Abstract
The mechanisms that are responsible for the development of myocardial fibrosis in the inflammatory cardiomyopathy are unknown. Previously we have generated lines of transgenic mice with cardiac restricted overexpression of tumor necrosis factor (MHCsTNF mice), a pro-inflammatory cytokine. The MHCsTNF mice develop a heart failure phenotype that is characterized by progressive myocardial fibrosis, as well as increase levels transforming growth factor-β (TGF-β) mRNA and protein. In order to determine whether TGF-β mediated signaling was responsible for the myocardial fibrosis observed in the MHCsTNF mice, we treated MHCsTNF and littermate control mice from 4 to 12 weeks of age with a novel orally available TGF-β receptor antagonist (NP-40208). At the time of terminal study myocardial collagen content was determined using the picrosirius red technique, and LV systolic and diastolic function were determined using the Langendorff method. Treatment with NP-40208 resulted in a significant decrease in the nuclear translocation of Smad 2/3, a decrease in heart-weight to body-weight ratio, decreased fibrillar collagen content and decreased LV chamber stiffness in the MHCsTNF mice when compared to diluent treated controls. Treatment with NP-40208 had no discernable effect on LV systolic function, nor any effect on fetal gene expression in the MHCsTNF mice. Taken together, these observations suggest that sustained pro-inflammatory signaling in the adult heart is associated with a pro-fibrotic phenotype that arises, at least in part, from TGF-β mediated signaling, with resultant activation of Smad 2/3, leading to increased myocardial fibrosis and increased LV diastolic chamber stiffness.
Keywords: Tumor necrosis factor, transforming growth factor, myocardial fibrosis, transgenesi
INTRODUCTION
Previous studies from this and other laboratories have shown that cardiac restricted ovexpression of tumor necrosis factor (TNF) consistently leads to the development of a heart failure phenotype characterized by left ventricular (LV) dilation and progressive myocardial fibrosis.1–3 Although the progressive LV dilation in these mice has been attributed to TNF-induced activation of matrix metalloproteinases (MMPs) with subsequent degradation of fibrillar collagen, the mechanisms that are responsible for the progressive myocardial fibrosis that accrues in these mice is not all understood. Given that TNF inhibits collagen gene expression and/or collagen synthesis in cardiac fibroblasts,4,5 the increased myocardial fibrosis observed in the transgenic models with cardiac restricted overexpression of TNF is unlikely to be a direct effect of TNF-mediated signaling. Of note, Feldman and colleagues have suggested the interesting possibility that MMP induced degradation products, or “matrikines,” are responsible for the progressive tissue fibrosis in their model of sustained TNF overexpression.6,7 A second possible explanation for the increased fibrosis observed in the TNF transgenic mice is that TNF mediated signaling increases the density of angiotensin type I receptors (AT1) on cardiac fibroblasts,8 and that this increase in AT1 receptor density sensitizes cardiac fibroblasts to the profibrotic actions of endogenous angiotensin II.5 Germane to the present discussion, we have shown that both transforming growth factor-β1 (TGF-β1) and TGF-β2 mRNA and protein levels are significantly increased in the hearts of the MHCsTNF transgenic mice relative to littermate controls, raising the interesting possibility that TGF-β mediated signaling was responsible for the fibrosis observed in the MHCsTNF mice.
TGF-β binds to at least three specific cell-surface receptors, referred to as receptor types I, II, and III, which exist in virtually all mammalian cells, including fibroblasts. The Type I (TβRI) and type II TGF-β (TβRII) receptors are transmembrane receptors with serine/threonine kinase activity, whereas the type III receptor is a membrane-anchored proteoglycan (betaglycan) with a short cytoplasmic domain that is unlikely to mediate the biologic actions of TGF-β, and likely acts to present TGF-β to other receptors. TGF-β mediated cellular signaling is initiated by binding of the ligand to a transmembrane type II TGF-β receptor (TβRII). After ligand activation, the TβRII receptor recruits the type I receptor TGF-β receptor (TβRI) into a heterotetrameric receptor signaling complex. The constitutively active TβRII activates TβRI by phosphorylating serine and threonine residues in a conserved stretch of glycine and serine residues that precede the receptor kinase domain. Phosphorylation of the TβRI kinase results in subsequent downstream signaling predominantly by phosphorylation of cytoplasmic mediators belonging to the Smad family, with resultant translocation of phosphorylated Smad2/3 to the nucleus. 9 Importantly, TGF-β can also signal through mitogen activated protein kinases (MAPK) in a Smad independent manner.
In order to determine whether TGF-β mediated signaling was responsible for the observed myocardial fibrosis observed in the in the MHCsTNF mice, we employed a novel orally available TβRI antagonist, NP-40208 (Scios Inc, Freemont, CA),10 that blocks TGF-β mediated signaling by inhibiting the intracellular kinase domain of TβRI. The results of this study suggest that TGF-β mediated signaling plays an important role in the structural remodeling and cardiac dysfunction that is observed in mice with cardiac-restricted overexpression of TNF.
