1. Introduction
A body of literature suggests that individuals with schizophrenia have deficits both in facial emotion recognition (for a recent review, see Kohler et al., 2010) and context processing (MacDonald et al., 2005a; Servan-Schreiber et al.,1996). Recently, there is evidence suggesting that context information can affect facial emotion recognition (Aviezer et al., 2009; Kim et al., 2004). Thus, individuals with schizophrenia may have deficits in facial emotion processing, at least in part, due to impairments in processing context information, as suggested by Green et al. (2007). In the current study, we designed an experimental paradigm to examine the influence of both emotional context and orienting attention to emotional context on facial emotion processing in schizophrenia.
Perceiving emotion displayed on faces is a key component of social interactions (Anthony, 1978; Charlesworth, 1982; Izard, 1971). Despite the extensive literature suggesting poor facial emotion recognition in schizophrenia (Addington & Addington, 1998; Baudouin et al., 2002; Fusar-Poli et al., 2009; Norton et al., 2009; Russell et al., 2003; Tremeau, 2006), the underlying mechanisms that lead to deficits of facial emotion recognition are not yet clear. One important factor could be the impact of context information. Although the majority of previous facial processing studies did not address the role of context on facial emotion recognition, presenting face stimuli in isolation (reviewed in Kohler et al., 2010), converging evidence suggests the strong influence of context on facial emotion recognition in a top-down manner (Bar, 2004; Barrett & Kensinger, 2010).
In the schizophrenia literature, it has been suggested that context processing is a core impairment that underlies multiple domains of cognitive impairments in schizophrenia (Cohen et al., 1999; Phillips & Silverstein, 2003). Given the evidence suggesting modulation of context information on facial emotion recognition in healthy participants (Bar, 2004), it is possible that aberrant or ineffective context processing could exacerbate impairments in facial emotion recognition in schizophrenia. However, there are only a few studies that directly examined the effect of context on facial emotion recognition in schizophrenia. For example, Huang et al. (2009) examined the effect of social context on the categorization of happy and angry facial expressions by presenting dyadic conversations containing different social contexts (e.g. praise). They found that the categorization of the faces in schizophrenia was less altered as a function of social context compared to healthy controls. Monkul et al. (2007) found that patients with schizophrenia showed less enhancement in emotional intensity judgments of sad and happy faces compared with a healthy control group. Taken together, these studies suggest that individuals with schizophrenia may have difficulty with integrating and processing context information, at least for judgments of some types of emotional expressions.
One important question that arises from previous studies of context effects on emotional face recognition is whether individuals with schizophrenia would be able to use such context information if they were provided with enhanced support for attending to and/or integrating such information. Recent studies suggested that individuals with schizophrenia can benefit from the provision of enhanced support for deep semantic processing of non-social stimuli, such as words (Bonner-Jackson et al., 2005, 2007, 2008). As such, it is conceivable that individuals with schizophrenia could process context information more effectively if they were oriented to attend to or process the context information.
The purpose of the current study was to test the following hypotheses: 1) individuals with schizophrenia will show impairments in the influence of emotional context on facial emotion ratings; 2) deficits on a non-emotion/social context processing task will predict deficits in the influence of context on facial emotion ratings in schizophrenia; 3) orienting individuals with schizophrenia to attend to the context information will increase the influence of context on facial emotion ratings in schizophrenia. To test these hypotheses, we administered a novel test of context influences on facial emotion ratings, along with a validated measure of non-social context processing, the Dot Probe Expectancy Task (MacDonald et al., 2005b) to a group of individuals with schizophrenia and a group of demographically similar controls.
2. Methods
2.1. Participants
Thirty-five medicated outpatients with DSM-IV-TR schizophrenia (SCZ) or schizoaffective disorder (n =3) and thirty-two matched healthy controls (CON) were included. Potential CON were excluded if they had current, past, or family history of Axis I psychotic disorder, including bipolar disorder or if they had depression with the past year. All participants were recruited through the Conte Center for the Neuroscience of Mental Disorders at Washington University. The exclusion criteria for participants in either SCZ or CON groups were the same as our prior work (Chung et al., 2010) (see Supplementary Methods for details). The groups were matched in terms of age, gender and parental education (see Table 1). However, personal education in CON was significantly higher than in SCZ [t (64) = 3.56, p = .001]. All participants signed a written informed consent prior to participation in the study. All experimental procedures were approved by the Washington University Institutional Review Board (IRB).
