronal subtypes in BAC-Cre driver lines.
Figure 1.
The major circuits of the basal ganglia are diagrammed in a sagittal plane. Corticostriatal inputs arise from two major subtypes, intertelencephalic neurons (IT), which provide bilateral inputs to the striatum, and pyramidal tract corticostriatal (PT), which project an axon ipsilatrally, with collaterals to the striatum, thalamus, subthalamic nucleus (STN), superior colliculus, pons, and to the spinal cord. Two main subtypes of projection neurons in the striatum give rise to the direct and indirect pathways. The direct pathway provides direct projections to the output nuclei of the basal ganglia, the internal segment of the globus pallidus (GPi) and the substantia nigra pars reticulata (SNr). The indirect pathway projects to the external segment of the globus pallidus (GPe), which connects indirectly through the STN to the GPi and SNr. The major output of the basal ganglia originates from GABAergic neurons in the GPi and SNr, which provide inhibitory input to the thalamus, superior colliculus and pedunculopontine nucleus (PPN).
Several general issues concerning functional Cre-expression emerged from analysis of the BAC-Cre driver lines described. First, for most lines, expression patterns obtained with both the Rosa26-EGFP and (CAG)dTomato reporter lines is similar. However, in some lines, additional expression is revealed with the Rosa26-(CAG)dTomato reporter, indicating that the Rosa26-(CAG)dTomato reporter may be more sensitive. Second, localized injections of AAV with Cre-dependent EGFP or dTomato expression constructs are used to determine whether such labeling occurs in the neuron population displaying Cre-dependent expression produced by crosses to the Rosa26-EGFP or (CAG)dTomato FLEX reporter lines. For most lines neuronal labeling produced with AAV-Cre dependent expression constructs corresponds to that predicted by expression produced with the reporter lines. In some lines, AAV-Cre dependent expression is produced in more neurons than observed from brains from the reporter lines. This discrepancy is most often observed with AAV-expression vector injections into Cre lines crossed to the Rosa26-EGFP reporter. The Rosa26-(CAG)dTomato reporter provides a more accurate predictor of labeling obtained with AAV-expression vector injections. An example of this type of discrepancy between the Rosa26 reporter lines and the efficacy of AAV-expression vector injection labeling is provided by the Syt17_NO14 BAC-Cre driver line described later in this study. A third issue is the absence of labeling with AAV-expression vector injections of neurons that display labeling with the Rosa26 reporters. This most likely occurs in BAC-Cre driver lines in which the gene promoter is transiently active during development but not in the adult. Additionally, lines used are heterozygous for the BAC-Cre construct to maintain uniform expression and to minimize possible affects of over-expression of an exogenous protein on neuron function.
Taken together these issues indicate the importance of characterizing BAC-Cre driver lines to be used for functional studies with multiple methods. In most cases analysis of expression with Rosa26-EGFP or (CAG)dTomato reporter lines provides an accurate predictor of neurons that express functional Cre, which will express injected viral constructs. However, injections of AAV or other viral expression vectors facilitates the characterization of BAC-Cre driver lines for use in functional studies. In this study we provide such analysis to identify BAC-Cre driver lines with functional Cre expression in identified neuron populations in the cerebral cortex and basal ganglia circuits.
Web-based database of BAC-Cre lines
A list of GENSAT BAC-Cre driver lines is provided on the website: http://www.gensat.org/cre.jsp. On this site, images of coronal and sagittal brain sections in the adult and at P7 show expression displayed with the Rosa26-EGFP reporter line. In addition, these lines may be obtained from the Mutant Mouse Regional Resource Center (MMRRC), with a link on the website for each line. Additional characterization provided cases of AAV-Cre dependent expression vector injections that label axonal projections are provided on a browser, which may be downloaded from the website: http://GENSATcreBrains.biolucida.net. Data on this website may be searched by specific BAC-Cre lines, neuronal subtypes or brain structure.
Cortical neurons
A list of GENSAT BAC-Cre lines with expression in the cerebral cortex is provided in Table 1. Most of these lines have expression in other brain regions, but here are characterized on the basis of cortical expression including expression in cortical layer and area determined with Cre-reporter expression and by neuron subtype determined by AAV-Cre-dependent expression vector injections to label axonal projection patterns.
Table 1.
BAC-Cre driver lines with expression in cortical pyramidal neurons. BAC-Cre driver lines with expression in pyramidal neurons in layers 2/3, 4,5 and 6 are categorized by layer, cortical area and subtype. The numbers of neurons displaying expression in different layers varies from line to line, with some expressing in most pyramidal neurons in a particular layer while others express in a subset in those layers. The cortical areas in which neurons are expressed are designated as either being distributed in most cortical areas or in select areas, such as in neocortical areas or in prefrontal areas, which for the most part are peri-allocortical.
| Gene | Line | Layer | Cortical areas | Subtype |
|---|---|---|---|---|
| Sepw1 | NP39 | 2/3 | most | IT |
| Grp | KH288 | 2/3/5 | prefrontal and MOs | IT |
| Rgs14 | SR63 | 2/3/5 | prefrontal (PL,AId) | IT |
| Lypd1 | NR149 | 2/3 | prefrontal(IL) | IT |
| Ccdc3 | PO24 | 2/3/5/6 | most | IT/(PT) |
| Arpp21 | OL90 | 2/3/5 | most | IT/PT |
| Cx3cl1 | RA63 | 2/3/(5)/6 | most | IT/CT |
| Plxnd1 | OG1 | 2/3/5 | most | IT/PT |
| Syt17 | NO14 | 2/3/5/6b | prefrontal/select others | IT/PT |
| Nts | RH4 | 2/3/5/6b | prefrontal | IT/PT |
| Rbp4 | KL100 | 5 | most | PT/IT |
| Dbp | MN120 | 5 | neocortex | PT/IT |
| Grm2 | MR90 | 5 | prefrontal/select others | PT/IT |
| Colgalt2 | NF107 | 5 | prefrontal/select others | PT/IT |
| Sema3e | OX2 | 5 | most | PT/IT |
| Gpr26 | KO250 | 5 | most | PT/IT |
| Sim1 | KJ18 | 5 | neocortex | PT |
| Chrna2 | OE25 | 5/6 | most | PT/CT |
| Rcan2 | ON50 | 5/6 | neocortex | PT/CT |
| Efr3a | NO108 | 5 | select areas | PT |
| Tlx3 | PL56 | 5 | neocortex | IT |
| Ebf2 | NP183 | 5 | neocortex | IT |
| Rxfp3 | RS38 | 5 | neocortex | IT |
| Ntsr1 | GN220 | 6 | neocortex | CT |
| Drd1a | FK164 | 6b | neocortex | CT |
| Drd3 | KI196 | 2/3 | somatosensory | IT |
| Gpr26 | KO273 | 4/5 | somatosensory | TBD |
| Grp | KH284 | 4/5 | somatosensory | TBD |
| Lamb3 | KH145 | 5 | somatosensory | TBD |
| Sim1 | KJ27 | 6 | somatosensory | CT |
In some lines expression is restricted to very specific cortical areas, such as particular prefrontal areas (i.e., prelimbic, PL; Agranular Insular, AId; infralimbic, IL) or in the somatosensory cortex. Subtypes of cortical pyramidal neurons determined by their axonal projections labeled with AAV-expression vector injections include intertelencephalic (IT), pyramidal tract (PT) neurons and cortico-thalamic (CT) neurons. Lines highlighted are those with the most select labeling of the highest percentage of particular cortical neuron subtypes. Full brain coronal image series for these lines are available on the website browser, which may be searched by BAC-Cre line name, layer, cortical area and neuron subtype.
