Abstract
The moss Physcomitrella patens is unique for the high frequency of homologous recombination, haploid state, and filamentous growth during early stages of the vegetative growth, which makes it an excellent model plant to study DNA damage responses. We used single cell gel electrophoresis (comet) assay to determine kinetics of response to Bleomycin induced DNA oxidative damage and single and double strand breaks in wild type and mutant lig4 Physcomitrella lines. Moreover, APT gene when inactivated by induced mutations was used as selectable marker to ascertain mutational background at nucleotide level by sequencing of the APT locus. We show that extensive repair of DSBs occurs also in the absence of the functional LIG4, whereas repair of SSBs is seriously compromised. From analysis of induced mutations we conclude that their accumulation rather than remaining lesions in DNA and blocking progression through cell cycle is incompatible with normal plant growth and development and leads to sensitive phenotype.
1. Introduction
Plants developed several strategies to protect integrity of their genome against various environmental stresses. Common denominator of most of them is oxidative stress mediated by reactive oxygen species (ROS). The origin of ROS within the cell could be a consequence of physical or chemical genotoxic treatment, as well as byproduct of internal oxygen metabolism often triggered by external stimuli as drought and salinity. To be able to cope with oxidative stress we have to assess all faces of this challenge for plants; in particular, how it affects genetic material of the cells and how eventual changes are temporarily or permanently expressed in plant phenotype. This is why we need a flexible and robust model system, which experimentally enables the use of reverse genetics for genotoxic and biochemical studies. In this paper we describe a novel system to be considered for genotoxicity testing in plants.
The moss Physcomitrella patens is an emerging model plant [1] with the following differences/advantages as compared to other plant test systems: efficient homologous recombination (enabling reverse genetics of virtually any gene), dominant haploid phase (enabling assessment of mutation phenotype), small size plantlets colonies with a quick and during early vegetative stage also filamentous growth, easy cultivation in inorganic media and several options of long term storage. Here we describe and validate a system of small protonemata fragments with high fraction of apical cells primarily developed for the purpose of genotoxicity testing. However, these one-day-old protonemata could be used as a substitute of protoplasts for other purposes, for example, for moss transformation [2].
APT (adenine phosphoribosyltransferase) is an enzyme of the purine salvage pathway that converts adenine into AMP and its loss of function generates plants resistant to adenine analogues, for example, 2-FA (2-Fluoroadenine) [3]. Mutational inactivation can be used as selectable marker for mutator genotyping as well as analysis of mutations in APT locus on nucleotide level [4–6].
This paper is an extension of our previous study of Physcomitrella knockout mutants of a key MRN (MRE11, RAD50, and NBS1) complex [6] with a pleiotropic effect on DSB repair in whole. We explore and validate the above outlined moss model system for genotoxicity testing in plants. We describe a parallel use of SCGE (single cell gel electrophoresis, comet) assay for detection of DNA damage and its repair and of APT assay with sequencing analysis of mutants. On example of lig4, mutated in a key component of nonhomologous DSB-end joining pathway (C-NHEJ), we show consequences of mis repair. For strengthening the model concept we also present preliminary results of pprad51AB sensitivity to genotoxin treatment and on ppku70 mutation rate.
2. Material and Methods
2.1. Plant Material
Physcomitrella patens (Hedw.) B.S.G. “Gransden 2004” wild type and pplig4 were vegetatively propagated as previously described [7]. The lig4 and ku70 mutants of C-NHEJ were generated by D. G. Schaefer, Neuchatel University, Switzerland, and F. Nogue, INRA, Paris, France, and kindly provided by F. Nogue. Detailed characteristic of this mutant will be published elsewhere. Mutant in both alleles of Physcomitrella RAD51 gene (pprad51AB, clone 721) is described elsewhere [8] and was kindly provided by B. Reiss, MPIZ, Cologne, Germany.
Individual plants were cultured as “spot inocula” on BCD agar medium supplemented with 1 mM CaCl2 and 5 mM ammonium tartrate (BCDAT medium) or as lawns of protonemal filaments by subculture of homogenized tissue on BCDAT agar medium overlaid with cellophane in growth chambers with 18/6 hours day/night cycle at 22/18°C.
One-day-old protonemal tissue for repair and mutation experiments were prepared from one-week-old tissue scraped from plates, suspended in 8 mL of BCD medium, and sheared by a T25 homogenizer (IKA, Germany) at 10 000 rpm for two 1-minute cycles and let 24 hours to recover in cultivation chamber with gentle shaking at 100 rpm. This treatment yielded suspension of 3–5 cell protonemata filaments, which readily settle for recovery. Settled protonemata could be handled without excessive losses by tweezers on glass Petri plates.
