Abstract
The accumulation of psychosine (galactosyl sphingosine) has been associated with the pathogenesis of Krabbe disease, however, the exact mechanism of its cytotoxicity remains unclear. Herein, we describe the synthesis of the unnatural enantiomer of erythrosphingosine, psychosine, and related derivatives thereof that would allow for the mechanistic elucidation of the toxicity of psychosine.
Keywords: carbohydrates, enantiomers, sphingosine, psychosine, glycosylation, glycosphingolipids
Introduction
Psychosine (galactosyl sphingosine) is a natural glycosphingolipid found in a variety of mammalian cells. While it typically exists in very low concentrations, elevated levels of psychosine are linked to the pathogenesis of Globoid Cell Leukodystrophy (GLD) or Krabbe disease.[1, 2] In GLD, the abnormal accumulation of psychosine is due to a genetic deficiency in galactosylceramide-β-galactosidase (also referred to as galactosyl ceramidase) activity, a degradative lysosomal enzyme.[3] Additionally, psychosine is highly cytotoxic,[4] and is known to lead to the death of oligodendrocytes, the myelin producing cells in the nervous system.[5] As a consequence, patients with GLD suffer from a number of neurological deficits and typically die before the age of five.[2] Despite its obvious relevance to the pathogenesis of GLD,[6] very little is understood about the mechanisms underlying psychosine's toxicity. Initially, it was presumed that the interactions between psychosine and specific protein partners were solely responsible for the resultant toxicity.[7] However, more recently it has been demonstrated that the accumulation of psychosine in the CNS may also interfere with membrane lipid raft (LR) function, significantly disrupting cellular signaling processes.[8] As such, it remains unclear which of these general mechanisms is responsible for the toxicity of psychosine, or whether both pathways are involved during different stages of the disease.
In order to resolve this fundamental question, we needed to discriminate between the two plausible mechanisms of toxicity. As such, an investigation of the enantiomer of natural psychosine (ent-psychosine, 1) was seen as a reliable approach whereby the two differing pathways could be distinguished. Due to the high stereochemical specificity of protein interactions, it can be assumed that the ent-psychosine would no longer be able to bind to any partner proteins. If psychosine toxicity is due to protein interactions, ent-psychosine would not exhibit any cytotoxic effect. However, if psychosine's toxicity is mediated through LRs within the cell membrane, the hydrophobic interactions between ent-psychosine and the achiral lipid membrane will be preserved, along with toxicity. Previously, Covey and others have made considerable progress elucidating the mechanisms of various signaling pathways by synthesizing and applying unnatural ent-steroids.[9] Hence, the synthesis of the unnatural ent-psychosine 1 and the investigation of its mode of action appealed to us as an important first step toward the elucidation of the psychosine toxicity pathways in GLD.
In spite of the considerable progress that has been made toward the synthesis of various glycosphingolipids and analogues thereof,[10] there still remains a challenge in obtaining such targets in high yields, with minimal synthetic steps and with complete stereochemical purity. These characteristics are essential since the interpretation of biological toxicity studies often hinge on the differential action of enantiomers. Herein, we report the synthesis of the unnatural ent-psychosine 1 from the chirally pure starting material (Figure 1). Unlike a number of previously reported approaches to the synthesis of enantio- and diastereomeric sphingosine analogues,[11] our synthetic target is derived from a readily available carbohydrate precursor of the L-series containing predetermined stereocenters.
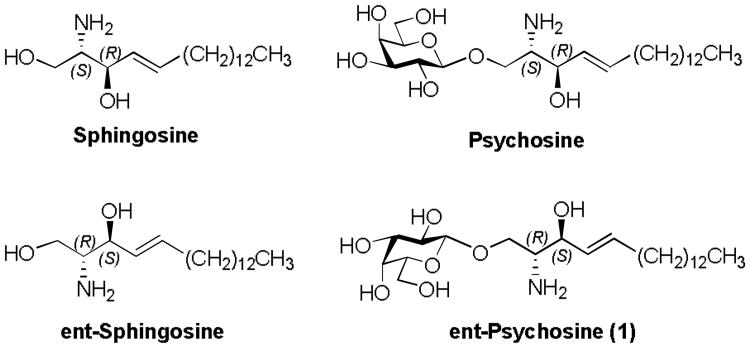
Figure 1. Sphingosine, Psychosine and enantiomers thereof.
Results and Discussion
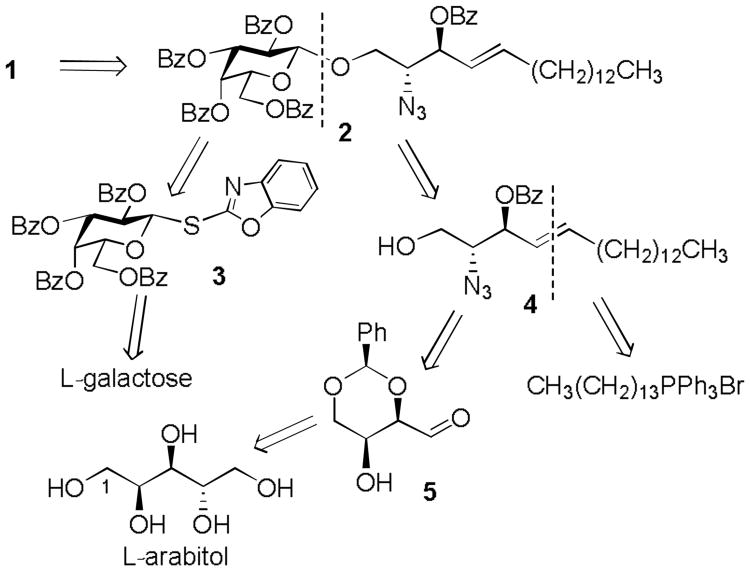
Ent-psychosine 1 is a complex glycosphingolipid that is comprised of the sphingosine enantiomer (ent-sphingosine) and L-galactose moieties (Figure 1). Synthetically, we envisaged that its fully protected precursor 2 could be assembled from a suitable L-galactose donor, such as S-benzoxazolyl (SBox) L-galactoside 3 (Scheme 1), while a partially protected enantiomeric sphingosine derivative 4 could serve as a suitable glycosyl acceptor. Although glycosyl donor 3 could be obtained in three steps from L-galactose, as previously described for the synthesis of its counterpart of the D-galacto series,[12, 13] the synthesis of acceptor 4 required careful retrosynthetic analysis. As depicted in Scheme 1, we decided that the most suitable option would be to base the synthesis on the inexpensive L-arabitol, which contains a predetermined stereochemistry at the C-2 and C-3 carbons that would be appropriate for our synthesis of ent-sphingosine. Subsequent regioselective 1,3-O-benzylidene protection, followed by oxidative cleavage with sodium periodate, could then provide our desired precursor 5.