Lines with expression in corticostriatal projection neurons
Virtually all areas of the cerebral cortex provide excitatory inputs to the striatum that target spiny projection neurons, as well as interneurons. Corticostriatal inputs from neocortical areas arise primarily from layer 5 pyramidal neurons, although layer2/3 neurons contribute to this projection as well. Two main subtypes of corticostriatal neurons are generally recognized (Wilson, 1987; Cowan and Wilson, 1994). One has ipsilateral projections to the striatum with collaterals that project to the pyramidal tract (PT corticostriatal neurons), as well as to the thalamus, zona incerta, subthalamic nucleus, superior colliculus, pontine nuclei and to the spinal cord through the pyramidal tract (Kita and Kita, 2012). Another subtype provides bilateral projections to the striatum with collaterals that connect primarily with other cortical areas but do not project to other subcortical areas (intertelencephalic IT corticostriatal neurons). BAC-Cre driver lines are characterized that preferentially express in each of these subtypes. Among these BAC-Cre driver lines there are variations related to the cortical area of origin, with some types showing expression throughout all cortical areas, others showing restriction to specific cortical areas, and others displaying gradients of expression across cortical regions.
Based on analysis of the axonal projection profiles from AAV-expression vector injections into the cerebral cortex, BAC-Cre driver lines with expression in corticostratal neurons are categorized into three types. First, those with expression in both PT and IT corticostriatal neurons, which include Rbp4_KL100, Dbp_MN120, Grm2_MR90 and Sema3e_OX2. Second, those with expression restricted to PT corticostriatal neurons, which include Sim1_KJ18, Chrna2_OE25, Efr3a_NO108 and Rcan2_ON50. Thrid, those with expression restricted to IT corticostriatal neuons, which include Tlx3_PL56, Ebf2_NP183, Sepw1_NP39 and Grp_KH288. The pattern of Cre-expression revealed with crosses of these lines to the Rosa26-EGFP reporter is provided in Supplementary Figure 1.
The BAC-Cre driver line Rbp4_KL100 may be considered a pan layer 5 line, displaying expression restricted to most layer 5 neurons throughout neocortical and peri-allocortical areas. AAV vector injections in primary sensorimotor, secondary motor and prelimibic cortical areas label axonal projections to the ipsilateral and contralateral cortex and striatum, to the thalamus, subthalamic nucleus, zona incerta, superior colliculus and other midbrain structures, the pontine nucleus and pyramidal tract, indicative of labeling of PT corticostriatal neurons (Figure 2). Expression in IT corticostriatal neurons is indicated by bilateral labeling of projections to the striatum and cerebral cortex.
Figure 2.
BAC-Cre line Rbp4_KL100 with expression in PT and IT corticostriatal neurons. Left column: Sections displaying Cre expression with Rosa26-EGFP reporter at coronal levels through the prefrontal cortex, nucleus accumbens, striatum, thalamus, subthalamic nucleus (STN) and zona incerta (ZI), superior colliculus, and pons. Second column: Injection of AAV-dTomato Cre-dependent expression vector into the secondary motor cortex labels neurons expressing Cre and their axonal projections (pseudo-colored yellow). Labeled axons project bilaterally to the striatum, indicating labeling of IT corticostriatal neurons, and to the ipsilateral thalamus, STN, ZI, superior colliculus and pons, indicting labeling of PT corticostriatal neurons. Injection site and axonal terminal projection areas are shown at progressively higher magnifications in columns to the right.
PT selective Layer 5 Cre lines
Four lines are described in which expression produced with AAV-vector injections is primarily restricted to layer 5 PT corticostriatal neurons. These include, Sim1_KJ18, Chrna2_OE25, Efr3a_NO108 and Rcan2_ON50 (Figure 3). Injections of AAV-Cre-dependent expression vectors into motor cortical areas in each of these lines label axonal projections restricted to the ipsilateral striatum, and labeling of axons in the fiber tracts that extend through the striatum into the internal capsule and cerebral peduncle to the pyramidal tracts. Labeled axon collaterals extend from these projections to target the thalamus, subthalamic nucleus and zona incerta, GPi and SNr and midbrain areas including the superior colliculus and pontine nuclei. These labeled axonal projections are characteristic of labeling of PT corticostriatal neurons.
Figure 3.
BAC-Cre lines with select expression in PT corticostriatal neurons. Four BAC-Cre driver lines, Sim1_KJ18, Chrna2_OE25, Efr3a_NO108 and Rcan2_ON50 are described in which AAV-dTomato expression vector injections into primary motor cortex selectively label PT corticostriatal neurons. Cre expression in these lines revealed by crosses to the Rosa26-EGFP reporter line display expression in layer 5 of the cortex, shown in the top row with the inset shown at higher magnification in the second row. AAV-dTomato injection sites into the motor cortex are shown in the third row displaying labeled neurons (pseudo-colored yellow). In each line, axonal projections are distributed to the ipsilateral striatum (caudate-putamen CP), with no evidence of projections to the contralateral hemisphere. Additionally, axonal projections are distributed through the internal capsule with axonal collaterals distributed to the thalamus, subthalamic nucleus (STN), superior colliculus (SC) and pontine nucleus (pons). This pattern of axonal projections is characteristic of pyramidal tract (PT) corticostriatal neurons.
For the most part, in each of these lines, AAV vector injections into neocortical areas display little evidence of labeling to the contralateral cortex or striatum, indicating preferential labeling of PT corticostriatal neurons. However, there are differences between these lines in the number and regional distribution of labeling of IT corticostriatal neurons. For the line Sim1_KJ18, expression demonstrated with the Rosa26 reporter shows labeling of a high percentage of deep layer 5 neurons, with some labeling of more superficial neurons. Labeled neurons are distributed in most neocortical areas with lesser or no labeling in peri-allocortical and prefrontal areas. Injections of AAV vectors into neocortical areas label relatively large numbers of deep layer 5 neurons with little labeling of more superficially distributed neurons. This suggests that the expression of Cre-recombinase in more superficial layer 5 neurons displayed with crosses to the reporter line is due to transient expression during development. Labeling of axonal projections with such injections display the pattern characteristic of PT corticostriatal neurons. Injections that involve the secondary motor cortex in the shoulder area result in some labeling of axons crossing to the contralateral striatum, in addition to the PT pattern of axonal labeling. The other PT selective BAC-Cre lines label a smaller number of deep layer 5 neurons. Whereas the line Rcan2_ON50 labels neurons restricted to neocortical areas, Chrna2_OE25 and Efr3a_NO108 label neurons distributed in throughout neocortical areas as well as in peri-allocortical and prefrontal areas.