METHODS
Mice
The studies described herein were performed on 12-week-old male with cardiac restricted overexpression of TNF (MHCsTNF) and littermate control mice lacking the TNF transgene. The characterization of the MHCsTNF mice used in these studies has been reported previously.1,11 Briefly, MHCsTNF mice develop progressive myocardial fibrosis from 8–12 weeks of age that is characterized by increased levels of TGFβ1 and TGFβ2 mRNA and protein.1 All mice housed under standard environmental conditions and maintained on commercial chow and tap water ad libitum. All studies conformed with the principles of the National Institutes of Health “Guide for the Care and Use of Laboratory Animals,” and were approved by the Baylor College of Medicine Animal Care and Use Committee.
TGF-β Receptor Inhibition
To determine the significance of TGF-β signaling MHCsTNF and littermate control mice were treated from 4 to 12 weeks of age with diluent or 100 mg/kg body weight/day NP-40208 (Scios Inc, Freemont, CA), a novel 2,4-disubstituted pteridine that inhibits the intracellular kinase domain of the type I TGF-β receptor (TβRI).10 In an in vitro kinase assay NP-40208 inhibits the TβRI kinase with an IC50 of 0.048 μmol/L. When tested at 50 nmol/L the compound has no effect on TβRII kinase (data provided by Scios Inc.). The dose of NP-40208 chosen for these studies was based on previous studies which showed that 50–150mg/kg body weight/day was sufficient to achieve adequate blood levels of NP-40208 (data provided by Scios Inc.). NP-40208 was dissolved in deionized water and administered through drinking water.
TGF-β Mediated Signaling
To determine the specific effects of the NP-40208 on TGF-β-mediated signaling, we examined protein levels of Smad2/3 in nuclear and cytoplasmic fractions of the MHCsTNF and littermate control mouse hearts. Hearts were excised, snap-frozen in liquid nitrogen and stored at 80 C° until protein extraction. After measuring frozen heart weight, nuclear and cytoplasmic proteins were isolated using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce; Rockford, IL) according to the manufacturer’s instructions. Protein levels for Smad2/3 were measured by Western blot analysis as described previously, 12 using a polyclonal Smad2/3 antibody (1:1000, Upstate, Lake Placid, NY). Western blots were incubated with an appropriate peroxidase-labeled secondary antibody (1:5000 dilution), and membranes were incubated with luminol using a commercially available kit (ECL plus, Amersham, IL), and exposed to chemiluminescent film. The films were scanned on a personal densitometer (Molecular Dynamics, Sunnyvale, CA) and band intensities were evaluated using ImageQuaNT 4.2a (Molecular Dynamics) software.
Cardiac Morphometrics and Myocardial Collagen Content
Cardiac hypertrophy was assessed by determining the heart-weight to body-weight ratio in littermate control and MHCsTNF mice at 12 weeks of age.1 Myocardial collagen content was determined using the picrosirius red technique, as described.1 Briefly, after completing the ex vivo studies described below, hearts were perfusion-fixed, embedded in paraffin and stained. The percent area of extracellular picrosirius red staining was computed from 3 random fields within the mid-myocardium in order to exclude large epicardial arteries and/or veins, as well as any cutting and/or compression artifacts.1
Left Ventricular Function and Stiffness
Hearts from diluent and NP-40208 treated littermate control and MHCsTNF mice were isolated and perfused in the Langendorff mode, as described.13 Briefly, mice were injected intraperitoneally with heparin (10,000U/Kg; Sigma, St Louis, MO) and anesthetized with Avertin (16 μl/g body weight of a 2.5% solution). The hearts were rapidly excised and perfused in a retrograde manner at a constant perfusion pressure of 80 mmHg with modified Krebs-Henseileit buffer containing (mmol): NaCl 118, NaHCO3 24, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.2, Glucose 11, Pyruvate 2. Buffer was oxygenated with 95% O2 and 5% CO2 at 37 °C, to yield a pH of 7.4. A thin cannula was pierced through the apex of the LV to vent thebesian drainage. A hand-made balloon (polyvinyl chloride film) connected to a polyethylene tube (PE50), was inserted into LV through mitral valve via an incision of left atrium and was connected to pressure transducer (ML844, ADinstruments, Colorado Spring CO). The hearts were paced from the right ventricle using Grass SD9 stimulator (Grass Instruments, Quincy, MA) at 420 beats per minute. Functional data were recorded at 1 KHz on a data acquisition system (PowerLab, ADinstruments, Colorado Spring CO). After 20 minutes of stabilization, the balloon was progressively inflated in 5-μL increments until LV developed pressure (LVDP) became asymptotic and then began to decline. LVDP was calculated as the difference between peak-systolic pressure and LV end-diastolic pressure (LVEDP). A final pressure-volume exponential relationship obtained as described.14 The data for LVEDP were plotted an a function of increasing balloon volumes, and were plotted and a best-fit exponential curve (P=b•exp(KcV), where V is volume, and P is LV pressure generated for each mouse. The volumes at a given pressure were averaged for animals in each group. The chamber stiffness constant (Kc) was determined by fitting the end-diastolic pressure volume curves from individual hearts to an exponential function.15,16 The LV chamber stiffness constant (Kc) was determined by fitting the end-diastolic pressure volume curves from individual hearts to an exponential function as described in detail elsewhere.15,16 In order to measure LV systolic function at a physiological filling pressure, the balloon was deflated to adjust LVEDP to 8–10mmHg, and LV systolic pressure and LVEDP were measured.