Scheme 1.
Retrosynthetic analysis of ent-psychosine 1.
To initiate this route, we first synthesized L-galactosyl donor 3 as shown in Scheme 2. Benzoylation of L-galactose resulted in the formation of per-benzoate 6, which was subjected to sequential anomeric bromination and the introduction of the SBox leaving group by the protocol developed in our laboratory for D-sugars (Scheme 2).[12] The resulting glycosyl donor 3 was obtained in 88% overall yield.
Scheme 2.
Synthesis of per-benzoylated SBox L-galactosyl donor 3.
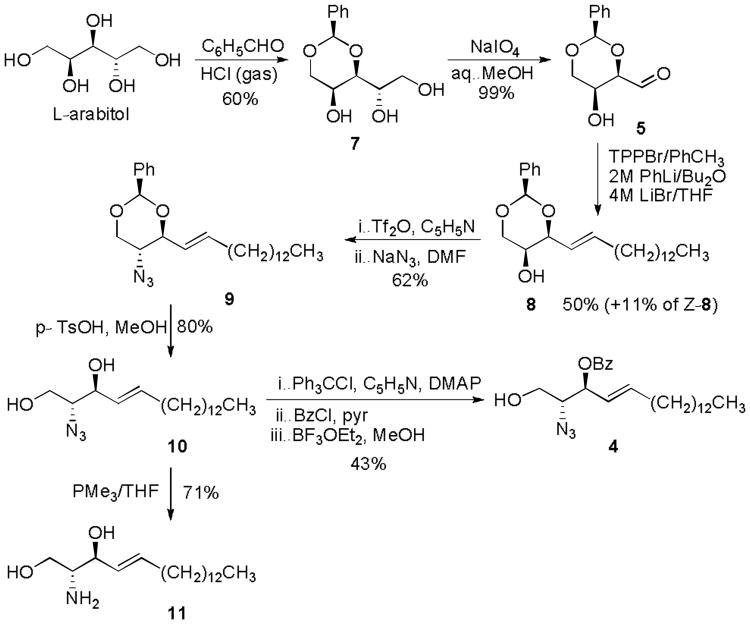
Among a plethora of available approaches,[14] for the synthesis of the ent-sphingosine precursor 4, we adapted a very effective strategy developed by Schmidt et. al., for the synthesis of natural sphingosine from D-arabitol.[15] Accordingly, L-arabitol was first converted to 1,3-O-benzylidene arabitol 7 in 60% yield, using benzaldehyde in the presence of HCl (gas, Scheme 3).[16] Compound 7 was then subjected to oxidative cleavage using sodium periodate. The resulting aldehyde 5, which was found to exist in the dimeric form, (as reported for similar analogous compounds),[17] was obtained in 99% yield. Next, the Wittig olefination of aldehyde 5 was accomplished using (1-tetradecyl)triphenylphosphonium bromide (TPPBr) in the presence of 2M PhLi/Bu2O and 4M LiBr/THF, to yield predominantly the (E)-isomer 8. It was found that the addition of LiBr, can benefit the preferential formation of the E-isomers,[18] and proved essential in obtaining a good isolated yield of 50% for compound 8. Nevertheless, this reaction also yielded the (Z)-isomer of 8 in 11% yield, however the stereoisomers were easily separable by column chromatography. The free hydroxyl group in compound 8 was then converted into an azide via sequential trifluoromethanesulfonation with triflic anhydride in pyridine, followed by treatment with sodium azide in DMF. The resulting compound 9 was isolated in 62% yield over two steps. Acetal cleavage of 9 was carried out using p-toluenesulfonic acid in methanol, and the resulting diol intermediate 10 was obtained in 80% yield. Lastly, compound 4 was obtained in 43% yield, via a three-step protocol involving sequential tritylation with trityl chloride, benzoylation with benzoyl bromide and detritylation using BF3-Et2O. The intermediate 10 was also deprotected using PMe3 in THF to afford ent-sphingosine 11 in 71% yield.
Scheme 3.
Synthesis of ent-sphingosine acceptor 4.
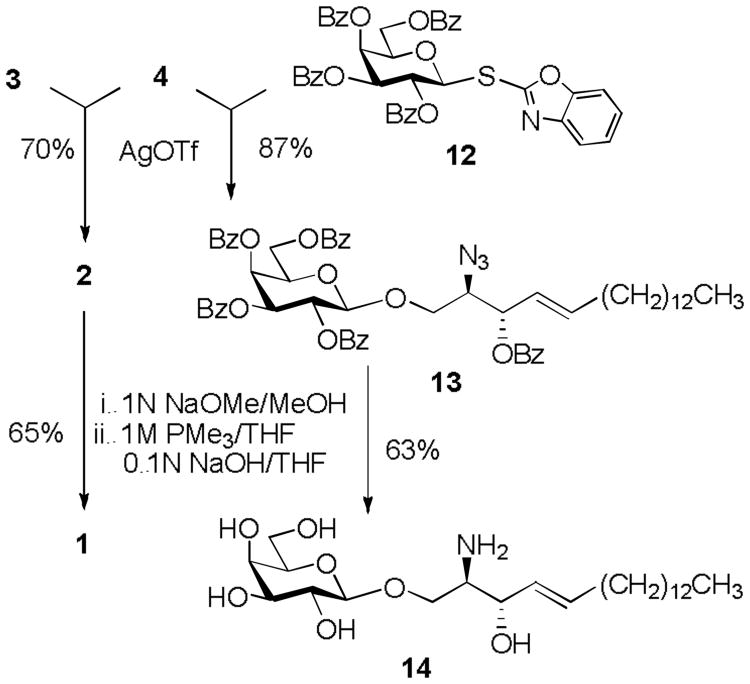
Having obtained the protected ent-sphingosine precursor 4, we investigated its capability as a glycosyl acceptor. For this purpose, it was subjected to glycosylation with SBox L- and D-galactosyl donors 3 and 12 (Scheme 4). These glycosylations, were promoted with silver(I) triflate, affording the corresponding glycosides 2 and 13 in 70% and 87% yield, respectively. Complete deprotection of psychosine precursors 2 and 13 was accomplished by deacylation in the presence of 1N NaOMe in MeOH, followed by azide reduction using PMe3 in THF. This sequence yielded the target ent-psychosine 1 in 65% yield, and its D-galactose diastereomer 14 in 63% yield. It should be noted that the use of PMe3 for the azide reduction step was found to be the most advantageous,[19] as other methods using H2S, HS(CH2)3SH, PPh3 or NaBH4 either failed or yielded the unprotected derivatives in lower yields.