IT corticostriatal Layer 5 and Layer 2/3 selective Cre lines
Three lines are described in which expression produced with AAV-vector injections is primarily restricted to IT corticostriatal neurons. These include Tlx3_PL56, Ebf2_NP183, Sepw1_NP39 and Gpr_KH288, (Figure 5). Tlx3_PL56 and Ebf2_NP183 displays expression in primary and secondary motor and sensory neocortical areas with less expression in prefrontal cortical areas. On the other hand, Grp_KH288 displays more restricted expression primarily in secondary motor and prefrontal cortical areas. AAV-Cre dependent tracer injections in Tlx3_PL56 and Ebf2_NP183 produced expression in primary motor layer 5 cortical neurons and labeled axonal projections bilaterally within the cerebral cortex and to the striatum. There was no evidence of labeled axons in the corticofugal fiber tracts within the striatum or of labeling to nonstriatal subcortical areas. AAV-Cre dependent tracer injections in Grp_KH288 that produced expression prelimbic cortical neurons produced labeling of bilateral cortical and striatal projections with no evidence of labeling to other subcortical structures. The pattern of axonal projections indicates that in both Tlx3_PL56, Ebf2_NP183 and Grp_KH288 Cre expression is restricted to IT corticostriatal neurons. Whether different subtypes of IT corticostriatal neurons are represented in the Tlx3_PL56 and Grp_KH288 Bac-Cre driver lines requires additional characterization (Otsuka and Kawaguchi, 2011).
Figure 5.
BAC-Cre lines with expression in prefrontal cortical areas. Four BAC-Cre lines, Colgalt2_NF107, Chrna2_OE25, Sepw_NP39 and Lypd1_NR49 display expression in prefrontal cortical areas revealed by crosses to the Rosa26-EGPF reporter (top row). AAV-dTomato expression vector injections label neurons in the medial prefrontal cortex are shown in the second row, with insets shown at higher magnification in the third row (pseudo-colored yellow). In Colgalt2_NF107, labeled prelimbic cortical neurons in layer 5 provide axonal projections bilaterally to the caudate-putamen (CP) and accumbens (ACC), indicating labeling of IT corticostriatal neurons. Of note, in this case the corticostritatal projections are homogenously distributed to both patch and matrix compartments. Additionally, axonal projections are labeled to the thalamus (not shown), zona incerta (ZI), subthalamic nucleus (STN), superior colliculus (SC) and pons (not shown), indicating labeling of PT corticostriatal neurons. In Chrna2_OE25, a subset of prelimbic layer 5 neurons are labeled, which project to the ipsilateral striatum, thalamus (not shown), ZI, STN, SC and pons (not shown), indicative of selective labeling of PT corticostriatal neurons. In Sepw1_NP39, labled prelimbic neurons in layer 2/3 provide bilateral projections to the CP, but no labeling to the thalamus, ZI, STN, SC or pons, indicating selective labeling of IT corticostriatal neurons. In Lypd1_NR149, labeled neurons in layer 2/3 of the infralimbic cortex, which extend axonal projections to the ipsilateral striatum, but not to the thalamus, STN, ZI, SC or pons, indicating selective labeling of IT corticostriatal neurons. In cases of prefrontal injections in Colgalt2_NF107, Sepw1_NP39 and Lypd1_NR149 there is labeling of axonal projections to the amygdala (amyg), which is not apparent in the injection case in Chrna2_OE25.
BAC-Cre driver lines with expression in cortical layers 2/3 include Sepw1_NP39, Rgs8_OK14, and Arpp21_OL90. Based on analysis of the Rosa26 reporter lines, Cre expression in the cortex is localized to layer 2/3 neurons throughout most cortical areas. Injections of AAV-Cre dependent tracers into motor cortical areas in Sepw1_NP39 labels neurons localized in layer 2/3, bilateral axonal projections within the cerebral cortex and bilateral projections to the striatum. There is no evidence of labeling of axonal projections to other subcortical regions, indicating that these neurons are IT corticostriatal neurons.
Prefrontal BAC-Cre driver lines
Prefrontal cortical areas in the mouse exhibit a peri-allocortical laminar organization distinct from neocortical areas. This raises the question as to whether BAC-Cre driver lines with selective expression in PT and IT corticostriatal neurons in neocortical areas are similar in prefrontal areas. In some lines that express in PT or IT corticostriatal subtypes, Cre expression is limited to neocortical areas and does not extend to prefrontal areas. BAC-Cre driver lines with expression in prefrontal cortical areas are characterized in terms of select labeling of PT and IT corticostriatal subtypes. In addition, lines are described that express in select prefrontal cortical areas. Examples of AAV-expression vector labeling of axonal projections for such lines is provided in Figure 5.
BAC-Cre driver line Colgalt2_NF107 displays reporter expression that is restricted to prefrontal cortical areas, with only sparse labeling in neocortical areas. Injections of AAV-expression vectors into the anterior cingluate and prelimbic areas result in labeling of projections bilaterally to cortical areas and the striatum, which is indicative of labeling of IT corticostriatal neurons. Axonal projections are also evident to the ipsilateral medial amygdala. In addition, axonal projections extend through the fiber fascicles through the striatum into the cerebral peduncle with collaterals extending into the thalamus, zona incerta, subthalamic nucleus, superior colliculus and into the pontine nuclei. These projections are ipsilateral to the injection site and indicate labeling of PT corticostriatal neurons. Thus Colgalt2_NF107 appears to express in both PT and IT corticostriatal neurons in the prefrontal cortex.
Some lines, in which PT corticostriatal neurons selectively express Cre, show limited labeling in prefrontal cortical areas (i.e., Sim1_KJ18 and Rcan2_ON50), while others display labeling in most neocortical and prefrontal areas (i.e., Chrna2_OE25 and Efr3a_NO108). Injections of AAV-expression vectors into medial prefrontal cortex in Chrna2_OE25 labels neurons with axonal terminal projections into the ipsilateral striatum, thalamus, zona incerta, suthalamic nucleus, superior colliculus and pontine nuclei. There is no evidence of labeled projectins to the contralateral hemisphere or to the ipsilateral amygdala. This pattern of axonal projections indicates selective labeling of PT corticostriatal neurons.
The two BAC-Cre lines with the most robust IT corticostriatal selective layer 5 expression in neocortical areas (Tlx3_PL56 and Ebf2_NP183) label few neurons in prefrontal areas. The Grp_KH288 line has expression selective for IT corticostriatal neurons in secondary motor areas, anterior cingulate areas, few neurons have expression in prelimbic, infralimbic and ventral prefrontal areas (Figure 4). The line Sepw1_NP39 has expression selective for layer 2/3 neurons throughout most neocortical (Figure 5) and periallocortical areas. Injections of AAV-expression vectors into the prelimbic cortex label neurons with axonal projections distributed bilaterally to homologous cortical areas and the striatum, as well as to the ipsilateral medial amygdala with no evidence of projections to subcortical areas, indicative of selective labeling of IT corticostriatal neurons (Figure 5).
Figure 4.