Table 1.
Clinical and Demographic Characteristics
| Schizophrenia (n = 35) | Healthy Controls (n = 32) | t or χ2 | p | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Age (years) | 37.03 (8.12) | 34.91 (8.95) | −1.02 | .31 |
| Sex (% male) | 54.3% | 56.3% | .03 | .87 |
| Race (% Caucasian) | 28.6% | 53.1% | 9.58 | .21 |
| Education | 13.06 (2.40) | 15.16 (2.40) | 3.56 | .001 |
| Parental education (years) | 14.00 (3.80) | 14.40 (2.62) | .48 | .63 |
| Medication (% Taking) | ||||
| Antipsychotics (%) | 94.29% | N/A | ||
| Typical antipsychotics | 8.57% | N/A | ||
| Atypical antipsychotics | 80% | N/A | ||
| Both typical and atypicals | 5.71% | N/A | ||
| SANS Global Anhedonia/Asociality | 1.29 (1.36) | |||
| Positive Symptoms | 2.69 (3.37) | N/A | ||
| Negative Symptoms | 5.49 (3.14) | N/A | ||
| Disorganization Symptoms | 2.03(1.84) | |||
Note. Positive symptoms consisted of the mean of the global scores for hallucinations and delusions; Negative symptoms consisted of the mean of the global scores for anhedonia/asociality, avolition/apathy, alogia, and affective flattening; Disorganization symptoms consisted of the mean of the global scores for inappropriate affect, positive formal thought disorder, and bizarre behavior.
Abbreviations: CON, healthy controls; SCZ, individuals with schizophrenia; SANS, scale for the assessment of negative symptoms
2.2. Diagnosis and Clinical Assessment
Clinical symptoms in individuals with SCZ were assessed with the Scales for the Assessment of Negative Symptoms [SANS; Andreasen (1983a)] and Positive Symptoms [SAPS; Andreasen (1983b)]. Clinical ratings were completed by one of two doctoral students in clinical psychology with the time scale of the past week. To measure the severity of clinical symptoms, we used negative, positive, and disorganized symptom summary scores from the SAPS and SANS (see Supplementary Methods for reliability information and details of clinical symptoms).
2.3. Measures
2.3.1. The Emotional Context Processing Task (ECPT)
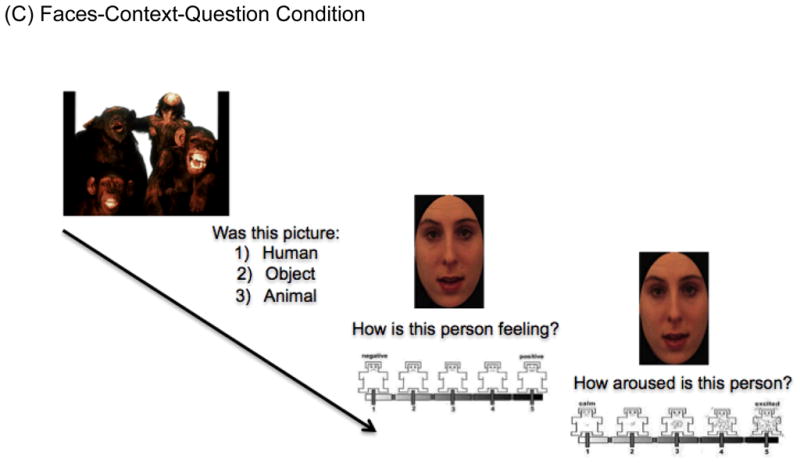
We designed a novel experimental task, ECPT using E-prime, inspired by the paradigm developed by Mobbs et al. (2006) (see Figure 1 note for details). The ECPT consisted of three conditions, the Faces-Only (FO), Faces-Context (FC), and Faces-Context-Question (FCQ) (30 trials each). While participants were presented only with morphed surprised faces in isolation in the FO condition, in the FC and FCQ conditions, each face was preceded by a positive, neutral or negative picture (10 for each). The goal of the FCQ condition was to determine whether enhancing attention to the picture would increase the influence of context on facial emotion ratings, by asking participants to answer a question about each emotional context picture (see Figure 1).
Figure 1. Example of the Emotional Context Processing Task.
