Abstract
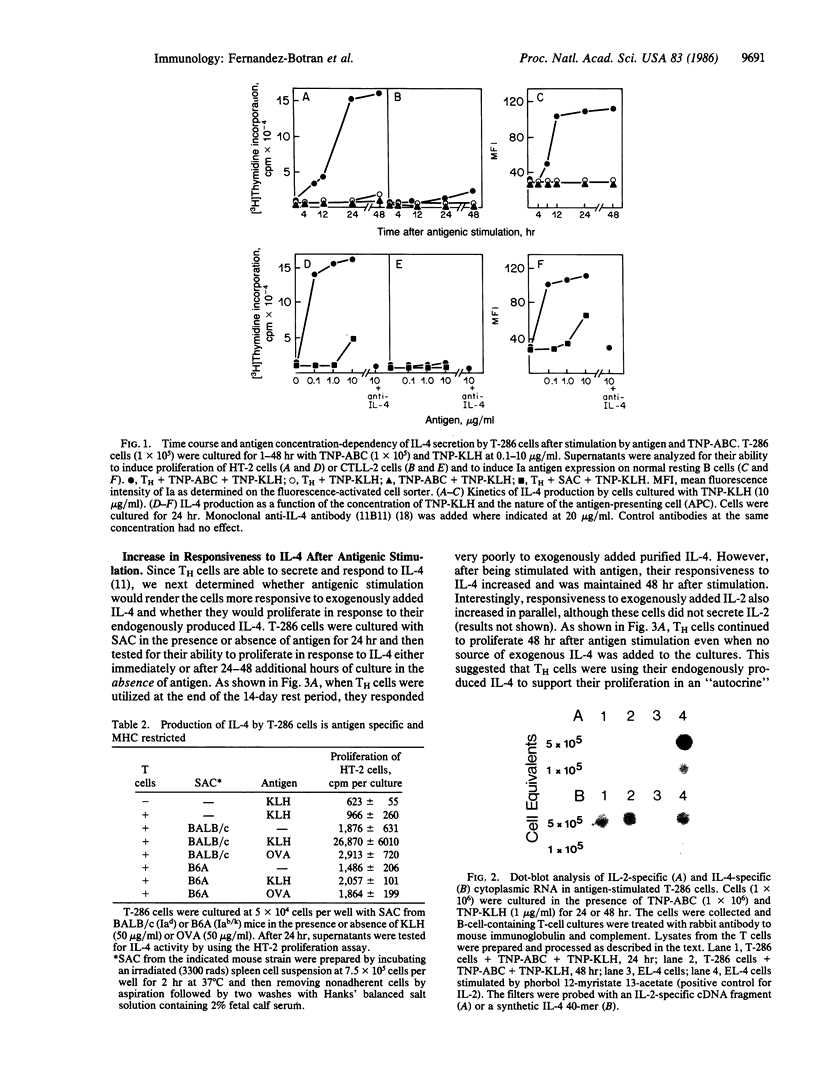
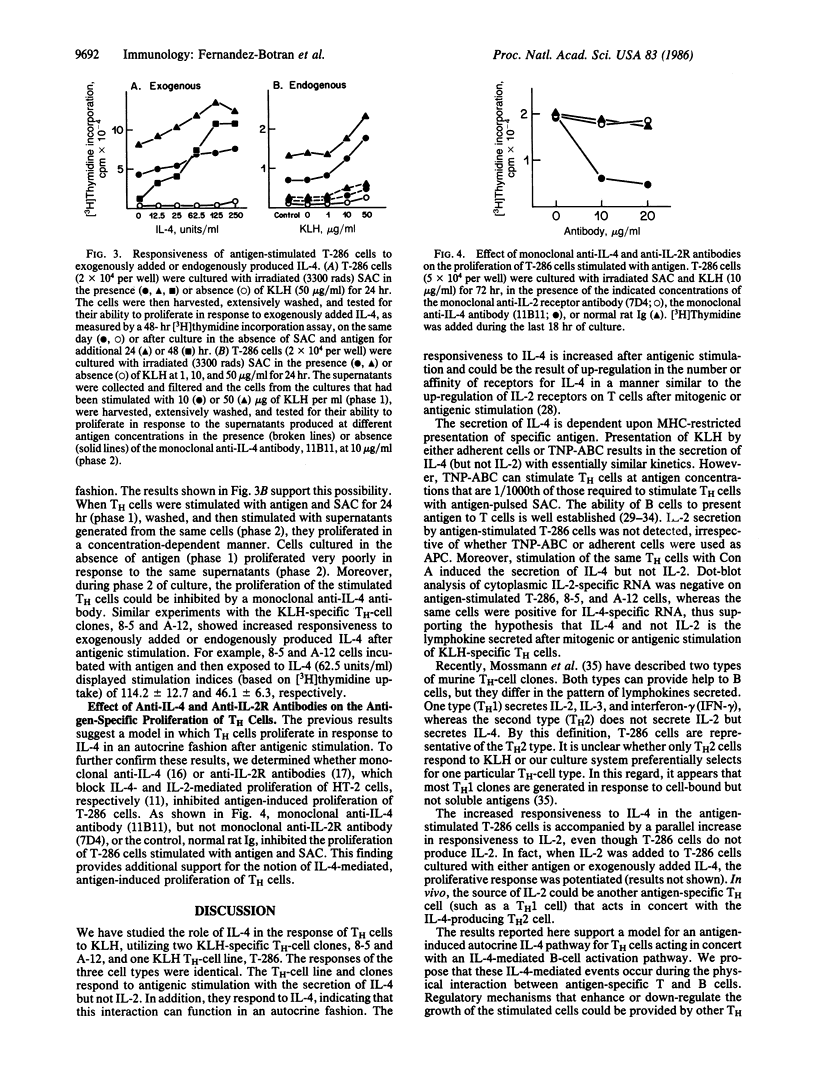
The role of interleukin 4 (IL-4) (previously called B-cell stimulatory factor 1) in the response of T helper (TH) cells to antigen presented by antigen-specific B cells or splenic adherent cells was investigated. Antigenic stimulation of either a keyhole-limpet-hemocyanin-specific TH-cell line or two keyhole-limpet-hemocyanin-specific T-cell clones resulted in the secretion of IL-4 but not interleukin 2 (IL-2). The secretion of IL-4 was first detected in the culture supernatant 6-8 hr after antigenic stimulation. Induction of IL-4 secretion was antigen specific and major histocompatibility complex restricted. Antigenic stimulation also resulted in increased responsiveness of the TH cells to exogenously added or endogenously produced IL-4. The antigen-induced proliferation of the TH cells could be inhibited by an anti-IL-4 antibody but not by an anti-IL-2-receptor antibody. These results suggest that IL-4 mediates the proliferation of some TH cells by an antigen-induced autocrine mechanism. Taken together with past results, these data indicate that, during T-cell-B-cell interactions involving some soluble protein antigens, IL-4 and not IL-2 is the critical lymphokine for activating resting B cells and inducing proliferation of the TH cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas A. K., Haber S., Rock K. L. Antigen presentation by hapten-specific B lymphocytes. II. Specificity and properties of antigen-presenting B lymphocytes, and function of immunoglobulin receptors. J Immunol. 1985 Sep;135(3):1661–1667. [PubMed] [Google Scholar]
- Asano Y., Hodes R. J. T cell regulation of b cell activation. Cloned Lyt-1+2-T suppressor cells inhibit the major histocompatibility complex-restricted interaction of T helper cells with B cells and/or accessory cells. J Exp Med. 1983 Oct 1;158(4):1178–1190. doi: 10.1084/jem.158.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. E., Gillis S., Smith K. A. Monoclonal cytolytic T-cell lines. J Exp Med. 1979 Jan 1;149(1):273–278. doi: 10.1084/jem.149.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. W., Word C. J., Jones S., Uhr J. W., Tucker P. W., Vitetta E. S. Double isotype production by a neoplastic B cell line. I. Cellular and biochemical characterization of a variant of BCL1 that expresses and secretes both IgM and IgG1. J Exp Med. 1986 Aug 1;164(2):548–561. doi: 10.1084/jem.164.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Studies on the capacity of B cells to serve as antigen-presenting cells. J Immunol. 