Abstract
The second extracellular loop (LFWQYFVGKRTVPPGECFIQFLSEPTITFGTAI, aa 205–237) of muscarinic acetylcholine 3 receptor (M3R) has been reported to be an epitope for autoantibodies generated during certain autoimmune disorders, including Sjögren's syndrome (SS). Autoantibodies against M3R228–237 have been shown to interfere with the function of M3R. However, few studies have been performed on the M3R205–227 peptide of the second extracellular loop. In the current study, we sought to investigate the effect of M3R208–227 peptide immunization on autoimmune response in NOD/LtJ mice. We synthesized the M3R208–227 peptide and immunized NOD/LtJ mice to investigate whether peptide-specific antibodies could be generated and whether immunization would lead to changes in autoimmune response in NOD/LtJ mice. Our results demonstrate that the secretions of Th-1, Th-2, and Th-17 cytokines are downregulated and lymphocytic infiltration is improved in the salivary glands and lacrimal glands following immunization with M3R208–227 peptide in NOD/LtJ mice, suggesting that peptide immunotherapy using the M3R208–227 peptide may represent a potential therapeutic alternative.
1. Introduction
The muscarinic acetylcholine 3 receptor (M3R) plays a key role in mediating exocrine protein secretion in the salivary and lacrimal glands. Autoimmune responses against M3Rs contribute to the development of sicca symptoms and autonomic dysfunction in patients with both primary and secondary Sjögren's syndrome (SS) [1, 2], which suggests that disorders related to M3R signaling in the salivary and lacrimal glands can lead to reduced secretions from these glands.
Iizuka et al. [3] demonstrated that the M3R reactive T helper cells play a crucial role in the pathogenesis of SS. The second extracellular loop (LFWQYFVGKRTVPPGECFIQFLSEPTITFGTAI, aa 205–237) of M3R has been reported to be an epitope for autoantibodies generated during certain autoimmune disorders [4–7], and autoantibodies against M3R205–237 have been shown to interfere with the function of M3R [4, 8–10]. Cavill et al. [11] reported that a short peptide sequence of 10 amino acids (EPTITFGTAI, aa 228–237) located at the COOH-terminal region of the second extracellular loop was shown to possess the strongest inhibitory activity, and the inhibitory activity of this short peptide was subsequently confirmed by Koo et al. [12]. However, few studies have been performed on the M3R205–227 peptide of the second extracellular loop. The study by Koo et al. showed that the M3R205–230 peptide does not bind to autoantibodies from patients with primary SS [12], and a separate study demonstrated that the M3R213–228-GST fusion peptide was antigenic only as a dimer [9]. Furthermore, the study by Naito et al. [13] demonstrated that VPPGECFKQFLSEPT (M3R 223I→K) and VPPGECFIAFLSEPT (M3R 224Q→A) for the anchor positions binding to HLA-DR B1*0901 were candidate altered peptide ligands of the second extracellular domain of M3R.
In the current study, we sought to investigate the effect of M3R208–227 peptide immunization on autoimmune response in NOD/LtJ mice. We synthesized the M3R208–227 peptide and immunized NOD/LtJ mice to investigate whether peptide-specific antibodies could be generated and whether immunization would lead to changes in autoimmune response in NOD/LtJ mice. Our results suggest that the secretions of Th-1, Th-2, and Th-17 cytokines are downregulated and lymphocytic infiltration is improved in the salivary glands and lacrimal glands following immunization with M3R208–227 peptide in NOD/LtJ mice, suggesting that peptide immunotherapy using the M3R208–227 peptide needs further studies.
2. Materials and Methods
2.1. Animals
Eight-week-old female NOD/LtJ mice were purchased from the Institute of Laboratory Animal Sciences at CAMS and PUMC (Beijing, China). The animals were maintained in a pathogen-free facility in the Animal Laboratory of Baotou Medical College. All procedures involving animals were performed according to the Research Animal Administration Guidelines of China and the Guidelines for the Care and Use of Laboratory Animals in China.
2.2. Peptides and Immunization
Four separate peptides, including the part of second extracellular loop (M3R208–227, QYFVGKRTVPPGECFIQFLS), C-terminal of the second extracellular loop (M3R228–237, EPTITFGTAI), the third extracellular loop (M3R514–527, NTFCDSCIPKTFWN), and the N-terminus of the murine M3R extracellular domain (MTLHSNSTTSPLFPNISSSW), were synthesized chemically via the solid-phase procedure and were purified by high-performance liquid chromatography (GL BioChem, Shanghai, China). The N-terminus of the murine M3R extracellular domain served as a control peptide (CP). NOD/LtJ mice were divided into either the M3R208–227, CP, or PBS groups (n = 8 per group) and received 100 μg of M3R208–227 or CP diluted in PBS and emulsified in complete Freund's adjuvant (CFA; Wako Pure Chemical Industries, Osaka, Japan). Injections of PBS were used as an additional control. Three weeks later, NOD/LtJ mice received another injection of M3R208–227 or CP diluted in PBS and emulsified in incomplete Freund's adjuvant (IFA; Wako Pure Chemical Industries, Osaka, Japan).
2.3. Antibody Titer Determination
The sera were collected every seven days after the second immunization. Ninety six well ELISA plates (JCHS, Shenzhen, China) were coated with M3R208–227, M3R228–237, and M3R514–527 peptide solutions (10 μg/mL in 0.1 M NaHCO3 (pH 8.6)) overnight at 4°C and were then blocked with 0.05% Tween 20 in PBS containing 5% dry milk for 2 h at 37°C. Sera diluted 1 : 50 in blocking buffer were incubated for 2 h at 37°C. The plates were then washed six times with 0.05% Tween 20 in PBS containing 5% dry milk, and an HRP-conjugated anti-mouse IgG antibody (Abcam, Cambridge, UK) diluted 1 : 1000 in blocking buffer was added for 1 h at 37°C. After washing, the reaction was developed with 2,2,-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma, St. Louis, MO) as a substrate. The optical density at 405 nm was measured using an ELISA reader (BioRad model 680).
