Abstract
GFP-chimeric mice are important tools to study the role of bone marrow-derived cells in eye physiology. A method is described to generate GFP-chimeric mice using whole-body, sub-lethal radiation (600 rad) of wild-type C57BL/6 recipients followed by tail vein injection of bone marrow cells derived from GFP+ (GFP-transgenic C57/BL/6-Tg(UBC-GFP)30 Scha/J) mice. This method yields stable GFP+ chimeras with greater than 95% chimerism (range 95–99%), achieved within one month of bone marrow transfer confirmed by microscopy and fluorescence-assisted cell sorting (FACS) analysis, with lower mortality after irradiation than prior methods. To demonstrate the efficacy of GFP+ bone marrow chimeric mice, the role of circulating GFP+ bone marrow–derived cells in myofibroblast generation after irregular photo-therapeutic keratectomy (PTK) was analyzed. Many SMA+ myofibroblasts that were generated at one month after PTK were derived from GFP+ bone marrow-derived cells. The GFP+ bone marrow chimeric mouse provides an excellent model for studying the role of bone marrow-derived cells in corneal wound healing, glaucoma surgery, optic nerve head pathology and retinal pathophysiology and wound healing.
1. Introduction
Many processes in the eye involve complex interactions between resident cells, progenitor and/or stem cells, inflammatory cells, soluble mediators (such as growth factors, cytokines and matrix metalloproteinases), and extracellular matrix components (Cotsarelis, 2006; Diegelmann et al., 1999; Kaur et al., 2009a and 2009b; Martins et al., 2012; Opalenik and Davidson, 2005; Singh et al., 2010 and 2012, Wilson, 2012). Cells that populate the wound site and participate in the wound healing response and tissue regeneration have traditionally been viewed as being derived primarily from adjacent uninjured cells in the tissue. However, numerous reports have provided evidence that cells seen in the peripheral blood (PB), such as hematopoietic stem cells (HSCs), endothelial progenitor cells (EPCs), circulating fibrocytes, BM-derived mesenchymal stem cells (MSCs), and rare tissue-derived mesenchymal stem cells also contribute to repair and regeneration of injured tissues (Stadelmann et al., 1998; Wu et al., 2010). For example, studies have shown that circulating bone marrow (BM)-derived cells plays critical roles in generation of cells involved in processes such as wound healing, fibrosis and malignancy (Bhawan and Majno, 1989; Chinnery et al., 2008; Direkze et al., 2003; Wilson et al., 2004; Kaneko et al., 2008; Santhiago et al., 2011; Sonoda et al., 2005; Barbosa et al., 2010). As an example, myofibroblasts involved in the healing of some types of corneal injuries are often generated from bone marrow-derived cells (Barbosa et al., 2010), although they may also be derived from corneal fibroblasts, and even epithelial cells (Direkze et al., 2003; Fathke et al., 2004; Kaur et al., 2009b; Novo et al., 2009; Saika et al., 2010; Wilson SE, 2012). The capacity to unambiguously monitor myofibroblasts derived from bone marrow-derived cells has contributed to our overall understanding of the corneal wound healing response and has similarly led to a better understanding of the role of bone marrow-derived cells in processes in other tissues in the eye.
The green fluorescent protein (GFP) produces the green bioluminescence of the jellyfish Aequorea Victoria (Shimomura, 2005). The expression of GFP in models such as C. elegans, Zebra fish, D. melanogaster attracted interest to this novel reporter as a potential in vivo marker (Cubitt et al., 1995). A modified method for generating greater than 95% chimerism in mice with GFP+ bone marrow cells was developed (Barbosa et al., 2010) since earlier methods (Ono et al., 1999; Hayakawa et al, 2003) showed stable chimerisation less than 80% in our hands and the mortality of mice after irradiation and injection of bone marrow was greater than 50% in the first few weeks. This model has important applications for studying the recruitment and function of bone marrow-derived cells in vivo after injury, surgery or therapy in the eye.
2. Materials, supplies and detailed methods
2.1. Animals
Six to eight week old C57BL/6 (Stock Number: 000664) or C57/BL/6-Tg(UBC-GFP)30 Scha/J (Stock Number: 004353) female mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All animals were treated in accordance with the tenets of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional animal care and use committee (IACUC) at the Cleveland Clinic.