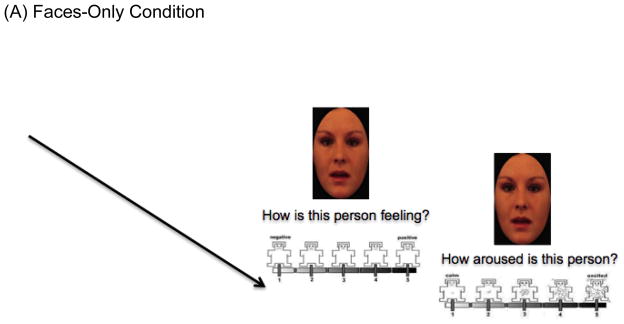
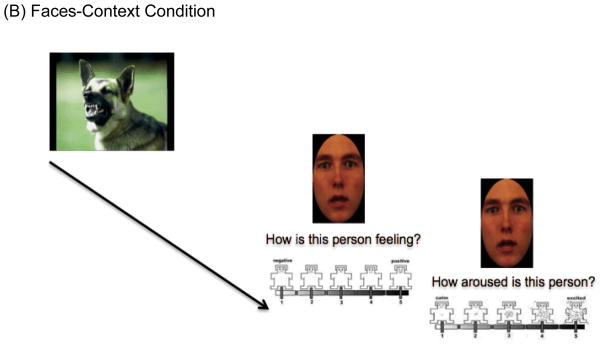
(A) Thirty-three faces from the Karolinska Directed Emotional Faces database [KDEF; Calvo & Lundqvist, 2008] and six faces from NimStim Set of Facial Expressions (Tottenham et al., 2009) were used as face stimuli. We generated morphs between neutral and surprised faces using the Morph Age 4.0.7 program. The same faces were used in all three conditions, and the order of task condition presentation was counterbalanced across participants. In every condition, the same valence and arousal questions were presented to participants with a five-point SAM (Bradley & Lang, 1994). During each of these ratings, the face stayed on the screen to reduce working memory load for SCZ group.
(B) Each face and emotional picture (10 positive, 10 neutral, 10 negative) taken from the International Affective Picture System (IAPS; Lang, 1999) were presented for 1500ms, respectively. Among sixty IAPS images, half of the pictures were used for the Faces-Context condition and half were used for the Faces-Context-Question condition with the pictures used for one conditions versus the other counterbalanced across subjects. Prior to starting, we provided the same instructions used in Mobbs et al. (2006) saying faces were captured using a camera when people were seeing different pictures (See Supplemental Materials for more details including valence and arousal ratings by both two groups).
(C) Participants were shown the contextual pictures prior to the faces, but were asked to answer a question about the contextual picture.
2.3.2. The Dot Pattern Expectancy Task (DPX)
To assess non-social/emotional context processing ability, we included the dot version of the AX-Continuous Performance Task (AX-CPT; Servan-Schreiber et al., 1996), the DPX task. Each dot pattern in this task refers to certain types of trials used in the AX-CPT (e.g., AX, BX). Participants are presented with pairs of cues and probes, and are asked to respond target when an “X” probe is presented, but only if it follows and “A” cue. As such, performance on this task depends on the representation and maintenance of context provided by the cue (A or not A) (see Supplemental Materials for additional DPX details). Performance of this task was measured by d’-context, error rates, response time (RT) for correct response. D’-context data was used in the main analysis, and the results of the other measures were reported in Supplementary Methods.
2.4. Data Analysis
To examine the effect of emotional context on facial emotion recognition, we conducted a repeated measures ANOVA with a group (CON, SCZ) as a between-subjects factor and context type (positive, negative, neutral context) as a within-subject factor. For the FCQ condition, we performed an analogous ANOVA with a group (CON, SCZ) as a between-subject factor and context type (positive, negative, neutral context), and the question (question, no-question) as within-subject factors. We also conducted follow-up analyses for the FC and FCQ conditions in which order (1st, 2nd, 3rd) was included as an additional between-subject factor (see Results in Supplementary Materials).
For the DPX, we used an independent samples t-test to compare d’-context across groups. In addition, we conducted Pearson’s correlation analysis within each group to examine whether individuals differences in non-social context processing were associated with altered valence scores in either the FC or the FCQ condition. In the correlation analysis, we used d’-context and percent change scores in the positive and negative context of the FC and FCQ conditions (See note in Table 2 for details).
Table 2.