2.4. Analysis of Cytokines in the Sera and Cell Supernatants
The levels of various cytokines in the sera were evaluated after the second immunization according to the instructions provided by the ELISA kits (IL-17, TGF-β 1, and IFN-γ: eBioscience, San Diego, CA; IL-4, IL-10, and IL-6: RayBiotech, Norcross, GA). The serum samples were diluted 1 : 50.
The spleen cells were obtained from the mice sacrificed in each group four weeks after the second immunization. Homogenized spleens from NOD/LtJ mice immunized with the M3R208–227 peptide were prepared as described previously with some modifications [3]. Following serum collection, the mice were sacrificed, and their spleens were isolated and pooled. Red blood cells were removed by treatment with a 0.16 M NH4Cl solution. Then, the homogenates were adjusted to 1 × 106 cells/mL and incubated with RPMI-1640 supplemented with 10% FBS (Gibco, Grand Island, NE), 1000 U/mL penicillin, 100 mg/mL streptomycin, and M3R208–227, CP, or PBS in an atmosphere of 5% CO2 at 37°C. The cell supernatants were obtained after 48 hours, at which time the levels of the various cytokines were measured using ELISA kits (IL-17, TGF-β 1, and IFN-γ: eBioscience, San Diego, CA; IL-4, IL-10, and IL-6: RayBiotech, Norcross, GA).
2.5. Histology
The salivary glands and lacrimal glands were obtained from the mice sacrificed in each group four weeks after the second immunization. Following sacrifice, the entire lacrimal glands of NOD/LtJ mice were surgically removed and placed in 10% phosphate-buffered formalin for 20 min. Paraffin-embedded sections were deparaffinized by immersion in xylene, followed by dehydration in ethanol. The tissue sections were prepared and stained with hematoxylin and eosin (H&E). Sections were observed at a 200x magnification under an Olympus IX71 microscope (Olympus, Shanghai, China). Images were obtained with DP2-BSW software (Olympus, Shanghai, China). Leukocytic infiltrations were analyzed with Image-Pro Plus 6.0 software (Media Cybernetics) in the histological sections of lacrimal glands. The lymphocytic infiltration (histological score) was graded using the method proposed by Greenspan et al. [14].
Immunofluorescence staining was performed as described previously with some modifications [3]. The sections were incubated with rat anti-mouse IFN-γ, rat anti-mouse IL-4, and rat anti-mouse IL-17 antibodies (Santa Cruz Biotechnology, CA) for 30 min. To detect primary antibodies, Alexa Fluor 546 goat anti-rat IgG antibody (Invitrogen, Carlsbad, CA) was added for 30 min. Sections were observed at a 100x magnification under an Olympus IX71 microscope (Olympus, Shanghai, China). Images were obtained with DP2-BSW software (Olympus, Shanghai, China).
2.6. Statistical Analysis
The data are expressed as the mean ± SD. The images were analyzed with Image-Pro Plus 6.0 software. Differences between groups were evaluated for statistical significance using the Student's t-test, and P values <0.05 were considered statistically significant.
3. Results
3.1. Generation of Peptide-Specific Antibodies
We performed ELISAs to determine whether peptide-specific antibodies could be detected in the sera after the second immunization. As shown in Figure 1, the serum titers of anti-M3R208–227 antibodies were higher in M3R208–227 peptide immunized NOD/LtJ mice than in the CP and PBS control groups, but no changes were observed in antibody titers against M3R228–237 and M3R514–527 in M3R208–227 peptide immunized NOD/LtJ mice (Figures 1(a), 1(b), and 1(c)). These results showed that peptide-specific antibodies were successfully and specifically generated only in animals immunized with the M3R208–227 peptide.
Figure 1.
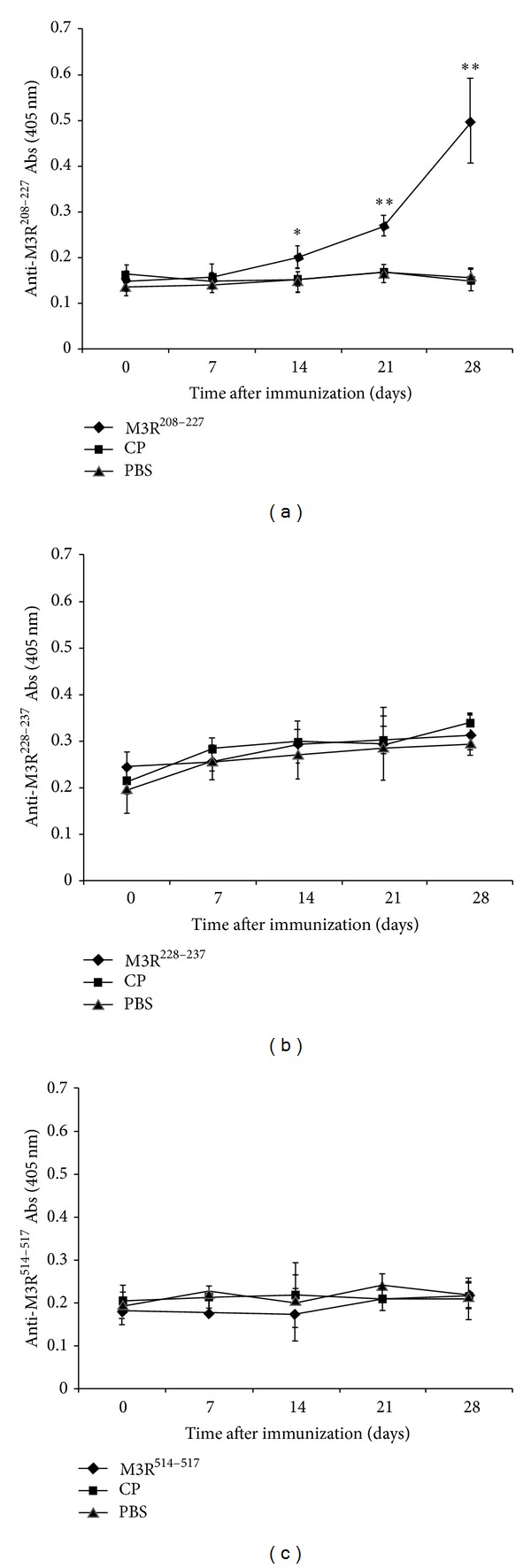
Immunization with the M3R208–227 peptide induced peptide-specific antibodies. NOD/LtJ mice received 100 μg M3R208–227 (rhombus) or CP (square) diluted in PBS and emulsified in incomplete Freund's adjuvant. PBS immunizations (triangle) were used as an additional control. Sera were obtained from the mice in each group every seven days after the second immunization, and these samples were diluted 1 : 50. Antibody titer determination was performed by ELISA. The results showed that peptide-specific antibodies were successfully induced in NOD/LtJ mice immunized with the M3R208–227 peptide (a), but no changes were observed in antibody titers against M3R228–237 (C-terminal) (b) and M3R514–527 (the third extracellular loop) (c) in M3R208–227 peptide immunized NOD/LtJ mice (mean ± SD; *P < 0.05; **P < 0.005).