BAC-Cre lines with select expression in IT corticostriatal neurons. Four BAC-Cre lines, Tlx3_PL56, Ebf2_NP183, Sepw1_NP39 and Grp_KH288 are described in which AAV-dTomato expression vector injections into primary and secondary motor cortical areas selectively label intertelencephalic (IT) corticostriatal neurons. Cre-expression revealed by crosses to the Rosa26-EGFP reporter line display expression in layer 5 in neocortical areas in Tlx3_PL56 and Ebf2_NP183, in layer 2/3 in all cortical areas in Sepw1_NP39 and layer 5 and layer 2/3 in secondary motor and prefrontal areas in Grp_KH288 (top 2 rows). AAV-dTomato injection sites label neurons in the cortex, which are shown in the third row (pseudo-colored yellow). In each of these lines, axonal projections are distributed bilaterally within the cerebral cortex and the caudate putamen (CP). Axonal projections from labeled corticostriatal neurons do not extend to the internal capsule, thalamus, subthalamic nucleus (STN), or superior colliculus. This patterns of axonal projections is characteristic of IT corticostriatal neurons.
Several BAC-Cre lines are identified that have layer selective expression restricted to specific prefrontal areas. An example of such a line is Lypd1_NR149, with expression localized to layer 2/3 of the infralimbic prefrontal cortex. Injections of AAV-expression vectors into the medial prefrontal cortex selectively label these neurons and axonal projections distributed to the medial striatum and to the medial amygdala (Figure 5). Interestingly both of these projections are only to the ipsilateral hemisphere. There is no evidence of labeling of axonal projections to non-striatal subcortical brain regions. This pattern of axonal projections indicates selective labeling of IT corticostriatal projection neurons. Other lines with expression restricted to specific prefrontal areas include Rgs14_ SR63 with expression in AId and prelimbic cortical areas.
BAC-Cre lines with expression somatosensory cortical areas
A number of BAC-Cre driver lines display expression as demonstrated with the Rosa26-EGFP reporter with expression that is somewhat restricted to specific layers within somatosensory cortical areas. These lines are listed in Table 1 and their patterns of expression are shown in Supplementary Figure 2.
BAC-Cre line with expression in reward circuits
The BAC-Cre driver line Syt17_NO14 displays expression in multiple brain areas associated with circuits involved in reward behavior (Thompson and Swanson 2010, Lammel et al., 2012). This line, crossed with the Rosa26-(CAG)dTomato reporter, displays expression in restricted cortical areas including the prelimbic, infralimbic, ventral prefrontal and piriform cortical areas, in the ventral CA1 hippocampus and dentate gyrus, intralaminar thalamic nuclei, select hypothalamic nuclei, and restricted to the dopamine neurons in the ventral tegmental area (with little expression in the lateral substantia nigra pars compacta dopamine neurons). The pattern of Cre expression demonstrated with the Rosa26-(CAG)dTomato reporter is more extensive than that with the Rosa26-EGFP reporter, which shows robust labeling of the VTA dopamine neurons, but only scattered neurons in the other areas. Injections of AAV-expression vectors labeled neurons and their axonal projections consistent with the expression pattern obtained with the Rosa26-(CAG)dTomato reporter (Figure 6).
Figure 6.
BAC-Cre line Syt17_NO14 with expression in reward circuits. Cre-induced expression is demonstrated in BAC-Cre line Syt17_NO14 by crosses to the Rosa26_(CAG)dTomato reporter line in coronal sections at the level of the prefrontal cortex, nucleus accumbens, striatum (caudate putamen, CP), thalamus, amygdala (amyg) and midbrain (pseudocolored green in left hand column). Injection cases of AAV-dTomato expression vectors into areas expressing Cre label neurons (top row) and their axonal projections, shown in column below in matched coronal levels (pseudocolored yellow). Injection case 385 labeled prelimbic cortical neurons, whch provide axonal projections bilaterally to the striatum (caudate putamen, CP), amygdala (amyg), indicative of labeling of IT corticostriatal neurons as well as labeling of projections to the STN, SC and pons, indicative of labeling of PT corticostriatal neurons. In this case there is retrograde labeling of intralaminar thalamic neurons. Injection case 006 labeled intralaminar thalamic neurons, which provide axonal projections to the prelimbic cortex, ipsilateral striatum (caudate putamen, CP and nucleus accumbens (Acc), and to retrosplenial cortical areas. Injection case 356 labeled ventral CA1 hippocampal neurons, which provide axonal projections to the prelimbic cortex, nucleus accumbens (Acc), lateral septal nucleus (LS), hypothalamus (hypo), and amygdala (amyg). Injection case 756 labeled ventral tegmental area dopamine neurons (VTA), which provide axonal projections to the prelimibic cortex, nucleus accumbens (ACC) and ventral caudate putamen (CP), and amygdala (amyg).
BAC-Cre driver lines in the thalamus
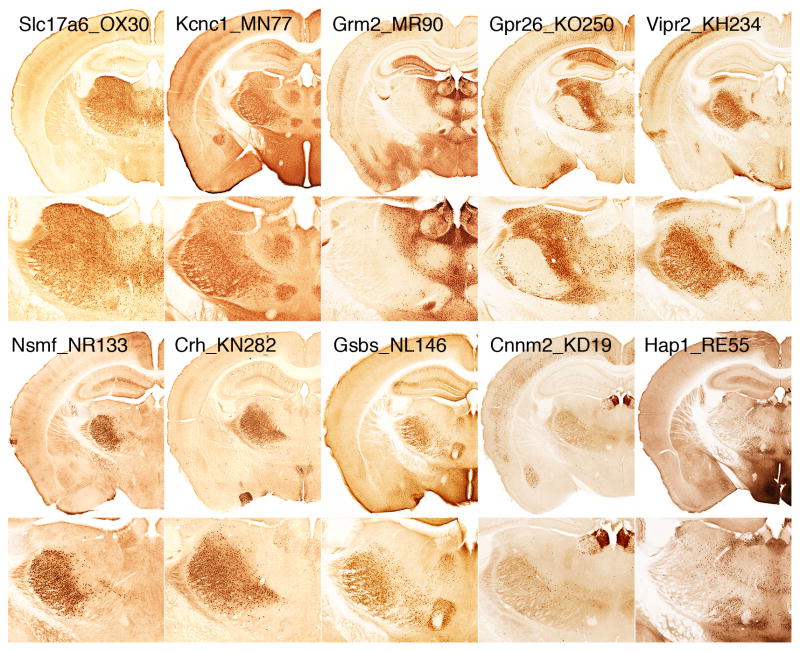
The dorsal thalamus is an important component of basal ganglia circuitry. Various thalamic nuclei are the target of the output of the basal ganglia, receive collateral inputs of corticostriatal PT neurons, and provide a major source of excitatory inputs to the striatum. A number of BAC-Cre driver lines are characterized with expression in the thalamus, which are listed in table 3. Figure 7, displays the Cre expression pattern with the Rosa26-EGFP reporter line for 10 of these lines. The BAC-Cre driver line Slc7a6_OX30, which uses the gene promoter for the vesiclular glutamate transporter subtype 2 (Vglut2), displays expression in most dorsal thalamic nuclei neurons. Other BAC-Cre driver lines display expression that is restricted to select dorsal thalamic nuclei.