Scheme 4.
Synthesis of ent-psychosine 1 and its D-galactose analogue 14.
Conclusions
In conclusion, we developed an efficient protocol for the synthesis of enantiomeric sphingosine and psychosine derivatives. The approach is based on sugar precursors, which eliminates the need for a stereocontrolled synthesis, and ensures complete enantiomeric purity of the end product. It is expected that the developed route would be suitable for the synthesis and application of other classes of natural and unnatural glycosphingolipids.[20] With the successful synthesis of ent-psychosine 1 and its D-galactose analogue 14, the next step will be the biological evaluation of these compounds, wherein the elucidation of psychosine's toxicity pathways in GLD afflicted mammalian nervous systems will be determined. This research is currently under way in our laboratories and will be reported in due course.
Experimental Section
General
Column chromatography was performed on silica gel 60 (70-230 mesh), reactions were monitored by TLC on Kieselgel 60 F254. The compounds were detected by examination under UV light and by charring with 10% sulfuric acid in methanol. Solvents were removed under reduced pressure at < 40 °C. ClCH2CH2Cl was distilled from CaH2 directly prior to application. Methanol was dried by refluxing with magnesium methoxide, distilled and stored under argon. Toluene was distilled over CaH2 under argon atmosphere and was refluxed for 2 h before application. THF was distilled over metallic sodium using benzophenone as indicator under argon atmosphere and was refluxed for 2 h before use. Anhydrous pyridine, anhydrous DMF, redistilled benzaldehyde were obtained from Sigma-Aldrich and used as is. (1-Tetradecyl) triphenylphosphonium bromide and L-galactose were purchased from TCI chemicals. Molecular sieves (3 Å), were crushed and activated in vacuo at 390 °C during 8 h in the first instance and then for 2-3 h at 390 °C directly prior to application. AgOTf was co-evaporated with toluene (3 × 10 mL) and dried in vacuo for 2-3 h directly prior to application. Optical rotations were measured at ‘Jasco P-1020’ polarimeter. Melting points were measured at Thomas Hoover capillary melting point apparatus. Unless noted otherwise, 1H-n.m.r. spectra were recorded in CDCl3 at 300 MHz (Bruker Avance), 13C-NMR spectra and two-dimensional experiments were recorded in CDCl3 at 75 MHz (Bruker Avance) or at 125 MHz (Bruker ARX-500). HRMS determinations were made with the use of JEOL MStation (JMS-700) Mass Spectrometer.
Benzoxazolyl 2,3,4,6-tetra-O-benzoyl-1-thio-β-L-galactopyranoside (3)
Benzoyl chloride (1.14 mL, 9.9 mmol) was added dropwise to a stirring mixture of L-galactose (0.255 g, 1.41 mmol) in dry pyridine (3.0 mL) under argon at rt. Upon completion (∼16 h), the reaction mixture was quenched by addition of methanol (∼5 mL), evaporated and co evaporated with toluene (3 × 25 mL). The residue was diluted with CH2Cl2 (50 mL), washed with water (20 mL), 1N HCl (20 mL), water (20 mL), NaHCO3 (2 × 20 mL) and water (2 × 20 mL). The organic layer was separated, dried, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (ethyl acetate- hexanes gradient elution) to afford 1,2,3,4,6-penta-O-benzoyl-L-galactopyranoside (6) as a white foam (0.98 g, 99% yield). HBr (30% v/v) in glacial AcOH (1.0 mL) was added to a stirred solution of pentabenzoate 6 (0.98 g, 1.4 mmol) in CH2Cl2 (5 mL) at rt. Upon completion (∼2 h), the reaction mixture was diluted with CH2Cl2 (50 mL), washed successively with ice-cold water (20 mL), sat. aq. NaHCO3 (2 × 20 mL) and cold water (3 × 20 mL). The organic layer was separated, dried, and concentrated in vacuo. The crude residue was purified by crystallization from anhydrous diethyl ether and hexanes to afford 2,3,4,6-tetra-O-benzoyl-α-L-galactopyranosyl bromide (0.83 g, 89%) as white crystals. Potassium benzoxazole-2-thiolate[12] (0.36 g, 1.9 mmol) was added to the stirring solution of the freshly prepared 2,3,4,6-tetra-O-benzoyl-α-L-galactopyranosyl bromide (0.83 g, 1.25 mmol) in dry acetone (8.0 mL) under argon at rt. Upon completion (∼3 h), the mixture was diluted with CH2Cl2 (30 mL) and washed successively with 1% aq. NaOH (2 × 15 mL), sat. aq. NaHCO3 (15 mL) and water (3 × 15 mL). The organic layer was separated, dried, and concentrated in vacuo. The residue was purified by column chromatography on silica gel (hexane–EtOAc gradient elution) to afford the title compound 3 (0.92 g, 99%). Analytical data for 3: Rf = 0.49 (3:7 ethyl acetate–hexanes); [α]D23 –130.77° (c 1, CHCl3); 1H NMR: δ, 4.46 (dd, 1H, H-6a), 4.54-4.61 (m, 2H, H-5, H-6b), 5.79 (dd, 1H, J2,3 = 8.8 Hz, J3,4 = 3.4 Hz, H-3), 5.97-6.07 (m, 3H, H-1, 2, 4), 7.20-7.51 (m, 16H, aromatic), 7.77 (dd, 2H, aromatic), 7.90 (m, 4H, aromatic), 8.08 (dd, 2H, aromatic) ppm; 13C NMR: δ, 68.4, 68.6, 72.8, 84.4, 110.2, 119.0, 124.7 (× 2), 128.6 (× 4), 128.7 (× 2), 128.8 (× 2), 128.9, 129.0, 129.4, 129.9 (× 3), 130.0 (× 2), 130.1 (× 2), 130.2 (× 2), 133.2, 133.5, 133.7, 133.8, 141.5, 152.0, 161.1, 165.4 (× 2), 165.6, 166.1 ppm; HR-FAB MS [M+H]+ calcd for C41H32NO10S 730.1747, found 730.1740.