2.2. Harvest of bone marrow cells from GFP+ mice (C57/BL/6-Tg(UBC-GFP)30 Scha/J)
The C57/BL/6-Tg(UBC-GFP)30 Scha/J transgenic mice express enhanced green fluorescent protein (GFP) under the transcriptional control of a human ubiqutin C promoter. Mice homozygous for the transgene are viable and fertile, and do not display any gross abnormalities other than the expression of the GFP marker in all nucleated cells. All hematapoetic cell types display distinct expression levels of GFP, thus allowing identification of different cells types by FACS analysis. GFP+ mice were euthanised, by CO2 inhalation. Long bones of both legs were removed and maintained in chilled 1X PBS (10X stock is 1.37M sodium chloride, 27mM potassium chloride, and 119mM phosphate buffer, endotoxin free) throughout the procedure. Care was taken to include the joints at both ends of the femur and tibia in each leg. Soft tissues and skin were removed and the femur and tibia separated at the knee joint by dissecting the adjoining connective tissue. Both ends of all bones were trimmed to allow insertion of a 26-gauge needle to flush the bone marrow with DMEM medium. Tissue populated with bone marrow cells and stem cells is typically found concentrated at the joints. This tissue was harvested by alternately flushing with medium and scratching the bone marrow cavity with the end of the needle. Clumps of bone marrow cells were teased out and gently dissociated using a one ml pipette to form a single-cell suspension. Single-cell suspensions were gently centrifuged at 1500 rpm for 10 minutes at 4°C to obtain a cell pellet. Red blood cells were lyzed by adding chilled sterile Milli-Q water followed by dilution with 10X PBS at a ratio of one part PBS to nine parts cell solution with immediate mixing. Cell suspensions were centrifuged again at 1500 rpm for 10 minutes at 4°C and resuspended in PBS at 4°C. Cell viability was verified by staining with 0.4% trypan blue and cells were suspended in PBS at 4°C at a final concentration of 20 ×106 cells/ml. Viability in the range of 90% to 95% should be obtained in the isolated cells.
2.3 Irradiation of recipient mice
C57BL/6 wild type mice were irradiation two hours before cell injection using a 137Cs Mark 1 irradiator (J.L. Sheperd & Associates, Glendale, CA). All recipient mice received a single 600 rads dose of whole-body irradiation. Irradiated animals were allowed to rest in cages with unlimited food and water for two hours prior to bone marrow injection.
2.4. GFP+ bone marrow transplantation in recipient mice
Irradiated recipient WT C57BL/6 mice were briefly placed under a UV lamp to allow dilation of the tail veins. Mice were immobilized using an animal holder and tail veins located. The tails were sterilized using 70% ethanol pads and 0.5 ml of bone marrow cell suspension (20 X 106 cells/ml) was injected intravenously per mouse using a 30-gauge needle. Mice were returned to their cages and monitored following injection. Animal mortality was observed within one to two weeks if bone marrow cells were not delivered in adequate numbers and with sufficient viability.