3.2. Reductions in the Levels of Cytokines in the Sera
Following the determination of antibody titers, the specificity of the Th-produced cytokines present in the sera was evaluated by comparing the cytokine profiles of animals immunized with the M3R208–227 peptide to those of animals immunized with CP or PBS. The cytokine concentrations were measured by ELISA. First, we measured the Th-17-associated cytokine IL-17 and the Th-1-associated cytokine IFN-γ, which are known to play crucial roles in the development of autoimmune disease. The results showed that the concentration of IFN-γ in the serum decreased following immunization with M3R208–227 as compared to the control groups. Meanwhile, the concentration of IL-17 was below the detection level (IL-17, P < 0.005; IFN-γ, P < 0.05) (Figure 2(a)).
Figure 2.
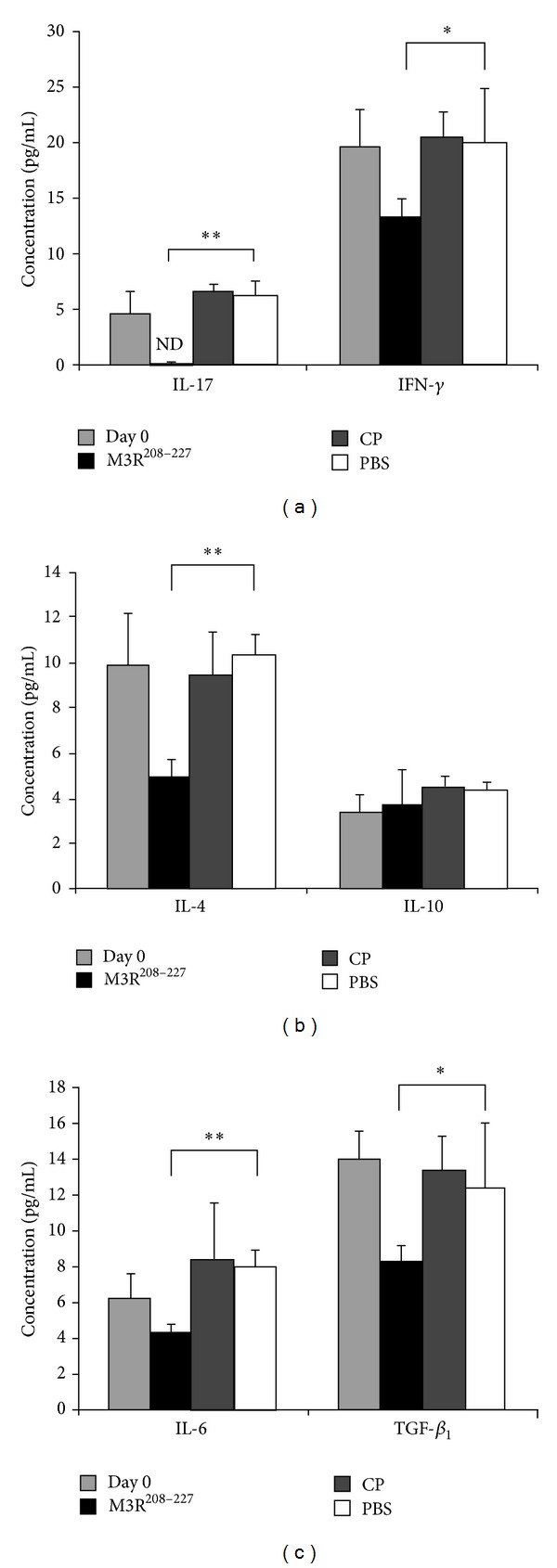
Downregulated expression of IFN-γ, IL-4, IL-17, IL-6, and TGF-β 1 in the serum following immunization with the M3R208–227 peptide. Sera were collected from mice in each group before immunization (light gray bar), and four weeks following the second immunization with M3R208–227 (black bar), CP (charcoal gray bar), or PBS (white bar), and the samples were diluted 1 : 50. The cytokine levels were measured using ELISA kits. (a) The results showed that the concentration of IFN-γ was significantly decreased in the sera from mice immunized with M3R208–227 in comparison to those in the sera of the control animals. On the other hand, the concentration of IL-17 was below the detection level (*P < 0.05; **P < 0.005). (b) These results showed that the concentration of IL-4 was significantly decreased in the sera of M3R208–227 peptide immunized mice as compared to the control groups (**P < 0.005). No significant decrease in IL-10 secretion was observed (P > 0.05). (c) The results demonstrated that the concentrations of TGF-β 1 and IL-6 were significantly decreased in the sera from mice immunized with M3R208–227 as compared to the controls (*P < 0.05; **P < 0.005).