TABLE 3.
BAC-Cre driver lines with expression in the striatum
| Gene | Line | Projection type | Striatal Region | compartment |
|---|---|---|---|---|
| Gng7 | KH71 | Direct/indirect | All | patch/matrix |
| Arpp21 | OL90 | Direct/indirect | All | patch/matrix |
| Pde1b | OZ114 | Direct/indirect | Dorsal | patch/matrix |
| Rasgrp1 | PO1 | Direct/indirect | Dorsal | patch/matrix |
| Rgs8 | OK14 | Direct/indirect | All | patch/matrix |
| Gpr88 | RX50 | Direct/indirect | All | patch/matrix |
| Drd1a | FK150 | Direct | All | patch/matrix |
| Drd1a | EY217 | Direct | Dorsal | patch/matrix |
| Drd1a | EY266 | Direct | Accumbens | patch/matrix |
| Gcm2 | OC103 | Direct | Scattered | patch/matrix |
| Drd2 | ER44 | Indirect | All | patch/matrix |
| Adora2a | KG126 | Indirect | All | patch/matrix |
| Plxnd1 | OG1 | Direct/indirect | Dorsal | Matrix-biased |
| Npyr1 | RL2 | Direct | All | Matrix-biased |
| Sepw1 | NP67 | Direct/indirect | All | Patch-biased |
| Syt6 | KI148 | Direct/indirect | All | Patch-biased |
| Dlg3 | KG118 | Direct/indirect | All | Patch-biased |
| Kcnip2 | OP11 | Direct/indirect | Lateral striatum | mixed patch/matrix |
| Gabarr3 | KC112 | Direct/indirect | Lateral striatum | patch/matrix |
| Drd3 | KI196 | Direct/indirect | Accumbens | patch/matrix |
Figure 7.
BAC-Cre lines with expression in thalamic nuclei. Immunohistochemical Cre-induced EGFP labeling produced by Rosa26_EGFP reporter in 10 lines with expression in various thalamic nuclei. Slc17a6_OX30 (gene also known as Vglut2) has Cre expression in most thalamic nuclei. Other lines show selective expression in thalamic nuclei: Kcnc1_MN77 (MD, VPM, VPL, SMT), Grm2_MR90 (AV,LD, IL, PVT, LH, MH), Gpr26_KO250 (LD, VAL, VM), Vipr2_KH234 (VPM, VPL), Nelf_NR133 (VPM, VPL), Crh_KN282 (VPM, VPL), Gsbs_NL146 (VPM, VPL, SMT), Cnnm2_KD19 (MH), Hap1_RE55 (LH, VM). See table 3 for complete listing.
In addition to inputs from the cerebral cortex, the other major source of excitatory inputs to the striatum arise from the intralaminar nuclei of the thalamus. Several BAC-Cre lines are identified with expression selectively in thalamic intralaminar nuclei, including Syt17_NO14, Plxnd1_OG1, Grp_KH288 and Syt17_NO14. Injections of AAV expression vectors into the intralaminar nuclei in each of these lines resulted in selective labeling of neurons in the intralaminar thalamic nucleus and axonal projections to the striatum and cortical areas (Figure 6 and Supplemental Figure 3).
Striatal circuits
The main neuron subtype in the striatum is the spiny projection neuron, which constitutes as much as 90% of the striatal neuron population. There are two main subtypes of striatal spiny projection neurons distinguished by their axonal projections (Kawaguchi et al., 1990). One gives rise to the striatal direct pathway as it projects to the output nuclei of the basal ganglia, the internal segment of the globus pallidus and substantia nigra, while the other gives rise to the indirect striatal pathway, which projects to the external segment of the globus pallidus, which through connections with the subthalamic nucleus provides indirect connections to the output nuclei of the basal ganglia. Direct and indirect striatal projection neurons are distinguished by their respective selective expression of the Drd1a and Drd2 dopamine receptors, as well as other neurochemical markers (Gerfen et al., 1990).
A list of the BAC-Cre driver lines with expression in the striatum is provided in Table 3. Lines are identified with expression in both direct and indirect pathway neurons, selective for either the direct or indirect pathway neurons, restricted to striatal regions and showing some preferential distribution in either the patch or matrix compartments.
Of particular utility are lines that differentially express Cre in striatal direct or indirect pathway neurons (Supplemental Figure 4). The Drd1a BAC-Cre driver lines provide labeling of direct pathway neurons and not indirect pathway neurons. The Drd1a BAC-Cre driver lines display different patterns of expression. Two Drd1a lines, FK150 and FK162, display expression that matches the full pattern of endogenous expression of the Drd1a gene, with expression throughout the striatum, in select cortical neurons, amygdala and hypothalamus. Other lines display expression in subsets of the full DRd1a expression pattern. Drd1a_EY217, displays expression throughout the dorsal striatum, with limited expression in the cortex and ventral striatum. Depending on the experimental paradigm different Drd1a BAC-Cre driver lines may be used for studies of the direct striatal pathway. BAC-Cre lines that label the indirect striatal pathway include Drd2-ER44 or Adora2a-KG126. While the Drd2_ER44 and Adora2a_KG126 lines label indirect and not direct spiny projection neurons, these genes are also expressed in striatal interneurons. The possibility that these BAC-Cre lines express in striatal interneurons must be taken into consideration.
Other BAC-Cre lines display expression in the striatum selective for the patch and matrix compartments (described below) or that display other patterns of restricted expression. These include Kcnip2_OP11 with expression in the lateral striatal matrix compartment and lateral striatal patches, Gabarr3_KC112 with expression restricted to a lateral region of the striatum and Sema3e_OX2 with expression restricted to the dorso-medial striatum. The BAC-Cre driver line Chrna2_NP306 is particularly interesting as it displays expression in striatal spiny projection neurons that are scattered throughout the striatum in a manner that there appears to be little overlap of dendritic arbors of labeled neurons.
Patch/Matrix BAC-Cre lines
Overlain on the homogenous organization of the direct and indirect striatal pathways there are two distinct macroscopic compartments, termed the patch/striosome and matrix compartment that are defined on the basis of expression of various neurochemical markers and by the organization of inputs from the cerebral cortex, thalamus and midbrain dopamine neurons and by their differential projections (Gerfen, 1992). Corticostriatal inputs to the patch and matrix compartments arise from distinct populations of layer 5 cortical neurons (Gerfen, 1989) and neurons in the patch and matrix compartments project respectively to dopamine neurons in the substantia nigra pars compacta (SNc) or to GABAergic neurons in the substantia nigra pars reticulata (SNr)(Gerfen, 1992). BAC-Cre driver lines are characterized that preferentially express in these patch/striosome and matrix circuits.
The BAC-Cre line, Plxnd1_OG1 displays expression in corticostriatal neurons projecting primarily to the striatal matrix compartment. Injections of AAV-Cre dependent expression vectors that label select neurons in layer 5 and some layer2/3 in the primary motor cortex, label axonal projections that are distributed primarily into the striatal matrix compartment, bilaterally (Figure 8). While there is substantial labeling of corticostriatal projections with cortical injections in this line, there is only sparse labeling of projections to other subcortical regions, including the thalamus, superior colliculus and some labeling of fibers extending through the internal capsule, cerebral peduncle to the pyramidal tract. This pattern of labeling suggests that the predominant matrix directed corticostriatal neurons are of the IT subtype.