2.2. Bleomycin Treatment and Sensitivity Assay
For treatments was used Bleomycin sulphate supplied as Bleomedac inj. (Medac, Hamburg, Germany). All solutions were prepared fresh prior treatment from weighted substance in BCDAT medium.
Protonemal growth was tested by inoculating explants of wild type and 5 mutant lines onto 6 × 4 multiwell plates organized to allow in line comparison of the effect of increasing Bleomycin concentrations. The wells were filled with 2 mL of standard growth BCDAT agar medium without or with 0.01, 0.1, and 1 μg mL−1 Bleomycin. The experiment was carried in 3 independent replicas and monitored up to 3 weeks for growth of “spot inocula.”
Treatment of one-day-old protonemata was performed on glass 5 cm Petri plates with the aid of bent tweezers to handle tissue and pipettes to remove excess liquids. Opening of yellow tips is generally small enough to avoid suction of moss filaments when drawing majority of liquid from tissue prior blotting of collected tissue on filter paper to remove the rest.
In dose-response and repair kinetic experiments, one-day-old protonemata were after the Bleomycin treatment thoroughly rinsed in water, blotted on filter paper, and either flash-frozen in liquid N2 (dose response and repair t = 0) or left to recover on plates in liquid BCDAT medium for the indicated repair times, before being frozen in liquid N2.
For induction and regeneration of apt mutants one-day-old protonemata were after Bleomycin treatment thoroughly rinsed with H2O, suspended in 2 mL of BCDAT medium, and spread on cellophane overlaid BCDAT agar plates, which were for selection supplemented with 2-FA (2-Fluoroadenine, Sigma-Aldrich, cat. Nr. 535087) and further incubated in growth chamber.
2.3. Detection of DNA Lesions
DNA single and double strand breaks were detected by a SCGE assay using either alkaline unwinding step A/N [9, 10] or fully neutral N/N protocol [11, 12] as previously described. In brief, approximately 100 mg of frozen tissue was cut with a razor blade in 300 μL PBS + 10 mM EDTA on ice and released nuclei transferred into Eppendorf tubes on ice. 70 μL of nuclear suspension was dispersed in 280 μL of melted 0.7% LMT agarose (GibcoBRL, cat. Nr. 15510-027) at 40°C and four 80 μL aliquots were immediately pipetted onto each of two coated microscope slides (in duplicate per slide), covered with a 22 × 22 mm cover slip and then chilled on ice for 1 min to solidify the agarose. After removal of cover slips, slides were immersed in lysing solution (2.5 M NaCl, 10 mM Tris-HCl, 0.1 M EDTA, and 1% N-lauroyl sarcosinate, pH 7.6) on ice for at least 1 hour to dissolve cellular membranes and remove attached proteins. The whole procedure from chopping tissue to placement into lysing solution takes approximately 3 minutes. After lysis, slides were either first incubated 10 minutes in 0.3 M NaOH, 5 mM EDTA, pH 13.5 solution to allow partially unwind DNA double helix to reveal SSBs (A/N protocol) or without unwinding step (N/N protocol) directly equilibrated twice for 5 minutes in TBE electrophoresis buffer to remove salts and detergents. Comet slides were then subjected to electrophoresis at 1 V cm−1 (app. 12 mA) for 3 minutes. After electrophoresis, slides were placed for 5 min in 70% EtOH, 5 min in 96% EtOH, and air-dried.
Comets were viewed in epifluorescence with a Nikon Eclipse 800 microscope stained with SYBR Gold (Molecular Probes/Invitrogen, cat. Nr. S11494) according to manufacture recommendation and evaluated by the LUCIA Comet cytogenetic software (LIM Inc., Czech Republic).
2.4. SCGE Assay Data Analysis
The fraction of DNA in comet tails (% tail-DNA, % T DNA) was used as a measure of DNA damage. Data for the wild type and the mutant pplig4 line analysed in this study were obtained in at least three independent experiments. In each experiment, the % T DNA was measured at seven time points: 0, 5, 10, 20, 60, 180, and 360 min after the treatment and in control tissue without treatment. Measurements included four independent gel replicas of 25 evaluated comets totalled in at least 300 comets analysed per experimental point. The percentage of damage remaining as plotted on Figure 2(b) after given repair time (t x) is defined as
| (1) |
Time-course repair data were analysed for one- or two-phase decay kinetic by Prism v.5 program (GrafPad Software Inc., USA).
Figure 2.