Correlations Between d’ from the DPX and Percent Change Scores from the Emotional Context Processing Task.
| Percent Change Scores (from FO condition) | Control (n = 30) | Schizophrenia (n = 30) | Correlation Comparison Across Groups |
|---|---|---|---|
| Negative FC | .15 | −.16 | Z = 1.15 |
| Positive FC | −.29 | −.02 | Z =−1.01 |
| Negative FCQ | .09 | −.36* | Z = 1.74 |
| Positive FCQ | −.05 | −.27 | Z = 0.84 |
Note. We excluded outlier data or cases showing extremely poor performance level (see Supplemental Materials). As such, the total number of participants included in the analysis of DPX was 31 out of 35 in SCZ group and 30 out of 32 in CON group. For the ECPT, the total number of participants was 33 out of 35 for SCZ and 32 for CON. Persons who completed both DPX and ECPT were 30 for each group; To calculate change scores, valence scores in the FO condition were subtracted from valence scores in the positive or negative context or context + question condition, which were divided by valence scores in the no context condition. For example, for the negative context only condition, the change score was ((FC Negative Valence – FO Valence)/FO Valence)*100); FO, Faces Only condition; FC, Faces-Context condition; FCQ, Faces-Context-Question condition; SCZ, individuals with schizophrenia; CON, healthy controls.
p < .05
3. Results
3.1. Does Emotional Context Alter Recognition of Faces?
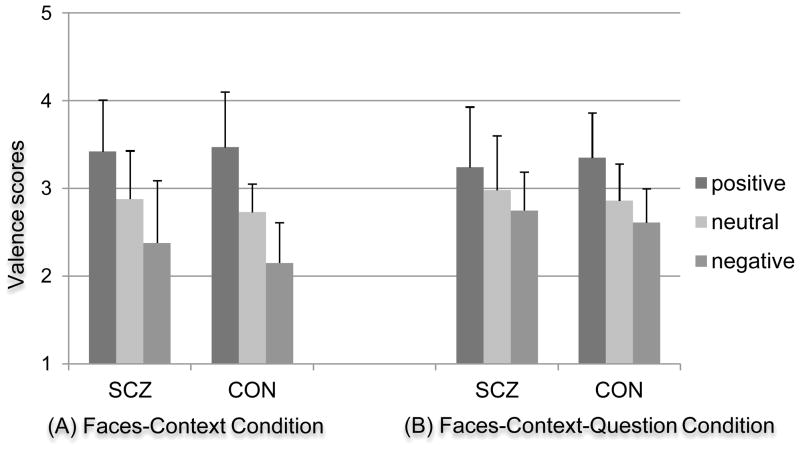
The valence ANOVA for the FC condition revealed a significant main effect of context [F (2, 126) = 78.62, p < .001, ηp2= .56], but neither a significant main effect of group [F (1, 63) = 1.71, p = .20 .05, ηp2 = .03] nor a significant group by context interaction [F (2, 126) = 1.18, p = .31, ηp2 = .02]. As shown in Figure 2(a), both groups showed altered valence scores as a function of context. Post hoc comparisons indicated that valence scores were significantly different between the positive and neutral context conditions [F (1, 63) = 69.68, p < .001, ηp2 = .53] and between the neutral and negative context conditions [F (1, 63) = 52.16, p < .001, ηp2 = .45]. In addition, a repeated measures ANOVA comparing the neutral FC condition to the FO condition, with group as a between subject factor indicated no significant main effect of condition [F (1, 63) = 2.79, p = .10, ηp2 = .04], group [F (1, 63) = 1.80, p = .18, ηp2 = .03] or group x condition interaction [F (1, 63) = .23, p = .64, ηp2 = .00]. Thus, the rating of the surprised faces were similar between CON and SCZ in the no-context and the neutral context conditions, but were altered by the positive and negative context conditions.
Figure 2. Valence Scores As a Function of Emotional Context.
Note. Valence scores ranging from one (negative) to five (positive) valence scores.
Abbreviations: SCZ, individuals with schizophrenia; CON, healthy controls.