Consequently, we analyzed the levels of Th-2-associated cytokines in the sera of NOD/LtJ mice. IL-4 is thought to inhibit the production of IFN-γ by Th-1 cells, and IL-10 is also an inhibitory cytokine, but recent research has shown that both of these cytokines are positively correlated with the pathogenic process of SS. Our data showed that immunization with the M3R208–227 peptide resulted in a significant reduction in IL-4, as compared to the control groups (P < 0.005). However, no trend toward decreased concentrations of IL-10 was observed (P > 0.05) (Figure 2(b)).
Moreover, recent data have shown that the levels of TGF-β 1 mRNA transcripts and protein were significantly higher in animals with an experimental model of dry eye [15]. As TGF-β 1 can synergize with IL-6 to induce the upregulation of the transcription factor RORγt, which promotes Th-17 differentiation and the secretion of IL-17 [16–19], we also evaluated the levels of IL-6 and TGF-β 1. These results demonstrated that both IL-6 and TGF-β 1 were downregulated in the sera of NOD/LtJ mice immunized with M3R208–227, which further confirmed the important role for IL-6 and TGF-β in Th-17 differentiation (IL-6, P < 0.005; TGF-β 1, P < 0.05) (Figure 2(c)).
3.3. Reductions in the Levels of Cytokines in Cell Supernatants
Here, we assessed the cytokine profiles of cell culture supernatants after spleen cells were cocultured with either the M3R208–227 peptide, CP, or PBS. Untreated spleen cells were used as an additional negative control. The results showed that the secretion of IL-17 in cell culture supernatant decreased following immunization with M3R208–227 as compared to the control groups (P < 0.005). However, in cell culture supernatant, no significant decrease in IFN-γ secretion was observed (P > 0.05) (Figure 3(a)). Our data also showed that immunization with the M3R208–227 peptide resulted in a significant reduction in IL-4 in cell culture supernatant, as compared to the control groups (P < 0.05). No trend toward decreased concentrations of IL-10 was observed (P > 0.05) (Figure 3(b)). The changes in the secretions of IL-6 and TGF-β 1 were also downregulated in cell culture supernatants of NOD/LtJ mice immunized with M3R208–227 compared to the control groups (IL-6, P < 0.005; TGF-β 1, P < 0.05) (Figure 3(c)).
Figure 3.
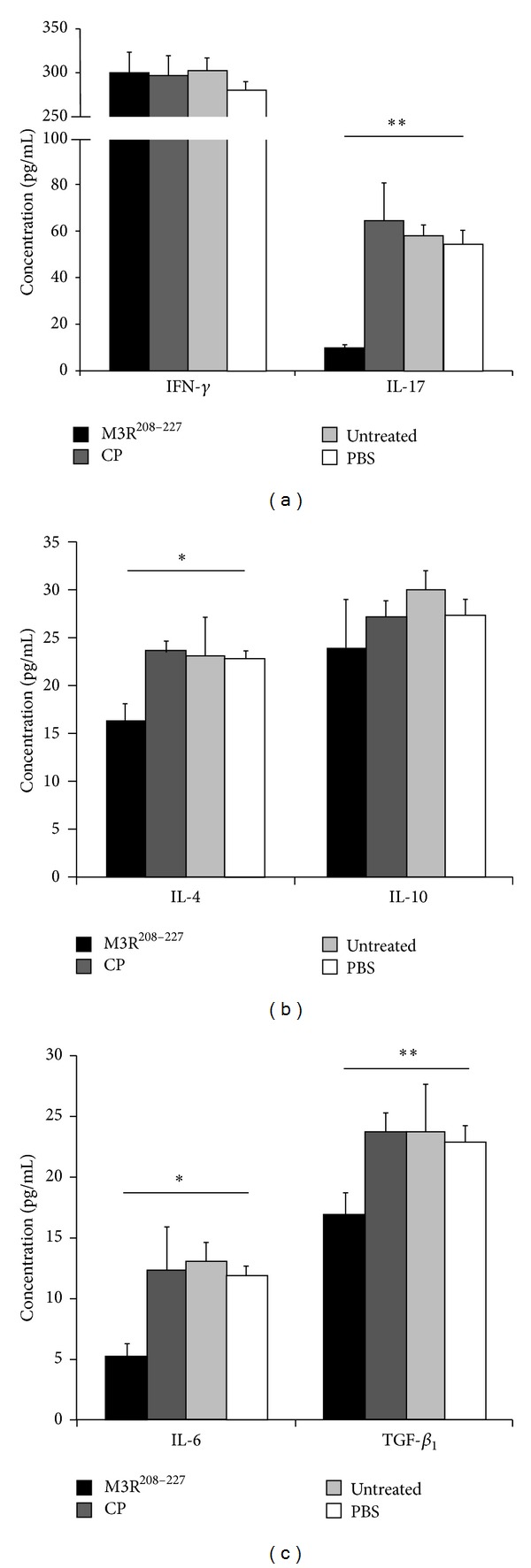
The secretions of IL-17, IL-4, IL-6, and TGF-β 1 were decreased in the cell supernatants following incubation with M3R208–227 peptide. Spleens were isolated from the NOD/LtJ mice immunized with the M3R208–227 peptide and the spleen cells were pooled. The homogenates were adjusted to 1 × 106 cells/mL and incubated with M3R208–227 (black bar), CP (charcoal gray bar), or PBS (white bar). Untreated spleen cells served as an additional control (light gray bar). Cell supernatants were collected after 48 hours of culture, and the cytokine concentrations were measured using ELISA kits. (a) The results indicated that the secretion of IL-17 was significantly reduced in the supernatants of cells that had been cocultured with the M3R208–227 peptide in comparison to those cocultured with CP and PBS or left untreated (**P < 0.005), although no significant decrease in IFN-γ was observed (P > 0.05). (b) These results showed that the secretion of IL-4 was also significantly decreased in the supernatants of cells cocultured with M3R208–227 as compared to those cocultured with CP and PBS or left untreated (*P < 0.05). No significant decrease in IL-10 secretion was observed (P > 0.05). (c) The results showed that the secretion of IL-6 and TGF-β 1 was significantly decreased in the supernatants of cells cocultured with M3R208–227 as compared to those cocultured with CP and PBS or left untreated (*P < 0.05; **P < 0.005).