Fetal Gene Expression
Northern blot analysis was performed on 15 μg of total RNA isolated from the hearts of transgenic mice and littermate control mice.17 The characterization of the probes for α-myosin heavy chain (α-MHC), β-myosin heavy and SERCA2a have been reported previously. 18
Statistical Analysis
Results are expressed as mean values ± SEM. Data were assessed with the use of a commercially available statistical software (STATVIEW version 5.0, SAS institute Inc., Cary, North Carolina). Differences at specific time points between groups were assessed using one-factor ANOVA, which was followed by Fisher’s PLSD test for post-hoc analysis where appropriate. A p value < 0.05 was considered statistically significant.
RESULTS
TGF-β Mediated Signaling
We have shown previously that TGF-β mRNA and protein levels are increased in the hearts of MHCsTNF mice as they develop myocardial fibrosis. 1 To determine whether there were TGF-β mediated alterations in Smad 2/3 signaling in the hearts of MHCsTNF mice, we performed Western blot analysis of Smad 2/3 protein levels in the cytoplasmic and nuclear extracts. Figure 1 highlights two important findings. First, although the cytoplasmic levels of Smad 2/3 were similar (p=0.33) in the MHCsTNF and littermate control mice, there was a significant (p=0.002) increase in the level of Smad 2/3 in the nuclear fraction of the MHCsTNF mouse hearts. The ratio of nuclear to cytoplasmic Smad 2/3 was significantly higher (p=0.018) in the MHCsTNF mice than in the littermate controls, consistent with as well as a significant, consistent with activation of the TGF-β signaling in the MHCsTNF mouse hearts. Second, treatment with the TβRI antagonist NP- 40208 significantly attenuated the increase in Smad 2/3 protein in the nuclear fraction compared to diluent treated MHCsTNF mice, as well as normalized the ratio of nuclear to cytoplasmic Smad 2/3 of the MHCsTNF mice when compared to diluent treated littermate controls. However, despite normalization of the nuclear to cytoplasmic ratio in the treated MHCsTNF mice, the absolute level of Smad 2/3 was still significantly greater (p <0.05) than that observed in the littermate control mice. NP-40208 had no effect on the cytoplasmic nor nuclear distribution of Smad 2/3 in the littermate control mice.
Figure 1.
Nuclear and cytoplasmic Smad 2/3 levels in littermate control and MHCsTNF mice. Panel A: Representative Western blots of Smad 2/3 in nuclear and cytoplasmic fractions in diluent treated littermate control, NP-40208 treated littermate control, diluent treated MHCsTNF, and NP-40208 treated MHCsTNF mice. Panel B: Results of group data for Smad 2/3 levels in nuclear and cytoplasmic fractions and the ratio of nuclear to cytoplasmic Smad 2/3 in diluent treated littermate control (n=3 hearts), NP-40208 treated littermate controls (n=3 hearts), diluent MHCsTNF (n = 3 hearts) mice, and NP-40208 treated MHCsTNF (n = 4 hearts) mice. (Key: * p <0.05 vs. diluent and NP-40208 treated littermate controls, † p < 0.05 vs. diluent treated MHCsTNF mice).
Cardiac Morphometrics
Figure 2 shows that the heart-weight to body-weight ratio was significantly greater in the MHCsTNF mice than in the littermate control mice (p<0.001), consistent with our previous findings. 1 Importantly, treatment with NP-40208 led to a small but significant decrease in the heart-weight to body-weight ratio in MHCsTNF mice (p=0.016), whereas NP-40208 had no effect on the heart-weight to body-weight ratio in the littermate control mice. However, treatment with NP-40208 did not abrogate cardiac hypertrophy in the MHCsTNF mice, insofar as the heart-weight to body-weight ratio was still significantly greater than that observed in littermate control mice.
Figure 2.
Heart-weight to body-weight ratio in diluent treated littermate controls (n=7), NP-4-2–8 treated littermate controls (n=6), diluent treated MHCsTNF (n=6) mice, and NP-40208 treated MHCsTNF (n=6) mice. (Key * p < 0.05 vs. diluent and NP-40208 treated littermate controls, † p < 0.05 vs. diluent treated MHCsTNF mice).