2.5 Fluorescence-activated cell sorting (FACS)
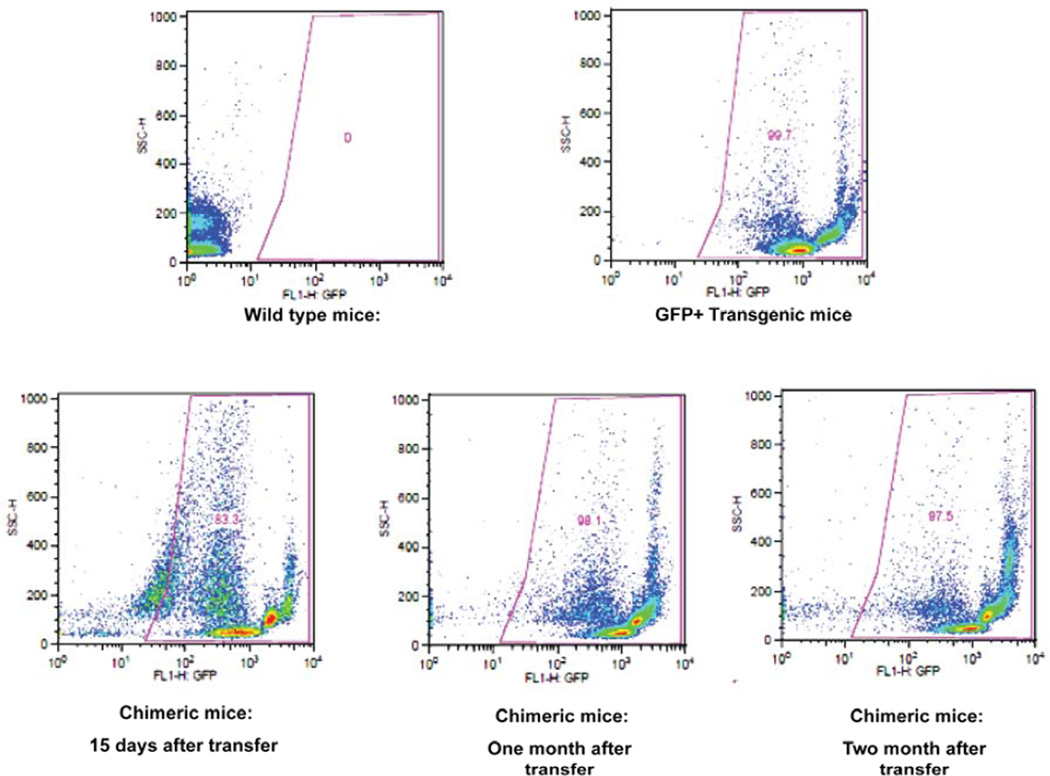
Twenty microliters of blood were collected directly from the tail vein using heparin-coated capillary tubes. Blood collected in capillary tubes was further diluted into one ml of PBS in FACS tubes and maintained at 4°C. Each sample was centrifuge for 10 min at 1500 rpm at 4°C and supernatant discarded. Red blood cells were lysed using water at 4°C and brought to 1X PBS by adding 10X PBS followed by centrifugation at 4°C for 10 min at 1500 rpm. The white blood cell pellet was washed once in staining buffer (filtered PBS with 1% BSA and 0.1% sodium azide) and resuspended in 200 μl of staining buffer. The samples were analyzed with a Becton–Dickinson FACS instrument (Franklin Lakes, New Jersey). Lymphocytes were gated on forward-scatter (FSC) and side-scatter (SSC) plots. All peripheral blood lymphocyte preparations were stained with 0.5 mg/ml propidium iodide for detection of procedure-induced cell death. Dead cells were gated on SSC vs FL2 dot-plots and excluded from further analysis. SSC vs. FL1 plots were analyzed to assess the percentage of GFP-positive cells in the peripheral blood lymphocytes of each chimeric mouse. Data shown is representative of 51 chimeric mice. FACS analysis of each chimeric mouse blood sample was performed to evaluate the efficiency of GFP chimerism. Twelve to fifteen chimeric mice were used for FACS analysis at each time point. These results show that more than 95% chimerism was obtained using the procedure described above (n = 51; Range 95 - 99%; Average 97.5%). Figure 1 represents the number of GFP-positive cells in chimeric mice one month after BM cell transfer via tail vein injections. It is recommended that animals that show less than 90% GFP chimerism after one month not be used in subsequent experiments. GFP chimerism was found to be already greater than 80% (average 84%) at 15 days after bone marrow transfer. In addition, GFP chimeras were found (Fig. 1) to be stable at two months after tail vein injections (average 96.5%). We found no increase in chimerism at time points three and six months after bone marrow transfer (data not shown).
Fig. 1. Flow cytometry analysis of stability and percentage of chimerization achieved by bone marrow transfer.
Peripheral blood preparations from WT (negative control), GFP+ transgenic (positive control) and chimeric mice were stained with propidium iodide and gated on live cell populations. Live cells were further analyzed on an SSC vs. FL1 (GFP+) plot. The upper panel shows the absence of GFP+ staining in peripheral blood from WT mice (left upper panel) and > 99% GFP+ staining in peripheral blood from GFP+ transgenics (GFP-transgenic C57/BL/6-Tg(UBC-GFP)30 Scha/J). The lower panel shows percentage of chimerization achieved in WT mice at 15 days after tail vein injection (left lower panel), at one month after tail vein injection (center lower panel) and 2 months after tail vein injection (right lower panel) of GFP+ bone marrow. Data shown is representative of 51 chimeras.