1,3-O-Benzylidene-L-arabitol (7)
Hydrogen chloride gas (generated by the dropwise addition of H2SO4 to NaCl) was bubbled through a mixture of L-arabitol (5.0 g, 32.0 mmol) and freshly distilled benzaldehyde (4.0 mL, 39 mmol) for 1 h until L-arabitol was completely dissolved. The reaction mixture was kept for an additional 16 h; after that, the resulting solidified mass was broken up and placed in desiccator containing KOH and H2SO4 and dried for 24 h in vacuo. The resulting solid was then triturated with diethyl ether (∼10 mL), mixed with 20% aq. NaHCO3 (∼20 mL). The solid containing compound 7 was filtered off and rinsed with water (10 mL) and diethyl ether (10 mL). The resulting solid was recrystallized from isopropanol to afford the title compound 7 as white crystals in 60% yield (4.5 g). Analytical data for 7 was essentially the same as reported previously.[21] 1H NMR (CD3OD): δ, 3.56 (m, 1H), 3.69 (br s, 1H), 3.71 (br s, 1H), 3.80 (br s, 2H), 4.00-4.13 (m, 2H), 5.50 (s, 1H, PhCH), 7.21-7.26 (m, 3H, aromatic), 7.41-7.44 (m, 2H, aromatic) ppm; 13C NMR (CD3OD): δ, 64.1, 64.2, 71.1, 73.9, 80.2, 102.7, 127.6 (× 2), 129.1, 129.9, 140.1 ppm.
(2S,3S,4E)-1,3-O-Benzylidene-4-octadecen-1,2,3-triol (8)
Compound 7 (2.7 g, 11 mmol) was dissolved in methanol (80 mL) and was cooled to 0 °C. A aqueous solution of NaIO4 (2.4 g, 11.0 mol) in water (30 mL) was added dropwise and the resulting mixture was stirred for 45 min. The reaction mixture was then concentrated in vacuo and the residue co evaporated with ethanol (3 × 20 mL). The residue was extracted with warm (∼ 40 °C) ethyl acetate (3 × 150 mL), the combined organic extract was washed with water (100 mL). The organic layer was separated, dried over MgSO4, and concentrated in vacuo to yield 2,4-O-benzylidene-L-threose (5, 2.3 g, 99% yield), which was used for the subsequent step without further purification. To a stirred suspension of 1-tetradecyl triphenylphosphonium bromide (12.2 g, 22.6 mmol) in dry toluene (100 mL) a solution of 2M PhLi in dibutyl ether (27 mL) was added followed by a solution of 4M LiBr in THF (14 mL) at -10 °C under argon. The resulting orange-red solution was stirred for 30 min at rt, after that it was cooled to -35 °C, and a solution of compound 5 (3.8 g, 18.0 mmol) in dry THF (27 mL) was added. The stirring reaction mixture was allowed to gradually warm to 0 °C during the period of 3 h. After that, methanol (∼10 mL) was added, the resulting mixture was poured in water (50 mL), and the resulting emulsion was vigorously stirred for 20 min, transferred into a separatory funnel and extracted with CH2Cl2 (2 × 250 mL). The combined organic extracts were washed with water (100 mL), separated, dried, and concentrated in vacuo. The crude residue was purified by column chromatography on silica gel (ethyl acetate - CH2Cl2 gradient elution). The title compound 8 was obtained as a white solid (3.5 g) in 50% yield. Z-isomer of 8 was also isolated in 11% yield. Analytical data for 8: Rf = 0.56 (3:7 ethyl acetate–hexanes); [α]D23 +3.21° (c 1, CHCl3); 1H NMR: δ, 0.85 (t, 3H, CH3), 1.20-1.36 (m, 22H, (CH2)11CH3), 2.04 (m, 2H, CH=CHCH2), 2.71 (d, 1H, OH, JCH,OH = 10.0 Hz), 3.47 (br d, 1H, CHOH), 4.00 (dd, 1H, ½ OCH2, J = 11.8 Hz, J = 1.2 Hz), 4.18 (d, 1H, ½ OCH2, J = 11.8 Hz, J = 1.8 Hz), 4.34 (d, 1H, CHCH=CH, J = 6.0 Hz), 5.56-5.65 (m, 2H, PhCH, CHCH=CH), 5.78-5.85 (m, 1H, CHCH=CH), 7.25-7.33 (m, 3H, aromatic), 7.47-7.50 (m, 2H, aromatic) ppm; 13C NMR: δ, 14.3, 22.8, 29.1, 29.4, 29.5, 29.6, 29.7, 29.8 (× 4), 32.1, 32.6, 66.5, 72.5, 80.8, 101.5, 126.1 (× 2), 126.2 (× 2), 128.4, 129.1, 135.2, 138.1 ppm; HR-FAB MS [M+Na]+ calcd for C25H40O3Na 411.2875, found 411.2852.