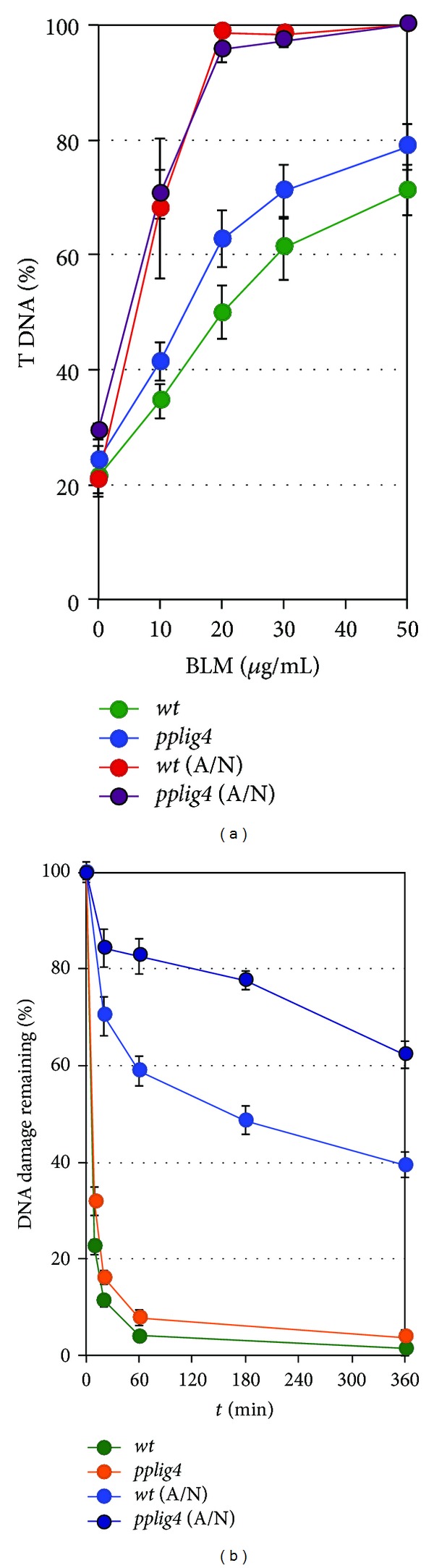
SSB and DSB repair kinetics determined by SCGE. One-day regenerated protonemal tissue from wild type and pplig4 lines was treated with Bleomycin for 1 h prior to nuclear extraction and the analysis. (a) Dose-response as the percentage of the free DNA moved by electrophoresis into comet tail (% T DNA) at the indicated Bleomycin concentrations. DSBs were determined by N/N protocol: green: wild type, blue: pplig4, whereas SSBs were determined by A/N protocol: red: wild type, dark purple: pplig4. (b) Repair kinetics is plotted as % of DSBs remaining over the 0, 5, 10, 20, 60, 180, and 360 min period of repair recovery. Maximum damage is normalised as 100% at t = 0 for all lines. SSBs were induced by 1-hour treatment with 2 μg mL−1 Bleomycin; bright blue: wild type, dark blue: pplig4, and determined by A/N protocol. DSBs were induced by 1-hour treatment with 30 μg mL−1 Bleomycin, green: wild type, orange: pplig4, and determined by N/N protocol. (Error bars-standard error).
2.5. Isolation and Analysis of apt Mutants after Bleomycin Treatment
Mutation rates were measured as the number of apt mutants that appeared as green foci of regenerating clones resistant to 2-FA (Figure 3). Treated protonemata were first incubated approximately 3 weeks on plates with 2 or 3 mM 2-FA until first green foci start to emerge. Then whole cellophane overlay was transferred to a new plate with 8 mM 2-FA and emerging clones were allowed to form colonies. Stable clones were then counted.
Figure 3.