2.6 Immunohistochemistry
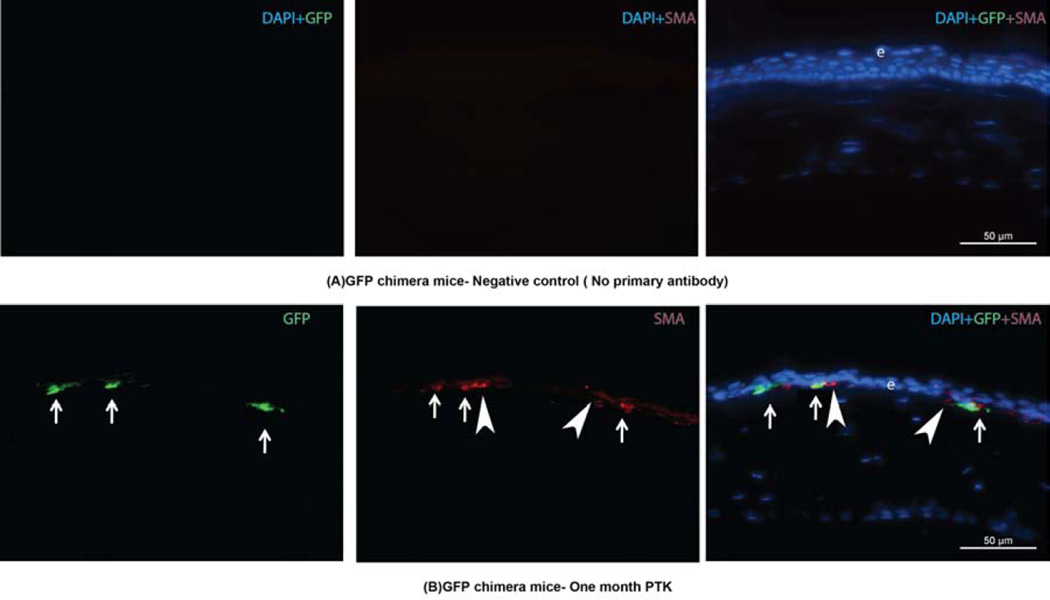
Immunohistochemical staining was performed on experimental and control tissue sections, as previously described (Barbosa et al., 2010; Singh et al., 2011). Although GFP+ cells can be detected directly with a fluorescent microscope, the intensity of the signal is markedly enhanced by immunohistochemical staining for the GFP. The sections were viewed and photographed with a Leica DM5000 microscope equipped with Q-Imaging Retiga 4000RV camera (Surrey, BC, Canada) and ImagePro software (Leica). All immunohistochemistry was performed at least three times to insure the results were consistent. For demonstration of bone marrow-derived cells contributing to corneal wound healing at one month after irregular PTK, the central corneal stoma was analyzed for alpha smooth muscle actin (SMA, a marker for myofibroblasts)+ and GFP+ cells. Many SMA+GFP+ and SMA+GFP− cells, as well as SMAGFP+ cells, were observed in the stroma, confirming the migration of donor GFP-positive bone marrow cells into the cornea and the differentiation of some of these cells into myofibroblasts after the corneal injury induced by irregular PTK (Fig. 2).
Fig. 2.
GFP+SMA+ immunohistochemistry images of corneal sections from GFP+ chimeric mice at one month after irregular PTK. In each row, the left lane shows GFP stained green; the middle lane shows SMA+ cells stained red; and the right lane is an overlay of DAPI, GFP and SMA staining. A: is immunohistochemistry in the cornea of a chimeric mouse at one month after irregular PTK where the primary antibodies were omitted (controls). B is immunohistochemistry for SMA and GFP proteins in the cornea of a chimeric mouse at one month after irregular PTK. The arrows indicate representative SMA+GFP+ positive cells that are myofibroblasts that differentiated in the cornea from bone marrow-derived cells. The arrowheads are SMA+ myofibroblasts that are GFP-, indicating they likely differentiated from cells derived from keratocytes in the cornea, although it cannot be excluded that one or both of these cells also differentiated from bone marrow-derived cells since it is not possible to achieve 100% chimerism. This section was selected to show SMA+ cells that are both GFP- and GFP+. When counts were performed in many fields on many sections in several corneas at one month after irregular PTK in chimeric mice, more than 90% of SMA+ cells were GFP+ (Barbosa, et al., 2010).
3.0 Potential Pitfalls and Trouble Shooting
3.1 GFP Bone marrow harvesting
It is critical to include the joints at both ends of the femur and tibia in each leg since many bone marrow-derived cells and stem cells have been found to be present in these joints. To increase the purity of the bone marrow tissue, soft tissues and skin should be removed from the femur and tibia separated at the knee joint and this is facilitated by freezing the adjoining connective tissue and dissecting away contaminating tissues. The harvested bone marrow cells can be filtered with a 40-micron cell strainer (BD Falcon, NC, USA) before red blood cell lysis and cell counting to eliminate any soft tissue or other debris from the final GFP bone marrow preparation. All steps need to be performed at 4°C after harvesting the bone marrow until the tail injection is performed to maintain cell viability.