(2R,3S,4E)-2-Azido-1,3-O-benzylidene-4-octadecen-1,3-diol (9)
To a stirred solution of compound 8 (1.5 g, 3.86 mmol) in CH2Cl2 (10.5 mL), pyridine (0.75 mL) was added followed by triflic anhydride (0.8 mL, 4.6 mmol) at -20 °C under argon. When TLC showed complete disappearance of the starting material (∼5 min), DMF (35 mL) was added followed by NaN3. (0.75g, 0.011 mmol). The external cooling was removed and the reaction mixture was stirred for 3 h at rt. After that, the reaction mixture was extracted with ethyl-acetate (3 × 150mL), the combined organic extracts were dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography on silica gel (ethyl acetate - hexanes gradient elution) to afford the title compound 9 as a colorless oil (0.98 g, 62%). Analytical data for 9: Rf = 0.55 (1:9 ethyl acetate–hexanes); [α]D24 +8.45° (c 1, CHCl3); 1H NMR: δ, 0.86 (t, 3H, CH3), 1.20-1.42 (m, 22H, (CH2)11CH3), 2.10 (m, 2H, CH=CHCH2), 3.45-3.50 (m, 1H, CHN3), 3.60 (dd, 1H, ½ OCH2, J = 11.8 Hz), 4.05 (m, 1H, CHCH=CH), 4.30-4.35 (m, 1H, ½ OCH2), 5.48-5.62 (m, 2H, PhCH, CHCH=CH), 5.94-6.01 (m, 1H, CHCH=CH), 7.34-7.38 (m, 3H, aromatic), 7.47-7.49 (m, 2H, aromatic) ppm; 13C NMR: δ, 14.3, 22.9, 28.9, 29.4, 29.6, 29.7, 29.8, 29.9 (× 4), 32.2, 32.7, 57.7, 69.3, 81.9, 101.3, 126.1, 126.6 (× 2), 128.5 (× 2), 129.2, 137.6, 137.9 ppm; HR-FAB MS [M+Na]+ calcd for C25H39N3O2Na 436.2940, found 436.2929
(2R,3S,4E)-2-Azido-4-octadecen-1,3-diol (10)
p-TsOH monohydrate (31 mg) was added to a stirred solution of compound 9 (0.98 g, 2.37 mmol) in a mixture of methanol (25 mL) and DCM (10 mL) at rt. Upon completion (∼24 h), solid NaHCO3 was added until neutral pH (∼7), the solid was filtered off and the filtrate was concentrated in vacuo. The crude residue was purified by column chromatography on silica gel (ethyl acetate - hexanes gradient elution) to afford the title compound 10 (0.6 g, 80% yield). Analytical data for 10: Rf = 0.41 (3:7 ethyl acetate–hexanes); [α]D24 +39.34° (c 1, CHCl3); 1H NMR: δ, 0.85 (t, 3H, CH3), 1.20-1.36 (m, 22H, (CH2)11CH3), 2.02-2.14 (m, 2H, CH=CHCH2), 2.29 (bs, 2H, 2 × OH), 3.44-3.50 (m, 1H, CHN3), 3.76 (bs, 2H, OCH2), 4.23 (m, 1H, CHCH=CH), 5.46-5.54 (m, 1H, CHCH=CH), 5.75-5.84 (m, 1H, CHCH=CH) ppm; 13C NMR: δ, 14.3, 22.9, 29.1, 29.4, 29.5, 29.7, 29.8, 29.9 (× 4), 32.1, 32.5, 62.7, 66.9, 73.8, 128.2, 136.2 ppm; HR-FAB MS [M+Na]+ calcd for C18H35N3O2Na 348.2627, found 348.2626
(2R,3S,4E)-2-Azido-3-benzoyloxy-4-octadecen-1-ol (4)
Trityl chloride (0.34 g, 1.22 mmol) was added to a stirring solution of compound 9 (0.305 g, 0.937 mmol) in pyridine (3.0 mL). The reaction mixture was stirred at rt for 16 h. Additional trityl chloride (0.3 g, 1.2 mmol), pyridine (1.0 mL), and DMAP (50 mg) were added and the reaction was stirred for 24 h. The resulting mixture was concentrated under reduced pressure, the residue was diluted with CH2Cl2 (125 mL), washed with water (50 mL), NaHCO3 (2 × 50 mL), and water (3 × 50 mL). The organic layer was separated, dried, and concentrated in vacuo. The residue was purified by column chromatography on silica gel (ethyl acetate - hexanes gradient elution) to afford (2R,3S,4E)-2-azido-1-trityloxy-4-octadecen-3-ol (0.60 g) as a white solid. The latter compound (0.54 g, 0.95 mmol) was dissolved in pyridine (4 mL), benzoyl chloride (0.22 mL, 1.9 mmol) was added dropwise, and the resulting reaction mixture was stirred under argon for 18 h at rt. After that, methanol (∼10 mL) was added, the mixture was concentrated in vacuo, and the residue was co-evaporated with toluene (2 × 25 mL). The residue was then diluted with CH2Cl2 (100 mL), washed with water (40 mL), 1N HCl (40 mL), water (40 mL), NaHCO3 (2 × 40 mL), and water (2 × 40 mL). The organic layer was separated, dried, an evaporated under reduced pressure. The residue was purified by column chromatography on silica gel (ethyl acetate - hexanes gradient elution) to afford (2R,3S,4E)-2-azido-3-benzoyloxy-1-trityloxy-4-octadecene as a colorless syrup (0.38 g, 60%). The latter compound (0.32 g, 4.76 mmol) was dissolved in methanol (4.0 mL), BF3-OEt2 (62 μL) was added dropwise, and the resulting mixture was stirred for 15 h at rt. After that, the reaction mixture was diluted with CH2Cl2 (25 mL), washed with water (10 mL), NaHCO3 (10 mL), and water (2 × 10 mL). The organic layer was separated, dried, and concentrated in vacuo. The residue was purified by column chromatography on silica gel (ethyl acetate – hexanes gradient elution) to afford the title compound 4 (0.15 g, 72 % yield) as a colorless syrup. Analytical data for 4: Rf = 0.56 (3:7 ethyl acetate–hexanes); [α]D23 +51.04° (c 1, CHCl3); 1H NMR: δ, 0.85 (t, 3H, CH3), 1.20-1.36 (m, 22H, (CH2)11CH3), 2.02-2.10 (m, 2H, CH=CHCH2), 3.58-3.64 (m, 1H, ½ OCH2), 3.71-3.80 (m, 2H, ½ OCH2, CHN3), 5.54-5.61 (m, 2H, CHCH=CH, CHCH=CH), 5.89-5.94 (m, 1H, CHCH=CH), 7.40-7.54 (m, 3H, aromatic), 8.02-8.05 (m, 2H, aromatic) ppm; 13C NMR: δ, 14.3, 22.9, 28.9, 29.3, 29.5, 29.6, 29.7, 29.8 (× 4), 32.1, 32.6, 62.2, 66.4, 74.8, 123.4, 128.7 (× 2), 129.9 (× 3), 133.5, 139.0, 165.7 ppm; HR-FAB MS [M+Na]+ calcd for C25H39N3O3Na 452.2889, found 452.2939.