Myocardial Collagen Content
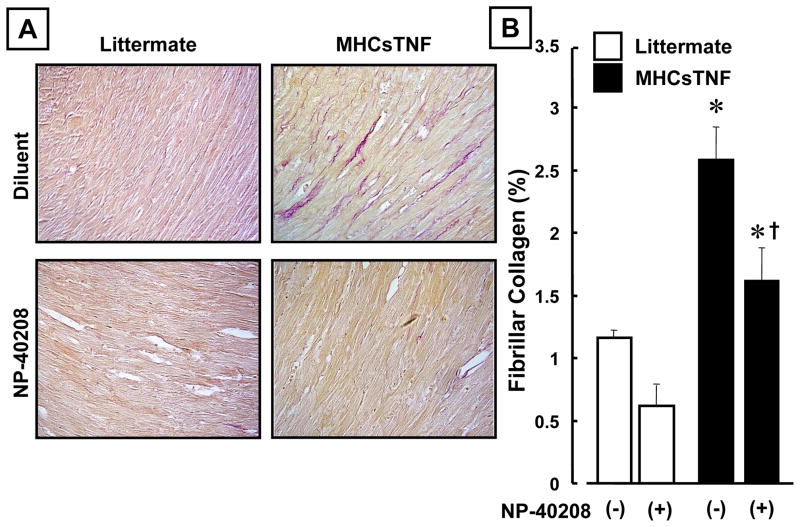
We have shown that myocardial fibrosis develops in the hearts of the MHCsTNF mice from 8–12 weeks of age.1 Treatment with NP-40208 attenuated the increase in myocardial collagen content in the MHCsTNF mice at 12 weeks of age (Figure 3A). The group data summarized in Figure 3B, show that treatment with NP-40208 significantly (p=0.007) attenuated the myocardial collagen content in the MHCsTNF mice when comparison to diluent treated MHCsTNF mice. However, the myocardial collagen content in the NP-40208 treated mice after 12 weeks of age was still significantly greater (p=0.014) than that obserbed in the littermate control mice at a comparable time point. Treatment with NP-40208 for 8 weeks resulted in a small, but non-significant decrease in myocardial collagen content in the littermate control mice.
Figure 3.
Effect of TβRI antagonism on myocardial fibrillar collagen content in littermate control and MHCsTNF mice. Panel A: Representative picrosirius red staining of myocardial samples from diluent treated littermate control, NP-40208 treated littermate control, diluent treated MHCsTNF, and NP-40208 treated MHCsTNF mice. Panel B: Results of group data for fibrillar collagen content (expressed as percentage of myocardial area) in diluent treated littermate control (n=5), NP-40208 treated littermate control (n=5), diluent treated MHCsTNF (n=8) mice, and NP-40208 treated MHCsTNF (n=7) mice. (Key * p < 0.05 vs. diluent and NP-40208 treated littermate controls, † p < 0.05 vs. diluent treated MHCsTNF mice).
Myocardial Function and Stiffness
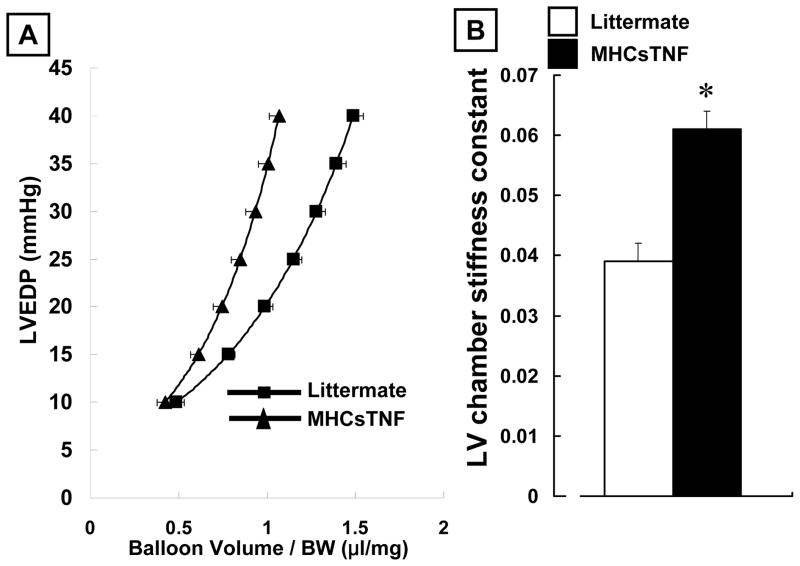
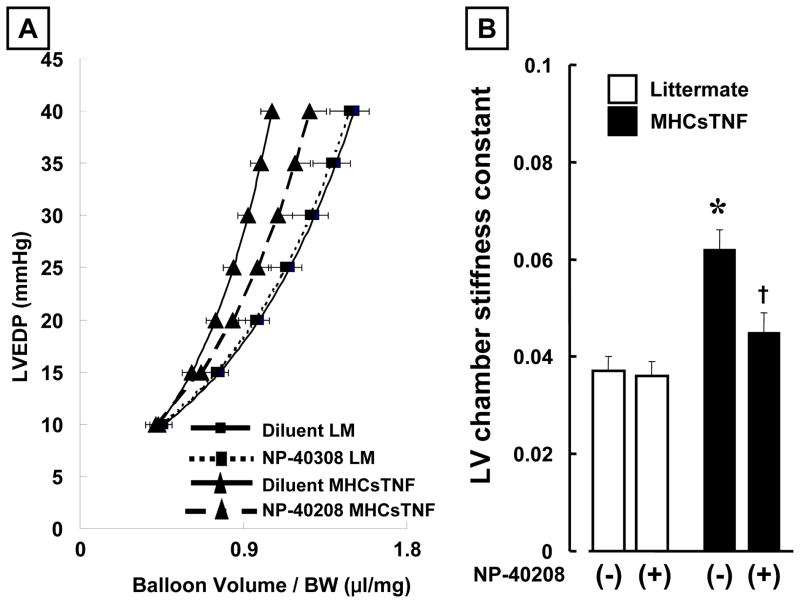
As shown in the Table, there was no significant difference in LV systolic pressure, LVEDP, nor LV developed pressure (LVDP) in the littermate control and MHCsTNF mice at baseline. Moreover, treatment with NP-40208 had no effect on LV systolic pressure, LVEDP, nor LV developed pressure (LVDP) in the littermate control and MHCsTNF mice. In contrast, Figure 4 shows that there was a significant increase in diastolic chamber stiffness in MHCsTNF hearts when compared to littermate control hearts, consistent with the increased interstitial myocardial collagen deposition observed in these mice. As illustrated, there was a significant (p < 0.05) leftward shift in the pressure-volume relationship (Figure 4A), and a significant (p < 0.05) increase in the LV chamber stiffness constant (Figure 4B) in the MHCsTNF hearts, when compared to littermate control mice. Consistent with the effects of NP-40208 on myocardial collagen content, treatment with NP-40208 resulted in a