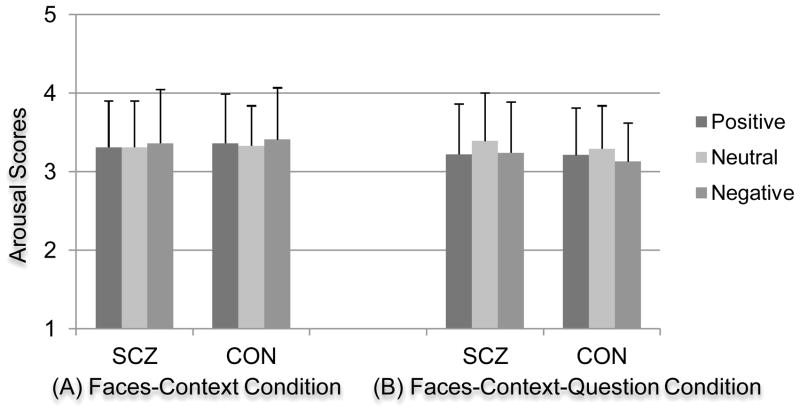
The arousal ANOVA for the FC condition revealed no significant main effect of context [F (2, 126) = .48, p = .62, ηp2 = .01] or group [F (1, 63) = .09, p = .76, ηp2 = .00], and no significant group by context interaction [F (2, 126) = .03, p = .97, ηp2 = .00]. Neither group showed an effect of emotional context on arousal scores (see Figure 3(a)).
Figure 3. Arousal Scores As a Function of Emotional Context.
Abbreviations: SCZ, individuals with schizophrenia; CON, healthy controls.
3.2. Does orienting attention to emotional context lead to better facial emotion ratings?
As presented in Figure 2(b), the ANOVA that included a within subjects question factor revealed a significant main effect of context [F (2,126) = 80.10, p < .001, ηp2= .56] and a significant main effect of the attention-orienting question [F (1,63) = 8.37, p < .01, ηp2= .12], which were modified by a significant interaction of context by question [F (2,126) =17.63, p < .001, ηp2= .22]. However, neither the interaction of group × question [F (1, 63)= .46, p = .50, ηp2= .01], group × context [F (2,126) = 2.03, p = .14, ηp2= .03], or the interaction of group × context × question [F (2,126) = .06, p = .94, ηp2= .00] were significant. The simple effects test to determine the source of the context by question interaction indicated that for both groups, including an attention-orienting question significantly reduced the effect of negative context on valence scores [F (1, 63) = 40.13, p < .001, ηp2= .39], but did not reduce the effects of positive and neutral contexts, respectively [F (1, 63) = 3.29, p = .08, ηp2= .05; F (1, 63) = 3.57, p = .06, ηp2= .05].
For the arousal scores of the ECPT, the ANOVA that included a within-subjects question factor revealed a significant main effect of question [F (1,63) = 15.80, p < .001, ηp2= .20]. However, there were no significant main effects of context [F (2, 126) = .93, p = .40, ηp2= .01] or group [F (1, 63) = .02, p =.90, ηp2= .00], and no significant interactions of context by group [F (2,126) = 1.37, p = .26, ηp2= .02], context by question [F (2, 126) = 1.48, p = .23, ηp2= .02] or context by question by group [F (2, 126) = .05, p =.95, ηp2= .00] (see Figure 3(b)).
3.3. Non-Social Context Processing Ability: DPX
An independent samples t-test indicated that the SCZ group had significantly lower d’-context (mean = 2.38) than the CON group (mean = 3.23) [t (53.51) = 3.81, p < .01], indicating sensitivity to non-social contextual information in the SCZ group. Errors and RTs for the DPX are presented in the Supplementary Results.
3.4. Does non-social context processing ability predict the influence of emotional context?
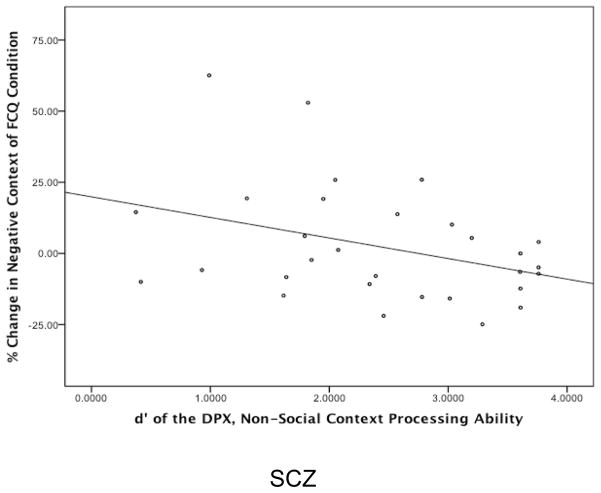
As shown in Table 2, percentage change scores in negative context for SCZ group were negatively correlated with d-’context, but only in the FCQ condition (p < .05). Thus, as shown in Figure 4, individuals with schizophrenia with better context processing rated faces as more negative in the negative context in the FCQ condition. However, percent change scores in the positive context condition was not significantly correlated with d’-context for either the FC or FCQ conditions. In contrast to the SCZ, the CON did not show any significant correlations between d’-context from the DPX and change scores in any emotional context condition (see Table 3). See Supplemental Results for analyses of the relationships of facial emotion processing and context processing to clinical symptoms.