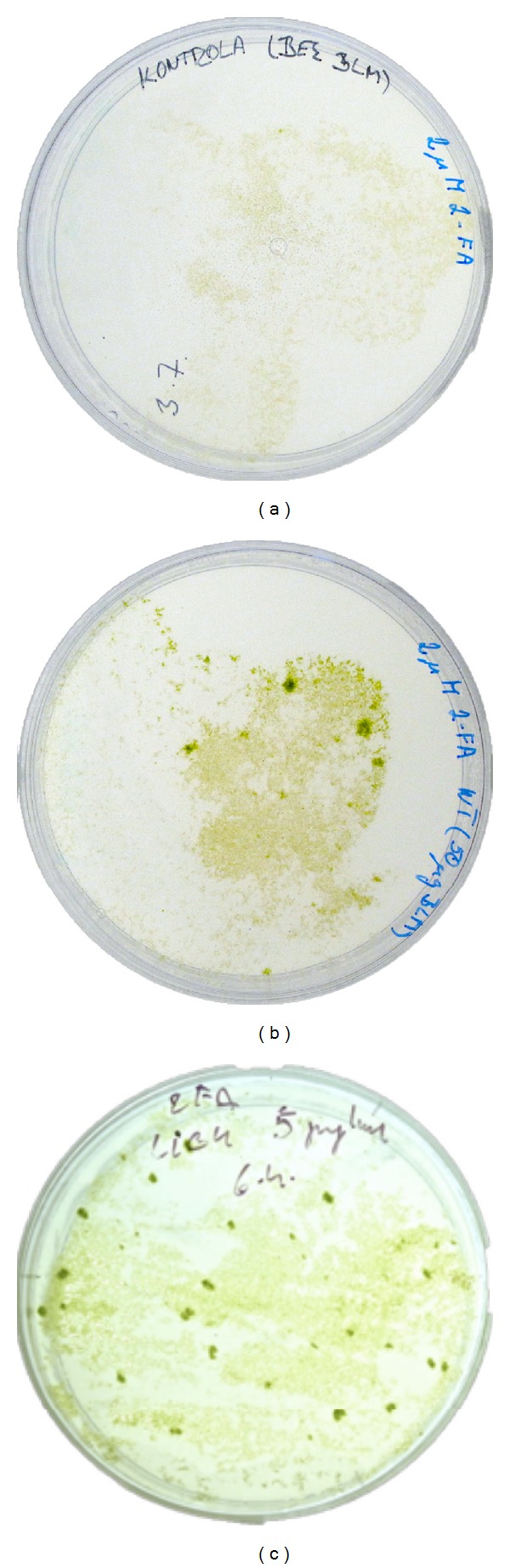
Plates with 2-FA resistant foci of wild type Physcomitrella (a and b) and pplig4 (c) after 3 weeks of selection. (a) Untreated Physcomitrella wild type, (b) 50 μg mL−1 Bleomycin treated wild type protonemata for 2 hours, and (c) 5 μg mL−1 Bleomycin treated pplig4 protonemata for 1 hour prior being spread on plates with BCDAT medium supplemented with 2 μM 2-FA and cultivated for 3 weeks.
Some clones were further propagated on plates with 8 mM 2-FA and their APT locus was subsequently PCR amplified and sequenced to identify the mutation(s) responsible for the resistance. Approximately 100 mg of tissue was used to isolate genomic DNA with DNeasy Plant Mini Kit (Qiagen, cat. Nr. 69104) using “ball” mill Retsch MM301 to homogenize the tissue in 2 mL round bottom Eppendorf tubes. APT locus was amplified from isolated genomic DNA with KOD Hot Start DNA Polymerase (Millipore/Novagen, cat. Nr. 71086), purified with the QIAquick PCR Purification Kit (Qiagen, cat. Nr. 28104) and used as a template for sequencing with BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, cat. Nr. 4337455). Locations of PCR primers used for APT amplification and sequencing are depicted on Supplementary Figure 2 and their sequences are listed in Supplementary Table 1 (see Supplementary Material available online at http://dx.doi.org/10.1155/2013/535049). To keep sequencing cost down only half volume of the BigDye Ready Reaction Mix was used in a standard sequencing reaction and combined with the same volume of Half-Term-Dye-Termination mixture (Sigma-Aldrich, cat. Nr. H1282).
2.6. Analysis of Sequencing Data
Sequences of each clone obtained on genetic analyser Prism 3130x1 (Applied Biosystems, USA) were stiched together with MacVector program Assembler 12.7.5 (MacVector, USA) into contigs and aligned to the latest annotated APT sequence Pp1s114_124V6.1 in the COSMOSS—the Physcomitrella patens resource database (https://www.cosmoss.org/).
3. Results and Discussion
In all experiments a model Physcomitrella patens has been used as one day recovered fragments of 3–5 cell size derived from the lawn of growing protonema filaments by extensive shearing. Such one-day-old protonemata represent a unique system among plants to study plant tissue with up to 50% of apical dividing cells. Convenient mechanical handling enables quick processing of tissue after the treatment to address short repair times and with fine tip tweezers also for uniform spotting to test sensitivity. In this respect one-day-old protonemata are preferred system to so far widely used protoplasts, which could be collected only by centrifugation. Another reason for using protonemata is possibility their mechanical disintegration by razor blade chopping for rapid release of nuclei for SCGE assay. In this way direct use of protoplasts for comet assay is obstructed by nearly instant regeneration of the cell wall within 4 hours after the release from cellulase treatment (unpublished observation), because cell wall prevents DNA movement from nuclei during electrophoresis.