rightward shift in the pressure-volume curve in the MHCsTNF mouse hearts when compared to untreated MHCsTNF hearts (Figure 5A), as well as a significant reduction (p < 0.05) in the LV chamber stiffness constant (Figure 5B). In comparison, treatment with NP-40208 had no effect on the pressure-volume relationship (Figure 5A), nor the chamber stiffness constant in the littermate control mice (Figure 5B). Importantly, the values for LV chamber stiffness in the NP-40208 treated MHCsTNF mice were not significantly different from those observed in treated and untreated littermate control mice (Figure 5B).
Table.
Functional Parameters in Littermate Control and MHCsTNF Hearts
| Littermate | MHCsTNF | p-value | |||
|---|---|---|---|---|---|
| Untreated (n=7) | Treated (n=6) | Untreated (n=7) | Treated (n=6) | ||
| Systolic LVP (mmHg) | 94.8 ± 2.4 | 98.8 ± 3.2 | 100.6 ± 2.4 | 100.2 ± 2.6 | 0.46 |
| LVEDP (mmHg) | 9.8 ±0.4 | 9.5 ± 0.7 | 8.6 ± 0.5 | 9.8 ± 0.3 | 0.23 |
| LVDP (mmHg) | 85.0 ± 2.4 | 8 9.1 ± 3.8 | 93.0 ± 3.0 | 90.4 ± 2.4 | 0.24 |
Key: LVP = left ventricular pressure, LVEDP = left ventricular end-diastolic pressure, LVDP = left ventricular developed pressure.
Figure 4.
Diastolic pressure volume relationship and LV chamber stiffness in littermate control and MHCsTNF mice. Panel A: Diastolic pressure-volume relationship for littermate control and MHCsTNF mice. Panel B: Results of group data for LV chamber stiffness constant in littermate control (n=10) and MHCsTNF (n=10) mice. (Key: LM = Littermate; * p<0.05 vs. diluent treated littermate controls; † p < 0.05 vs. diluent treated MHCsTNF mice)
Figure 5.
Effect of TβIR antagonism on diastolic pressure volume relationship and LV chamber stiffness in littermate control and MHCsTNF mice. Panel A: Diastolic pressure-volume relationship for diluent treated littermate control (n=7 hearts), NP-40208 treated littermate controls (n= 6 hearts), diluent treated MHCsTNF (n=7 hearts), and NP-40208 treated MHCsTNF (n=6 hearts) mice. Panel B: Results of group data for chamber stiffness constant in diluent treated littermate control (n=7 hearts), NP-40208 treated littermate control (n=6), diluent treated MHCsTNF (n=7), and NP-40208 treated MHCsTNF (n=6) hearts. (Key: LM = littermate; *p <0.05 vs. diluent and NP-40208 treated littermate controls, † p < 0.05 vs. diluent treated MHCsTNF mice).
Fetal Gene Expression
As shown in Figure 6, Serca2a and α-MHC mRNA expression were both reduced, and the expression of β-MHC was increased in MHCsTNF mice when compared to littermate controls. Treatment with NP-40208 had no effect on the level of expression of Serca2a, α-MHC and β-MHC mRNA in the MHCsTNF mice.
Figure 6.