3.4. Reductions in the Levels of IL-17 and IFN-γ in the Lacrimal Glands
To further observe whether the concentrations of cytokines decreased in the lacrimal glands, we also examined the production of IFN-γ, IL-4, and IL-17 in the lacrimal glands of NOD/LtJ mice by immunofluorescence staining. Whole lacrimal glands from NOD/LtJ mice were removed, fixed, embedded, and incubated with primary antibodies, followed by a secondary goat anti-rat IgG antibody. The result of immunofluorescence staining showed that the production of IL-17 and IFN-γ decreased in the lacrimal glands of NOD/LtJ mice immunized with the M3R208–227 peptide. However, the production of IL-4 was not observed in the lacrimal glands of NOD/LtJ mice in all groups (Figure 4).
Figure 4.
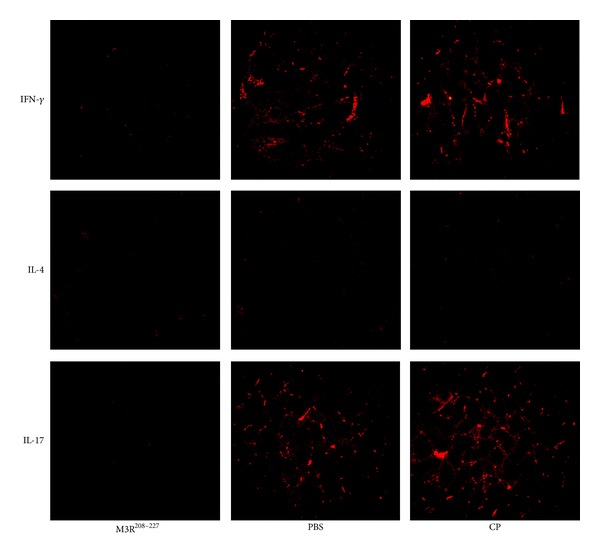
Reductions in the levels of IL-17 and IFN-γ in the lacrimal glands following immunization with the M3R208–227 peptide. To further observe whether the concentrations of cytokines decreased in the lacrimal glands, we also examined the production of IFN-γ, IL-4, and IL-17 in the lacrimal glands of NOD/LtJ mice by immunofluorescence staining. Whole lacrimal glands from NOD/LtJ mice were removed, fixed, embedded, and incubated with primary antibodies, followed by a secondary goat anti-rat IgG antibody. The results of immunofluorescence staining demonstrated that the production of IL-17 and IFN-γ decreased in the lacrimal glands of NOD/LtJ mice immunized with the M3R208–227 peptide. In contrast, the production of IL-4 was not observed in the lacrimal glands of NOD/LtJ mice in all groups.
3.5. Improved Lymphocytic Infiltration in the Salivary Glands and Lacrimal Glands
To evaluate lymphocytic infiltration in the salivary glands and lacrimal glands, whole salivary glands and lacrimal glands from NOD/LtJ mice were removed, fixed, embedded, and stained with hematoxylin and eosin (H&E). Lymphocytic infiltration was decreased in the salivary glands and lacrimal glands of NOD/LtJ mice immunized with M3R208–227. In contrast, marked lymphocytic infiltration was observed in the salivary glands (Figure 5) and lacrimal glands (Figure 6) of animals in the control groups (P < 0.05).
Figure 5.
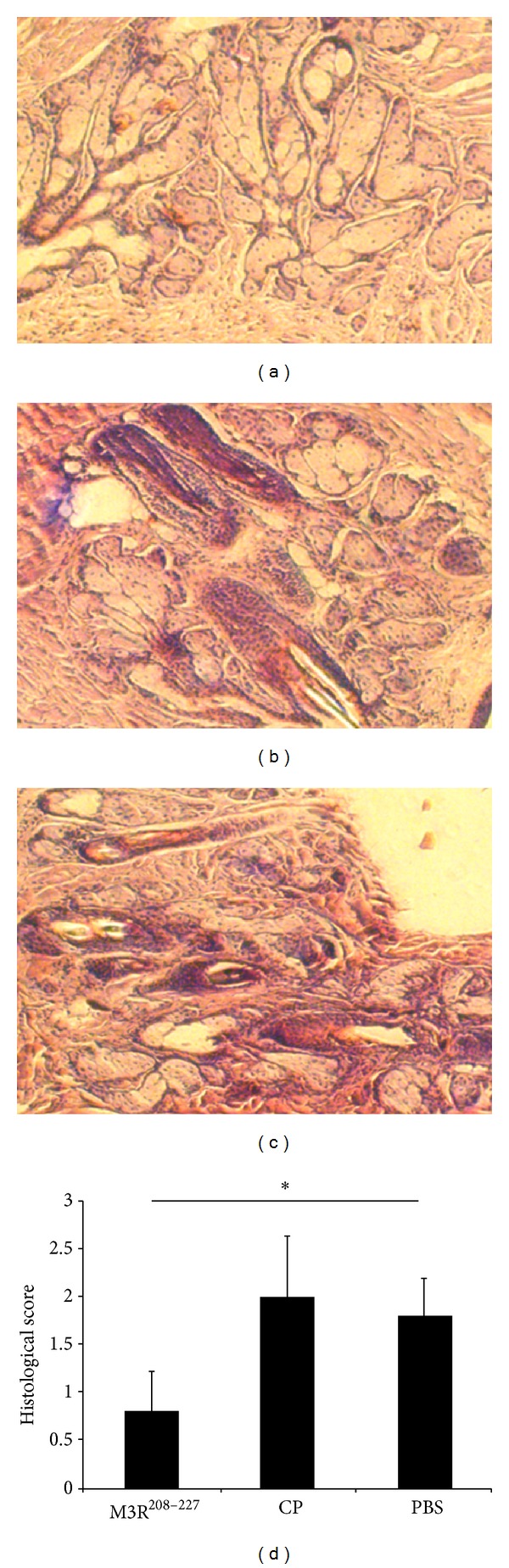
Lymphocytic infiltration was improved in the salivary glands following immunization with the M3R208–227 peptide. Whole salivary glands of NOD/LtJ mice were surgically removed from each mouse and placed in 10% phosphate-buffered formalin for 20 min. After the samples were fixed, embedded, and stained with hematoxylin and eosin (H&E), sections were observed under a microscope. The histological analysis suggested that immunization with M3R208–227 could reduce the lymphocytic infiltration in the salivary glands of NOD/LtJ mice. (a) Tissue from a NOD/LtJ mouse immunized with the M3R208–227 peptide. (b) Tissue from a NOD/LtJ mouse immunized with CP. (c) Tissue from a NOD/LtJ mouse immunized with PBS. (d) Mean grade (histological score) of inflammatory lesions in salivary glands of NOD/LtJ mouse immunized with the M3R208–227 peptide as compared to the controls (*P < 0.05).