3.2. Transfer into recipient irradiated mice
Experience in developing and using the current methods has shown that the time from harvesting to transfer of GFP bone marrow cell into the irradiated mice is critical. Tail vein injection of the harvested GFP+ bone marrow cells should be completed within two to three hours after the recipient mouse irradiation step. One should insure that precise volume and number of bone marrow cells (0.5 ml at 20 X 106 cells/ml) is injected into the tail vein of the recipient mice so that optimal chimerism is achieved. Injection of lower cell numbers typically results in a lower percentage of chimerism and/or animal death. Bone marrow injections in higher PBS volumes increase difficulty of the procedure as well as potential of mortality while making no additional impact on increasing the efficiency of bone marrow engraftment.
Conclusion and Clinical applications
Using these methods, chimeric mice with greater than 95% (range 95–99%) chimerism with GFP+ bone marrow cells were generated. In our experience with earlier models reported by Ono et al (1999) and Hayakawa et al (2003), chimerism greater than 95% was infrequently achieved, and often levels of 70% to 80% were obtained. In addition, the use of lower doses of radiation in the current method resulted in a significant reduction in animal mortality during the early post-transfer period, without diminishing the level of chimerization. However, the results in generation of GFP+ chimeric mice often vary between different strains of mice (Carstea et al., 2009; Keskintepe et al., 2007).
The GFP+ bone marrow chimeric mice model provides a powerful model to explore the roles of bone marrow-derived cells in the cornea, retina, and optic nerve head, and would likely be an interesting avenue to investigate other ocular injuries such as those cause by infection by herpes simplex virus and other pathogens, as well as the response to surgeries such as glaucoma filtration surgeries complicated by fibrosis, corneal transplantation with penetrating keratoplasty and lamellar endothelial transplantation methods where complications such as opacity or rejection are noted, or scarring and repair in the retina associated with macular degeneration or disorders such as proliferative diabetic retinopathy.
Highlights.
Method to generate GFP+ bone marrow chimeras at greater than 95% chimerization in mice
Optimal model to study the role of bone marrow-derived cells in corneal, retinal and glaucoma surgery wound healing and disease
Example of application to corneal myofibroblast generation from bone marrow-derived precursors
Acknowledgements
Supported in part by US Public Health Service grants EY10056 and EY015638 from the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, New York, NY. We wish to thank Dr. Marcony R Santhiago for assistance with phototherapeutic keratectomy in the mice and Vandana Agrawal for her technical assistance. We would like to thank the flow cytometry core facility at the Lerner Research Institute, The Cleveland Clinic, for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Proprietary interest statement: None of the authors have any proprietary or financial interests in the topics discussed in this manuscript.
References
- Barbosa FL, Chaurasia SS, Cutler A, Asosingh K, Kaur H, de Medeiros FW, Agrawal V, Wilson SE. Corneal myofibroblast generation from bone marrow-derived cells. Exp. Eye Res. 2010;91:92–96. doi: 10.1016/j.exer.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhawan J, Majno G. The myofibroblast. Possible derivation from macrophages in xanthogranuloma. Am. J. Dermatopathol. 1989;11:255–258. doi: 10.1097/00000372-198906000-00010. [DOI] [PubMed] [Google Scholar]
- Carstea AC, Pirity MK, Dinnyes A. Germline competence of mouse ES and iPS cell lines: Chimera technologies and genetic background. World J Stem Cells. 2009;31;1(1):22–29. doi: 10.4252/wjsc.v1.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery HR, Humphries T, Clare A, Dixon AE, Howes K, Moran CB, Scott D, Zakrzewski M, Pearlman E, McMenamin PG. Turnover of bone marrow-derived cells in the irradiated mouse cornea. Immunology. 2008;125(4):541–548. doi: 10.1111/j.1365-2567.2008.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G. Epithelial stem cells: a folliculocentric view. J. Invest. Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien RY. Understanding, improving and using green fluorescent proteins. Trends Biochem. Sci. 1995;20(11):448–455. doi: 10.1016/s0968-0004(00)89099-4. [DOI] [PubMed] [Google Scholar]