Fetal gene expression in littermate control and MHCsTNF mice. Representative Northern blots for Serca2a, alpha-myosin heavy chain (α-MHC) and beta-myosin heavy chain (β-MHC) in diluent treated littermate control, NP-40208 treated littermate control, diluent treated MHCsTNF mice and NP-40208 treated MHCsTNF mice.
DISCUSSION
The results of this study suggest that the TGF-β mediated signaling contributes to LV structural remodeling (fibrosis) and diastolic dysfunction in mice with cardiac-restricted overexpression of TNF (MHCsTNF). The following three lines of evidence support this statement. First, we observed increased nuclear localization of Smad 2/3 protein in the MHCsTNF mice when compared to littermate control mice, consistent with TGF-β induced Smad 2/3 activation (Figure 1). Importantly, treatment with NP-40208, a selective TβR1 antagonist, normalized the nuclear to cytoplasmic ratio of Smad2/3 when compared to littermate control mice (Figure 1); however, it bears emphasis that the absolute level of nuclear Smad2/3protein was still significantly greater in the NP-40208 mice MHCsTNF when compared to diluent treated littermate controls, raising the possibility of TGF-β-independent Smad activation in the MHCsTNF, as has been reported recently for angiotensin II (reviewed in 19). In this regard, we have reported previously that there is increased activation of the rein angiotensin system, as well as increased angiotensin II peptide levels in the hearts of the MHCsTNF mice as they age.20 That said, we cannot exclude the formal possibility that increased dosage and/or duration of treatment with NP-40208 might have further attenuated nuclear localization of Smad 2/3 in the MHCsTNF mice. Second, we observed that the increased myocardial fibrosis observed in the MHCsTNF mice was sensitive to inhibition with NP-40208 (Figure 3). Treatment with NP-40208 resulted in a significant attenuation of myocardial fibrosis in the MHCsTNF mouse hearts when compared to diluent treated MHCsTNF mice. However, the level of myocardial fibrosis in the NP-40208 treated mice was still significantly greater than that observed in diluent treated littermate control mice, consistent with the observation that Smad 2/3 nuclear translocation was not completely abrogated by NP-40208. Treatment with NP-40208 resulted in a small but significant decrease in the heart-weight to body-weight ratio of the NP-40208 treated MHCsTNF mice compared to diluent treated MHCsTNF mice (Figure 2). Nonetheless, the decrease the heart-weight to body-weight ratio was relatively modest (~ 10%) compared to the more robust (~ 40%) decrease in myocardial collagen in the NP-40208 treated MHCsTNF, suggesting that the cardiac hypertrophy observed in the MHCsTNF mice was not due to increased myocardial fibrosis alone. Finally, treatment of the MHCsTNF mice with NP-40208 for 8 weeks prevented the leftward shift of the pressure-volume relationship, as well as the increased LV chamber stiffness observed in diluent treated MHCsTNF mice (Figure 5). Indeed the values for LV chamber stiffness in the NP-40208 treated MHCsTNF mice were not different from those observed in diluent treated littermate control mice. Taken together, these observations suggest that sustained pro-inflammatory signaling in the adult heart is associated with a pro-fibrotic phenotype that arises, at least in part, from TβRI mediated signaling, with resultant activation of Smad 2/3, leading to increased myocardial fibrosis and increased LV diastolic chamber stiffness. Interestingly, treatment with NP-40208 had no discernable effect on expression of the fetal gene pattern observed in the MHCsTNF mice (Figure 6), suggesting that the major pathophysiological role of TGF-β signaling in the MHCsTNF mice involves remodeling of the extracellular matrix.