Figure 4. The Relationship Between Non-Social Context Processing and Altered Valence Scores in the Negative Context Condition.
Abbreviations: SCZ, individuals with schizophrenia; DPX, Dot Patterns Expectancy Task; FCQ, Face Context Question condition of the Emotional Context Processing Task.
4. Discussion
The purpose of this study was to examine the effect of both emotional context and enhanced support for processing emotional context on facial emotion ratings in schizophrenia compared to healthy controls. Contrary to our expectations, we found that individuals with schizophrenia showed an intact influence of context information on facial emotion ratings. However, in schizophrenia, better processing of non-social context was associated with a stronger influence of context on valence ratings of faces in the negative context condition. These results suggest that in schizophrenia, similar mechanisms may influence the process of context for both social and non-social information.
We found that pairing surprised faces with emotional context pictures led to altered valence ratings of the faces as a function of context, with no significant group differences. These results are not consistent with previous studies suggesting deficits in processing social context information on various facial emotion recognition (Huang et al., 2009; Monkul et al., 2007) and other social cognitive processing tasks among individuals with schizophrenia (Green et al., 2007, 2008). However, one major difference between the current study and previous studies was the type of context information used. While we presented static IAPS pictures including both social and non-social information separately from the faces, many previous studies presented the emotional cue directly in either more dynamic social information or more social interactive contexts [e.g., famous baseball player was reaching out a crying boy in the audience from (Monkul et al., 2007)]. In these studies, individuals with SCZ showed an impaired ability to use the social context to interpret the target person’s emotion and/or intention (Green et al., 2008; Penn et al., 2002). These findings may indicate that impairments in facial emotion recognition for schizophrenia may result, in part, from impairments in the processing of social interactive cues, and not necessarily from the processing of the emotional content of context information.
Furthermore, we also examined whether the provision of enhanced support for allocating attention to emotional context would strengthen the influence of context on facial emotion ratings in schizophrenia. Contrary to our expectations, the attention-orienting question reduced the effect of emotional context on both valence and arousal ratings of faces for both two groups, particularly in the negative context condition. Making a categorical judgment about the emotional context picture may have triggered emotion-cognition interactions for both groups. Prior studies have found that cognitive task performance reduced responses to emotional stimuli similar to those used in the current study (Blair et al., 2007; Kellermann et al., 2011; Pauly et al., 2008). In addition, the presence of emotional pictures also disrupts cognitive performance as well (Blair et al., 2007; Pauly et al., 2008).
We also investigated whether individual differences in non-social/emotional context processing ability would be related to the influence of emotional context on facial emotion processing. Consistent with prior research, we found that as a group, individuals with schizophrenia were impaired in the use of context information on the DPX. Further, the individuals with schizophrenia having better non-social/emotional context processing ability were more influenced by negative context in facial valence ratings, while the controls did not show any significant relationship between non-social and emotional context processing (see Table 2). Interestingly, however, this relationship was significant only in the FCQ, attention-allocating condition, in which the influence of context on facial emotion ratings in both groups was actually reduced. In other words, individuals with schizophrenia having the best non-social context processing showed the least reduction in emotion context effects in the FCQ condition, at least for the negative context. We found no significant association between non-social and positive emotion context processing. The finding of an association between non-social and emotional context processing for schizophrenia is in the line with our previous research (Chung et al., 2010), which showed that individuals with schizophrenia who had better non-social/emotional context processing abilities were also better at judging the mental state of an individual. Together, the findings of the current and past researches suggest that at least among individuals with schizophrenia, non-social/emotional context processing abilities may contribute to at least some aspects of social cognitive function in this illness.