3.1. Sensitivity to Bleomycin Treatment
Moss wild type and pplig4, mre11, nbs1, rad51AB, and rad50 [6, 8] mutant lines were analysed for their sensitivity to radiomimetic Bleomycin in chronic “survival” assay when test plates with various concentrations of Bleomycin were inoculated with equal tissue “spots” of one-day-old protonemata and incubated up to 3 weeks (Figure 1). Only rad51AB and rad50 strains displayed one order of a magnitude higher sensitivity in comparison to other tested lines. The survival growth of ppmre11 is somehow in contradiction with pervious results of Kamisugi et al. [6], but one has to realize different assay conditions, for example, acute versus chronic exposure and protoplast cells versus protonemata. In protonema tissue under permanent genotoxic stress mre11 express phenotype similar to wild type, nbs1, and also lig4. One can speculate that 3′ to 5′ exonuclease and endonuclease activity associated with MRE11 is dispensable for tissue survival, but proteins RAD50 and RAD51 supporting DNA structure are not. Kozak et al. [12] previously showed crucial role of structural maintenance of chromosome complex SMC5/6 in the repair of Bleomycin induced DSBs. In this context RAD50s have similar structural role in MRN complex as SMCs in the structure of the SMC5/6 complex [13]. Both these complexes can function in tethering of broken ends in close proximity.
Figure 1.
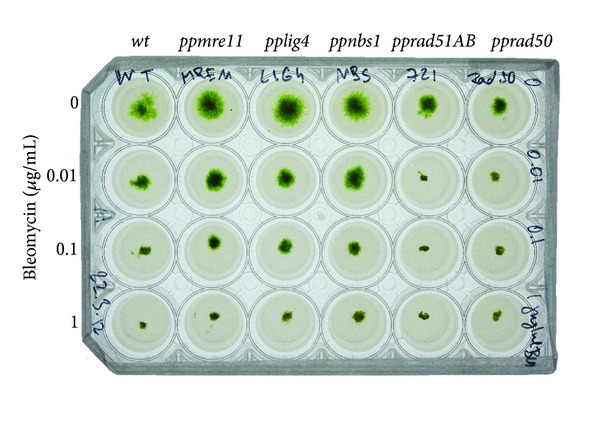
Sensitivity of the Physcomitrella patens repair mutants mre11, lig4, nbs1, rad51AB, and rad50 to chronic exposure of Bleomycin. Physcomitrella explants were inoculated as “spot inocula” onto BCDAT-agar plates supplemented with 0, 0.01, 0.1, and 1 μg mL−1 Bleomycin and photographed 10 days after inoculation.
3.2. Induction of DNA Lesions and Their Repair
Bleomycin, an ionizing radiation mimicking agent, functions as a catalyst activated by interaction with DNA and attachment of Fe2+ to produce oxygen radicals leading to lesions as SSBs, DSBs, AP-sites, and damaged bases [14, 15], which all could be readily detected by SCGE [16]. DNA breaks and other lesions converted to breaks lead to DNA fragmentation and nucleoid unwinding allowing relaxed DNA to move in electric field from nuclei out to form a “comet” like object in which increased quantity of fragmented DNA in comet tail (% T DNA) is proportional to breakage. DSBs are detected by an N/N assay when pH of lysing and electrophoretic solutions is kept under pH 10, whilst for the detection of SSBs DNA after the lysis is allowed to unwind DNA double-helix in alkali [17] to separate individual strands and expose their fragmentation (A/N protocol [9]).
Bleomycin fragmentation of genomic DNA by induction of SSBs and DSBs is documented on Figure 2(a). Ten times higher efficiency to induce SSBs than DSBs is in agreement with generally accepted ratio of 1 : 10, DSBs versus SSBs, induced by ionizing radiation. Evidently this also applies for Bleomycin treatment of Physcomitrella. The background level of genomic DNA damage in wild type and pplig4 is similar, between 20 and 25% T DNA, indicating that the repair defect has no significant effect on natural levels of genomic DNA fragmentation. Nevertheless, in comparison with wild type, pplig4 is vulnerable to Bleomycin induction of DSBs and SSBs.
In both wild type and pplig4 lines, the Bleomycin induced DSBs are repaired with a rapid, biphasic kinetics (Figure 2(b)). Half-lives of DSB survival τ 1/2 1.5 min for wild type and 2.5 min for pplig4 are similar to τ 1/2 2.9 min for pprad50, τ 1/2 4.1 min for ppmre11, and τ 1/2 1.9 min for ppnbs1 previously reported in [6].