Figure 6.
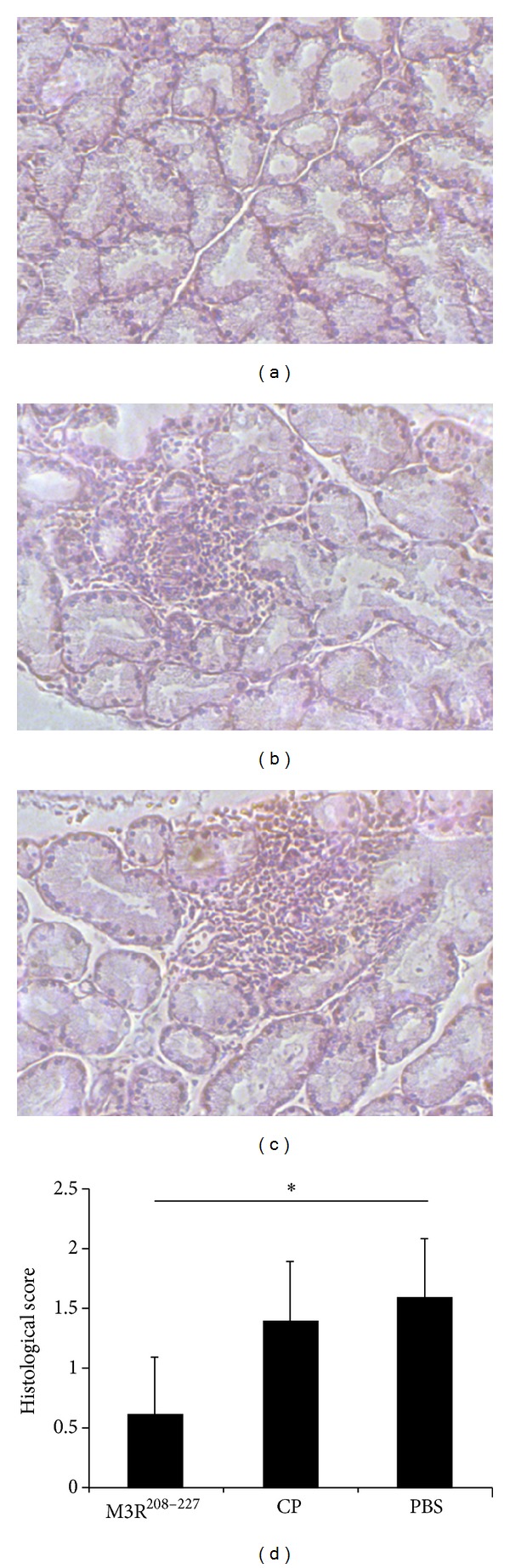
Lymphocytic infiltration was improved in the lacrimal glands following immunization with the M3R208–227 peptide. Whole lacrimal glands of NOD/LtJ mice were surgically removed from each mouse and placed in 10% phosphate-buffered formalin for 20 min. After the samples were fixed, embedded, and stained with hematoxylin and eosin (H&E), sections were observed under a microscope. The histological analysis suggested that immunization with M3R208–227 could reduce the lymphocytic infiltration in the lacrimal glands of NOD/LtJ mice. (a) Tissue from a NOD/LtJ mouse immunized with the M3R208–227 peptide. (b) Tissue from a NOD/LtJ mouse immunized with CP. (c) Tissue from a NOD/LtJ mouse immunized with PBS. (d) Mean grade (histological score) of inflammatory lesions in lacrimal glands of NOD/LtJ mouse immunized with the M3R208–227 peptide as compared to the controls (*P < 0.05).
4. Discussion
Elias et al. [20] demonstrated that pep277, a peptide of the human 60 kDa heat-shock protein, was found to be a therapeutic agent to arrest the autoimmune process in NOD mice. In addition, He et al. [21] demonstrated that mucosal administration of α-fodrin, a 120 kd autoantigen in Sjögren's syndrome, effectively inhibited the progression of experimental Sjögren's syndrome autoimmunity. In this study, we aimed to evaluate the effect of M3R208–227 peptide immunization on autoimmune response of NOD/LtJ mice. As immunization with M3R208–227 peptide conjugated to an immunogenic carrier protein such as KLH may induce some uncertain immunological responses and immunization with the entire second extracellular loop tends to be pathogenic and, in addition, Naito et al. [13] demonstrated that VPPGECFKQFLSEPT (M3R 223I→K) and VPPGECFIAFLSEPT (M3R 224Q→A) were candidate altered peptide ligands of the second extracellular domain of M3R, we immunized NOD/LtJ mice intradermally with the M3R208–227peptide. The ELISA results showed that anti-M3R208–227 antibodies were induced successfully and specifically in M3R208–227 immunized animals as compared to the CP and PBS groups. Autoantibodies against M3R228–237 and M3R514–527 were reported in patients with Sjögren's syndrome [10, 11], but no changes were observed in antibody titers against M3R228-237 and M3R514–527 in M3R208–227 peptide immunized NOD/LtJ mice, suggesting that generations of autoantibodies against M3R228–237 and M3R514–527 may not be regulated by immunization with M3R208–227 peptide in NOD/LtJ mice.