Figure 8. BAC-Cre lines with preferential expression in Patch/Matrix circuits.
Left panels: In lines Plxnd1_OG1 and Colgalt2_NF107, Cre-induced EGFP expression produced with Rosa26_EGFP reporter is shown in the cortex (top row) and striatum (bottom row). Injections of AAV-dTomato expression vectors in Plxnd1_OG1 labels neurons in the somatosensory cortex that provide projections selectively distributed in the striatal matrix compartment (pseudo-colored yellow), whereas injections in Colgalt2_NF107 labels neurons in the prelimbic cortex, which in this case distribute projections to the striatal patch compartment. Right Panels: Striatal patch/matrix projections to the substantia nigra. (top row) Cre-induced EGFP expression produced with Rosa26_EGFP reporter displays expression in neurons selectively in the striatal matrix (Plxnd1_OG1) and patch (Sepw1_NP67) compartments. Injections of AAV-dTomato expression vectors into the striatum in line Pxnd1_OG1 selectively labels neurons in the matrix compartment with axonal projections that terminate in the substantia nigra pars reticulata, which does not contain dopamine neurons (labeled red). In contrast, injections into the striatum in line Sepw1_NP67 labels neurons preferentially in the patch compartment, which provide axonal projections to the substantia nigra that target the dopamine neurons in the pars compacta (labeled red) with some terminals in the pars reticulata.
To date there have not been any BAC-Cre driver lines identified with expression strictly restricted to corticostriatal neurons selectively projecting to the patch/striosome compartment. One line, Colgalt2_NF107, displays Cre expression that has some selectivity for such neurons. Expression in this line displayed with the Rosa26-reporter lines shows somewhat preferential labeling of corticostriatal terminals in the patch/striosome compartment, although there is clear labeling in both compartments. Injections of AAV vectors in this line reveal both patterns of labeling. In some cases (Figure 5), injections into the medial prefrontal cortex label axonal projections that are distributed bilaterally to the striatum in a somewhat homogenous pattern. On the other hand, other injections into the medial prefrontal cortex label corticostriatal axons that display a preferential distribution in the striatal patch/striosome compartment (Figure 8). Similar patterns of preferential patch projections are also seen with injections in the prefrontal and motor cortical areas in Syt17_NO14. This suggests that use of these lines may provide selective labeling of corticostriatal neurons with selective inputs to the patch/striosome compartment.
The BAC-Cre line Plxnd1_OG1 also displays expression in the striatum, restricted to spiny projection neurons in the matrix compartment. Injections of AAV vectors into the striatum selectively label neurons in the matrix compartment and axonal projections distributed in the GPe and into the substantia nigra. Axonal labeling in the substantia nigra is restricted to the pars reticulata (SNr) with no terminals directed to the location of dopamine neurons in the substantia nigra pars compacta (SNc). This pattern of labeling suggests labaling of both striatal direct and indirect pathway neurons in the matrix compartment. Expression in Plxnd1_OG1is somewhat restricted to the dorsal half of the striatal matrix compartment. Another Bac-Cre line, Npyr1_RL2, also selectively labels striatal matrix neurons, which are distributed through all regions of the striatum, including the ventral regions (Supplemental Figure 5).
Three lines are characterized with preferential labeling in striatal patch neurons, Sepw1_NP67, Syt6_KI148 and Dlg3_KG118 determined with the relationship to the neurochemical patch marker mu opiate receptor immunohistochemistry. In each of these lines there is some labeling of scattered neurons in the striatal matrix compartment. Injections of AAV- vectors into the striatum in Sepw1_NP67, label neurons preferentially localized in the striatal patch compartment, with some scattered neurons in the matrix. Labeling of axonal projections to the substantia nigra show preferential labeling terminating in the location of dopamine neurons in the substantia nigra pars compacta (SNc). In this case there is some labeling of axonal terminals in the SNr, which may be attributed to labeling of some axons of patch neurons and the scattered neurons in the striatal matrix. The patterns of labeling of axonal projections of striatal patch/strisome (Sepw1_NP67) and matrix neurons (Plxnd1_OG1) is consistent with the established differential projections of these striatal compartments to the SNc and SNr (Gerfen, 1992; Fujiyama et al., 2011; Watabe-Uchida et al., 2012).
Additional BAC-Cre driver lines
The GENSAT BAC-Cre driver line collection contain lines that display selective expression in many brain areas related to cortical and basal ganglia circuits in addition to those described above. Information on these lines is available on the GENSAT websites:: http://www.gensat.org/cre.jsp and http://GENSATcreBrains.net. A list of these lines is provided in Table 4. Among these are those with expression in the amygdala, hippocampus, in neurotransmitter specific neurons, including dopamine, noradrenergic, serotonergic and cholinergic neurons and in components of the basal ganglia circuits including the habenula, subthalamic nucleus(STN), and the superior colliculus (SC). Lines that display Cre expression in the intermediate layer of the superior colliculus, which is the target of the output substantia nigra pars reticulata are shown in Supplementary Figure 6. Additionally there are lines that express in GABAergic and peptidergic interneurons in the cerebral cortex and striatum. A more complete collection of GABAergic interneurons has been provided in a recent study (Taniguchi et al., 2011).
TABLE 4.