Contrary to DSBs, SSBs are repaired far less efficiently. Slow SSB repair might be common feature of plants since Donà et al. [18] recently observed similar phenomenon in Medicago truncata cell culture irradiated with different doses of γ-ray. The SSB repair kinetic in wild type Physcomitrella is clearly biphasic and in this respect parallels repair of MMS induced SSBs in Arabidopsis [19]. In comparison to DSBs, substantially smaller fraction of SSBs is repaired with fast kinetics; the defect even more manifested in pplig4. It suggests an important role for LIG4 in the repair of DNA lesions like modified basis, AP sites that are usually detected as SSBs and are repaired via BER (base excision repair). It is noteworthy that LIG3, the ligase finishing BER pathway, is not represented in plants. We showed earlier that principal substitute for LIG3 in Arabidopsis is LIG1 [19]. The repair kinetic of MMS induced SSBs in atlig1 posed an exceptional route. After the treatment the number of breaks continues to increase during the first hour of repair and after 3 hours returns to the level at the end of treatment. Then repair continues similarly as in the wild type (see Figure 4 in [19]). Because atlig1 is an RNAi line with only 40% of remaining LIG1 activity, such repair course is a consequence of unbalanced BER due to attenuated ligation step. Evidently the knockout mutation in pplig4 does not have such severe effect on repair of SSBs; nevertheless, the defect clearly shows that LIG4 is also involved in the repair of SSBs in plants.
3.3. Induction and Analysis of apt Mutants
The mutator phenotype was assessed as the loss of function of the APT gene [4] due to presence or error prone repair of endogenous DNA damage in the wild-type moss and lig4, mre11, and rad50 repair mutant lines.
We found dramatic, over two orders of magnitude, variation of mutator phenotype in response to mutagenic treatment. While wild type Physcomitrella with low mutator phenotype needed 2 hours and 50 μg mL−1 Bleomycin treatment to induce any apt clone, in pprad50 with high mutator phenotype 1 hour treatment with only 0.1 μg mL−1 Bleomycin was enough for massive induction of apt clones. Other lines, pplig4 and ppmre11, assumed as having “moderate” mutator phenotype, were mutagenized either with 5 or, respectively, 1 μg mL−1 Bleomycin for 1 hour. Mutagenesis and clone selection in Physcomitrella wild type and pplig4 is depicted on Figure 3. For comparison we normalised the yield of 2-FA resistant clones to 1 μg mL−1 Bleomycin treatment per 1 g dry tissue weight in each line as “relative number of ppapts.” The values of these normalised yields range from 9 in wild type to 875 for pprad50 (see Supplementary Figure 1 where are plotted summarized results of Bleomycin mutagenesis in Physcomitrella wild type and lig4, ku70, rad50, mre11, and nbs1 mutants).
Randomly picked apt clones from selection plates were further propagated on 2-FA media to provide enough material for isolation of genomic DNA and sequencing analysis of APT locus. Results of sequencing analysis are pictured in Figure 4 and detailed annotations of identified mutations are summarized in Supplementary Table 2. In total were analysed 5 clones of Physcomitrella wild type, 4 clones of pplig4, 3 clones of ppmre11, and 6 clones of pprad50 and identified 48 mutations. Mutations were according to assumed mechanism of formation classified as reversions, single base insertion or deletion, and insertions or deletions larger than 2 bases either in coding (exons) or noncoding regions of APT locus.
Figure 4.
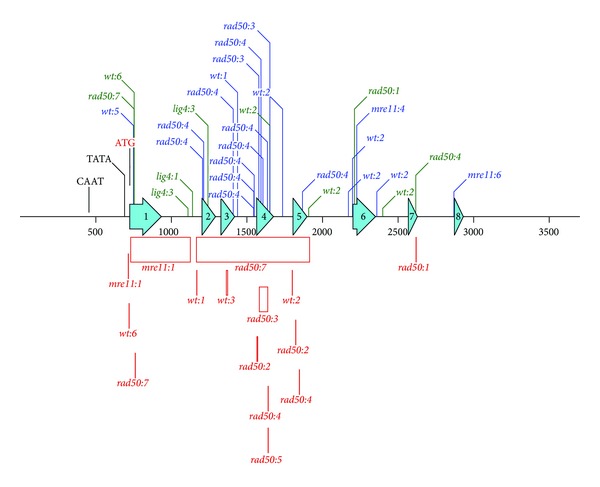
Map of identified mutations within APT locus. Bleomycin induced mutations are identified by color (blue: substitutions, green: insertions, and red: deletions) and tagged according to background as wt, lig4, mre11, and rad50 and the number of line in which mutation was detected. Deletions are shown as boxes of size proportional to their length. On the locus map are depicted 500 nucleotide size markers and eight turquoise hollow arrows representing exons of APT CDS. Detailed description of each mutation is provided in Supplementary Table 2.