The IFN-γ and IL-17 have been shown to play crucial roles in the pathogenesis of SS [22–27]. Therefore, we measured the concentrations of IL-17 and IFN-γ in the sera, cell supernatants, and lacrimal glands from M3R208–227 immunized mice and found that these concentrations were decreased in comparison to the control-immunized mice. This decrease in the levels of IL-17 and IFN-γ suggested that the activities of the secretions of IL-17 and IFN-γ were inhibited following M3R208–227 immunization in NOD/LtJ mice.
Then, we evaluated the Th-2 cytokines IL-4 and IL-10 and found that the activity of IL-4 was significantly decreased in the serum and cell culture supernatants of M3R208–227 immunized mice, although decreases in IL-10 were not observed. One important function of IL-4 is to promote the differentiation of CD4+ T cells into Th-2 cells, which antagonizes the production of IFN-γ by Th-1 cells. However, recent data have shown that IL-4 was required to regulate the synthesis of pathogenic autoantibodies against M3R by B lymphocytes in the NOD.IL-4-gene knockout mouse model of Sjögren's syndrome [28]. In the current study, the production of IL-4 was not observed in the lacrimal glands of NOD/LtJ mice in all groups, suggesting that IL-4 may be more required to regulate the B-cell autoimmune response in this animal model. IL-10 is a cytokine capable of inhibiting the synthesis of other proinflammatory cytokines, but the secretion of IL-10 has also been shown to be elevated in patients with SS [29, 30]. However, no changes in the concentration of IL-10 were observed in the current study, suggesting that IL-10 may not be regulated following immunization with M3R208–227.
The lymphocytic infiltration in the salivary glands and lacrimal glands in M3R208–227 treated mice compared to control peptide and PBS was measured four weeks after the second immunization. Jonsson et al. [31] demonstrated that salivary gland inflammation does not necessarily correlate with salivary flow, so we graded the lymphocytic infiltration by using histological score instead of measurement of salivary flow. Lower histological scores showed that tissue from the M3R208–227 immunized mice revealed at that time point less infiltrates in the salivary glands and lacrimal glands after immunization, suggesting that the effect of M3R208–227 immunization may induce a delay in disease symptoms in NOD/LtJ mice.
The secretions of Th-1, Th-2, and Th-17 cytokines and leukocyte infiltration may have been reduced in the NOD/LtJ mice after immunization with M3R208–227. These changes in cytokine secretion and leukocyte infiltration observed could be interpreted in many different ways. The most likely explanation could be that the administration of M3R208–227 induced some other antigen-specific T or B regulatory cells that secreted other cytokines which could have immunomodulatory activity. Antigen-specific regulatory cells may suppress autoimmune response by inhibiting the generation of Th-1, Th-2, and Th-17 cells. In addition, the study by Tsuboi et al. [32] indicated that the presence of several different B-cell epitopes within M3R could influence salivary secretion, so it is possible that peptide-specific antibodies may also relate to this process.
In conclusion, immunization with the M3R208–227 peptide reduced the secretions of Th-1, Th-2, and Th-17 cytokines and leukocyte infiltration in NOD/LtJ mice, suggesting that immunotherapy with the M3R208-227 peptide needs further studies.
Disclosure
The paper has not been published and is not under consideration for publication elsewhere.
Conflict of Interests
None of the authors has any potential financial conflict of interests related to this paper. No financial or other relationships could lead to a conflict of interests.
Authors' Contribution
Lin Yang, Jinzhe Ju, Wei Zhang, Fengfeng Lv, Chunyan Pang, Guoan Yang, and Yongfu Wang contributed equally to this work. Lin Yang performed most of the experiments. Wei Zhang and Jinzhe Ju participated in experiments in ELISA test. Chunyan Pang and Fengfeng Lv participated in experiments in animal test. Lin Yang, Guoan Yang, and Yongfu Wang conceived the study and participated in the design and in the interpretation of results. Lin Yang drafted the paper. All authors read and approved the final paper.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (no. 30860316), the College Scientific Research Project of Inner Mongolia (no. NJZY11178) and the Nature Science Foundation of Inner Mongolia (no. 20011MS1133).