BAC-Cre driver lines with expression in other cortical- and basal ganglia-related structures
| Gene | Line | Brain region or neuron type |
|---|---|---|
| Amygdala | ||
| Htr2a | KM208 | Amygdala (Central nucleus) |
| Klk8 | NP157 | Amygdala(central nucleus) |
| Arhgef6 | NL189 | Amygdala (basolateral nucleus) |
| Rasgrp1 | PO1 | Amygdala (basolateral nucleus) |
| Nts | RH4 | Amygdala (basolateral nucleus) |
| Drd1a | EY266 | Amygdala (intercalated nucleus) |
| Hippocampus | ||
| Chrna3 | NO196 | Dentate gyrus |
| Chrna5 | NP306 | Hippocampus/dentate gyrus |
| Dlg3 | KG118 | Hippocampus (CA2/3) |
| Drd3 | KI196 | Hippocampus (CA1)/dentate gyrus |
| Epyc | KR363 | Hippocampus (CA1) |
| Lypd1 | NR149/NR151 | Hippocampus |
| Rasgrp1 | PO14 | Hippocampus |
| Rbp4 | KL100 | Dentate gyrus |
| neurotransmitter specific | ||
| Chat | GM24/GM53 | Cholinergic neurons |
| Dbh | KH212 | Noradrenergic neurons |
| TH | FI12/FI172 | Catecholaminergic neurons |
| Slc18a2 | OZ13/OZ14 | Catecholaminergic neurons |
| Slc6a3(DAT) | SG56/SG62 | Dopaminergic neurons |
| Slc6a4(SERT) | ET33/ET127 | Serotonergic neurons |
| habenula | ||
| Chrna3 | NO196 | Habenula, LH and MH |
| Cnnm2 | KD18 | Habenula, MH |
| A830010M20Rik | KJ227 | Habenula, MH |
| subthalamic nucleus | ||
| Gabarr3 | KC112 | STN |
| Cort | IM42 | STN |
| superior colliculus | ||
| Pde1c | IT146 | SC, intermediate layer, medial |
| Chrna3 | NO196 | SC, intermediate layer |
| Chrna7 | NP366 | SC, intermediate and superficial layers |
| Lypd1 | SE5 | SC, intermediate layer |
| interneurons | ||
| Npy | RH26 | interneurons |
| Htr3a | NO152 | interneurons |
| Vip | PH13 | interneurons |
DISCUSSION
The GENSAT project has produced over 250 BAC-Cre driver lines that are available to the research community (Gong et al., 2007). These lines utilize BAC constructs that are modified by replacing the coding sequence for the protein of the gene of interest with Cre-recombinase. An advantage of this approach is that the BAC constructs in most cases contain the entire promoter region of the gene, which is essential for regulating expression of its coding sequence (Heintz, 2001). In most cases, Cre-expression mimics the expression of the endogenous gene. On the other hand, for some BAC-Cre driver constructs, the expression pattern in a specific line displays expression patterns that might not be predicted from the endogenous gene expression, most likely due to the genomic site that the BAC construct is inserted. Nonetheless, such lines often produce expression in specific neuron populations and are stable. Additionally, the use of BAC constructs to drive Cre expression allows targeted neurons to be functionally manipulated without affecting the function of the endogenous gene. The utility of these lines for use in combination with other techniques to address functional questions is dependent on determining the specific neuron subtypes in which Cre-recombinase is functionally expressed. Expression data provided with Rosa26- reporter lines provides valuable information, but is limited by inherent limitations due to transient expression of some genes during development and the sensitivity of the reporter lines used. In this study we provide additional characterization based on injection of Cre-dependent AAV-expression vectors to identify specific subtypes of neurons. Data from such injections is predictive of the neurons in a given BAC-Cre line that will be infected with similar AAV-vectors with Cre-dependent constructs to express proteins used to functionally activate or record physiologic activity. Additionally, data provided by AAV-expression vectors allows for the identification of neuron subtypes on the basis of their axonal projections.
In this study, over 30 BAC-Cre lines are described with expression in the cerebral cortex. The specific cortical subtypes that express functional Cre varies in these lines determined with AAV-expression vector injections. Some lines have expression that is specific for particular layers and for the IT and PT layer 5 subtypes. These linclude Sepw1_NP39 for layer 2/3, Tlx3_PL56 for layer 5 IT corticostriatal neurons, Sim1_KJ18 for layer 5 PT corticostriatal neurons and Ntsr1_GN220 for layer 6 corticothalamic neurons. Other lines label subsets of the neuron subtypes or label subsets of neurons in multiple layers. These lines may be useful for identifying additional subtypes of cortical neurons or for determining specific connections between neurons in different cortical layers. These BAC-Cre driver lines provide research tools for addressing questions concerning the functional role of specific neuron circuits in behavior.
Recent studies have begun to elaborate principles of functional connectivity within the cerebral cortical circuits (Molyneaux et al, 2007). Patterns of connectivity between pyramidal neurons suggest the organization of excitatory networks that segregate functional pathways for processing different types of information (Otsuka and Kawaguchi, 2011; Morishima et al., 2011). These networks are formed by connections between cortical areas that differentially target layer 2/3 neurons and PT and IT layer 5 neurons (Mao et al., 2011), by differential connections from layer 2/3 neurons onto PT and IT layer 5 neurons (Anderson et al., 2010), by differential probability of connections between PT and IT layer 5 neurons (Brown and Hestrin, 2009) and differential connections of layer 5 neurons with the thalamus (Hooks et al., 2013). These distinct functional channels of excitatory networks are suggested to differentially regulate the output of PT and IT corticostriatal neurons (Morita et al 2012; Shepherd 2013).
Patterns of segregated excitatory circuits are proposed to underlie the integration of sensory and motor representations within motor cortical areas involved in sensorimotor learning (Huber et al., 2012). One of the findings of this study is that intermingled neurons of a particular cortical subtype differentially encode sensory and motor information. This suggests that there are functionally distinct subtypes of particular types of cortical neurons. Other studies have identified subtypes of PT and IT corticostriatal neurons based on their dendritic morphology and physiologic characteristics (Otsuka and Kawaguchi, 2011; Morishima et al., 2011). The BAC-Cre driver lines described in this study with selective expression in PT and IT corticostriatal neurons display differences that suggest different subtypes of these neurons may express functional Cre in different lines. Such differences are apparent both within particular cortical areas and also between cortical areas. Further characterization of these subtypes of Cre-expressing PT and IT neurons is necessary to determine whether they correspond to subtypes with distinct physiologic and connectional properties.
The specificity of connections between subtypes of cortical pyramidal neurons provides a neuroanatomical substrate for processing distinct types of information in functional channels within the organization of cerebral cortical circuits. Understanding how such channels affect behavior depends on determining how the output of these channels are organized through connections to their targets in subcortical circuits. The maintenance of segregation of different cortical inputs through the connections of the striatal patch and matrix compartments established the existence of functional channels at a macroscopic level in corticostriatal circuits (Gerfen, 1992). As discussed above, recent studies suggest that specificity between connections within the cerebral cortex establish distinct functional channels for PT and IT corticostriatal neurons. The report that PT and IT corticostriatal neurons selectively project to direct and indirect striatal neurons, respectively, suggests that the segregation of excitatory networks in cerebral cortical circuits extends into the striatum (Lei et al., 2004). These studies suggest that a general principle of the organization of functional excitatory circuits within the cortex is the high degree of specificity in the patterns of connectivity of distinct subtypes of cortical pyramidal neurons. The availability of BAC-Cre driver lines that allow targeting of subtypes of cortical neurons provide research tools for determining not only the specificity of intracortical connections but the specificity of connections to defined neurons in the striatum. Such studies may then be extended to understanding of how functional excitatory cortical circuits affect behavior.
EXPERIMENTAL PROCEDURES
Mice were maintained in an IACUC approved NIH shared animal facility and all experimental procedures were conducted in accordance with NIH ethical guidelines under an Animal Study Protocol approved by the NIMH, DIRP ACUC.
Mice
BAC-Cre transgenic mouse lines were created using BAC engineering techniques as described previously (Gong et al., 2002; 2007). BAC vectors containing the target gene of interest were modified by insertion of an intron containing a Cre-recombinase cassette followed by by a polyadenylation sequence to terminate transcription of the fusion transcript immediately after the recombinase gene into the BAC vector at the initiating ATG cdon in the first coding exon of the selected gene. This was done using a shuttle vector (pLD53.SC-Cre) carrying the appropriate selectable markers and a small homology arm from the locus being targeted to guide recombination. To characterize functional Cre-expression, mice were crossed to the Rosa26/stop/EGFP reporter line (Soriano, 1999). Brains from mice carrying both the BAC-Cre construct and Rosa26_EGFP transgene were processed for immunohistochemical detection of EGFP using either peroxidase or indirect fluorescent labeling. Some lines were also crossed to the Rosa26_CAG-tdTomato Ai9 reporter line (B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J,from the Allen Institute for Brain Science, Madisen et al., 2010). Detection of functional reporter expression in brain sections was provided either with direct fluorescent observation or with immunohistochemical detection of tdTomato.