(2R,3S,4E)-2-Amino-4-octadecen-1,3-diol (11)
Compound 10 (48 mg, 0.15 mmol) was dissolved in THF (7.0 mL), 0.1 M NaOH (1 mL) was added and the resulting mixture was stirred for 20 min. A 1M solution of PMe3 in THF (0.15 mL) was then added dropwise and the reaction mixture was stirred for 16 h at rt. After that, it was neutralized with 0.1 N aq. HCl (∼0.5 mL) and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (packed in 5% solution of methanol: ammonia (40:1 wt/wt) in DCM) to afford the title compound 11 (31 mg, 70% yield) as a white solid. Analytical data for 11: [α]D25 -9.23° (c 1, CH3OH); 1H NMR (CD3OD): δ, 0.83 (t, 3H, CH3), 1.15-1.36 (m, 22H, (CH2)11CH3), 1.98-2.05 (m, 2H, CH=CHCH2), 2.68-2.80 (br m, 1H, CHN), 3.41-3.63 (br m, 2H, OCH2), 3.92-3.96 (m, 1H, CHCH=CH), 5.38-5.45 (m, 1H, CHCH=CH), 5.63-5.72 (dd, 1H, CHCH=CH) ppm; 13C NMR (CD3OD): δ, 14.6, 23.9, 30.5 (× 2), 30.6, 30.7, 30.8 (× 2), 30.9 (× 3), 33.2, 33.6, 58.2, 63.8, 74.6, 130.6, 135.6 ppm; HR-FAB MS [M+Na]+ calcd for C18H38NO2 300.2897, found 300.2899.
(2R,3S,4E)-2-Azido-3-benzoyloxy-1-(2,3,4,6-tetra-O-benzoyl-β-L-galactopyranosyl)oxy-4-octadecene (2)
A mixture of the glycosyl donor 3 (0.22 g, 0.31 mmol), glycosyl acceptor 4 (0.12 g, 0.28 mmol), and freshly activated molecular sieves (3Å, 0.67 g) in ClCH2CH2Cl (2.0 mL) was stirred under argon for 1.5 h. Freshly conditioned AgOTf (0.24 g, 0.92 mmol) was added and the reaction mixture was stirred for 15 min at rt. After that, it was diluted with CH2Cl2 (15 mL), the solid was filtered-off and the residue was washed with CH2Cl2 (3 × 5 mL). The combined filtrate (35 mL) was washed with sat. aq. NaHCO3 (15 mL) and water (2 × 15 mL), the organic phase was separated, dried, and concentrated in vacuo. The residue was purified by column chromatography on silica gel (ethyl acetate - hexane gradient elution) to afford the title compound 2 as a white foam (0.2 g, 70%). Analytical data for 2: Rf = 0.55 (3:7 ethyl acetate–hexanes); [α]D27 –44.49° (c 1, CHCl3); 1H NMR: δ, 0.83 (t, 3H, CH3), 1.20-1.30 (m, 22H, (CH2)11CH3), 1.85-1.90 (m, 2H, CH=CHCH2), 3.62-3.72 (m, 1H, ½ OCH2), 3.92-4.05 (m, 2H, ½ OCH2, CHN3), 4.29-4.40 (m, 2H, H-6a, 6b), 4.45-4.65 (m, 1H, H-5), 4.85 (d, 1H, J1,2 = 7.9 Hz, H-1), 5.39-5.50 (m, 1H, CHCH=CH), 5.55-5.65 (m, 2H, H-3, CHCH=CH), 5.66-5.74 (m, 1H, CHCH=CH), 5.75-5.87 (dd, 1H, J1,2 = 7.9 Hz, J2,3 = 10.3 Hz, H-2), 5.97 (br d, 1H, J3,4 = 3.1 Hz, H-4), 7.12-8.11 (m, 25H, aromatic) ppm; 13C NMR: δ, 14.3 (× 2), 22.9 (× 2), 28.8, 29.3, 29.4 (× 2), 29.5, 29.7, 29.8 (× 4), 32.0 (× 2), 32.5, 62.0, 63.6, 68.1 (× 2), 69.8, 71.5, 71.7, 74.8, 101.4, 122.8, 128.3 (× 2), 128.4 (× 2), 128.6 (× 3), 128.8, 128.9, 129.0, 129.1, 129.3, 129.5, 129.8 (× 2), 129.9 (× 5), 130.1, 130.2, 133.2, 133.3, 133.4, 133.7, 139.1, 165.1 (× 2), 165.7 (× 2), 166.1 ppm. HR-FAB MS [M+Na]+ calcd for C59H65N3O12Na 1030.4466, found 1030.4497.