The current study also had several limitations. First, it is possible that antipsychotic medications may have improved facial emotion recognition ability for schizophrenia, which may have precluded us from finding group differences in the influence of emotional context on face processing. However, many prior studies have found facial emotion recognition deficits among medicated individuals with schizophrenia (Herbener et al., 2005; Penn et al., 2009). Second, we cannot rule out a possibility that presenting the same faces in three conditions for the ECPT may have affected facial emotion ratings. However, we did find significant effects of emotional context on face ratings in both groups regardless of the order in which the context condition was presented (1st, 2nd or 3rd), suggesting that this was not a confound. Third, we did not exclude patients with schizophrenia who had comorbid mood or anxiety diagnoses, and it is possible that the presence of such affective symptoms could have influenced face processing among the individuals with schizophrenia.
In summary, the current findings suggest that individuals with schizophrenia have at least some intact influence of context information on emotional face ratings, and that they demonstrate a relationship between better non-social context processing ability and a stronger influence of negative context information on face ratings. In the line with prior research (Chung et al., 2010), these results suggest that facial emotion processing may share similar mechanisms with the processing of non-social context, at least among individuals with schizophrenia.
Supplementary Material
Acknowledgments
Role of funding source
This material is based upon the funding from Department of Psychology in Washington University in St.Louis.
Contributor Information
Yu Sun Chung, Email: yschung@wustl.edu, Washington University in St.Louis, Psychology, Campus box1125, One Brookings Drive, St.Louis, MO 63130, United States, Phone: 314 9358547, Fax: 314 9358790
Deanna M. Barch, Email: dbarch@artsci.wustl.edu, Professor, Washington University, Editor-in-Chief, Cognitive, Affective and Behavioral Neuroscience, Director, Conte Center for the Neuroscience of Mental Illness, Department of Psychology, Psychiatry, and Radiology, Box 1125, One Brookings Drive, St. Louis, MO 63130, Phone: 314-935-8729 or 314-362-2608, Fax: 314-935-8790
References
- Addington J, Addington D. Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophr Res. 1998;32:171–81. doi: 10.1016/s0920-9964(98)00042-5. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City: 1983a. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; Iowa City: 1983b. [Google Scholar]
- Anthony BJ. Piagetian egocentrism, empahty, and affect discrimination in children at high risk for psychosis. In: Anthony EJ, Koupernik C, Chiland C, editors. Child and His Family: Vulnerable Children. Vol. 4. 1978. pp. 359–379. [Google Scholar]
- Aviezer H, Bentin S, Hassin RR, Meschino WS, Kennedy J, Grewal S, Esmail S, Cohen S, Moscovitch M. Not on the face alone: perception of contextualized face expressions in Huntington’s disease. Brain. 2009;132:1633–44. doi: 10.1093/brain/awp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M. Visual objects in context. Nat Rev Neurosci. 2004;5:617–29. doi: 10.1038/nrn1476. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Kensinger EA. Context is routinely encoded during emotion perception. Psychol Sci. 2010;21:595–9. doi: 10.1177/0956797610363547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin JY, Martin F, Tiberghien G, Verlut I, Franck N. Selective attention to facial emotion and identity in schizophrenia. Neuropsychologia. 2002;40:503–511. doi: 10.1016/s0028-3932(01)00114-2. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJ. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–40. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Jackson A, Haut K, Csernansky JG, Barch DM. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biol Psychiatry. 2005;58:47–55. doi: 10.1016/j.biopsych.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Jackson A, Csernansky JG, Barch DM. Levels-of-processing effects in first-degree relatives of individuals with schizophrenia. Biol Psychiatry. 2007;61:1141–7. doi: 10.1016/j.biopsych.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A, Yodkovik N, Csernansky JG, Barch DM. Episodic memory in schizophrenia: the influence of strategy use on behavior and brain activation. Psychiatry Res. 2008;164:1–15. doi: 10.1016/j.pscychresns.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Lundqvist D. Facial expressions of emotion (KDEF): identification under different display-duration conditions. Behav Res Methods. 2008;40:109–15. doi: 10.3758/brm.40.1.109. [DOI] [PubMed] [Google Scholar]