TGF–β Signaling and Myocardial Fibrosis
TGF-β provokes a classical set of transcriptional responses that define the “profibrotic” phenotype, including increased myofibroblast transformation, increased collagen synthesis and decreased collagen degradation.21 Indeed several studies have demonstrated that TGF-β promotes collagen synthesis and/or deposition in cultured cardiac fibroblasts in vitro.22–24 Moreover, TGF-β antagonism with neutralizing antibodies, soluble TGF-β receptors, and over expression decorin or dominant negative TGF-β receptors has resulted in attenuation of tissue fibrosis can be attenuated in a variety of different tissues including the heart.25–27 Further, expression of Smad7, an endogenous inhibitor of TGF-β induced signaling, attenuates experimentally induced lung and kidney fibrosis.28,29 Taken together, these observations suggest that sustained activation of TGF-β can lead to pathological tissue fibrosis and/or tissue dysfunction in a variety of organs, including the heart. The results of the present study both complement and extend these previous studies by showing for the first time that an orally available TβR1 antagonist, NP-40208, is sufficient to attenuate myocardial fibrosis, as well as decrease myocardial stiffness in the adult heart. Further, the present study provides provisional evidence that increased Smad 3 signaling is responsible for the increased myocardial fibrosis observed in the MHCsTNF mouse hearts, insofar treatment with NP-40208 resulted in a significant attenuation of Smad 2/3 nuclear translocation in the MHCsTNF mice. This statement notwithstanding, we cannot exclude a potential contributory role for Smad independent pro-fibrotic mechanisms, including activation of extracellular signal-regulated kinase,30 the Rho/Rho-kinase signaling pathway,31 and connective tissue growth factor. 32,33
Although TGF-β mediated signaling has been shown to be important in myocardial fibrosis, it is worth noting a recent study in transgenic mice with cardiac restricted overexpression of a constitutively active TGF-β1 mutant, which showed that the expression of this constitutively active mutant did lead to the development of ventricular fibrosis.34 Moreover, fibrosis was not observed in hearts engrafted with skeletal myoblasts expressing high levels of TGF-β1.35 While these latter two observations do not necessarily obviate a potentially important role for TGF-β as a mediator of cardiac fibrosis, they do suggest that TGF-β per se may be insufficient to promote fibrosis in the adult heart, and that the profibrotic actions of TGF-β may be the result of important time-dependent interactions between TGF-β and the complex milieu of growth factors and inflammatory cytokines (TNF, IL-1β) that are expressed following tissue injury. Germane to the present discussion, we have shown previously that the myocardial fibrosis observed in the MHCsTNF mice is sensitive to treatment with losartan, an angiotensin type 1 receptor antagonist.20 These latter observations are consistent with the point of view that TGF-β may be one of several important mediators that act in concert during the development the progressive myocardial fibrosis that occurs in the setting of sustained pro-inflammatory signaling.
In summary, this study suggests that the TGF-β pathway plays an important role in the development of the progressive myocardial fibrosis and LV chamber stiffness that occurs in the setting of sustained pro-inflammatory signaling. The results of the present study also raise the interesting possibility that the TGF-β pathway may represent a therapeutic target in inflammatory cardiomyopathies.
Acknowledgments
The authors would like to acknowledge the technical assistance of Dorellyn Lee and Feng Gao. This research was supported by research funds from Scios, Inc. and the N.I.H. (P50 HL-O6H and RO1 HL58081, and RO1 HL73017).
Reference List
- 1.Sivasubramanian N, Coker ML, Kurrelmeyer K, et al. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;2001:826–831. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 2.Kubota T, McTiernan CF, Frye CS, et al. Dilated cardiomyopathy in transgenic mice with cardiac specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 3.Bryant D, Becker L, Richardson J, et al. Cardiac Failure in transgenic mice with myocardial expression of tumor necrosis factor-α (TNF) Circulation. 1998;97:1375–1381. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 4.Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000;86:1259–1265. doi: 10.1161/01.res.86.12.1259. [DOI] [PubMed] [Google Scholar]
- 5.Peng J, Gurantz D, Tran V, et al. Tumor necrosis factor-alpha-induced AT1 receptor upregulation enhances angiotensin II-mediated cardiac fibroblast responses that favor fibrosis. Circ Res. 2002;91:1119–1126. doi: 10.1161/01.res.0000047090.08299.d5. [DOI] [PubMed] [Google Scholar]
- 6.Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res. 2000;46:214–224. doi: 10.1016/s0008-6363(00)00003-1. [DOI] [PubMed] [Google Scholar]
- 7.Li YY, Feng YQ, Kadokami T, et al. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti- tumor necrosis factor alpha therapy. Proc Natl Acad Sci U S A. 2000;97:12746–12751. doi: 10.1073/pnas.97.23.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurantz D, Cowling RT, Villarreal FJ, et al. Tumor necrosis factor-alpha upregulates angiotensin II type 1 receptors on cardiac fibroblasts. Circ Res. 1999;85:272–279. doi: 10.1161/01.res.85.3.272. [DOI] [PubMed] [Google Scholar]
- 9.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagawa T, Li JH, Garcia G, et al. TGF-beta induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int. 2004;66:605–613. doi: 10.1111/j.1523-1755.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Moody MR, Engel D, et al. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation. 2000;102:1690–1696. doi: 10.1161/01.cir.102.14.1690. [DOI] [PubMed] [Google Scholar]