1981 Mar;126(3):1075–1079. [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Fernandez-Botran R., Krammer P. H., Diamantstein T., Uhr J. W., Vitetta E. S. B cell-stimulatory factor 1 (BSF-1) promotes growth of helper T cell lines. J Exp Med. 1986 Aug 1;164(2):580–593. doi: 10.1084/jem.164.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein K., Eisenman J., Mochizuki D., Shanebeck K., Conlon P., Hopp T., March C., Gillis S. Purification to homogeneity of B cell stimulating factor. A molecule that stimulates proliferation of multiple lymphokine-dependent cell lines. J Exp Med. 1986 Jun 1;163(6):1405–1414. doi: 10.1084/jem.163.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson P. C. Antiimmunoglobulin-treated B cells respond to a B cell differentiation factor for IgG1. J Exp Med. 1986 Jul 1;164(1):303–308. doi: 10.1084/jem.164.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi T., Chesnut R. W., Grey H. M. B cells as antigen-presenting cells: the requirement for B cell activation. J Immunol. 1983 Jul;131(1):109–114. [PubMed] [Google Scholar]
- Kimoto M., Fathman C. G. Antigen-reactive T cell clones. I. Transcomplementing hybrid I-A-region gene products function effectively in antigen presentation. J Exp Med. 1980 Oct 1;152(4):759–770. doi: 10.1084/jem.152.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Finkelman F. D., Sarma C., Ohara J., Serrate S. Recombinant interferon-gamma inhibits the B cell proliferative response stimulated by soluble but not by Sepharose-bound anti-immunoglobulin antibody. J Immunol. 1985 Oct;135(4):2513–2517. [PubMed] [Google Scholar]
- Mosmann T. R., Bond M. W., Coffman R. L., Ohara J., Paul W. E. T-cell and mast cell lines respond to B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5654–5658. doi: 10.1073/pnas.83.15.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Myers C. D., Sanders V. M., Vitetta E. S. Isolation of antigen-binding virgin and memory B cells. J Immunol Methods. 1986 Aug 21;92(1):45–57. doi: 10.1016/0022-1759(86)90502-8. [DOI] [PubMed] [Google Scholar]
- Noelle R., Krammer P. H., Ohara J., Uhr J. W., Vitetta E. S. Increased expression of Ia antigens on resting B cells: an additional role for B-cell growth factor. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6149–6153. doi: 10.1073/pnas.81.19.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Ohara J., Lahet S., Inman J., Paul W. E. Partial purification of murine B cell stimulatory factor (BSF)-1. J Immunol. 