- Charlesworth WR. An ethological approach to research on facial expressions. In: Izard CE, editor. Measuring Emotions in Infants and Children. New York: Cambridge University Press; 1982. pp. 317–334. [Google Scholar]
- Chung YS, Mathews JR, Barch DM. The Effect of Context Processing on Different Aspects of Social Cognition in Schizophrenia. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–33. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–32. [PMC free article] [PubMed] [Google Scholar]
- Green MJ, Waldron JH, Coltheart M. Emotional context processing is impaired in schizophrenia. Cogn Neuropsychiatry. 2007;12:259–80. doi: 10.1080/13546800601051847. [DOI] [PubMed] [Google Scholar]
- Green MJ, Waldron JH, Simpson I, Coltheart M. Visual processing of social context during mental state perception in schizophrenia. J Psychiatry Neurosci. 2008;33:34–42. [PMC free article] [PubMed] [Google Scholar]
- Herbener ES, Hill SK, Marvin RW, Sweeney JA. Effects of antipsychotic treatment on emotion perception deficits in first-episode schizophrenia. Am J Psychiatry. 2005;162:1746–8. doi: 10.1176/appi.ajp.162.9.1746. [DOI] [PubMed] [Google Scholar]
- Huang J, Chan RC, Lu X, Tong Z. Emotion categorization perception in schizophrenia in conversations with different social contexts. Aust N Z J Psychiatry. 2009;43:438–45. doi: 10.1080/00048670902817646. [DOI] [PubMed] [Google Scholar]
- Izard CE. The Face of Emotion. New York: Appleton Century; 1971. [Google Scholar]
- Kellermann TS, Sternkopf MA, Schneider F, Habel U, Turetsky BI, Zilles K, Eickhoff SB. Modulating the processing of emotional stimuli by cognitive demand. Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16:1730–45. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–19. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. International affective picture system (IAPS): Instruction manaul and affective ratings. Gainesville: University of Florida; 1999. [Google Scholar]
- MacDonald AW, 3rd, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Stenger VA, Cohen JD. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005a;162:475–84. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Goghari VM, Hicks BM, Flory JD, Carter CS, Manuck SB. A convergent-divergent approach to context processing, general intellectual functioning, and the genetic liability to schizophrenia. Neuropsychology. 2005b;19:814–21. doi: 10.1037/0894-4105.19.6.814. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Weiskopf N, Lau HC, Featherstone E, Dolan RJ, Frith CD. The Kuleshov Effect: the influence of contextual framing on emotional attributions. Soc Cogn Affect Neurosci. 2006;1:95–106. doi: 10.1093/scan/nsl014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkul ES, Green MJ, Barrett JA, Robinson JL, Velligan DI, Glahn DC. A social cognitive approach to emotional intensity judgment deficits in schizophrenia. Schizophr Res. 2007;94:245–52. doi: 10.1016/j.schres.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Norton D, McBain R, Holt DJ, Ongur D, Chen Y. Association of impaired facial affect recognition with basic facial and visual processing deficits in schizophrenia. Biol Psychiatry. 2009;65:1094–8. doi: 10.1016/j.biopsych.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Pauly K, Seiferth NY, Kellermann T, Backes V, Vloet TD, Shah NJ, Schneider F, Habel U, Kircher TT. Cerebral dysfunctions of emotion-cognition interactions in adolescent-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2008;47:1299–310. doi: 10.1097/CHI.0b013e318184ff16. [DOI] [PubMed] [Google Scholar]
- Penn DL, Ritchie M, Francis J, Combs D, Martin J. Social perception in schizophrenia: the role of context. Psychiatry Res. 2002;109:149–59. doi: 10.1016/s0165-1781(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Penn DL, Keefe RS, Davis SM, Meyer PS, Perkins DO, Losardo D, Lieberman JA. The effects of antipsychotic medications on emotion perception in patients with chronic schizophrenia in the CATIE trial. Schizophr Res. 2009;115:17–23. doi: 10.1016/j.schres.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci. 2003;26:65–82. doi: 10.1017/s0140525x03000025. discussion 82–137. [DOI] [PubMed] [Google Scholar]
- Russell JA, Bachorowski JA, Fernandez-Dols JM. Facial and vocal expressions of emotion. Annu Rev Psychol. 2003;54:329–49. doi: 10.1146/annurev.psych.54.101601.145102. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context. A test of a theoretical model. Arch Gen Psychiatry. 1996;53:1105–12. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremeau F. A review of emotion deficits in schizophrenia. Dialogues Clin Neurosci. 2006;8:59–70. doi: 10.31887/DCNS.2006.8.1/ftremeau. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.