- 12.Nakano M, Knowlton AA, Yokoyama T, et al. Tumor necrosis factor-α induced expression of heat shock protein 72 in adult feline cardiac myocytes. Am J Physiol. 1996;270:H1231–H1239. doi: 10.1152/ajpheart.1996.270.4.H1231. [DOI] [PubMed] [Google Scholar]
- 13.Sakata Y, Dong JW, Vallejo JG, et al. Toll-like receptor 2 modulates left ventricular function following ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H503–H509. doi: 10.1152/ajpheart.00642.2006. [DOI] [PubMed] [Google Scholar]
- 14.Hilfiker-Kleiner D, Hilfiker A, Fuchs M, et al. Signal Transducer and Activator of Transcription 3 Is Required for Myocardial Capillary Growth, Control of Interstitial Matrix Deposition, and Heart Protection From Ischemic Injury. Circ Res. 2004 doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- 15.Hilfiker-Kleiner D, Kaminski K, Kaminska A, et al. Regulation of proangiogenic factor CCN1 in cardiac muscle: impact of ischemia, pressure overload, and neurohumoral activation. Circulation. 2004;109:2227–2233. doi: 10.1161/01.CIR.0000127952.90508.9D. [DOI] [PubMed] [Google Scholar]
- 16.Trueblood NA, Xie Z, Communal C, et al. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res. 2001;88:1080–1087. doi: 10.1161/hh1001.090842. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Seta Y, Baumgarten G, et al. Functional significance of hemodynamic overload-induced expression of leukemia-inhibitory factor in the adult mammalian heart. Circulation. 2001;103:1296–1302. doi: 10.1161/01.cir.103.9.1296. [DOI] [PubMed] [Google Scholar]
- 18.Dibbs ZI, Diwan A, Nemoto S, et al. Targeted overexpression of transmembrane tumor necrosis factor provokes a concentric cardiac hypertrophic phenotype. Circulation. 2003;108:1002–1008. doi: 10.1161/01.CIR.0000085203.46621.F4. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, et al. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Flesch M, Hoper A, Dell’Italia L, et al. Activation and functional significance of the renin-angiotensin system in mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2003;108:598–604. doi: 10.1161/01.CIR.0000081768.13378.BF. [DOI] [PubMed] [Google Scholar]
- 21.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1) Mol Genet Metab. 2000;71:418–435. doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- 22.Sigel AV, Centrella M, Eghbali-Webb M. Regulation of proliferative response of cardiac fibroblasts by transforming growth factor-beta 1. J Mol Cell Cardiol. 1996;28:1921–1929. doi: 10.1006/jmcc.1996.0185. [DOI] [PubMed] [Google Scholar]
- 23.Agocha A, Lee HW, Eghbali-Webb M. Hypoxia regulates basal and induced DNA synthesis and collagen type I production in human cardiac fibroblasts: effects of transforming growth factor-beta1, thyroid hormone, angiotensin II and basic fibroblast growth factor. J Mol Cell Cardiol. 1997;29:2233–2244. doi: 10.1006/jmcc.1997.0462. [DOI] [PubMed] [Google Scholar]
- 24.Butt RP, Laurent GJ, Bishop JE. Collagen production and replication by cardiac fibroblasts is enhanced in response to diverse classes of growth factors. Eur J Cell Biol. 1995;68:330–335. [PubMed] [Google Scholar]
- 25.Kuwahara F, Kai H, Tokuda K, et al. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106:130–135. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Wang Y, Hyde DM, et al. Reduction of bleomycin induced lung fibrosis by transforming growth factor beta soluble receptor in hamsters. Thorax. 1999;54:805–812. doi: 10.1136/thx.54.9.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isaka Y, Brees DK, Ikegaya K, et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996;2:418–423. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- 28.Nakao A, Fujii M, Matsumura R, et al. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest. 1999;104:5–11. doi: 10.1172/JCI6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terada Y, Hanada S, Nakao A, et al. Gene transfer of Smad7 using electroporation of adenovirus prevents renal fibrosis in post-obstructed kidney. Kidney Int Suppl. 2002;61 (Suppl 1):94–98. doi: 10.1046/j.1523-1755.2002.0610s1094.x. [DOI] [PubMed] [Google Scholar]
- 30.Finlay GA, Thannickal VJ, Fanburg BL, et al. Transforming growth factor-beta 1-induced activation of the ERK pathway/activator protein-1 in human lung fibroblasts requires the autocrine induction of basic fibroblast growth factor. J Biol Chem. 2000;275:27650–27656. doi: 10.1074/jbc.M000893200. [DOI] [PubMed] [Google Scholar]
- 31.Kataoka C, Egashira K, Inoue S, et al. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 2002;39:245–250. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- 32.Chen MM, Lam A, Abraham JA, et al. CTGF Expression is Induced by TGF- beta in Cardiac Fibroblasts and Cardiac Myocytes: a Potential Role in Heart Fibrosis. J Mol Cell Cardiol. 2000;32:1805–1819. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 33.Ohnishi H, Oka T, Kusachi S, et al. Increased expression of connective tissue growth factor in the infarct zone of experimentally induced myocardial infarction in rats. J Mol Cell Cardiol. 1998;30:2411–2422. doi: 10.1006/jmcc.1998.0799. [DOI] [PubMed] [Google Scholar]
- 34.Long CS, Brown RD. The cardiac fibroblast, another therapeutic target for mending the broken heart? J Mol Cell Cardiol. 2002;34:1273–1278. doi: 10.1006/jmcc.2002.2090. [DOI] [PubMed] [Google Scholar]
- 35.Koh GY, Kim SJ, Klug MG, et al. Targeted expression of transforming growth factor-beta 1 in intracardiac grafts promotes vascular endothelial cell DNA synthesis. J Clin Invest. 1995;95:114–121. doi: 10.1172/JCI117627. [DOI] [PMC free article] [PubMed] [Google Scholar]