AAV-expression vector injections
To trace axonal projections of Cre-expressing neurons in the BAC-Cre transgenic lines, the EGFP and tdTomato AAV Cre-dependent expression vectors, AAV2/1.CAG.FLEX.EGFP.WPRE.bGH and AAV2/1.CAG.FLEX.tdTomato.WPRE.bGH were injected into selected brain areas (Harris et al, 2012). Animals were killed 7–21 days following injections, and their brains processed for immunohistochemical localization of EGFP and/or tdTomato.
Microscopic imaging
Brains were frozen sectioned at 40 or 80 μm and free floating sections processed with standard peroxidase or fluorescent immunohistochemical techniques. Slide mounted sections were imaged on a Zeiss or Olympus microscope with a Ludl motorized stage controlled with Neurolucida software (Microbrightfield, Inc., Burlington, VT). Imaging was done with a 10x objective, with an Hamamtsu Orca Flash 4 camera, with each coronal section containing between 80–200 tiles merged together with Neurolucida software.
Supplementary Material
TABLE 2.
BAC-Cre lines with expression in thalamic nuclei.
| Gene | Line | Thalamic nuclei |
|---|---|---|
| Grp | KH288 | IL |
| Plxnd1 | OG1 | IL |
| Syt17 | NO14 | IL |
| Slc17a6 | OX30 | all |
| Kcnc1 | MN77 | VPM, VPL, SMT, LD, MD |
| Grm2 | MR90 | AV, LD, IL, PVT, LH, MH |
| Gpr26 | KO250 | LD, VAL, VM |
| Vipr2 | KH234 | VPM, VPL |
| Nsmf | NR133 | VPM, VPL |
| Crh | KN282 | VPM, VPL |
| Cnnm2 | KD19 | MH |
| Hap1 | RE55 | LH, VM |
| Chrna3 | NO196 | LH and MH |
| Gsbs | NL146 | VPM, VPL, SMT |
Thalamic nuclei abbreviations: AV (anteroventral nucleus), IL (intralaminar nucleus), LD (laterodorsal nucleus), LH (lateral habenula), PVT (paraventricular nucleus), MD (mediodorsal nucleus), MH (medial habenula), VPL (ventral posterolateral nucleus), VPM (ventral posteromedial nucleus) SMT (submedial nucleus)
Acknowledgments
Funding support: to CRG (NIMH IRP project MH002497-24); to NH: (GENSAT contract NIH/NINDS N271200723701C; NIH/NINDS MMRRC Subaward 201224636; NIH/NIDA 1P30 DA035756-01; NIH/NIMH P50 MH090963 P2; Howard Hughes Medical Institute Investigator Award). We would like to acknowledge the excellent technical support and expertise of Michelle Tenace, Scott Stavrou, Laura Kus, Shiaoching Gong and Nicholas Didkovsky. We would also like to thank the NINDS GENSAT staff, Laura Mamounas, Coryse St. Hillaire-Clarke, and Amelie Gubitz.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CT, Sheets PL, Kiritani T, Shepherd GM. Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nat Neurosci. 2010;13:739–44. doi: 10.1038/nn.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature. 2009;457:1133–6. doi: 10.1038/nature07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–71. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, et al. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur J Neurosci. 2011;33:668–77. doi: 10.1111/j.1460-9568.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR. D1 and D2 dopamine receptor regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: Striatal patch -matrix organization is related to cortical lamination. Science. 1989;246:385–388. doi: 10.1126/science.2799392. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The Neostriatal Mosaic: Multiple Levels of Compartmental Organization. Trends in Neuroscience. 1992;15: 133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Wilson CJ. The Basal Ganglia. In: Hokfelt T, Swanson LW, editors. Handbook of Chemical Neuroanatomy. Elsevier; 1996. pp. 365–462. [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–25. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–23. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Wook Oh S, Zeng H. Adeno-associated viral vectors for anterograde axonal tracing with fluorescent proteins in nontransgenic and cre driver mice. Curr Protoc Neurosci. 2012;Chapter 1(Unit 1.20.1) doi: 10.1002/0471142301.ns0120s59. [DOI] [PubMed] [Google Scholar]
- Heintz N. BAC to the future: the use of bac transgenic mice for neuroscience research. Nat Rev Neurosci. 2001;2:861–70. doi: 10.1038/35104049. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Mao T, Gutnisky DA, Yamwaki N, Svoboda K, Sheperd GMG. Organization of cortical and thalamic input to Pyramidal Neurons in Mouse Motor Cortex. J Neuroscience. 2013;33: 748–760. doi: 10.1523/JNEUROSCI.4338-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZH, Zeng H. Genetic approaches to neural circuits in the mouse. Annu Rev Neurosci. 2013;36:183–215. doi: 10.1146/annurev-neuro-062012-170307. [DOI] [PubMed] [Google Scholar]
- Huber D, Gutnisky DA, Peron S, O’Connor DH, Wiegert JS, Tian L, Oertner TG, Looger LL, Svoboda K. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484:473–8. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci. 1990;10:3421–38. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritani T, Wickersham IR, Seung HS, Shepherd GM. Hierarchical connectivity and connection-specific dynamics in the corticospinal-corticostriatal microcircuit in mouse motor cortex. J Neurosci. 2012;32:4992–5001. doi: 10.1523/JNEUROSCI.4759-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Kita H. The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. J Neurosci. 2012;32:5990–9. doi: 10.1523/JNEUROSCI.5717-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–6. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neuroscience. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye K, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci. 2004;24:8289–99. doi: 10.1523/JNEUROSCI.1990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–60. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–40. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L, Svoboda K. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron. 2011;72:111–23. doi: 10.1016/j.neuron.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–37. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Morita K, Morishima M, Sakai K, Kawaguchi Y. Reinforcement learning: computing the temporal difference of values via distinct corticostriatal pathways. Trends Neurosci. 2012;35:457–67. doi: 10.1016/j.tins.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Morishima M, Morita K, Kubota Y, Kawaguchi Y. Highly differentiated projection-specific cortical subnetworks. J Neurosci. 2011;31:10380–91. doi: 10.1523/JNEUROSCI.0772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T, Kawaguchi Y. Cell diversity and connection specificity between callosal projection neurons in the frontal cortex. J Neurosci. 2011;31:3862–70. doi: 10.1523/JNEUROSCI.5795-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM. Corticostriatal connectivity and its role in disease. Nat Rev Neurosci. 2013;14:278–91. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc Natl Acad Sci U S A. 2010;107:15235–9. doi: 10.1073/pnas.1009112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–62. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–73. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Morphology and synaptic connections of crossed corticostriatal neurons in the rat. J Comp Neurol. 1987;263:567–80. doi: 10.1002/cne.902630408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.