(2R,3S,4E)-2-Azido-3-benzoyloxy-1-(2,3,4,6-tetra-O-benzoyl-β-D-galactopyranosyl)oxy-4-octadecene (13)
The title compound was obtained as a white foam in 87% from building blocks 4 and 12[12] as described for the synthesis of 2. Analytical data for 13: Rf = 0.55 (3:7 ethyl acetate – hexanes); [α]D24 +80.06° (c 1, CHCl3); 1H NMR: δ, 0.84 (t, 3H, CH3), 1.15-1.35 (m, 22H, (CH2)11CH3), 1.95-2.04 (m, 2H, CH=CHCH2), 3.50-3.62 (m, 1H, ½ OCH2), 3.90-4.02 (m, 1H, CHN3), 4.05-4.18 (m, 1H, ½ OCH2), 4.32-4.40 (m, 2H, H-6a, 6b), 4.60-4.75 (m, 1H, H-5), 4.91 (d, 1H, J1,2 = 7.9 Hz, H-1), 5.42-5.48 (m, 2H, CHCH=CH, CHCH=CH), 5.58-5.62 (br dd, 1H, H-3), 5.79-5.85 (m, 2H, H-2, CHCH=CH), 5.99 (br d, 1H, H-4), 7.15-7.60-8.11 (m, 25H, aromatic) ppm; 13C NMR: δ, 14.3 (× 2), 22.9 (× 2), 28.8, 29.4, 29.5 (× 2), 29.6, 29.8, 29.9 (× 4), 32.1 (× 2), 32.5, 62.1, 64.5, 68.2, 69.4, 69.8, 71.6, 71.8, 74.8, 102.1, 123.1, 128.5 (× 2), 128.6 (× 2), 128.7 (× 3), 128.8, 128.9, 129.2, 129.4, 129.6, 129.9 (× 3), 130.0 (× 5), 130.2, 133.4 (× 2), 133.5, 133.8, 138.8, 165.3, 165.5, 165.7, 165.8, 166.2 ppm; HR-FAB MS [M+Na]+ calcd for C59H65N3O12Na 1030.4466, found 1030.4497
(2R, 3S, 4E)-2-Amino-1-(β-L-galactopyranosyl)oxy-3-hydroxy-4-octadecene (1)
To a stirred solution of 2 (173 mg, 0.171 mmol) in dry methanol (3 mL) 1M NaOMe was added (∼0.4 mL, pH = 10) and the resulting mixture was stirred for 20 h at rt. After that it was neutralized by addition of Dowex (H+), the resin was filtered off and rinsed successively with methanol (5 × 5 mL). The filtrate was concentrated in vacuo and the crude residue (93 mg) was dissolved in THF (13.0 mL), 0.1 M NaOH (1.9 mL) was added and the resulting mixture was stirred for 20 min. A 1M solution of PMe3 in THF (0.19 mL) was then added dropwise and the reaction mixture was stirred for 16 h at rt. After that, it was neutralized with 0.1 N aq. HCl and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (packed in 5% solution of methanol: ammonia (40:1 wt/wt) in CH2Cl2) to afford the title compound 1 (58 mg, 65% yield) as a white solid. Analytical data for 1: [α]D26 +1.46° (c 1, CH3OH); 1H NMR (CD3OD): δ, 0.82 (t, 3H, CH3), 1.20-1.34 (m, 22H, (CH2)11CH3), 1.97-2.02 (m, 2H, CH=CHCH2), 2.80-2.89 (m, 1H, CHN3), 3.35-3.50 (m, 3H, H-2, 4, 5), 3.62-3.81 (m, 5H, H-3, 6a, 6b, OCH2), 3.90-3.95 (m, 1H, CHCH=CH), 4.14 (d, 1H, J1,2 = 7.2 Hz, H-1), 5.38-5.48 (m, 1H, CHCH=CH), 5.65-5.76 (m, 1H, CHCH=CH) ppm; 13C NMR (CD3OD): δ, 14.6, 23.9, 30.4, 30.5, 30.6, 30.7, 30.8 (× 2), 30.9 (× 3), 33.2, 33.5, 56.7, 62.7, 70.0, 70.5, 72.6, 73.2, 74.9, 76.9, 104.9, 129.9, 136.1 ppm; HR-FAB MS [M+H]+ calcd for C24H48NO7 462.3431, found 462.3434.
(2R, 3S, 4E)-2-Amino-1-(β-D-galactopyranosyl)oxy-3-hydroxy-4-octadecene (14)
The title compound was obtained as a white solid in 63% from 13 as described for the synthesis of 1. Analytical data for 14: [α]D22 -6.20° (c 1, CH3OH); 1H NMR (CD3OD): δ, 0.82 (t, 3H, CH3), 1.20-1.34 (m, 22H, (CH2)11CH3), 1.97-2.04 (m, 2H, CH=CHCH2), 2.80-2.90 (m, 1H, CHNH2), 3.38-3.50 (m, 4H, H-2, 4, 5, ½ OCH2), 3.64-3.78 (m, 3H, H-3, 6a, 6b), 3.92-4.01 (m, 2H, CHCH=CH, ½ OCH2), 4.14 (d, 1H, J1,2 = 7.2 Hz, H-1), 5.40-5.45 (m, 1H, CHCH=CH), 5.65-5.76 (m, 1H, CHCH=CH) ppm; 13C NMR (CD3OD): δ, 14.6, 23.9, 30.4, 30.5, 30.6, 30.7, 30.8 (× 2), 30.9 (× 3), 33.2, 33.5, 56.7, 62.7, 70.0, 70.5, 72.6, 73.2, 74.9, 76.9, 104.9, 129.9, 136.1 ppm; HR-FAB MS [M+H]+ calcd for C24H48NO7 462.3431, found 462.3434.
Acknowledgments
This work was supported by awards from the National Science Foundation (CHE-0547566 to AVD), American Heart Association (0855743G to AVD), National Institutes of Health (HD055461 to MSS), and the Hunter's Hope Foundations (to MSS).