- Diegelmann RF, Evans MC. Wound Healing: and overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow transplanted mice. Stem Cells. 2003;21:514–520. doi: 10.1634/stemcells.21-5-514. [DOI] [PubMed] [Google Scholar]
- Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22:812–822. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Nishiguchi KM, Nakamura M, Kachi S, Terasak H. Characteristics of bone marrow–derived microglia in the normal and injured retina. Invest. Ophthalmol. Vis. Sci. 2008;49:4162–4168. doi: 10.1167/iovs.08-1738. [DOI] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Agrawal V, Wilson SE. Corneal myofibroblast viability: opposing effects of IL-1 and TGF beta-1. Exp. Eye Res. 2009a;89:152–158. doi: 10.1016/j.exer.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Agrawal V, Wilson SE. Expression of PDGF receptor-alpha in corneal myofibroblasts in situ. Exp. Eye Res. 2009b;89:432–434. doi: 10.1016/j.exer.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskintepe L, Norris K, Pacholczyk G, Dederscheck SM, Eroglu A. Derivation and comparison of C57BL/6 embryonic stem cells to a widely used 129 embryonic stem cell line. Transgenic Res. 2007;16(6):751–758. doi: 10.1007/s11248-007-9125-8. [DOI] [PubMed] [Google Scholar]
- Hayakawa J, Migita M, Ueda T, Shimada T, Fukunaga Y. Generation of a chimeric mouse reconstituted with green fluorescent protein-positive bone marrow cells: a useful model for studying the behavior of bone marrow cells in regeneration in vivo. Int J Hematol. 2003;77:456–462. doi: 10.1007/BF02986613. [DOI] [PubMed] [Google Scholar]
- Martins VL, Caley M, O'Toole EA. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 2013;351:255–268. doi: 10.1007/s00441-012-1410-z. [DOI] [PubMed] [Google Scholar]
- Novo E, di Bonzo LV, Cannito S, Colombatto S, Parola M. Hepatic myofibroblasts: a heterogeneous population of multifunctional cells in liver fibrogenesis. Int. J. Biochem. Cell Biol. 2009;41:2089–2093. doi: 10.1016/j.biocel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Ono K, Takii T, Onozaki K, Ikawa M, Okabe M, Sawada M. Migration of exogenous immature hematopoietic cells into adult mouse brain parenchyma under GFP-expressing bone marrow chimera. Biochem Biophys Res Commun. 1999;262:610–614. doi: 10.1006/bbrc.1999.1223. [DOI] [PubMed] [Google Scholar]
- Opalenik SR, Davidson JM. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J. 2005;19:1561–1563. doi: 10.1096/fj.04-2978fje. [DOI] [PubMed] [Google Scholar]
- Santhiago MR, Singh V, Barbosa FL, Agrawal V, Wilson SE. Monocyte development inhibitor PRM-151 decreases corneal myofibroblast generation in rabbits. Exp Eye Res. 2011;93:786–789. doi: 10.1016/j.exer.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O. The discovery of aequorin and green fluorescent protein. J Microsc. 2005;217:1–15. doi: 10.1111/j.0022-2720.2005.01441.x. 2005. [DOI] [PubMed] [Google Scholar]
- Singh V, Agrawal V, Santhiago MR, Wilson SE. Stromal fibroblast bone marrow-derived cell interactions: implications for myofibroblast development in the cornea. Exp. Eye Res. 2012;23(98):1–8. doi: 10.1016/j.exer.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Santhiago MR, Barbosa FL, Agrawal V, Singh N, Ambati BK, Wilson SE. Effect of TGFb and PDGF-B blockade on corneal myofibroblast development in mice. Exp. Eye Res. 2011;93:810–817. doi: 10.1016/j.exer.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda KH, Nakao S, Nakamura T, Oshima T, Qiao H, Hisatomi T, Kinoshita S, Ishibashi T. Cellular events in the normal and inflamed cornea. Cornea. 2005;24(8):S50–S54. doi: 10.1097/01.ico.0000178736.35297.9d. [DOI] [PubMed] [Google Scholar]
- Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176(2A Suppl):26S–38S. doi: 10.1016/s0002-9610(98)00183-4. [DOI] [PubMed] [Google Scholar]
- Wilson SE. Corneal myofibroblast biology and pathobiology: Generation, persistence, and transparency. Exp Eye Res. 2012;99:78–88. doi: 10.1016/j.exer.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Netto MV, Perez V, Possin D, Huang J, Kwon R, Alekseev A. RANK, RANKL, OPG, and M-CSF expression in stromal cells during corneal wound healing. Invest. Ophthalmol. Vis. Sci. 2004;45:2201–2211. doi: 10.1167/iovs.03-1162. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhao RC, Tredget EE. Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells. 2010;28:905–915. doi: 10.1002/stem.420. [DOI] [PMC free article] [PubMed] [Google Scholar]