Most of the identified mutations are as expected localised within CDS, in particular within exon 4 that is annotated as coding for adenine salvage activity (see Figure 4). Nevertheless, in wt:1, lig4:1, lig4:2, and mre11:6 apt clones, mutations were identified only in the noncoding region and their contribution to mutated APT phenotype has to be established. Majority of mutations in CDS are point mutations (base substitution, single base insertions, and deletions) and it is difficult to dissect the route of their formation. Some of single base deletions could come from classical or altered NHEJ repair of DSBs [20], but more likely they represent along with other point mutations outcome of processing base oxidative damage. Interesting point is that only single base insertions were identified in APT CDS of pplig4. Insertion of extra base might imply defect in BER repair of oxidative damage in the absence of LIG4 and could be associated with defective repair of SSBs in pplig4.
Long deletions are clearly associated with NHEJ repair of DSBs, because, besides one rather short (8 bp) deletion in wt:3 clone, all appear in clones derived from mre11 and rad50 background. This supports our working hypothesis that MRN-unsupervised repair generates more severe forms of genomic damage [6].
Only one 4 base insertion was identified in noncoding region of wt:2.
4. Conclusions
We validated the use of regenerating one-day-old protonemal tissue of Physcomitrella patens for complex analysis of genotoxic stress by parallel study of DNA damage, its repair, and mutagenic consequences in wild type and lig4 mutant plants. From experimental point of view we developed a novel model system where 3–5 cell protonemata filaments with up to 50% of apical cells can substitute and surplus protoplasts use. Bleomycin was used to model DNA oxidative genotoxic stress with all its consequences as SSBs and DSBs, which can be followed by SCGE. We confirmed in Physcomitrella as previously in Arabidopsis rapid DSB repair even in the absence of LIG4, the key ligase of major DSB repair pathway by NHEJ mechanism [12]. Moreover, we showed crucial role of LIG4 in the repair of SSBs by BER mechanism, where it can substitute along with LIG1 [19] in plants missing LIG3. We selected and analysed by sequencing 2-FA resistant clones with Bleomycin mutated APT locus and found out that mutation spectra of lig4 mutant reflects rather the defect of SSB than DSB repair. Nevertheless, as previously described [6], we interpret that mutations due to the error prone repair in pplig4 rather than unrepaired lesions within DNA and interfering with progression through the cell cycle are responsible for pplig4 sensitive phenotype.
Supplementary Material
Supplementary Figure 1: Normalized mutation frequencies in Physcomitrella wild type and lig4, ku70, rad50, mre11and nbs1 mutant lines.
Supplementary Figure 2: Map and localization of APT primers used for rescue of APT locus and sequencing and which are listed in Supplemental Table1.
Supplementary Table 2: Mutations (location and description) identified in the APT locus of Physcomitrella wild type and lig4, mre11 and rad50 mutant lines.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgments
Czech Science Foundation (13-06595S) and Ministry of Education, Youth, and Sport CR (LD13006) supported this work. The authors appreciate Dr. Andrew Cuming's help with sequences and mutations analysis.
Abbreviations
- A/N:
Comet assay with alkaline unwinding step
- AP:
Apurinic/apyrimidinic (site)
- APT:
Adenine phosphoribosyltransferase
- BER:
Base excision repair
- CDS:
Coding DNA sequence
- DSB(s):
DNA double-strand break(s)
- 2-FA:
2-Fluoroadenine
- HR:
Homologous recombination
- MMS:
Methyl methanesulfonate
- NHEJ:
Nonhomologous end joining
- N/N:
Neutral comet assay
- ROS:
Reactive oxygen species
- SCGE:
Comet assay
- SSB(s):
DNA single-strand break(s)
- τ1/2:
Half-life.