1985 Oct;135(4):2518–2523. [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Oliver K., Noelle R. J., Uhr J. W., Krammer P. H., Vitetta E. S. B-cell growth factor (B-cell growth factor I or B-cell-stimulating factor, provisional 1) is a differentiation factor for resting B cells and may not induce cell growth. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2465–2467. doi: 10.1073/pnas.82.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pure E., Isakson P. C., Kappler J. W., Marrack P., Krammer P. H., Vitetta E. S. T cell-derived B cell growth and differentiation factors. Dichotomy between the responsiveness of B cells from adult and neonatal mice. J Exp Med. 1983 Feb 1;157(2):600–612. doi: 10.1084/jem.157.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin E. M., Mond J. J., Ohara J., Paul W. E. Interferon-gamma inhibits the action of B cell stimulatory factor (BSF)-1 on resting B cells. J Immunol. 1986 Sep 1;137(5):1573–1576. [PubMed] [Google Scholar]
- Rabin E. M., Ohara J., Paul W. E. B-cell stimulatory factor 1 activates resting B cells. Proc Natl Acad Sci U S A. 1985 May;82(9):2935–2939. doi: 10.1073/pnas.82.9.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B., Abbas A. K. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984 Oct 1;160(4):1102–1113. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm N. W., Leibson H. J., Zlotnik A., Kappler J., Marrack P., Cambier J. C. Interleukin-induced increase in Ia expression by normal mouse B cells. J Exp Med. 1984 Sep 1;160(3):679–694. doi: 10.1084/jem.160.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideras P., Bergstedt-Lindqvist S., Severinson E. Partial biochemical characterization of IgG1-inducing factor. Eur J Immunol. 1985 Jun;15(6):593–598. doi: 10.1002/eji.1830150612. [DOI] [PubMed] [Google Scholar]
- Snow E. C., Noelle R. J., Uhr J. W., Vitetta E. S. Activation of antigen-enriched B cells. II. Role of linked recognition in B cell proliferation to thymus-dependent antigens. J Immunol. 1983 Feb;130(2):614–618. [PubMed] [Google Scholar]
- Snow E. C., Vitetta E. S., Uhr J. W. Activation of antigen-enriched b cells. I. Purification and response to thymus-independent antigens. J Immunol. 1983 Feb;130(2):607–613. [PubMed] [Google Scholar]
- Tony H. P., Parker D. C. Major histocompatibility complex-restricted, polyclonal B cell responses resulting from helper T cell recognition of antiimmunoglobulin presented by small B lymphocytes. J Exp Med. 1985 Jan 1;161(1):223–241. doi: 10.1084/jem.161.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Ohara J., Myers C. D., Layton J. E., Krammer P. H., Paul W. E. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985 Nov 1;162(5):1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]