References
- 1.Igisu H, Suzuki K. Science. 1984;224:753–755. doi: 10.1126/science.6719111. [DOI] [PubMed] [Google Scholar]
- 2.Wenger D, Suzuki K, Suzuki Y. In: Galactosylceramide Lipidosis: Globoid Cell Leukodystrophy (Krabbe Disease) Scriver C, Beaudet A, Sly W, Valle D, Childs B, Kinzler K, Vogelstein B, editors. Vol. 3. McGraw-Hill Medical Publishing Division; New York: 2001. pp. 2669–3694. [Google Scholar]
- 3.Svennerholm L, Vanier MT, Mansson JE. J Lipid Res. 1980;21:53–64. [PubMed] [Google Scholar]
- 4.Taketomi T, Nishimura K. Jpn J Exp Med. 1964;34:255–265. [PubMed] [Google Scholar]
- 5.Nagara H, Ogawa H, Sato Y, Kobayashi T, Suzuki K. Brain Res. 1986;391:79–84. doi: 10.1016/0165-3806(86)90009-x. [DOI] [PubMed] [Google Scholar]
- 6.Giri S, Khan M, Nath N, Singh I, Singh AK. J Neurochem. 2008;105:1820–1833. doi: 10.1111/j.1471-4159.2008.05279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Igisu H, Nakamura M. Biochem Biophys Res Commun. 1986;137:323–327. doi: 10.1016/0006-291x(86)91213-1. [DOI] [PubMed] [Google Scholar]
- 7.Haq E, Contreras MA, Giri S, Singh I, Singh AK. Biochem Biophys Res Commun. 2006;343:229–238. doi: 10.1016/j.bbrc.2006.02.131. [DOI] [PubMed] [Google Scholar]; Haq E, Giri S, Singh I, Singh AK. J Neurochem. 2003;86:1428–1440. doi: 10.1046/j.1471-4159.2003.01941.x. [DOI] [PubMed] [Google Scholar]; Hannun YA, Bell RM. Science. 1987;235:670–674. doi: 10.1126/science.3101176. [DOI] [PubMed] [Google Scholar]
- 8.White AB, Givogri MI, Lopez-Rosas A, Cao H, van Breemen R, Thinakaran G, Bongarzone ER. J Neurosci. 2009;29:6068–6077. doi: 10.1523/JNEUROSCI.5597-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pathirathna S, Brimelow BC, Jagodic MM, Krishnan K, Jiang X, Zorumski CF, Mennerick S, Covey DF, Todorovic SM, Jevtovic-Todorovic V. Pain. 2005;114:429–443. doi: 10.1016/j.pain.2005.01.009. [DOI] [PubMed] [Google Scholar]; Li P, Bracamontes J, Katona BW, Covey DF, Steinbach JH, Akk G. Mol Pharmacol. 2007;71:1582–1590. doi: 10.1124/mol.106.033407. [DOI] [PubMed] [Google Scholar]; Covey DF. Steroids. 2009;74:577–585. doi: 10.1016/j.steroids.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vankar YD, Schmidt RR. Chem Soc Rev. 2000;29:201–216. [Google Scholar]; Ishida H. In: Glycolipid synthesis. Ernst B, Hart GW, Sinay P, editors. Vol. 1. Wiley-VCH; Weinheim, New York: 2000. pp. 305–318. [Google Scholar]
- 11.Selected references: Garigipati RS, Freyer AJ, Whittle RR, Weinreb SM. J Am Chem Soc. 1984;106:7861–7867.Hino T, Nakakyama K, Taniguchi M, Nakagawa M. J Chem Soc, Perkin Trans. 1986;1:1687–1690.Hudlicky T, Nugent T, Griffith W. J Org Chem. 1994;59:7944–7946.Nugent TC, Hudlicky T. J Org Chem. 1998;63:510–520. doi: 10.1021/jo971335a.Lee JM, Lim HS, Chung SK. Tetrahedron: Asymmetry. 2002;13:343–347.Lee HK, Kim EK, Pak CS. Tetrahedron Lett. 2002;43:9641–9644.Raghavan S, Rajender A. J Org Chem. 2003;68:7094–7097. doi: 10.1021/jo034157w.Kobayashi J, Nakamura M, Mori Y, Yamashita Y, Kobayashi S. J Am Chem Soc. 2004;126:9192–9193. doi: 10.1021/ja047597t.Shimizu M, Ando H, Niwa Y. Lett Org Chem. 2005;2:512–514.Merino P, Jimenez P, Tejero T. J Org Chem. 2006;71:4685–4688. doi: 10.1021/jo060465t.Morales-Serna JA, Diaz Y, Matheu MI, Castillon S. Synthesis. 2009:710–712.
- 12.Kamat MN, Rath NP, Demchenko AV. J Org Chem. 2007;72:6938–6946. doi: 10.1021/jo0711844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demchenko AV, Kamat MN, De Meo C. Synlett. 2003:1287–1290. [Google Scholar]
- 14.Selected references: Fujita S, Sugimoto M, Tomita K, Nakahara Y, Ogawa T. Agric Biol Chem. 1991;55:2561–2569.Yadav JS, Vidyanand D, Rajagopal D. Tetrahedron Lett. 1993;34:1191–1194.Compostella F, Franchini L, De Libero G, Palmisano G, Ronchetti F, Panza L. Tetrahedron. 2002;58:8703–8708.Van den Berg RJBHN, Korevaar CGN, Van der Marel GA, Overkleeft HS, van Boom JH. Tetrahedron Lett. 2002;43:8409–8412.Lu X, Bittman R. Tetrahedron Lett. 2005;46:1873–1875.
- 15.Zimmermann P, Schmidt RR. Liebigs Ann Chem. 1988;4:663–667. [Google Scholar]
- 16.Haskins WT, Hann RM, Hudson CS. J Am Chem Soc. 1943;65:1663–1667. [Google Scholar]
- 17.Schmidt RR, Zimmermann P. Tetrahedron Lett. 1986;27:481–484. [Google Scholar]; Wild R, Schmidt RR. Tetrahedron: Asymmetry. 1994;5:2195–2208. [Google Scholar]
- 18.Wang Q, Deredas D, Huynh C, Schlosser M. Chem Eur J. 2003;9:570–574. doi: 10.1002/chem.200390061. [DOI] [PubMed] [Google Scholar]; Reitz AB, Nortey SO, Jordan AD, Mutter MSM., BE J Org Chem. 1986;51:3302–3308. [Google Scholar]
- 19.Noti C, Paz JL, Polito L, Seeberger PH. Chem Eur J. 2006;12:8664–8686. doi: 10.1002/chem.200601103. [DOI] [PubMed] [Google Scholar]
- 20.Hakomori S. Biochim Biophys Acta. 2008;1780:325–346. doi: 10.1016/j.bbagen.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wennekes T, van den Berg RJBHN, Boot RG, Van der Marel GA, Overkleeft HS, Aerts JMFG. Angew Chem Int Ed. 2009;48:8848–8869. doi: 10.1002/anie.200902620. [DOI] [PubMed] [Google Scholar]
- 21.Drew KN, Church TJ, Basu B, Vuorinen T, Seriani AS. Carbohydr Res. 1996;284:135–143. [Google Scholar]