References
- 1.Cove DJ, Knight CD, Lamparter T. Mosses as model systems. Trends in Plant Science. 1997;2(3):99–105. [Google Scholar]
- 2.Šmídková M, Holá M, Angelis KJ. Efficient biolistic transformation of the moss Physcomitrella patens . Biologia Plantarum. 2010;54(4):777–780. [Google Scholar]
- 3.Gaillard C, Moffatt BA, Blacker M, Laloue M. Male sterility associated with APRT deficiency in Arabidopsis thaliana results from a mutation in the gene APT1. Molecular & General Genetics. 1998;257(3):348–353. doi: 10.1007/s004380050656. [DOI] [PubMed] [Google Scholar]
- 4.Trouiller B, Schaefer DG, Charlot F, Nogué F. MSH2 is essential for the preservation of genome integrity and prevents homeologous recombination in the moss Physcomitrella patens . Nucleic Acids Research. 2006;34(1):232–242. doi: 10.1093/nar/gkj423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trouiller B, Charlot F, Choinard S, Schaefer DG, Nogué F. Comparison of gene targeting efficiencies in two mosses suggests that it is a conserved feature of Bryophyte transformation. Biotechnology Letters. 2007;29(10):1591–1598. doi: 10.1007/s10529-007-9423-5. [DOI] [PubMed] [Google Scholar]
- 6.Kamisugi Y, Schaefer DG, Kozak J, et al. MRE11 and RAD50, but not NBS1, are essential for gene targeting in the moss Physcomitrella patens . Nucleic Acids Research. 2012;40(8):3496–3510. doi: 10.1093/nar/gkr1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight CD, Cuming AC, Quatrano RS. Moss gene technology. In: Gilmartin PM, Bowler C, editors. Molecular Plant Biology Volume 2. Vol. 2. Oxford, UK: Oxford University Press; 2002. pp. 285–299. [Google Scholar]
- 8.Markmann-Mulisch U, Wendeler E, Zobell O, Schween G, Steinbiss H-H, Reiss B. Differential requirements for RAD51 in Physcomitrella patens and Arabidopsis thaliana development and DNA damage repair. Plant Cell. 2007;19(10):3080–3089. doi: 10.1105/tpc.107.054049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelis KJ, Dusinska M, Collins AR. Single cell gel electrophoresis: detection of DNA damage at different levels of sensitivity. Electrophoresis. 1999;20(10):2133–2138. doi: 10.1002/(SICI)1522-2683(19990701)20:10<2133::AID-ELPS2133>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 10.Menke M, Chen I-P, Angelis KJ, Schubert I. DNA damage and repair in Arabidopsis thaliana as measured by the comet assay after treatment with different classes of genotoxins. Mutation Research. 2001;493(1-2):87–93. doi: 10.1016/s1383-5718(01)00165-6. [DOI] [PubMed] [Google Scholar]
- 11.Olive PL, Banáth JP. The comet assay: a method to measure DNA damage in individual cells. Nature Protocols. 2006;1(1):23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- 12.Kozak J, West CE, White C, da Costa-Nunes JA, Angelis KJ. Rapid repair of DNA double strand breaks in Arabidopsis thaliana is dependent on proteins involved in chromosome structure maintenance. DNA Repair. 2009;8(3):413–419. doi: 10.1016/j.dnarep.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Murray JM, Carr AM. Smc5/6: a link between DNA repair and unidirectional replication? Nature Reviews Molecular Cell Biology. 2008;9(2):177–182. doi: 10.1038/nrm2309. [DOI] [PubMed] [Google Scholar]
- 14.Steighner RJ, Povirk LF. Bleomycin-induced DNA lesions at mutational hot spots: implications for the mechanism of double-strand cleavage. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(21):8350–8354. doi: 10.1073/pnas.87.21.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgakilas AG. Processing of DNA damage clusters in human cells: current status of knowledge. Molecular BioSystems. 2007;4(1):30–35. doi: 10.1039/b713178j. [DOI] [PubMed] [Google Scholar]
- 16.Georgakilas AG, Holt SM, Hair JM, Loftin CW. Measurement of oxidatively-induced clustered DNA lesions using a novel adaptation of single cell gel electrophoresis (comet assay) In: Bonifacino SJ, et al., editors. Current Protocols in Cell Biology. chapter 6. John Wiley & Sons; 2010. p. p. 6.11. [DOI] [PubMed] [Google Scholar]
- 17.Ahnström G, Erixon K. Radiation induced strand breakage in DNA from mammalian cells. Strand separation in alkaline solution. International Journal of Radiation Biology and Related Studies in Physics, Chemistry, and Medicine. 1973;23(3):285–289. doi: 10.1080/09553007314550311. [DOI] [PubMed] [Google Scholar]
- 18.Donà M, Ventura L, Balestrazz A, et al. Dose-dependent reactive species accumulation and preferential double-strand breaks repair are featured in the γ-ray response in Medicago truncatula cells. Plant Molecular Biology Reporter. 2013 [Google Scholar]
- 19.Waterworth WM, Kozak J, Provost CM, Bray CM, Angelis KJ, West CE. DNA ligase 1 deficient plants display severe growth defects and delayed repair of both DNA single and double strand breaks. BMC Plant Biology. 2009;9, article 79 doi: 10.1186/1471-2229-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charbonnel C, Allain E, Gallego ME, White CI. Kinetic analysis of DNA double-strand break repair pathways in Arabidopsis. DNA Repair. 2011;10(6):611–619. doi: 10.1016/j.dnarep.2011.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Normalized mutation frequencies in Physcomitrella wild type and lig4, ku70, rad50, mre11and nbs1 mutant lines.
Supplementary Figure 2: Map and localization of APT primers used for rescue of APT locus and sequencing and which are listed in Supplemental Table1.
Supplementary Table 2: Mutations (location and description) identified in the APT locus of Physcomitrella wild type and lig4, mre11 and rad50 mutant lines.