References
- 1.Waterman SA, Gordon TP, Rischmueller M. Inhibitory effects of muscarinic receptor autoantibodies on parasympathetic neurotransmission in Sjögren’s syndrome. Arthritis and Rheumatism. 2000;43:1647–1654. doi: 10.1002/1529-0131(200007)43:7<1647::AID-ANR31>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 2.Dawson L, Tobin A, Smith P, Gordon T. Antimuscarinic antibodies in Sjögren’s syndrome. Arthritis and Rheumatism. 2005;52:2984–2995. doi: 10.1002/art.21347. [DOI] [PubMed] [Google Scholar]
- 3.Iizuka M, Wakamatsu E, Tsuboi H, et al. Pathogenic role of immune response to M3 muscarinic acetylcholine receptor in Sjögren’s syndrome-like sialoadenitis. Journal of Autoimmunity. 2010;35(4):383–389. doi: 10.1016/j.jaut.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Robinson CP, Brayer J, Yamachika S, et al. Transfer of human serum IgG to nonobese diabetic Igμ null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjögren’s syndrome. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(13):7538–7543. doi: 10.1073/pnas.95.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goin JC, Borda E, Leiros CP, Storino R, Sterin-Borda L. Identification of antibodies with muscarinic cholinergic activity in human Chagas’ disease: pathological implications. Journal of the Autonomic Nervous System. 1994;47(1-2):45–52. doi: 10.1016/0165-1838(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 6.Eftekhari P, Salle L, Lezoualc'h F, et al. Anti-SSA/Ro52 autoantibodies blocking the cardiac 5-HT4, serotoninergic receptor could explain neonatallupus congenital heart block. European Journal of Immunology. 2000;30:2782–2790. doi: 10.1002/1521-4141(200010)30:10<2782::AID-IMMU2782>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Magnusson Y, Marullo S, Hoyer S, et al. Mapping of a functional autoimmune epitope on the β1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. Journal of Clinical Investigation. 1990;86(5):1658–1663. doi: 10.1172/JCI114888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen K, Broyer J, Cha S, Diggs S, Yasunari U, Hilal G. Evidence for antimuscarinic acetylcholine receptor antibody-mediated secretory dysfunction in NOD mice. Arthritis and Rheumatism. 2000;43:2297–2306. doi: 10.1002/1529-0131(200010)43:10<2297::AID-ANR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 9.Kovács L, Marczinovits I, György A, et al. Clinical associations of autoantibodies to human muscarinic acetylcholine receptor 3(213-228) in primary Sjögren’s syndrome. Rheumatology. 2005;44:1021–1025. doi: 10.1093/rheumatology/keh672. [DOI] [PubMed] [Google Scholar]
- 10.Marczinovits I, Kovács L, György A, et al. A peptide of human muscarinic acetylcholine receptor 3 is antigenic in primary Sjögren’s syndrome. Journal of Autoimmunity. 2005;24:47–54. doi: 10.1016/j.jaut.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Cavill D, Waterman SA, Gordon TP. Antibodies raised against the second extracellular loop of the human muscarinic M3 receptor mimic functional autoantibodies in Sjögren’s syndrome. Scandinavian Journal of Immunology. 2004;59(3):261–266. doi: 10.1111/j.0300-9475.2004.01395.x. [DOI] [PubMed] [Google Scholar]
- 12.Koo N-Y, Li J, Hwang S-M, et al. Functional epitope of muscarinic type 3 receptor which interacts with autoantibodies from Sjögren’s syndrome patients. Rheumatology. 2008;47(6):828–833. doi: 10.1093/rheumatology/ken064. [DOI] [PubMed] [Google Scholar]
- 13.Naito Y, Matsumoto I, Wakamatsu E, et al. Altered peptide ligands regulate muscarinic acetylcholine receptor reactive T cells of patients with Sjögren’s syndrome. Annals of the Rheumatic Diseases. 2006;65(2):269–271. doi: 10.1136/ard.2005.039065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjogren’s syndrome in labial salivary gland biopsies. Oral Surgery Oral Medicine and Oral Pathology. 1974;37(2):217–229. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- 15.de Paiva CS, Hwang CS, Pitcher JDI, et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjögren’s syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology. 2009;49(2):246–258. doi: 10.1093/rheumatology/kep357.kep357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 17.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-β induces development of the T H17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Elias D, Reshef T, Birk OS, van der Zee R, Walker MD, Cohen IR. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(8):3088–3091. doi: 10.1073/pnas.88.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He J, Zhao J, Li Z. Mucosal administration of alpha-fodrin inhibits experimental Sjögren's syndrome autoimmunity. Arthritis Research and Therapy. 2008;10(2, article R44) doi: 10.1186/ar2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen CQ, Yin H, Lee BH, Carcamo WC, Chiorini JA, Peck AB. Pathogenic effect of interleukin-17A in induction of Sjögren’s syndrome-like disease using adenovirus-mediated gene transfer. Arthritis Research and Therapy. 2010;12(6, article R220) doi: 10.1186/ar3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nature. 2008;453(7198):1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren’s syndrome: findings in humans and mice. Arthritis and Rheumatism. 2008;58(3):734–743. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren’s syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. Journal of Immunology. 2008;181(4):2898–2906. doi: 10.4049/jimmunol.181.4.2898. [DOI] [PubMed] [Google Scholar]
- 26.de Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunology. 2009;2(3):243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konno A, Takada K, Saegusa J, Takiguchi M. Presence of B7-2+ dendritic cells and expression of Th1 cytokines in the early development of sialodacryoadenitis in the IQI/Jic mouse model of primary Sjögren’s syndrome. Autoimmunity. 2003;36(4):247–254. doi: 10.1080/0891693031000141077. [DOI] [PubMed] [Google Scholar]
- 28.Brayer JB, Cha S, Nagashima H, et al. IL-4-dependent effector phase in autoimmune exocrinopathy as defined by the NOD.IL-4-gene knockout mouse model of Sjögren’s syndrome. Scandinavian Journal of Immunology. 2001;54(1-2):133–140. doi: 10.1046/j.1365-3083.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 29.Bertorello R, Cordone MP, Contini P, et al. Increased levels of interleukin-10 in saliva of Sjögren’s syndrome patients: correlation with disease activity. Clinical and Experimental Medicine. 2004;4(3):148–151. doi: 10.1007/s10238-004-0049-9. [DOI] [PubMed] [Google Scholar]
- 30.Anaya J-M, Correa PA, Herrera M, Eskdale J, Gallagher G. Interleukin 10 (IL-10) influences autoimmune response in primary Sjögren’s syndrome and is linked to IL-10 gene polymorphism. Journal of Rheumatology. 2002;29(9):1874–1876. [PubMed] [Google Scholar]
- 31.Jonsson MV, Delaleu N, Brokstad KA, Berggreen E, Skarstein K. Impaired salivary gland function in NOD mice: association with changes in cytokine profile but not with histopathologic changes in the salivary gland. Arthritis and Rheumatism. 2006;54(7):2300–2305. doi: 10.1002/art.21945. [DOI] [PubMed] [Google Scholar]
- 32.Tsuboi H, Matsumoto I, Wakamatsu E, et al. New epitopes and function of anti-M3 muscarinic acetylcholine receptor antibodies in patients with Sjögren’s syndrome. Clinical and Experimental Immunology. 2010;162(1):53–61. doi: 10.1111/j.1365-2249.2010.04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


